Abstract
Objectives
Perioperative cardiovascular events remain an important factor that affects surgery outcome. We assessed if asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, predicts perioperative risk, and if pre-operative supplementation with L-arginine/L-citrulline improves the plasma L-arginine/ADMA ratio.
Methods
In this prospective study, planned thoracic and/or abdominal surgery patients were randomized to receive L-arginine/L-citrulline (5 g/day) or placebo 1 to 5 days before surgery. We measured perioperative plasma ADMA and L-arginine levels. The primary outcome was a 30-day combined cardiovascular endpoint.
Results
Among 269 patients, 23 (8.6%) experienced a major adverse cardiovascular event. ADMA and C-reactive protein were significantly associated with the incidence of cardiovascular complications in the multivariable-adjusted analysis. The L-arginine plasma concentration was significantly higher on the day of surgery with L-arginine/L-citrulline supplementation compared with placebo. In patients with high pre-operative ADMA, there was a non-significant trend towards reduced incidence of the primary endpoint with L-arginine/L-citrulline supplementation (six vs. nine events).
Conclusions
ADMA is a predictor of major adverse cardiovascular complications in the perioperative period for patients who are undergoing major abdominal and/or thoracic surgery. Supplementation with L-arginine/L-citrulline increased the L-arginine plasma concentration, enhanced the L-arginine/ADMA ratio, and induced a trend towards fewer perioperative events.
Keywords: Perioperative risk, nitric oxide, asymmetric dimethylarginine, American Society of Anesthesiologists score, biomarker, L-arginine, L-citrulline
Introduction
Perioperative cardiovascular complications have remained a major threat for the outcome of major surgery.1 However, complications affect only a small portion of surgical patients.2 The risk of major cardiovascular events is significantly increased during the perioperative period in patients with pre-existing cardiovascular disease, and screening for such pre-existing conditions has long been part of pre-operative risk checks.3 However, even in patients without clinically apparent cardiovascular disease, the risk of experiencing a first major cardiovascular event is increased during the immediate postoperative period.4 Established clinical risk scores such as the American Society of Anesthesiologists (ASA) score and the Revised Cardiac Risk Index (RCRI) are routinely being used to improve the identification of patients with an elevated risk.5 However, these clinical scores are not sufficient to distinguish between high and low-risk patients,6,7 and new markers are needed to improve pre-operative risk assessment.
The L-arginine–nitric oxide (NO) pathway is a crucial signaling pathway for cardiovascular functional integrity.8 The production and action of NO, which is formed in the healthy vascular endothelium from L-arginine, is impaired in the very early stages of cardiovascular diseases, and a lack of biologically active NO is widely believed to contribute to the pathogenesis of various cardiovascular diseases (for review, cf.9).
We and others have identified asymmetric dimethylarginine (ADMA), which is an endogenous competitive inhibitor of NO synthesis, as a risk marker of cardiovascular disease and mortality in diverse patient populations with, for example, end-stage renal disease10 and coronary artery disease.11 In the general population, a high ADMA concentration predicts total mortality independently of other established cardiovascular risk factors.12,13 Because ADMA competes with L-arginine at the level of the NO synthase, the L-arginine/ADMA ratio is an appropriate estimate of “true” substrate availability for NOS; this ratio is independently associated with mortality.12 L-arginine, when administered orally, has an extremely short half-life.14 Conversely, oral administration of L-citrulline, which is converted into L-arginine by argininosuccinate synthase and argininosuccinate lyase activity, causes a more extended elevation of plasma L-arginine levels.15 The combination of L-arginine and L-citrulline has been shown to result in a larger increase in NO production compared with either of the amino acids given alone.16
In a previous study, our group reported that high ADMA levels were associated with perioperative cardiovascular complications in a surgical population with low-to-medium estimated perioperative risk.17 Based on improved bioanalytical methodology, we aimed to extend our previous findings to a surgical population with medium-to-high estimated risk in this study.
Several pharmacotherapeutic strategies for reducing perioperative risk have been proposed and subsequently tested in clinical trials, such as beta receptor blockers18 and statins,19 but clinical trials have yielded varying results.5 Based upon the dynamics of NO synthase inhibition by ADMA, which competitively displaces the substrate L-arginine from the catalytic site of the enzyme, we hypothesized that pre-operative supplementation with L-arginine might reduce the ADMA-related risk.20 We, therefore, performed a pilot clinical intervention study using dietary L-arginine in the immediate pre-operative period and measured the time course of ADMA and L-arginine in plasma from the pre-operative visit until day 3 post-surgery. Major adverse cardiovascular events were recorded until day 30 post-surgery.
Patients and methods
Patients and study design
There were 269 patients who took part in this study, which was performed as a single-center, prospective, randomized, double-blind study. Patients and investigators were blinded to the study group because the study products were prepared at the local pharmacy by personnel who were independent of any other study procedures. The local ethics committee approved the study protocol (Ethics Committee of the Hamburg Board of Physicians, PV 3466), and the study was conducted in accordance with the Principles of Good Clinical Practice and the Declaration of Helsinki. All study participants provided written informed consent before they were included in the study. The study was registered in the German Clinical Trial Register (trial no. DRKS00016797) and is searchable at http: //apps.who.int/trialsearch/.
Patients were eligible for the study if they had been scheduled for medium-to-high risk thoracic (non-cardiac), abdominal, or two cavity surgery, were between 30 and 75 years old, were classified as having an ASA Score of II to IV, and had signed the informed consent form. Medium-to-high surgery-related risk was defined in accordance with the 2014 ESC/ESA guidelines on cardiovascular risk assessment and management in non-cardiac surgery.21 Major exclusion criteria were myocardial infarction/acute coronary syndrome or stroke within the last 3 months (other types of ischemic vascular disease were permitted), emergency surgery, cardiac surgery, and presumed life expectancy of <6 months.
Study participants were recruited from patients who routinely visited the outpatient clinic at the Department of Anesthesiology in advance of their planned surgical intervention. Patients visited the clinic 1 to 5 working days in advance of their scheduled date of surgery.
Clinical assessment and follow-up
Non-fasting blood samples to measure plasma L-arginine and ADMA concentrations were taken at the time of study enrollment (baseline value), in the morning before surgery (pre-operative value), and on days 1 and 3 after surgery. Safety parameters were assessed from the routine blood draws when the patient was entered into the study, on the morning before surgery, and at hospital discharge. All clinical evaluations and risk classifications (ASA score22 and RCRI23) were performed in accordance with widely used protocols by trained anesthesiologists within the week preceding surgery. The RCRI accounts for ischemic heart disease, heart failure, diabetes, impaired renal function (serum creatinine >2 mg/dL or dialysis treatment), high-risk surgery, and history of cerebrovascular disease.
After surgery was complete, patients were monitored daily for the duration of their in-patient treatment, and all clinical events, changes in laboratory parameters, and clinical diagnostic tests that were scheduled based on routine clinical testing were documented. No additional clinical testing was performed.
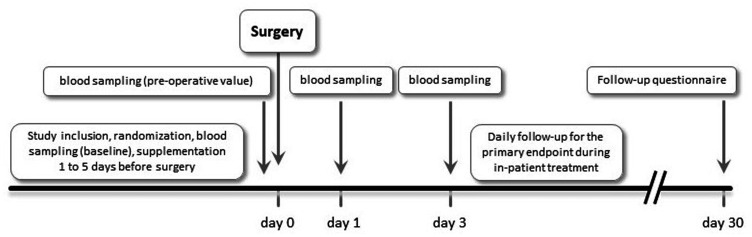
After discharge, patients were followed-up by telephone using a structured questionnaire at day 30 after surgery; all serious adverse events occurring during that period were recorded and validated. The time course of the study is schematically represented in Figure 1.
Figure 1.
Schematic representation of study timelines.
The primary end point was a composite end point that was defined by the occurrence of death (any cause), myocardial infarction or acute coronary syndrome, decompensated congestive heart failure, severe arrhythmia, major thrombosis, or embolism (cerebrovascular or pulmonary) from the beginning of surgery until 30 days after surgery.
Diagnosis of myocardial infarction was based on new persistent ST-segment (≥1 mm in ≥2 leads) or T-wave changes, new Q waves, and angiographic evidence or a positive troponin test. Acute coronary syndrome was defined as severe precordial chest pain lasting >30 minutes that was unresponsive to standard treatment and that was accompanied by ischemic electrocardiogram changes and identification of stenosis in coronary angiography, but that had negative cardiac enzymes and no development of Q waves. Diagnosis of decompensated congestive heart failure was based on dyspnea and rales indicative of pulmonary edema and confirmed by chest radiograph, and/or acute requirement of intravenous positive inotropic drugs or diuretics. Deep vein thrombosis was defined as loss of deep vein compressibility visualized by ultrasound. Pulmonary embolism suspected by shortness of breath in combination with jugular vein congestion and/or elevation of D-dimer >190 µg/L was to be confirmed by spiral computed tomography, magnetic resonance imaging, or ventilation perfusion scanning. Severe arrhythmia was defined as any of the following: primary cardiac arrest requiring resuscitation, atrial arrhythmia (e.g., atrial fibrillation or flutter requiring cardioversion or drug intervention for frequency control or stroke prevention), sustained or polymorphic ventricular tachycardia, or third-degree atrioventricular block.
Quantification of ADMA and L-arginine concentrations
Non-fasting blood samples were drawn from an antecubital vein. Samples were centrifuged immediately and stored at −20°C until analysis. L-Arginine and ADMA were quantified using a validated liquid chromatography–tandem mass spectrometry method (LC-MS/MS) as described before.24 Briefly, 25 µL of ethylenediamine tetraacetic acid (EDTA) plasma were spiked with stable isotope-labeled L-arginine and ADMA as internal standards. Proteins were precipitated with 100 µL of methanol. After filtering the samples through a 0.22-µm hydrophilic membrane (Multiscreen HTS™, Millipore, Molsheim, France), compounds were derivatized to their butylester derivatives with butanolic 1 N HCl, and analyzed by LC-MS/MS (Varian 1200 MS, Agilent Technologies, Santa Clara, CA, USA). The analytical range of the method was validated from 0.05 to 4 µmol/L for ADMA and from 0.5 to 250 µmol/L for L-arginine, and mean coefficients of variation were ≤5% for both analytes. All other laboratory values including C-reactive protein (CRP) were measured using routine clinical laboratory methods.
Dietary supplementation with L-arginine/L-citrulline
Beginning immediately after the pre-surgery visit and ending in the morning of the day of surgery, study participants were requested to ingest five capsules, two times per day. Each capsule contained 333 mg of L-arginine and 167 mg of L-citrulline, resulting in a daily dose of 5 g of L-arginine/L-citrulline, or corresponding placebo (lactose powder). The individual treatment period was determined by the time that elapsed between the pre-surgery visit in the anesthesiology outpatient clinic and admission for surgery, both of which were not standardized in the protocol, and were driven by clinic routine. The first dose of study medication was taken in the evening after the pre-surgery visit and the last dose was taken in the morning of surgery. Twelve patients were scheduled for same-day surgery. These patients took a single dose of 5 g L-arginine/L-citrulline on the morning of surgery.
Statistical analyses
All statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). Data are presented as median with the 25th and 75th percentiles, or as the mean with standard deviation (SD). Differences between the groups were tested for significance using the non-parametric Mann–Whitney U test. Odds ratios with 95% confidence intervals were computed using a univariate logistic regression model with the primary endpoint as the dependent variable. The Chi-square test was used to test for significant differences in the incidence of the primary endpoint in patients with high or low ADMA. ADMA and L-arginine concentrations over time were examined using repeated measures two-way ANOVA followed by Tukey’s multiple comparisons test. For all tests, p < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
The present study included 269 patients between 30 and 75 years of age (mean ± SD, 56.7 ± 11.3 years). The baseline demographic and anthropometric characteristics of the patients are listed in Table 1. The proportion of men was higher in the L-arginine/L-citrulline group compared with the placebo group (83 [58.5%] vs. 53 [41.7%]; p < 0.01), and the proportion of patients with high surgical risk was slightly, but significantly, higher in the L-arginine/L-citrulline group compared with the placebo group (p < 0.05). There were no other significant differences were between both groups with respect to demographics, risk factors, chronic illness, and pre-operative risk scores (Table 1). Additionally, 179 patients underwent general surgery (66.6%), 47 underwent trans-abdominal gynecological surgery (17.5%), and 43 had transabdominal urological interventions (15.9%).
Table 1.
Baseline patient characteristics.
| All | L-arginine/L-citrulline group | Placebo group | ||
|---|---|---|---|---|
| Demographics | ||||
| N | 269 | 142 | 127 | |
| Age | years | 56.7 ± 11.3 | 57.2 ± 11.1 | 56.3 ± 11.5 |
| Sex | Male (N [%]) | 136 [50.6] | 83 [58.5] | 53 [41.7]* |
| BMI | kg/m2 | 28.3 ± 8.4 | 28.6 ± 9.1 | 28.0 ± 7.7 |
| Cardiovascular risk factors | ||||
| Hypertension | N [%] | 120 [44.6] | 59 [41.5] | 61 [48.0] |
| Diabetes mellitus | N [%] | 42 [15.6] | 21 [14.8] | 21 [16.5] |
| Chronic renal failure | N [%] | 9 [3.3] | 2 [1.4] | 7 [5.5] |
| Smoking | N [%] | 78 [29.0] | 40 [28.2] | 28 [29.9] |
| CHD | N [%] | 23 [8.6] | 14 [9.9] | 9 [7.1] |
| History of MI | N [%] | 12 [4.5] | 6 [4.2] | 6 [4.7] |
| Dyslipidemia | N [%] | 46 [17.1] | 24 [16.9] | 22 [17.3] |
| Arrhythmia | N [%] | 36 [13.4] | 15 [10.6] | 21 [16.5] |
| Pre-operative risk stratification | ||||
| ASA Score 2/3/4 | N | 133/128/8 | 70/69/3 | 63/59/5 |
| % | 49.4/47.6/3.0 | 49.3/48.6/2.1 | 49.6/46.5/3.9 | |
| RCRI 0/1/2/3/4 | N | 34/189/38/7/1 | 15/102/19/6 | 19/87/19/1/1 |
| % | 12.6/70.3/14.1/2.6/0.4 | 10.6/71.8/13.4/4.2 | 15/68.5/15/0.8/0.8 | |
| Surgical risk group 1/2 | N | 79/190 | 31/111 | 48/79* |
| % | 29.4/70.6 | 21.8/78.2 | 37.8/62.2 | |
| Medication (N [%]) | ||||
| Angiotensin blockers | 31 [11.5] | 16 [11.3] | 15 [11.8] | |
| Beta blockers | 61 [22.7] | 30 [21.1] | 31 [24.4] | |
| Loop diuretics | 50 [18.6] | 27 [19.0] | 23 [18.1] | |
| Calcium channel blockers | 18 [6.7] | 8 [5.6] | 10 [7.9] | |
| Statins | 30 [11.2] | 16 [11.3] | 14 [11.0] | |
| Platelet aggregation inhibitors | 40 [14.9] | 20 [14.1] | 20 [15.7] | |
| Insulin | 14 [5.2] | 11 [7.7] | 4 [3.1] | |
Data are presented as N [%], except for age and BMI, which are presented as the mean ± SD.
*p < 0.05 between groups. Differences between treatment groups were assessed using Mann–Whitney U test and Kruskal–Wallis test, as appropriate.
BMI, body mass index; ASA, American Society of Anesthesiologists; RCRI, Revised Cardiac Risk Index; MI, myocardial infarction; CHD, coronary heart disease; SD, standard deviation.
There were 142 patients who were randomized to receive L-arginine/L-citrulline supplementation, and 127 patients who were randomized to receive the corresponding placebo. Supplementation with study product was initiated at the pre-surgery visit and continued until the morning of the surgical intervention, which corresponded to a variable period of 1 to 5 days. The cumulative doses of L-arginine/L-citrulline or placebo, respectively, were between 2.5 and 25 g (median [interquartile range, IQR], 18 [14; 32] g).
Baseline concentrations of L-arginine and ADMA
At baseline, the plasma ADMA concentration in the study cohort was (median [IQR]) 0.61 (0.54–0.68) µmol/L, and the L-arginine concentration was 59.5 (45.0–80.2) µmol/L. Compared with the healthy reference cohorts, ADMA and L-arginine concentrations were within the respective normal ranges.25,26 The calculated L-arginine/ADMA ratio was 98.0 (73.0–131.5).
There were no significant differences between the L-arginine/L-citrulline and placebo groups for the baseline ADMA concentrations (0.61 [0.53–0.69] vs. 0.61 [0.54–0.67] µmol/L), L-arginine levels (58.4 [46.7–79.1] vs. 62.0 [44.2–81.0] µmol/L), and the L-arginine/ADMA ratio (96.5 [72.8–132.0] vs. 98.0 [74.0–131.0]).
Perioperative ADMA and L-arginine plasma concentrations over time
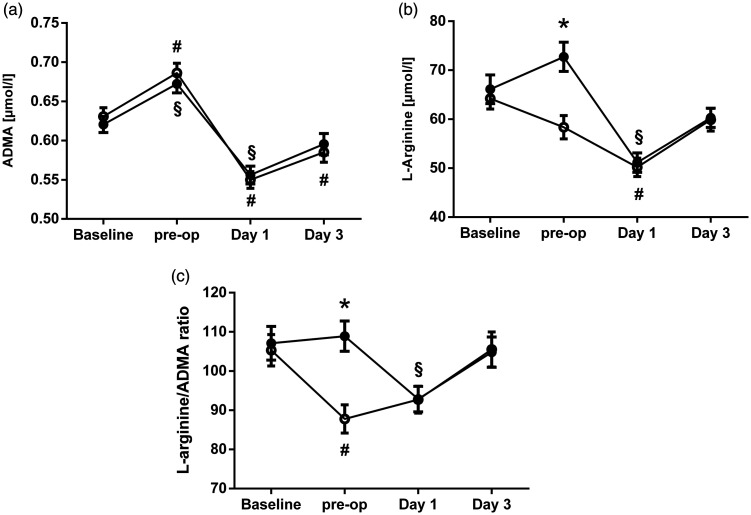
The ADMA plasma concentration significantly increased from 0.61 (0.54–0.68) µmol/L to 0.68 (0.59–0.76) µmol/L between baseline and the day of surgery (p < 0.001). After surgery, the ADMA concentration sharply decreased to 0.54 (0.47–0.62) µmol/L (p < 0.001) and returned to baseline level until day 3 (0.57 [0.49–0.68] µmol/L). There was no significant difference in the ADMA concentration over time between the L-arginine/L-citrulline and placebo groups (Figure 2a).
Figure 2.
Perioperative plasma concentrations over time for (a) ADMA, (b) L-arginine, and (c) the L-arginine/ADMA ratio in the L-arginine/L-citrulline group (filled circles) and the placebo group (open circles). Data are presented as the mean ± SD. *p < 0.001 for comparison between L-arginine/L-citrulline vs. placebo group; §p < 0.05 vs. baseline within the L-arginine/L-citrulline group; #p < 0.05 vs. baseline within the placebo group.
ADMA, asymmetric dimethylarginine; SD, standard deviation.
A significant difference in the L-arginine plasma concentration over time was observed between the L-arginine/L-citrulline and placebo groups (Figure 2b). While the L-arginine plasma concentration decreased significantly from baseline to the day of surgery in the placebo group (62.0 [44.2–81.0] to 56.9 [41.6–70.8] µmol/L; p = 0.02), it slightly increased in the L-arginine/L-citrulline group during the same period (58.4 [46.9–79.1] to 69.4 [51.5–89.4] µmol/L; p = 0.05), and there was a significant difference in L-arginine plasma concentration between both groups on the day of surgery (p < 0.001).
After surgery, the L-arginine plasma concentration sharply decreased in both groups, and the difference between the groups was no longer detectable on day 1 after surgery. Until day 3 after surgery, the L-arginine concentration had almost returned to baseline values (59.5 [45.0–80.2] µmol/L in the L-arginine/L-citrulline group vs. 57.4 [43.1–73.7] µmol/L in the placebo group).
The L-arginine/ADMA ratio was 98 (73–132) at baseline. Until the day of surgery, the L-arginine/ADMA ratio decreased significantly in the placebo group (from 98 [74–131] to 82 [66–110]; p = 0.003), while it remained stable in the L-arginine/L-citrulline group (from 97 [73–132] to 106 [75–139]; Figure 2c). The difference in the L-arginine/ADMA ratio on the day of surgery was statistically significant (p < 0.001). After surgery, L-arginine/ADMA ratios in both groups were not significantly different and largely paralleled each other.
Prospective follow-up for perioperative complications
There were 23 patients (8.6% of the study population) who reached the primary composite endpoint. Table 2 shows the incidence of the different primary endpoint components. There was no significant difference in age, sex, laboratory markers, or ASA or RCRI classification between patients who did or did not reach the primary endpoint (Table 3). All patients who reached the primary endpoint were classified as RCRI <3. However, 22 of 23 patients who reached the primary endpoint underwent high-risk surgery. Patients who reached the primary endpoint had a significantly longer duration of hospital treatment (21.0 ± 13.0 vs. 12.9 ± 12.5 days; p = 0.003).
Table 2.
Incidence of the components of the primary endpoint.
| Endpoint | All | L-arginine/L-citrulline group | Placebo group | p |
|---|---|---|---|---|
| Total | 23 | 10 | 13 | n.s. |
| Death | 2 | 2 | 0 | n.s. |
| Myocardial infarction | 1 | 1 | 0 | n.s. |
| Stroke | 1 | 0 | 1 | n.s. |
| Thromboembolism | 14 | 5 | 9 | n.s. |
| Angina pectoris | 3 | 0 | 3 | n.s. |
| Decompensated heart failure | 2 | 2 | 0 | n.s. |
n.s., not significant.
Table 3.
Pre-operative risk scores and laboratory values in patients with and without the primary endpoint.
| Primary endpoint | No endpoint | P | |
|---|---|---|---|
| N | 23 | 246 | |
| ASA score 2/3/4 (N) | 7/16/ 0 | 126/112/8 | n.s. |
| RCRI 0/1/2/3/4 (N) | 1/20/2/0/0 | 33/169/36/7/1 | n.s. |
| ASA score >2 (N [%]) | 16 [69] | 120 [49] | n.s. |
| RCRI <3 (N [%]) | 23 [100] | 238 [96.7] | n.s. |
| Serum creatinine (mg/dL) | 0.90 (0.73; 1.15) | 0.90 (0.80; 1.10) | n.s. |
| INR | 0.98 (0.95; 1.04) | 0.98 (0.95; 1.02) | n.s. |
| CRP (mg/L) | 3.00 (0.01; 21.75) | 0.01 (0.01; 10.00) | n.s. |
| Hemoglobin (g/dL) | 13.2 (12.6; 14.1) | 13.7 (12.2; 14.4) | n.s. |
| ADMA (µmol/L) | 0.69 (0.62; 0.76) | 0.67 (0.58; 0.76) | n.s. |
Data are displayed as N [%] or median (25th; 75th percentiles).
Differences between groups were assessed using Mann–Whitney U test or Kruskal–Wallis test, as appropriate.
ASA, American Society of Anesthesiologists; RCRI, Revised Cardiac Risk Index; INR, international normalized ratio; CRP, C-reactive protein; ADMA, asymmetric dimethylarginine; n.s., not significant.
Association of ADMA and L-arginine plasma concentrations with the incidence of the primary endpoint
Patients with ADMA concentration above the median on the day of surgery reached the primary endpoint significantly more often compared with those with ADMA below the median (OR 2.52, 95% CI 1.03–6.16, p = 0.04). In a stepwise regression model, including traditional risk factors such as gender, age, RCRI ≥3, and postoperative CRP, only ADMA concentration above the median and CRP remained significantly associated with the primary endpoint (Table 4).
Table 4.
Stepwise logistic regression for the primary endpoint.
| Model | Odds Ratio (95% CI) | p | |
|---|---|---|---|
| ADMA concentration> 0.68 µmol/L | yes vs. no | 2.53 (1.01–6.32) | 0.04 |
| CRP | 1 mg/L | 1.01 (1.00–1.01) | 0.01 |
| Sex | male vs. female | 1.51 (0.60–3.80) | n.s. |
| RCRI > 2 | yes vs. no | 0.01 (0.00–0.01) | n.s. |
ADMA, asymmetric dimethylarginine; CRP, C-reactive protein; RCRI, Revised Cardiac Risk Index; n.s., not significant.
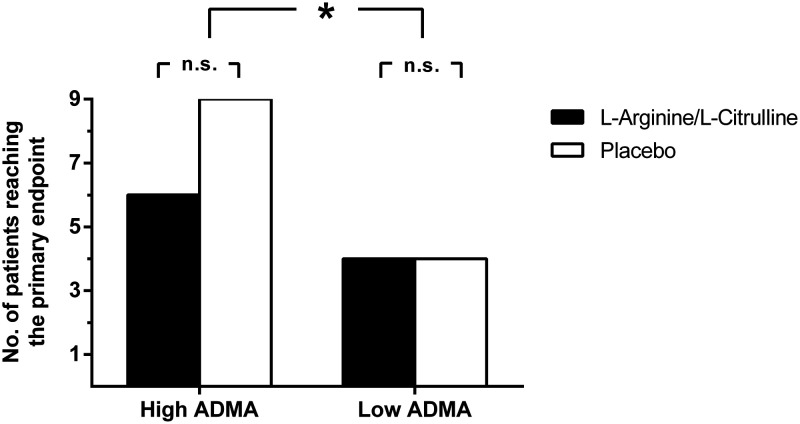
There was no overall significant difference in the incidence of the primary endpoint between the L-arginine/L-citrulline and placebo groups (N = 10 and N = 13, respectively). However, we observed opposing trends for patients with low and high ADMA concentration. In patients with high ADMA, there was an insignificant trend towards an improved outcome with L-arginine/L-citrulline supplementation, while this trend was absent in patients with low ADMA (Figure 3).
Figure 3.
Incidence (absolute number of patients) of the primary endpoint in patients with high or low ADMA, stratified according to supplementation group. High and low ADMA denotes ADMA levels above or below the median as determined pre-operatively. The primary endpoint was a combined cardiovascular endpoint comprising death from any cause, myocardial infarction or acute coronary syndrome, decompensated congestive heart failure, severe arrhythmia, major thrombosis, or embolism (cerebrovascular or pulmonary) during the period from the beginning of surgery until 30 days after surgery. *p < 0.05 for the incidence of the primary endpoint between the groups with high and low ADMA, respectively.
ADMA, asymmetric dimethylarginine.
Thirty-five patients in our study reached an L-arginine/ADMA ratio of 150 or above, which was associated with low cardiovascular risk in previous prospective studies.12 Additionally, 75% of these patients (N = 26) were treated with L-arginine/L-citrulline, while only nine of these patients (25%) received placebo (p = 0.006).
Discussion
The present study has two key findings. First, elevated ADMA is a risk factor for major cardiovascular complications that occur in the perioperative period. The prediction of perioperative risk by ADMA significantly adds to the risk prediction that is obtained using the currently applied routine risk scores. Second, pre-operative supplementation with L-arginine/L-citrulline enhances the plasma L-arginine/ADMA ratio, which might contribute to diminished cardiovascular risk. Although still a trend, this possible risk reduction becomes more apparent in patients with elevated ADMA, indicating a possible role for disrupted NO metabolism in the pathophysiology of perioperative cardiovascular complications.
A broad range of biomarkers relating to organ dysfunction and traditional cardiovascular risk factors has been investigated to identify patients who are at risk of perioperative cardiovascular complications.27 Among them are troponin T28 and B-type natriuretic peptide.29 In the clinical routine, renal function markers, coagulation tests, and blood cell count are the main clinical laboratory values that were considered, in addition to standardized clinical scoring systems such as the ASA score and the RCRI.5,21
We have previously reported that patients with high ADMA at the time of surgery are at increased risk of major cardiovascular events in the peri- and postoperative period.17 In that study, 402 surgical patients were included with a mean baseline ADMA of 0.8 ± 0.2 µmol/L. ADMA was identified as an independent risk predictor with an odds ratio of 1.33 (1.12–1.59) per 0.1 µmol/L increase in ADMA concentration in a fully adjusted model. Our present data fully support these findings in a slightly younger cohort of patients with higher risk based on the pre-operative ASA and RCRI scores. Patients with ADMA above the median (0.61 µmol/L) had a significantly elevated risk of experiencing the primary endpoint (OR = 2.52 (1.03–6.16; p < 0.04) compared with those with an ADMA concentration below the median. Taken together, these two studies strongly support the hypothesis that high circulating ADMA concentrations may contribute to the pathophysiology of major perioperative cardiovascular complications. Since publication of our previous study, other investigators have reproduced our finding in various other populations. In a prospective study of 100 pediatric patients who underwent cardiac surgery involving cardio-pulmonary bypass (CABG), elevated pre-operative ADMA was significantly associated with length of hospital stay, length of intensive care unit (ICU) treatment, and prolonged mechanical ventilation.30 Another study with 158 adult patients who were undergoing CABG surgery showed that postoperative myocardial infarction and early perioperative mortality were significantly associated with high pre-operative ADMA concentration.31 The latter group later extended their findings in a prospective cohort study of 191 consecutive on-pump and off-pump CABG patients.32 They confirmed that elevated ADMA at baseline was associated with an elevated risk of postoperative morbidity and mortality.
ADMA is an endogenous, competitive inhibitor of NO synthesis.9 Previous studies from our group showed that ADMA is significantly associated with impaired endothelium-dependent vasodilation in human subjects in a manner that is reversible with L-arginine.33 Using stable isotope-labeling techniques, we further showed that there is an inverse correlation between ADMA concentration and the rate of conversion of 15N2-L-arginine into 15N-nitrate by NO synthase, both in vitro and in vivo.34,35 Our present study strongly suggests that ADMA-mediated inhibition of nitric oxide synthesis may be one major underlying cause of perioperative cardiovascular event rate and mortality, which is consistent with the previous published studies that are cited above. This hypothesis is further supported by the occurrence of post-operative dysfunction of endothelium-dependent, NO-mediated vasodilation after major colon cancer surgery.36 In that study, endothelial dysfunction significantly correlated with the L-arginine/ADMA ratio. A large array of prospective studies analyzed the role of ADMA as a predictor of cardiovascular events and mortality across a broad risk spectrum, ranging from advanced cardiovascular and metabolic disease, end-stage renal disease, and ICU-treated patients with multi-organ failure to population-based cohorts (for review cf.37,38).
The present study is the first to report L-arginine and ADMA concentrations over time during the perioperative period in non-cardiac surgery patients. We found that the L-arginine concentration sharply decreased from baseline to day 1 after surgery and recovered slowly after the surgery. There was also a concurrent increase in ADMA concentration from baseline to the day of surgery, followed by a decrease after surgery. The antagonistic changes in L-arginine and ADMA concentrations may contribute to unfavorable outcomes after surgery because a reduction in L-arginine and an increase in ADMA both negatively affect NO production rate. Thus, the L-arginine/ADMA ratio was used as a marker of substrate availability for NO synthase.20,39 The L-arginine/ADMA ratio was identified as an independent risk marker of total mortality in the population-based Framingham Offspring cohort.12 Additionally, a low L-arginine/ADMA ratio is associated with endothelial dysfunction after major colon surgery.36
Being a semi-essential amino acid, L-arginine plasma concentration largely depends on dietary intake. The perioperative decrease in L-arginine may, thus, be a result of strict fasting in the immediate perioperative period. A recent study in patients who were undergoing colorectal surgery found similar decreases in L-arginine and increases in ADMA concentrations during the perioperative period.40 In another perioperative clinical trial, Visser and co-workers assessed the effects of defined, consistent perioperative enteral and parenteral nutrition compared with no-nutrition control in patients who were undergoing CABG.41 They observed a significantly enhanced myocardial L-arginine/ADMA ratio and improved myocardial glucose metabolism in the two nutrition groups. Consistent with these results, our present data suggest that maintaining the L-arginine/ADMA ratio at a normal level might contribute to the reduction in major perioperative complications. The L-arginine/ADMA ratio reference levels in a healthy population were determined to be 74.3 (95% CI 71.1–77.3) to 225 (95% CI 222–228) µmol/L.25 Compared with these values, the median of the baseline L-arginine/ADMA ratio in the present study was at the low end of the reference range, and a significant number of patients (n = 81) were far below this value on the day of surgery. The L-arginine/ADMA ratio alone is a marker of cardiovascular risk, as shown in the population-based, prospective Framingham Offspring cohort.12 In that study, there was a significant decrease in the total mortality risk for patients with an L-arginine/ADMA ratio above the population mean (149.4 ± 44.4). In our present study, only 35 patients reached an L-arginine/ADMA ratio ≥150 on the day of surgery, among whom 26 patients (75%) were treated with L-arginine/L-citrulline. This resulted in a significantly higher L-arginine/ADMA ratio on the day of surgery, and it may provide an explanation for the trend towards fewer major perioperative events in the L-arginine/L-citrulline group among patients with high ADMA levels. Our study was not powered to show the clinical superiority of L-arginine/L-citrulline supplementation over placebo; therefore, the results of this pilot study need confirmation in a formal clinical trial.
The benefit of supplementation with L-arginine has been debated, based upon controversial results of clinical trials. However, many studies that produced negative results had an insufficient increase in L-arginine plasma concentration during L-arginine supplementation,42 which was possibly related to the conversion of L-arginine to ornithine by arginase43 and the short half-life of L-arginine, which was determined to be only 1 hour;14,44 this necessitated multiple daily doses to observe a constant effect throughout the day. In our present study, we used a combination of L-arginine plus L-citrulline because L-citrulline was shown to cause a prolonged increase in plasma L-arginine concentration.15,16 The extended increase in L-arginine plasma concentration after taking a combination of L-arginine and L-citrulline allows for twice daily administration (RB, unpublished observations). Consistent with pathophysiological considerations, L-arginine supplementation was shown to improve NO-mediated vascular function primarily in subjects with high ADMA levels.45,46 This assumption is corroborated by the results of the present study, which show a favorable trend with L-arginine/L-citrulline supplementation in patients with elevated ADMA concentration only.
It is an intriguing hypothesis that only a few days of pre-operative L-arginine/L-citrulline supplementation generate a trend towards an improved outcome during a period of 30 days after surgery. The only significant difference in the L-arginine/ADMA ratio was on the day of surgery; before and after surgery, both groups were almost identical. Therefore, this short time window may be sufficient to reduce the perioperative complication rate, given that the intake protocol ensures a long enough intake duration of the study product.
The high variability and the predominantly short intake period for the study product between the pre-surgery visit and the day of surgery was the major flaw in this proof-of-concept pilot study. This was because we could not modify the routine clinical workflows in this study, and clinical processes tended to continuously shorten the pre-operative period. The median intake of L-arginine/L-citrulline was 18 (14–32) g, which roughly corresponds to a mean of only six to seven doses. However, we speculate that a defined period of L-arginine/L-citrulline supplementation before surgery might significantly contribute to reducing the risk of major perioperative complications that are associated with high ADMA levels.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work has been funded by the German Interdisciplinary Association of Intensive Care and the Faculty of Medicine of the University of Hamburg, Germany.
ORCID iD
Juliane Hannemann https://orcid.org/0000-0003-3499-4827
References
- 1.Sankar A, Beattie WS, Wijeysundera DN. How can we identify the high-risk patient? Curr Opin Crit Care 2015; 21: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearse RM, Harrison DA, James P, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care 2006; 10: R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smilowitz NR, Gupta N, Ramakrishna H, et al. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med 2015; 373: 2258–2269. [DOI] [PubMed] [Google Scholar]
- 5.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014; 130: 2215–2245. [DOI] [PubMed] [Google Scholar]
- 6.Acheampong D, Guerrier S, Lavarias V, et al. Risk factors contributing to cardiac events following general and vascular surgery. Ann Med Surg (Lond) 2018; 33: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridley S. Cardiac scoring systems–what is their value? Anaesthesia 2003; 58: 985–991. [DOI] [PubMed] [Google Scholar]
- 8.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993; 329: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 9.Böger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res 2003; 59: 824–833. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Bode-Böger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 2001; 358: 2113–2117. [DOI] [PubMed] [Google Scholar]
- 11.Schnabel R, Blankenberg S, Lubos E, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res 2005; 97: e53–e59. [DOI] [PubMed] [Google Scholar]
- 12.Böger RH, Sullivan LM, Schwedhelm E, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation 2009; 119: 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leong T, Zylberstein D, Graham I, et al. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women: 24-year follow-up of the population study of women in Gothenburg. Arterioscler Thromb Vasc Biol 2008; 28: 961–967. [DOI] [PubMed] [Google Scholar]
- 14.Bode-Böger SM, Böger RH, Galland A, et al. L-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship. Br J Clin Pharmacol 1998; 46: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwedhelm E, Maas R, Freese R, et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 2008; 65: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita M, Hayashi T, Ochiai M, et al. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem Biophys Res Commun 2014; 454: 53–57. [DOI] [PubMed] [Google Scholar]
- 17.Maas R, Dentz L, Schwedhelm E, et al. Elevated plasma concentrations of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine predict adverse events in patients undergoing noncardiac surgery. Crit Care Med 2007; 35: 1876–1881. [DOI] [PubMed] [Google Scholar]
- 18.Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64: 2406–2425. [DOI] [PubMed] [Google Scholar]
- 19.Sanders RD, Nicholson A, Lewis SR, et al. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7: Cd009971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Böger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 2004; 134: 2842S–2847S; discussion 2853S. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol 2014; 31: 517–573. [DOI] [PubMed] [Google Scholar]
- 22.Menke H, Klein A, John KD, et al. Predictive value of ASA classification for the assessment of the perioperative risk. Int Surg 1993; 78: 266–270. [PubMed] [Google Scholar]
- 23.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043–1049. [DOI] [PubMed] [Google Scholar]
- 24.Schwedhelm E, Maas R, Tan-Andresen J, et al. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 851: 211–219. [DOI] [PubMed] [Google Scholar]
- 25.Lüneburg N, Xanthakis V, Schwedhelm E, et al. Reference intervals for plasma L-arginine and the L-arginine: asymmetric dimethylarginine ratio in the Framingham Offspring Cohort. J Nutr 2011; 141: 2186–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwedhelm E, Xanthakis V, Maas R, et al. Asymmetric dimethylarginine reference intervals determined with liquid chromatography-tandem mass spectrometry: results from the Framingham offspring cohort. Clin Chem 2009; 55: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012; 380: 1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307: 2295–2304. [DOI] [PubMed] [Google Scholar]
- 29.Rodseth RN, Biccard BM, Le Manach Y, et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol 2014; 63: 170–180. [DOI] [PubMed] [Google Scholar]
- 30.Hassinger AB, Wainwright MS, Lane JC, et al. Elevated preoperative serum asymmetrical dimethylarginine (ADMA) is associated with poor outcomes after pediatric cardiac surgery. Intensive Care Med 2012; 38: 1697–1704. [DOI] [PubMed] [Google Scholar]
- 31.Plicner D, Mazur P, Sadowski J, et al. Asymmetric dimethylarginine and oxidative stress following coronary artery bypass grafting: associations with postoperative outcome. Eur J Cardiothorac Surg 2014; 45: e136–e141. [DOI] [PubMed] [Google Scholar]
- 32.Plicner D, Stoliński J, Wąsowicz M, et al. Preoperative values of inflammatory markers predict clinical outcomes in patients after CABG, regardless of the use of cardiopulmonary bypass. Indian Heart J 2016; 68: S10–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Böger RH, Bode-Böger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 1998; 98: 1842–1847. [DOI] [PubMed] [Google Scholar]
- 34.Böger RH, Sydow K, Borlak J, et al. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 2000; 87: 99–105. [DOI] [PubMed] [Google Scholar]
- 35.Böger RH, Tsikas D, Bode-Böger SM, et al. Hypercholesterolemia impairs basal nitric oxide synthase turnover rate: a study investigating the conversion of L-[guanidino-(15)N(2)]-arginine to (15)N-labeled nitrate by gas chromatography–mass spectrometry. Nitric Oxide 2004; 11: 1–8. [DOI] [PubMed] [Google Scholar]
- 36.Ekeloef S, Larsen MH, Schou-Pedersen AM, et al. Endothelial dysfunction in the early postoperative period after major colon cancer surgery. Br J Anaesth 2017; 118: 200–206. [DOI] [PubMed] [Google Scholar]
- 37.Böger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med 2006; 38: 126–136. [DOI] [PubMed] [Google Scholar]
- 38.Böger RH, Maas R, Schulze F, et al. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality–an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res 2009; 60: 481–487. [DOI] [PubMed] [Google Scholar]
- 39.Mochizuki S, Ono J, Yada T, et al. Systemic nitric oxide production rate during hemodialysis and its relationship with nitric oxide-related factors. Blood Purif 2005; 23: 317–324. [DOI] [PubMed] [Google Scholar]
- 40.Ragina N, Davis G, Doorly M, et al. Arginine/asymmetric dimethylarginine ratio in colorectal surgery. J Clin Med Res 2017; 9: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visser M, Davids M, Verberne HJ, et al. Nutrition before, during, and after surgery increases the arginine: asymmetric dimethylarginine ratio and relates to improved myocardial glucose metabolism: a randomized controlled trial. Am J Clin Nutr 2014; 99: 1440–1449. [DOI] [PubMed] [Google Scholar]
- 42.Schulman SP, Becker LC, Kass DA, et al. L-arginine therapy in acute myocardial infarction: the Vascular Interaction with Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA 2006; 295: 58–64. [DOI] [PubMed] [Google Scholar]
- 43.Wilson AM, Harada R, Nair N, et al. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation 2007; 116: 188–195. [DOI] [PubMed] [Google Scholar]
- 44.Tangphao O, Grossmann M, Chalon S, et al. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol 1999; 47: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Böger RH. L-Arginine therapy in cardiovascular pathologies: beneficial or dangerous? Curr Opin Clin Nutr Metab Care 2008; 11: 55–61. [DOI] [PubMed] [Google Scholar]
- 46.Böger RH, Ron ES. L-Arginine improves vascular function by overcoming deleterious effects of ADMA, a novel cardiovascular risk factor. Altern Med Rev 2005; 10: 14–23. [PubMed] [Google Scholar]