Abstract
Background:
Current management of ulcerative colitis (UC) is aimed to treat active disease and to maintain remission. For patients in whom conventional treatment is no longer effective, biological or small molecule therapy may be an option. The aim was to assess the cost-effectiveness of induction and maintenance treatment up to 1 year of UC with infliximab (IFX), adalimumab (ADA), golimumab, vedolizumab (VDZ) and tofacitinib (TFB) compared with standard of care (SoC) in Poland.
Methods:
A hybrid decision tree/Markov model was used to estimate the expected costs and effects of four biologics, TFB and placebo in patients with the diagnosis of moderate to severe UC who had an inadequate response, lost response, or were intolerant to a conventional therapy. Prior exposure to anti-TNF was considered. At the beginning of the maintenance phase, the decision to continue biological therapy was determined by the achievement of response at the end of induction. Efficacy data were obtained from a network meta-analysis using placebo as the common comparator. Costs were presented in 2018 Polish zloty (PLN) and outcomes included quality-adjusted life-years (QALYs). The analysis was performed from the Polish public payer’s perspective and lifetime horizon was set.
Results:
In anti-TNF naïve, IFX and VDZ were characterized by the most favourable incremental cost-effectiveness ratios (ICURs) compared with SoC, PLN211,250.78 and PLN361,694.61/QALY (€49,589.38 and €84,904.84/QALY), respectively. In anti-TNF-exposed population the most effective treatment was TFB. Both ADA and VDZ were more effective than SoC; however, ICUR values were much above the cost-effectiveness threshold. The incorporation of biosimilars reversed the ranking of treatments in relation to the growing ICUR.
Conclusion:
Although ICUR values for all biological therapies exceeded the acceptability threshold in Poland, for anti-TNF-naïve UC patients IFX and for anti-TNF-exposed UC patients VDZ are currently the most cost-effective alternatives.
Keywords: biological therapy, biosimilars, cost effectiveness, tofacitinib, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic, idiopathic, remitting and relapsing inflammatory bowel disease (IBD), usually affecting adults of 30–40 years old and causing disability.1 The incidence and prevalence of UC has been increasing over time.2 The disease involves the rectum, and may extend proximally in a contiguous pattern to affect the left part of the colon or the entire colon. Patients have abdominal cramps, urgency or tenesmus, diarrhoea, rectal bleeding, fatigue and weight loss, depending on the extent and severity of the disease.1 Although progress has been made in the overall disease management, no medical cure has been discovered.3 Treatment of UC is aimed at resolving symptoms quickly (induction phase). After achieving this, the long-term goal is to prevent further flares (maintenance phase). Lifelong medical treatment is required.1 Currently, aminosalicylates are the first-line therapy for induction and maintenance of remission in active, mild to moderate disease. In the absence of response, as in moderate to severe UC, corticosteroids are started, and when disease relapses on tapering steroids, it becomes an indication for immunosuppresants.4
Until recently, surgery was the only remaining choice for patients with chronic active disease who failed standard treatment or when it was not tolerated. Over the last decade, biological therapies have significantly improved the care of UC patients. Infliximab (IFX) was the first biologic approved for moderate to severe disease, then, two more tumour necrosis factor (TNF) antagonists, adalimumab (ADA) and golimumab (GOL), and anti-integrin agent vedolizumab (VDZ) received regulatory approval.5 They have shown good efficacy and safety profiles in various clinical trials.6–14 Recently, tofacitinib (TFB), an oral small molecule Janus kinase inhibitor, proved to be more effective than placebo in the induction and maintenance of remission in adults with moderate to severe UC.15 This resulted in its regulatory approval first by the US Food and Drug Administration and then by the European Medicines Agency.
Although biologics and small molecules are able to modify the natural history of the disease, one-third of patients do not attain a primary response and, among those who respond, up to a half lose their response over time.16,17 With the anticipated availability of several different biologics and small molecules for management of moderate to severe UC with variable efficacy and safety profiles, acquiring comparative evidence to position them in treatment course, as first-line (in anti-TNF-naïve patients) and second-line (in patients with prior anti-TNF exposure), is important to inform patient management decisions.18 On the other hand, the use of biologics constitutes a heavy burden for the public payer, so their use may be limited in many countries. In Poland, patients with moderate to severe UC who do not respond to conventional therapy have the option of receiving induction and maintenance treatment for up to 1 year with IFX or VDZ, the latter also in the case of non-response to IFX. Therefore, we aimed to conduct a comparative assessment of the cost-effectiveness of biologics and TFB used as induction and maintenance therapy in patients with moderate to severe active UC in Poland.
Methods
Model structure
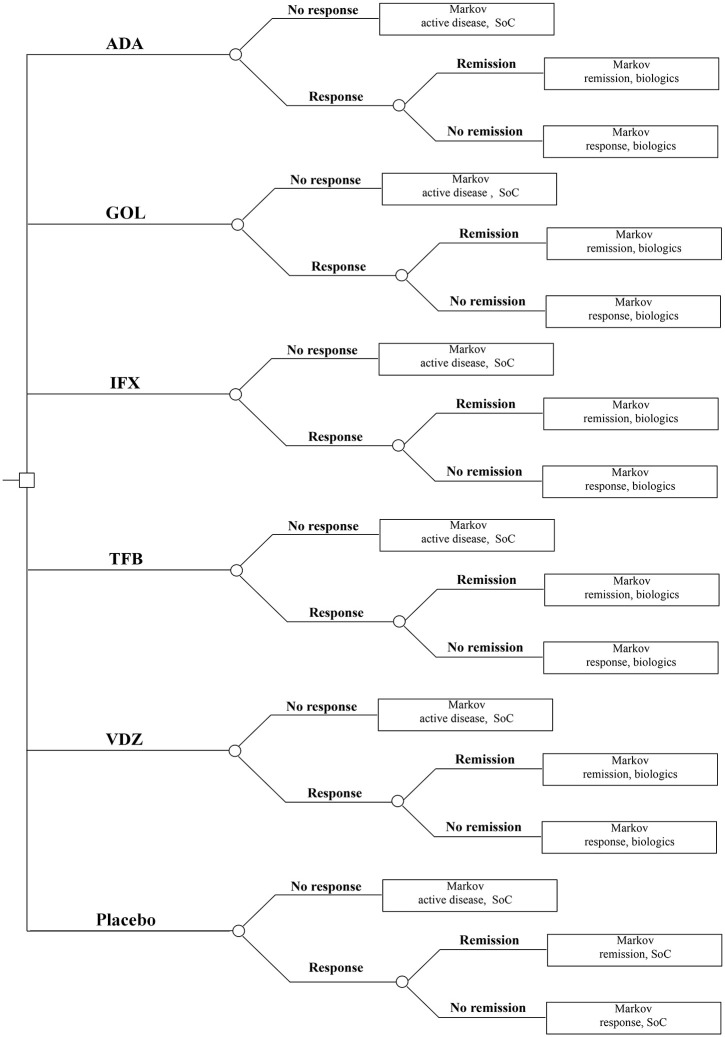
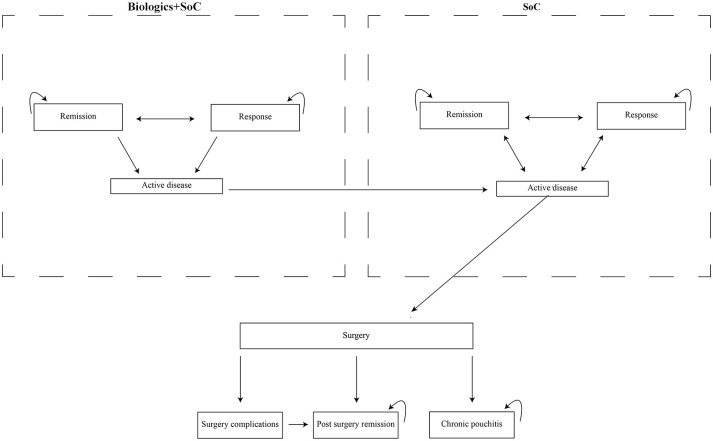
A hybrid decision tree/Markov model was developed (Figures 1 and 2).
Figure 1.
Decision tree structure for ulcerative colitis induction phase.
ADA, adalimumab; GOL, golimumab; IFX, infliximab; SoC, standard of care; TFB, tofacitinib; VDZ, vedolizumab
Figure 2.
Markov model structure for ulcerative colitis maintenance phase.
SoC, standard of care
The induction phase was modelled by the decision tree, and the maintenance phase was modelled with the Markov structure. The model was simulated (cohort simulation) in Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA). In both phases, the cycle length was 8 weeks. The model included the following health states: on biological treatment – active disease, response and remission; on standard of care – active disease, response and remission, surgery, post-surgery remission, surgery complications and chronic pouchitis. The Mayo score was used to assess disease activity. This is a composite index calculated as the sum of four items: stool frequency, rectal bleeding, endoscopic results and general physician assessment. Values can range from 0 to 12 points, with higher scores indicating more severe disease activity. Patients entered the model with moderate to severe disease, defined as a Mayo score of 6–12 points, which is consistent with the randomized controlled trials (RCTs) included in the network meta-analysis by Singh et al.6–15 Remission is defined as the result of the Mayo score ⩽2 points without individual subscore >1 point. The response is defined as a reduction in the total Mayo score of at least three points and at least 30%, accompanied by a decrease in rectal bleeding by at least one point or an absolute subscore for rectal bleeding of 0 or 1. Because remission is a subset of the broader category of response, we have excluded those who achieved remission from responders. After the first cycle, the response to induction treatment was evaluated and biological treatment was continued only in responders. Patients who did not respond during the induction phase, who lost their response during the maintenance phase or who discontinued due to adverse events changed to standard of care (5-aminosalicylates, glucocorticosteroids, immunosuppressants). Patients starting induction with standard of care proceeded similarly to patients treated biologically. However, it was assumed that patients who did not respond continued to receive the standard of care. Patients with active disease receiving standard of care might have undergone colectomy during any cycle; patients receiving biological therapy must have undergone at least one standard of care cycle before transiting to surgery.
Patients
The population consisted of adult patients with moderate to severe active UC (Mayo score ⩾ 6):
With inadequate response or loss of response to standard treatment [including glucocorticoids (GCSs), azathioprine (AZA) or 6-mercaptopurine (6-MP)];
Intolerant or having contraindications to treatment with GCS, AZA or 6-MP.
We included anti-TNF-naïve patients, as well as those with previous exposure to anti-TNF treatment or an anti-TNF treatment failure (no response, loss of response, adverse events (AEs) occurrence leading to treatment discontinuation).
The patient population had an average age of 40.4 years, 61% were males, and an average weight of 76.3 kg. This was estimated based upon the pooled patient populations of RCTs included in the network meta-analysis by Singh et al.6–15
Intervention
For biological therapies and TFB we considered only data for dosage and administration as approved in the respective Summary of Product Characteristics (Table 1).
Table 1.
Dosage and administration of comparator treatments in moderate to severe ulcerative colitis.
| Induction phase | Maintenance phase | |
|---|---|---|
| IFX | 5 mg/kg IV at weeks 0, 2, 6 | 5 mg/kg IV q8w |
| ADA | 160 mg SC at week 0, 80 mg at week 2, 40 mg at weeks 4, 6 | 40 mg SC eow |
| GOL | 200 mg SC at week 0, 100 mg at week 2 and 100 mg (⩾80 kg)/50 mg (<80 kg) at week 6 | 100/50 mg SC q4w |
| VDZ | 300 mg IV at weeks 0, 2, 6 | 300 mg IV q8w |
| TFB | 10 mg PO bid for 8 weeks | 5 mg PO bid |
ADA, adalimumab; bid, twice daily; eow, every other week; GOL, golimumab; IFX, infliximab; IV, intravenously; PO, per os; q4w, every 4 weeks; q8w, every 8 weeks; SC, subcutaneously; TFB, tofacitinib; VDZ, vedolizumab.
Maintenance treatment with biologicals was restricted to 1 year.
Clinical efficacy
Treatment efficacy was presented as the probability of response and/or remission during the induction phase (8 weeks) and the likelihood of remaining in remission or response at the end of the maintenance phase (at 1 year). To estimate relative remission rates due to lack of data from head-to-head clinical trials comparing treatments one with each other, we decided to use the results of the network meta-analysis by Singh et al. in which each drug’s key RCTs were included.18 To estimate relative response rates, we adopted Bucher’s method of adjusted indirect comparisons, extracting data on clinical response rates after 6–8 weeks and after 30–54 weeks from the aforementioned clinical trials. We used the ITC software (Indirect Treatment Comparison program, Canadian Agency for Drugs and Technologies in Health, Ottawa, Ontario, Canada). The estimates for response and remission rates for each treatment are presented in Table 2.
Table 2.
Response and remission rates at the end of induction and maintenance phases for each treatment.
| Induction phase (6–8 weeks) |
Maintenance phase (30–54 weeks) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naïve |
Exposed |
Response rate (%) | OR versus SoC | Remission rate (%) | OR versus SoC | |||||||
| Response rate (%) | OR versus SoC | Remission rate (%) | OR versus placebo | Response rate (%) | OR versus SoC | Remission rate (%) | OR versus SoC | |||||
| SoC | 33.6 | 9.8 | 24.6 | 3.3 | 23.5 (27.5)* | 11.8 (12.8)* | ||||||
| IFX | 68.1 | 4.22 | 30.8 | 4.1 | 45.2 | 2.68 | 28.2 | 2.93 | ||||
| ADA | 40 | 1.32 | 16.1 | 1.77 | 32 | 1.44 | 4.4 | 1.36 | 36.8 | 1.9 | 25.1 | 2.51 |
| GOL | 51.9 | 2.13 | 23 | 2.75 | 48 | 2.44 | 41.5 | 4.84 | ||||
| VDZ | 61.6 | 3.17 | 31.6 | 4.26 | 38.9 | 1.95 | 10.1 | 3.3 | 61.3 | 4.17 | 38.7 | 4.3 |
| TFB | 56.1 | 2.53 | 18.9 | 2.15 | 55.2 | 3.78 | 28.8 | 11.88 | 61.4 | 4.2 | 38 | 4.18 |
Remission rates at the end of induction and maintenance phase were obtained from the network meta-analysis by Singh et al.18 This meta-analysis included 14 randomized placebo-controlled trials with biologic or small molecule therapy in moderate to severe ulcerative colitis patients.6–15 Response rates were calculated independently based on the data extracted from these trials and with the use of indirect treatment comparisons method. Appropriate maintenance remission and response rates refer to patients who responded in the induction phase. They were estimated separately in groups with infliximab and adalimumab as well as with golimumab, vedolizumab and tofacitinib due to the differences in qualifying patients to the maintenance therapy, thus the two different values of these ratios for standard of care.
The values for golimumab, vedolizumab and tofacitinib are shown in parentheses.
ADA, adalimumab; GOL, golimumab; IFX, infliximab; OR: odds ratio; SoC, standard of care; TFB, tofacitinib; VDZ, vedolizumab.
We assumed that the transition probabilities after discontinuation of biological treatment (due to lack of response or AEs) and beyond the first year of treatment were the same as those estimated for the first year on treatment with only standard of care.
To derive the probability of colectomy, we used data from the study of Solberg et al.19 In this study, a group of 843 patients with IBD was followed up to 10 years, assessing the cumulative rate of colectomy at 9.8% (95% confidence interval 7.4–12.4). The likelihood of surgical complications was calculated based on the study by Arai et al. as well as the likelihood of chronic pouchitis.20 With the exception of chronic pouchitis, it was assumed that all surgical complications are transient and resolve within 8 weeks so that patients achieve post-surgical remission.
Safety
Biologically treated patients may discontinue treatment due to AEs. Data on discontinuation during the maintenance phase were obtained from the network meta-analysis by Singh et al.18 The incidence of AEs and serious AEs, including serious infections, was similar in patients treated biologically and on placebo and therefore not included in the model.
Mortality
There is no evidence that the lives of patients with ulcerative colitis are shorter, thus the probability of death was calculated on the basis of the life expectancy table for the general Polish population (www.stat.gov.pl).
Utility
We undertook a systematic review of studies presenting valuations of states relating to different levels of UC control and post surgery. We chose the values reported by Woehl et al.21 In this study, the EQ-5D utility scores were obtained from 180 patients with active UC. UC disease severity groups were classified according to Simple Colitis Activity Index (SCAI-2), that is, mean utilities were reported for remission, mild disease and moderate to severe disease. Patients with post-operative remission reported utilities that were lower than in post-treatment remission. The utility of chronic pouchitis was derived from the study by Arseneau et al.,22 in which, instead of the EQ-5D questionnaire, a scenario-based time-trade off method was used.
Costs
All the costs were presented in 2018 Polish zloty (PLN). The direct medical costs considered in the model included biological treatment acquisition and administration costs, standard care costs and direct health care costs associated with each health state. The actual unit prices of biological therapies were established upon the decree of the Minister of Health. Since TFB has only recently been registered in UC in Europe and is not currently reimbursed in Poland in any indication, we calculated respective willingness-to-pay estimates. As IFX and VDZ are administered intravenously, it was assumed (according to the Polish practice) that 1-day hospitalization is required, the cost of which is consistent with their administration cost. For ADA, GOL and TFB, the administration cost equals 0. The dosage of medicines used in standard of care was based on expert opinion. It was assumed that in the active phase, 80% of patients take mesalazine, 20% sulphasalazine, 40% AZA, 10% 6-MP and 100% prednisone, whereas while in remission/response patients do not take GCS. For patients undergoing biological therapy, it was assumed that the cost of standard of care is half that of standard of care alone. This mostly reflects the methodology of primary RCTs and similar assumptions have been made previously.23 The health state costs relating to the use of elective and emergency endoscopy, hospitalizations and consultant visits were calculated based on the resource use estimation by Tsai et al.24 The unit costs included in the decree of the President of the National Health Fund were applied. It was assumed that all patients undergoing surgical procedure will have restorative proctocolectomy, which will be performed in two stages in 70% of patients and in three stages in 30% of patients. Patients who develop surgical complications will require admission on a gastroenterology or general surgery ward. Clinical parameters, utility values and costs used in the model are presented in Table 3.
Table 3.
Clinical inputs of the model.
| Discontinuation due to adverse events |
Reference | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | SoC | IFX | ADA | GOL | VDZ | TFB | |
| Rate of AEs leading to discontinuation (%) | 7.6 | 5.1 | 9.8 | 3.1 | n/a | 4.8 | Singh et al.18 |
| Surgery probabilities | |||||||
| Probability of surgery (%) | 9.8/10 years | Solberg et al.19 | |||||
| Probability of surgery-related complications (%) | 47.3 | Arai et al.20 | |||||
| Probability of chronic pouchitis (%) | 5 | Arai et al.20 | |||||
| Health state utilities | |||||||
| Parameter | Active disease | Response | Remission | Post-surgery remission | Chronic pouchitis | ||
| Utility weight | 0.41 | 0.76 | 0.87 | 0.71 | 0.4 | Woehl et al.21 | |
| Intervention treatment costs | |||||||
| Parameter | IFX | ADA | GOL | VDZ | TFB | ||
| Acquisition cost per cycle – induction phase (PLN) | 12,180.43 | 13,299.2 | 23,331.2 | 22,113 | n/a | Decree of the Minister of Health | |
| Acquisition cost per cycle – maintenance phase (PLN) | 4060.14 | 6649.6 | 8622.4 | 7371 | n/a | ||
| Administration cost (PLN) | 486.72 | 0 | 0 | 486.72 | 0 | Decree of the President of NHF | |
| Standard of care costs | |||||||
| Acquisition cost per cycle – active disease (PLN) | 423.07 | Decree of the Minister of Health, expert opinion | |||||
| Acquisition cost per cycle – remission/response (PLN) | 211 | ||||||
| Surgery costs | |||||||
| Cost of surgery (PLN) | 19,030.2 | Decree of the President of NHF, expert opinion | |||||
| Cost of surgery-related complications (PLN) | 6490 | ||||||
| Health state resource use (per cycle) and costs | |||||||
| Parameter | Unit cost (PLN) | Active disease | Response | Remission | Post-surgery remission | Chronic pouchitis | |
| Hospitalization | 4358.46 | 0.05 | 0.05 | 0.05 | 0 | 0.5 | Decree of the President of NHF, Tsai et al.24 |
| Consultant visit | 159 | 1 | 0.69 | 0.31 | 0.23 | 0.27 | |
| Emergency endoscopy | 359 | 0.13 | 0.08 | 0.03 | 0.18 | 0.1 | |
| Elective endoscopy | 359 | 0.12 | 0.04 | 0 | 0.08 | 0.02 | |
| Indirect costs | |||||||
| Active disease (PLN/year) | 20,802 | Stawowczyk et al.25 | |||||
| Remission (PLN/year) | 6895 | ||||||
Unit costs for all resource use were obtained from the decree of the President of National Health Fund, while data on resource use expressed per 8-week cycle come from Tsai et al.24 PLN: €1 = PLN4.26, based on the average exchange course from the year 2018.
ADA, adalimumab; AE, adverse event; GOL, golimumab; IFX, infliximab; n/a, not available; NHF, National Health Fund; PLN, Polish zloty; SoC, standard of care; TFB, tofacitinib; VDZ, vedolizumab.
Economic analysis
The main outcome of this study was the incremental cost utility ratio (ICUR) for each of the four biologics compared with standard of care. It was calculated as the difference in total costs for each treatment and standard of care, then divided by the difference in effectiveness expressed in quality adjusted life years (QALYs). Additionally, in groups, ICUR was estimated, finding the difference in costs and effectiveness between individual drugs. The analysis was performed from the Polish public payer’s perspective (National Health Fund), lifetime horizon was set, outcomes and costs were discounted at an annual rate of 3.5% and 5%, respectively. Furthermore, an analysis from the societal perspective was carried out and indirect costs generated by patients in remission and with active disease were included. The data were taken from a Polish survey on 202 UC patients assessing absenteeism, presenteeism and costs of leaving earlier the labour market.25
Sensitivity analysis
To test the robustness of the model assumptions and parameters, we examined the effects of changing parameters in one-way sensitivity analyses:
The 95% confidence intervals’ upper/lower values for probabilities of response and remission for each treatment at the end of induction and maintenance phases;
Probabilities of response and remission for each treatment at the end of induction and maintenance phases assessed based upon pairwise head-to-head comparisons of IFX, ADA, GOL, VDZ and TFB versus standard of care using meta-analysis of direct evidence from RCTs;
The dosage of GOL was assumed to be 200 mg at week 0, 100 mg at week 2 and 50 mg every 4 weeks beginning at week 6;
No acquisition cost in the cases of IFX and VDZ was assumed;
Biosimilars of IFX and ADA were taken into account;
Health state utility values based on the study by Punekar et al.26/Swinburn et al.;27
UC health state costs doubled/halved;
Cost of surgery doubled/halved;
Time horizon = 10/30 years.
Results
Base-case analysis
First-line pharmacotherapy for moderate to severe UC
The results of the base-case analysis in the anti-TNF-naïve population are presented in Table 4.
Table 4.
Base-case results (first-line pharmacotherapy).
| Cost (PLN) | Incremental cost (versus SoC) | QALY | Incremental QALY (versus SoC) | ICUR versus SoC (payer’s perspective) (PLN/QALY) | ICUR versus SoC (societal perspective) (PLN/QALY) | ICUR in between treatments (payer’s perspective) (PLN/QALY) | |
|---|---|---|---|---|---|---|---|
| SoC | 113,149.36 | 7.823 | |||||
| ADA | 139,425.85 | 26,274.53 | 7.859 | 0.037 | 705,725.33 | 685,766.64 | |
| IFX | 141,300.94 | 28,150.05 | 7.955 | 0.133 | 211,250.78 | 183,421.06 | 19,548.78* |
| GOL | 158,127.01 | 43,828.48 | 7.908 | 0.087 | 524,973.37 | 476,715.64 | |
| VDZ | 159,940.45 | 46,785.59 | 7.952 | 0.130 | 361,694.61 | 332,069.00 | 68,394.43** |
| TFB | 7.922 | 0.099 |
PLN: €1 = PLN4.26, based on the average exchange course from the year 2018.
IFX versus ADA.
VDZ versus GOL.
ADA, adalimumab; GOL, golimumab; ICUR, incremental cost-effectiveness ratio; IFX, infliximab; PLN, Polish zloty; QALY, quality adjusted life year; SoC, standard of care; TFB, tofacitinib; VDZ, vedolizumab.
In anti-TNF-naïve populations IFX treatment instead of standard of care resulted in the largest number of additional years of life in full health, namely 0.133. Considering the cost of therapy, for each drug ICUR, when compared with standard of care, exceeded the cost-effectiveness threshold adopted in Poland, but it was again the most favourable in the case of IFX.
Second-line pharmacotherapy for moderate to severe UC
The results of the base-case analysis in the anti-TNF-exposed population are presented in Table 5.
Table 5.
Base-case results (second-line pharmacotherapy).
| Cost (PLN) | Incremental cost | QALY | Incremental QALY | ICUR (payer’s perspective) (PLN/QALY) | ICUR (societal perspective) (PLN/QALY) | |
|---|---|---|---|---|---|---|
| SoC | 113,503.27 | 7.772 | ||||
| ADA | 137,103.43 | 23,600.16 | 7.806 | 0.034 | 689,441.39 | 666,041.80 |
| VDZ | 151,757.95 | 38,254.68 | 7.838 | 0.066 | 581,005.48 | 551,180.65 |
| TFB | 7.918 | 0.146 |
PLN: €1 = PLN4.26, based on the average exchange course from the year 2018.
ADA, adalimumab; ICUR, incremental cost-effectiveness ratio; PLN, Polish zloty; QALY, quality adjusted life year; SoC, standard of care; TFB, tofacitinib; VDZ, vedolizumab.
In anti-TNF-exposed populations, TFB treatment instead of standard of care resulted in the largest number of additional years of life in full health, namely 0.146. Willingness-to-pay for TFB in this population has been estimated at PLN3467.75/QALY, which means that for this price, the choice of TFB compared with standard of care will translate into ICUR not exceeding the cost-effectiveness threshold adopted in Poland. Both ADA and VDZ were more effective than standard of care; however, ICUR values were much above the cost-effectiveness threshold.
Sensitivity analysis results
Results of various one-way sensitivity analyses are presented in Table 6.
Table 6.
Sensitivity analysis results.
| Naïve (ICUR versus SoC) (PLN/QALY) |
Exposed (ICUR versus SoC) (PLN/QALY) |
|||||
|---|---|---|---|---|---|---|
| IFX | ADA | GOL | VDZ | ADA | VDZ | |
| Pairwise | 211,250.78 | 705,725.34 | 524,973.37 | 361,694.61 | 689,441.39 | 581,005.48 |
| Upper values | 166,050.07 | 351,310.16 | 338,628.21 | 240,041.91 | 299,714.36 | 263,715.49 |
| Lower values | 284,336.92 | 4,369,167.69 | 924,075.11 | 761,332.31 | Dominated | 4,258,790.97 |
| GOL (lower maintenance dose) | 211,459.12 | 706,642.98 | 437,194.22 | 360,013.17 | 689,441.39 | 581,005.48 |
| No acquisition cost | 188,049.34 | 706,642.98 | 505,407.39 | 337,250.47 | 689,441.39 | 544,467.79 |
| Biosimilars of IFX and ADA* | 147,722.87 | 197,733.31 | 505,407.39 | 360,013.17 | 192,446.93 | 581,005.48 |
| Utilities Punekar et al.26 | 205,088.46 | 683,437.56 | 489,931.11 | 348,791.08 | 673,373.24 | 565,315.93 |
| Utilities Swinburn et al.27 | 255,236.52 | 735,591.97 | 608,113.49 | 432,470.60 | 730,828.45 | 708,422.59 |
| HS costs ×2 | 209,362.52 | 704,398.26 | 503,094.43 | 357,768.73 | 688,209.67 | 579,074.28 |
| HS costs ×0.5 | 212,507.42 | 707,765.34 | 506,563.87 | 361,135.39 | 690,057.25 | 581,971.08 |
| Surgery cost ×2 | 211,068.39 | 706,372.71 | 505,013.52 | 359,625.95 | 689,114.42 | 580,587.75 |
| Surgery cost ×0.5 | 211,654.49 | 706,778.12 | 505,604.32 | 360,206.78 | 689,604.88 | 581,214.35 |
| Time horizon = 10 years | 199,914.99 | 768,940.80 | 513,195.51 | 357,255.57 | 752,231.44 | 586,570.34 |
| Time horizon = 30 years | 209,318.08 | 716,768.09 | 506,038.85 | 359,173.21 | 699,613.61 | 581,179.40 |
PLN: €1 = PLN4.26, based on the average exchange course from the year 2018.
Acquisition costs of biosimilars of IFX and ADA are presented in 2019 Polish zloty.
ADA, adalimumab; GOL, golimumab; HS, health state; ICUR, incremental cost-effectiveness ratio; IFX, infliximab; PLN, Polish zloty; QALY, quality adjusted life year; SoC, standard of care; VDZ, vedolizumab.
Results were the most sensitive to changes in remission and response rates at the end of the induction and maintenance phases. If other assumptions changed, ICUR values for each drug changed minimally. In any case, the ranking of the therapies according to the criterion of the most favourable ICUR remained unchanged: in anti-TNF-naïve populations: IFX > VDZ > GOL > ADA and in anti-TNF-exposed populations: VDZ > ADA. The exception was the use of biosimilars of IFX and ADA; here it was as follows: in anti-TNF-naïve populations: IFX > ADA > VDZ > GOL, and in anti-TNF-exposed populations: ADA > VDZ.
Discussion
In this study, we assessed the relative cost-effectiveness of induction and maintenance treatment up to 1 year with anti-TNF agents (IFX, ADA and GOL), anti-integrin agent (VDZ) and Janus kinase inhibitor TFB in anti-TNF-naive and -exposed patients with moderate to severe active UC in Poland. In anti-TNF-naïve populations, IFX and VDZ were the most effective treatments. Taking into account costs and a lifetime horizon IFX had the most favourable incremental cost–utility ratio. Treatment with IFX was cost-effective compared with ADA, likewise treatment with VDZ when compared with GOL (in both cases ICUR was much below the cost-effectiveness threshold in Poland in 2018 – at the level of PLN134,514/QALY).
The entry of biosimilars of IFX and ADA onto the market has created hope for a significant reduction in the cost of biological therapy and an increase of its availability. Indeed, pharmacoeconomic analysis not only confirms their cost-effectiveness in relation to the standard of care, but also changes the ranking of treatments in relation to the growing ICUR. However, efficacy and immunogenicity remain a concern, particularly in patients switching from the reference biologic to the biosimilar.28
In anti-TNF-exposed populations the most effective treatment was TFB. Due to the fact that this drug is currently not reimbursed in Poland in any indication, it was impossible to estimate its cost and calculate ICUR. While the use of TFB seems promising because of its high effectiveness as second-line therapy and the possibility of oral administration, the side effects, and especially the risk of pulmonary embolism, associated with the dose of 10 mg twice daily, can be severely restrictive.29 In this population, both ADA and VDZ were more effective than placebo, but in both cases incremental cost-effectiveness ratios significantly exceeded the acceptability threshold.
The lack of head-to-head RCTs makes comparisons among the biologic therapies difficult. Therefore, we assumed that information derived from indirect treatment comparisons using the placebo arm from each trial as a ‘bridge’ may be useful to facilitate decision-making. We identified two recent systematic reviews and network meta-analyses comparing efficacy and safety of IFX, ADA, GOL, VDZ and TFB in moderate-to-severe UC.18,30 We decided to derive clinical inputs from the study of Singh et al.18 as it was the only one assessing the comparative efficacy of different agents in patients with prior anti-TNF exposure. This study combines the evidence from 14 trials including 4212 biologic-naïve and -exposed patients.6–15 Because maintenance trials of biologics had different designs (treat straight-through with IFX and ADA versus re-randomizing responders to induction therapy with GOL, VDZ and TFB), separate analyses were performed taking into account different study patterns. Therefore, we also decided to carry out a comparative economic assessment in a group with IFX and ADA, as well as in a group with GOL, VDZ and TFB, taking different placebo rates in both groups. In fact, the results of the VARSITY study, the only RCT directly comparing two biological agents in IBD, in this case ADA and VDZ in patients with moderately to severely active UC, are already known. Patients were randomized 1:1 to either: (1) active VDZ intravenous (IV) infusions (300 mg)/placebo subcutaneous (SC) injections; or (2) placebo IV infusions/active ADA SC injections (160/80/40 mg). VDZ was shown to be superior to ADA in achieving clinical remission (31.3% versus 22.5%, p < 0.05) and endoscopic mucosal healing (39.7% versus 27.7%, p < 0.05) at week 52, while VDZ and ADA were both generally safe and well-tolerated.31
While we observe the increasing availability for biologic treatment, we acknowledge that about one-third of patients do not achieve a clinically relevant response to the induction series (primary failure), and about half of patients with initial response lose effect in the maintenance phase (secondary failure).16,17 Therefore, the study of the comparative effectiveness of different agents as second-line therapy becomes highly relevant in clinical practice. Unfortunately, there have been no trials of IFX or GOL as second-line agents, which limits inference on their effectiveness in this setting. Furthermore, in the ULTRA-2 study patients qualified to second-line therapy with ADA were anti-TNF-experienced (who by definition may have responded to prior anti-TNF therapy)9 in contrast to studies with VDZ and TFB where they were required to have had treatment failure.14,15 It is very likely that anti-TNF therapy-failure population is more difficult to treat than anti-TNF therapy-experienced population, which is why conclusions from the analysis should be made with caution.
We identified three pharmacoeconomic analyses evaluating the cost-effectiveness of IFX, ADA and GOL in induction and maintenance treatment up to 1 year in moderate to severe UC in Polish populations. All three analyses were performed by the same authors.25,32,33 Comparing with our analysis, patient characteristics were the same, the model that was built is very similar, an 8-week cycle for the maintenance phase was assumed, the analysis was done from the public payer’s (National Health Fund) and societal perspective, taking on the same discount rates for outcomes and costs. The time horizon was different; in the studies of Stawowczyk et al. it was 30 years, in our lifetime. The same set of utility values was adopted. All analyses were done in the conditions of Polish health care, so the unit costs were similar, but we noted some differences in terms of resource use: the cost of concomitant standard of care was not included in case of biologically treated patients in the studies of Stawowczyk et al., different dosage of drugs were used in standard of care (yet its cost in the maintenance phase was calculated as almost the same) and, above all, they did not take into account the cost attributed to individual health states. However, it seems that all these differences are too small to justify the quite divergent results of cost-effectiveness analysis. And so, for IFX plus standard of care the incremental cost–utility ratio versus standard of care alone we estimated was PLN211,250.78/QALY gained in contrast to PLN402,420/QALY gained as assessed by Stawowczyk et al. For ADA plus standard of care the incremental cost-utility ratio versus standard of care alone we estimated was PLN705,725.33/QALY gained in contrast to PLN324,270/QALY gained as assessed by Stawowczyk et al. And for GOL plus standard of care the incremental cost–utility ratio versus standard of care alone we estimated PLN524,973.37/QALY gained in contrast to PLN391,252/QALY gained as assessed by Stawowczyk et al. Another difference is the ranking of the therapies according to the criterion of the most favourable ICUR: in our assessment it looks like the following: IFX > GOL > ADA; according to Stawowczyk et al.: ADA > GOL > IFX. In the absence of head-to-head trials, the use of an indirect treatment comparisons method, including network meta-analysis, enables integrating data and ranking therapies to better inform decision makers, doctors and patients about their relative efficacy and cost-effectiveness. Of note, Stawowczyk et al. took outcome data from single studies, for IFX: ACT1 and ACT2,6 for ADA: ULTRA29 and for GOL: PURSUIT-SC, PURSUIT-M,11,12 while we, like Singh et al.,18 have integrated data from all the studies that met prespecified inclusion criteria and estimated the comparative efficacy of individual therapies in relation to others. If we had not done so, we could have come to misleading conclusions about which drug is more beneficial in a given population based on the separately performed cost-effectiveness analyses.
Searching the literature, we managed to find the study of Wilson et al.23 This is the cost-effectiveness analysis comparing VDZ with anti-TNF drugs. Clinical inputs on efficacy and safety were derived from the network meta-analysis performed for four biological agents (IFX, ADA, GOL and VDZ) by Vickers et al.34 They adjusted for different study designs (including randomization methods) to allow the inclusion of all the maintenance studies in their analysis, which, however, was criticized in later publications. TFB has not been included. In comparison with ours, their model included other health states: mild disease instead of response, additionally post-surgery complications were incorporated. The proportion of hospitalizations was estimated based on the study of Loftus et al.35 Other sets of utility values were used: for pre-surgery health states – coming from the GEMINI 1 trial,14 for post-surgery health states – coming from the study of Punekar et al.26 The cost of management of adverse effects was accounted for. The analysis was carried out for a lifetime time horizon, from an extended payer’s perspective, and adopting a different discount rate (3.5%) for costs. In terms of results, we can only compare incremental cost–utility ratios for VDZ versus GOL in the anti-TNF-naïve population. We, due to different randomization methods, decided not to compare VDZ with IFX and ADA. Vickers et al. did not carry out the study in anti-TNF-exposed populations. The ICUR we calculated was PLN68,494.43 (€16,055.03)/QALY gained, which made the replacement of GOL with VDZ a cost-effective strategy, while according to Wilson et al. VDZ appeared to even dominate (be more effective and less costly) over GOL.
Limitations
Our study is not without limitations. First of all, due to the lack of head-to-head clinical trials, when comparing the efficacy and safety of biologic drugs and small molecules, we decided to use the indirect treatment comparisons method. It is possible that the estimates of clinical remission and response rates obtained in this way will be verified by direct evidence in the future. Second, thorough comparative analysis across all agents was limited to induction phase. Due to the differences in trial design of maintenance therapy, we relied on network meta-analyses separately carried out in groups, which hindered comparative economic assessment. Third, comparing biologic drugs as second-line therapy also had many shortcomings. Comparison was possible for only three of them: ADA, VDZ and TFB. These studies had different inclusion criteria (anti-TNF-experienced versus anti-TNF-failure patients), which may have had an impact on efficacy of second-line interventions. In addition, data on the number of prior anti-TNF agents to which the patient was exposed were not consistent in the studies. Current trials did not use therapeutic drug monitoring to understand the plausible mechanism of failure of initial biologic intervention, which approach is becoming widely accepted in real clinical practice. Fourth, we conducted a comparative cost-effectiveness assessment in the Polish setting. Nevertheless, the results could be adopted by health care systems in other countries as clinical practice is shaped by international multicentre trials and the differences mainly relate to the cost of pharmacotherapy and unit costs of other resources used. However, each time, this should be deliberated on very carefully and a number of questions regarding transferability would have to be answered – what differences exist, how likely will they be to impact the results and in which direction, and will the final conclusion be changed? For example, the cost of purchasing individual therapies in Poland and the UK will be different, and so will be the cost of administering them – in Poland, administration of IFX and VDZ requires 1-day hospitalization, whereas in the UK these drugs are administered on an outpatient basis. This favours the use of ADA, GOL and TFB in Poland. Fifth, we did not include treatment related AEs as separate health states in the model. However, we included AEs leading to treatment discontinuation and patients in whom they occurred were receiving standard of care in subsequent cycles. Sixth, some assumptions about utility values had to be made.
Nevertheless, our study has many strengths; above all, it goes along with the complexity of the issue. It included all modern therapies registered in the EU in the population of patients with moderate to severe UC. Clinical effectiveness assessment was carried out separately in two subpopulations: anti-TNF naïve and anti-TNF exposed. The use of network meta-analysis and indirect treatment comparison methods in combination with costs enabled the positioning of treatments according to the growing ICUR. We included biosimilars as well as indirect costs in our analysis.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: The research did not involve human participants nor animals. The study received exemption from ethics approval and the need for informed consent was waived by the Bioethics Committee at Wroclaw Medical University, Poland.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Pawel Petryszyn  https://orcid.org/0000-0003-2397-7028
https://orcid.org/0000-0003-2397-7028
Contributor Information
Pawel Petryszyn, Department of Clinical Pharmacology, Wroclaw Medical University, Borowska 211, Wroclaw 50-556, Poland.
Pawel Ekk-Cierniakowski, Warsaw School of Economics, Warsaw, Poland.
Grzegorz Zurakowski, Department of Clinical Pharmacology, Wroclaw Medical University, Wroclaw, Poland.
References
- 1. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017; 389: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54.e42. [DOI] [PubMed] [Google Scholar]
- 3. Farrell RJ, Peppercorn MA. Ulcerative colitis. J R Soc Med 2016; 2: 86–88. [Google Scholar]
- 4. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 5. Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis. Ann Intern Med 2014; 160: 704–711. [DOI] [PubMed] [Google Scholar]
- 6. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- 7. Jiang XL, Cui HF, Gao J, et al. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J Clin Gastroenterol 2015; 49: 582–588. [DOI] [PubMed] [Google Scholar]
- 8. Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011; 60: 780–787. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012; 142: 257–265.e3. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol 2014; 49: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 85–95. [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 96–109.e1. [DOI] [PubMed] [Google Scholar]
- 13. Hibi T, Imai Y, Senoo A, et al. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study). J Gastroenterol 2017; 52: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 16. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012; 10: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 17. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 2011; 33: 987–995. [DOI] [PubMed] [Google Scholar]
- 18. Singh S, Fumery M, Sandborn WJ, et al. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther 2018; 47: 162–175. [DOI] [PubMed] [Google Scholar]
- 19. Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN study). Scand J Gastroenterol 2009; 44: 431–440. [DOI] [PubMed] [Google Scholar]
- 20. Arai K, Koganei K, Kimura H, et al. Incidence and outcome of complications following restorative proctocolectomy. Am J Surg 2005; 190: 39–42. [DOI] [PubMed] [Google Scholar]
- 21. Woehl A, Hawthorne A, McEwan P. The relation between disease activity, quality of life and health utility in patients with ulcerative colitis. Gut 2008; 57: A153. [Google Scholar]
- 22. Arseneau KO, Sultan S, Provenzale DT, et al. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis? Clin Gastroenterol Hepatol 2006; 4: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 23. Wilson MR, Bergman A, Chevrou-Severac H, et al. Cost-effectiveness of vedolizumab compared with infliximab, adalimumab, and golimumab in patients with ulcerative colitis in the United Kingdom. Eur J Health Econ 2018; 19: 229–240. [DOI] [PubMed] [Google Scholar]
- 24. Tsai HH, Punekar YS, Morris J, et al. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther 2008; 28: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 25. Stawowczyk E, Kawalec P, Pilc A. Cost-effectiveness analysis of 1-year treatment with golimumab/standard care and standard care alone for ulcerative colitis in Poland. PLoS One 2016; 11: e0160444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Punekar Y, Hawkins N. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis. Eur J Health Econ 2010; 11: 67–76. [DOI] [PubMed] [Google Scholar]
- 27. Swinburn P, Elwick H, Bean K, et al. The impact of surgery on health related quality of life in ulcerative colitis. Gut 2012; 61: A237. [Google Scholar]
- 28. Danese S, Gomollon F. ECCO position statement: the use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD). J Crohns Colitis 2013; 7: 586–589. [DOI] [PubMed] [Google Scholar]
- 29. Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019; 50: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonovas S, Lytras T, Nikolopoulos G, et al. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther 2017; 47: 454–465. [DOI] [PubMed] [Google Scholar]
- 31. Sands BE, Peyrin-Biroulet L, Loftus E V, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019; 381: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 32. Stawowczyk E, Kawalec P, Pilc A. Cost-utility analysis of infliximab with standard care versus standard care alone for induction and maintenance treatment of patients with ulcerative colitis in Poland. Pharmacotherapy 2016; 36: 472–481. [DOI] [PubMed] [Google Scholar]
- 33. Stawowczyk E, Kawalec P, Pilc A. Cost-utility analysis of 1-year treatment with adalimumab/standard care and standard care alone for ulcerative colitis in Poland. Eur J Clin Pharmacol 2016; 72: 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vickers AD, Ainsworth C, Mody R, et al. Systematic review with network meta-analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS One 2016; 11: e0165435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loftus EV, Delgado DJ, Friedman HS, et al. Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the United States. Am J Gastroenterol 2008; 103: 1737–1745. [DOI] [PubMed] [Google Scholar]