Abstract
Limb ischemia/reperfusion (I/R) can induce inflammation, causing acute lung injury. The Toll-like receptor 4 (TLR4)/NF-κB pathway plays an important role in acute and chronic inflammatory disorders. Several studies have demonstrated the efficacy of acupuncture in lung inflammatory injury. The aim of the present study was to elucidate the mechanism underlying the protective effect of electroacupuncture (EA) against lung injury induced by limb I/R. EA applied at the Zusanli and Sanyinjiao acupoints attenuated lung injury and decreased the secretion of inflammatory factors such as tumor necrosis factor-α, interleukin (IL)-1, IL-6 and myeloperoxidase. Moreover, the expression levels of TLR4 and NF-κB were suppressed by EA. Thus, the present findings suggested that EA can reduce pulmonary inflammation induced by limb I/R injury, possibly via the inhibition of the TLR4/NF-κB pathway.
Keywords: electroacupuncture, limb ischemia/reperfusion injury, Toll-like receptor 4, NF-κB
Introduction
Ischemia/reperfusion (I/R) injury of the limb occurs as a result of exposure to a large degree of blood flux in vascular reperfusion during hypoxia. I/R usually occurs following various types of trauma, including vascular injury, surgical procedures and extreme pressure (1). Limb I/R can damage the source limb, as well as remote organs. The lung is highly sensitive to inflammatory reactions and prone to acute injury during reperfusion (2). Lung inflammation involves complex mechanisms, including inflammatory reaction, oxidative stress and immune dysfunction (3,4).
It is crucial to identify that endogenous proteins that are produced after lung injury at the early phase of I/R (5). Toll-like receptors (TLRs) are a class of pattern recognition receptors expressed on immune and non-immune cell surfaces (6). In particular, TLR4 has been demonstrated to play a major role in the development of lung injury (7,8). Moreover, Activation of TLR4 stimulates downstream NF-κB, resulting in an increased release of downstream inflammatory cytokines, including interleukin 1 (IL)-1, IL-6 and tumor necrosis factor-α (TNF-α) (9).
Acupuncture is a traditional therapeutic technique. A variant of acupuncture, electroacupuncture (EA), has an anti-inflammatory effect on lung injury caused by inflammatory diseases (10). Our previous study suggested that EA pre-treatment could reduce the inflammatory reactions in patients with lung injury associated with limb I/R, as well as the associated release of inflammatory cytokines (11). However, the underlying mechanism remains unknown. Gong et al (12) demonstrated that EA attenuated limb I/R-induced lung injury via the p38 mitogen-activated protein kinase/nuclear factor erythroid 2-like 2/heme oxygenase 1 pathway. However, whether EA also reduces lung injury induced by limb I/R via the suppression the TLR4/NF-κB pathway has not yet been elucidated. Therefore, in the present study, a rat model of limb I/R-induced lung injury was used in order to assess whether EA applied at Zusanli (ST36) and Sanyinjiao (SP6) can inhibit inflammation and achieve a protective effect. In addition, the hypothesis that EA exerts an anti-inflammatory effect via the suppression of the TLR4/NF-κB pathway was also tested.
Materials and methods
Animals
Male Sprague-Dawley rats aged 7–8 weeks and weighing 250–300 g were acquired from The Animal Center of Wenzhou Medical University. Rats were housed in a temperature-controlled chamber with a 12-h light/dark cycle at 24°C and 50–60% relative humidity. All rats were provided standard chow and tap water ad libitum until they were made to fast for 12 h before experiments. All animal experiments were approved by The Animal Research Ethics Committee of Wenzhou Medical University and were performed in accordance with the guidelines of The National Institutes of Health for the Care and Use of Laboratory Animals (13).
Establishment of the limb I/R injured rat model
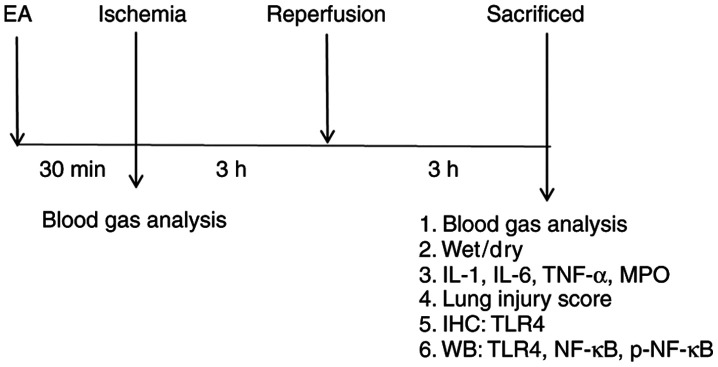
The rats (n=32) were randomly divided into four groups of eight rats each. The lower limb I/R method was adapted from a previous report (14). The treatment schedule is presented in Fig. 1. All rats in the study survived the treatment procedures. At the end of the experiment, the rats were intraperitoneally injected with 1 g/kg urethane and sacrificed by piercing of the heart and bleeding until cessation of breathing and heartbeat. In the I/R group, A rubber band (size 13 mm) above the greater trochanter was applied to interrupt the arterial blood for 3 h and then followed by 3 h of reperfusion; no drugs or EA treatment were administered. The band was put in place without being fastened in the sham group. In the sham EA (SEA) group, acupuncture needles were inserted 2–3 mm deep at the ST36 and SP6 acupoints, without stimulation. In the EA group, the rats received EA for 30 min before I/R, and the stimulation parameters comprised dispersed waves of 2 and 15 Hz at 1 mA. The ST36 acupoint is located 5 mm below the head of the fibula under the knee joint, and 2 mm lateral to the anterior tubercle of the tibia in the rat (15). SP6 is located 10 mm above the hindlimb medial malleolus front of the tibia and fibula (15). EA was performed using a LH202H Hans® Acupoint Nerve Stimulator (Hans Therapy, Co., Ltd.).
Figure 1.
Diagram of the experimental protocol. EA, electroacupunture; IL, interleukin; TNF-α, tumor necrosis factor-α; MPO, myeloperoxidase; IHC, immunohistochemistry; WB, western blotting; TLR4, Toll-like receptor 4; p-, phosphorylated.
Blood gas analysis
Left common carotid artery blood gas analysis (I-STAT 300, Abbott Pharmaceutical Co. Ltd.) was performed to measure the arterial partial pressure of oxygen (PaO2), base excess and hemoglobin (Hb) content. The derived variables included the arterial-alveolar oxygen tension ratio (a/A), alveolar-arterial oxygen tension difference (A-aO2) and respiratory index (RI).
Lung tissue wet/dry weight ratio
The right upper tissue of the lung in each rat was removed and weighed immediately after removal (wet weight). Sections were desiccated in an oven at 60°C for 2 days until a stable dry weight was reached. The wet/dry weight ratio was then calculated.
Histological analysis and lung injury score
Lungs were perfused with cold saline, followed by 4% paraformaldehyde. After fixation at 4°C for 24 h, the tissues were embedded in paraffin, cut into 5-µm sections, then stained with hematoxylin and eosin at room temperature for 5 min. The stained lung sections were observed under a light microscope (Leica Microsystems GmbH) at ×400 magnification by an experienced pathologist blinded to the protocols of the present study. Lung injury scores were assessed in a blinded manner and determined based on four independent parameters: i) Alveolar edema; ii) hemorrhage; iii) infiltration of inflammatory cells; and iv) thickened alveolar septum (16).
Measurement of lung IL-1, IL-6, TNF-α and myeloperoxidase (MPO) concentrations
The concentrations of IL-1, IL-6, TNF-α and MPO in the rat lung were measured using ELISA kits according to the manufacturer's instructions (Shanghai Boyun Biotechnology Co., Ltd.). The catalogue numbers for the ELISA kits were IL-1 (BP-E30419), IL-6 (BP-E30646), TNF-α (BP-E30635) and MPO (BP-E31651). The absorbance value at 450 nm was determined with a multifunctional microplate reader (Thermo Fisher Scientific, Inc.).
Immunohistochemistry
TLR4 expression in lung tissues was evaluated using immunohistochemistry. The lung samples were fixed in 4% paraformaldehyde at room temperature for 24 h. The next day the lungs were dehydrated in graded ethanol solution, embedded in paraffin for three times an hour at 55°C and embedded in a small stainless steel container. Sections were cut at 5 µm and were heated in an oven for 1 h, deparaffinized in xylene, rehydrated in graded ethanol solutions and microwaved at 100°C in sodium citrate buffer for 20 min. The slides were cooled to room temperature and incubated for 10 min in 3% H2O2. The sections were blocked using 5% donkey serum (Beijing Solarbio Science & Technology Co., Ltd.) at room temperature for 1 h, then incubated at 4°C overnight with primary antibody against TLR4 (1:100; cat. no. AF7017; Affinity Biosciences). The sections were subsequently washed three times with PBS every 5 min and incubated with 100 µl enzyme-labeled goat anti-rabbit IgG polymer (cat. no. PV-9001; OriGene Technologies, Inc.) for 30 min at 37°C. The samples were then incubated with 3,3′-diaminobenzidine substrate for 30 sec at room temperature. After dehydration and drying, the sections were mounted with neutral gum. Subsequently, the slides were examined under a light microscope (magnification, ×200).
An immunohistochemical score (IHS) was used to evaluate TLR4 expression (17)
The IHS accounts for the percentage of immunoreactive cells (quantity score) and the staining intensity (staining intensity score). The quantity score was assigned follows: i) No staining was scored as 0; ii) 1–10% of cells stained as 1; iii) 11–50% as 2; iv) 51–80% as 3; and v) 81–100% as 4. Staining intensity was rated on a scale of 0–3, where: i) 0 was negative; ii) 1 was weak; iii) 2 was moderate; and iv) 3 was strong. When there was multifocal immunoreactivity and there were significant differences in staining intensities between foci, the average of the least intense and most intense staining was recorded. The raw data were converted to the IHS by multiplying the quantity and staining intensity scores.
Western blot analysis
The lung tissues were dissociated using RIPA buffer (Beijing Solarbio Science & Technology Co., Ltd.) with protease (1:100) and phosphatase inhibitors (1:50). Extracts were homogenized, then centrifuged at 12,000 × g for 30 min at 4°C. Protein concentration was measured in the supernatant using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Inc.). Samples were boiled for 5 min at 95°C in 5X loading buffer. Protein (50 µg) was then separated by SDS-PAGE on 10% gels and then electro-transferred to a 0.22-µm PVDF membrane (Bio-Rad Laboratories, Inc.) for 1.5 h. The membranes were subsequently blocked with 5% bovine serum albumin (Biosharp Life Sciences) for 1 h at room temperature. Membranes were then incubated with primary antibodies on a table concentrator overnight at 4°C. Primary antibodies against β-actin (1:8,000; cat. no. AP0060; Bioworld Technology, Inc.), TLR4 (1:1,000; cat. no. AF7017; Affinity Biosciences), NF-κB (1:1,000; cat. no. AF5006; Affinity Biosciences) and phosphorylated (p)-NF-κB (1:1,000; cat. no. AF2006; Affinity Biosciences) were used. After washing the membranes three times with Tris-buffered saline plus 0.1% Tween-20, a horseradish peroxidase-conjugated secondary antibody (1:5,000; cat. no. BL003A; Biosharp Life Sciences) was added for 1.5 h at room temperature. The membranes were subsequently washed three times with Tris-buffered saline plus 0.1% Tween-20 and were visualized by electrochemiluminescence (cat. no. K-12045-D10; Advansta, Inc.). Images were analyzed by Image Lab Analysis System v6.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± SD from ≥6 independent experiments. Statistical analysis was conducted using one-way ANOVA, followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference. Analysis was performed using the SPSS v 19.0 software (IBM Corp.).
Results
EA pre-treatment at ST36 and SP6 alleviates limb I/R-induced lung injury in rats
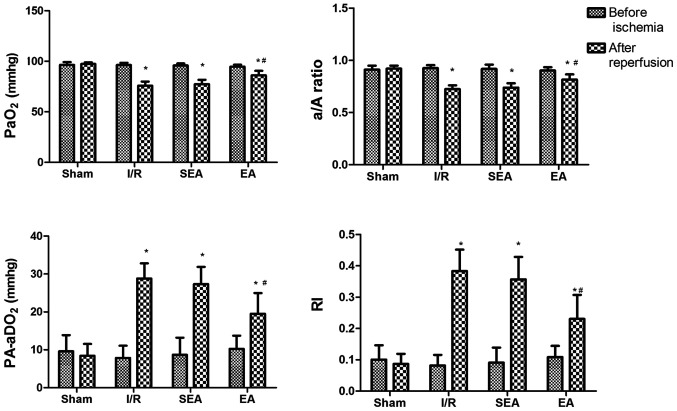
The protective effect of EA on lung injury induced by limb I/R was examined by blood gas analysis (PaO2, A-aO2, a/A ratio and RI). Before ischemia, the analyzed parameters did not differ between the sham, I/R, SEA and EA groups. Following 3 h of reperfusion, rats in the I/R, SEA and EA groups displayed significant pulmonary dysfunction, compared with the sham group, indicating that I/R-induced injury had been successfully established in this model. However, in the EA group, pulmonary oxygenation was significantly improved, compared with the I/R group, demonstrating the protective effect of EA pre-treatment against limb I/R-induced lung injury (Fig. 2).
Figure 2.
Blood gas analysis. PaO2, A-aO2, a/A ratio and RI were measured before and after ischemia. Data are presented as the mean ± SD. N=8 in each group. *P<0.05 vs. sham; #P<0.05 vs. I/R. PaO2, arterial partial pressure of oxygen; a/A, arterial-alveolar oxygen tension ratio; A-aO2, alveolar-arterial oxygen tension difference; RI, respiratory index; EA, electroacupuncture; I/R, ischemia/reperfusion; SEA, sham electroacupunture.
EA pre-treatment alleviates pulmonary injury in I/R-injured rats
To investigate the effect of EA pre-treatment on pulmonary inflammation induced by limb I/R injury, the wet/dry weight ratio and lung injury scores were compared between the sham, I/R, SEA and EA groups. The wet/dry weight ratio was significantly increased in the I/R and SEA groups compared with sham group. However, pre-treatment with E/A significantly reduced the wet/dry weight ratio compared with the I/R group (Fig. 3). Moreover, increased lung injury scores, edema of the lung interstitium and destruction of the alveolar architecture were observed in the I/R and SEA groups. However, lung injury scores and histopathology were significantly improved with EA pre-treatment, compared with the I/R group (Fig. 4). These results demonstrated that EA pre-treatment could exert an anti-inflammatory effect on limb I/R mediated lung injury.
Figure 3.
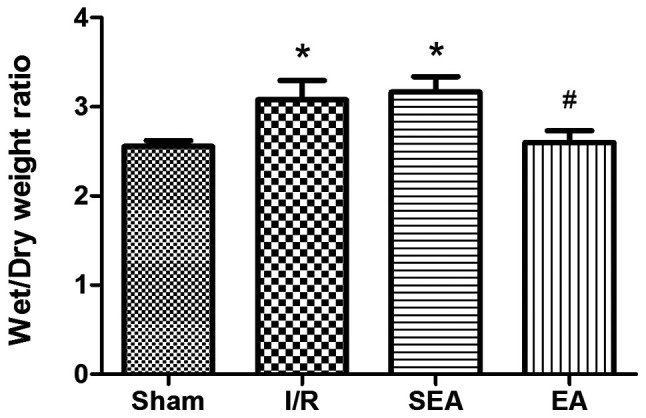
Wet/dry weight ratios. Wet/dry weight ratios were measured in each of the rats in all groups. Data are presented as the mean ± SD. N=8 in each group. *P<0.05 vs. sham; #P<0.05 vs. I/R. EA, electroacupuncture; I/R, ischemia/reperfusion; SEA, sham electroacupunture.
Figure 4.
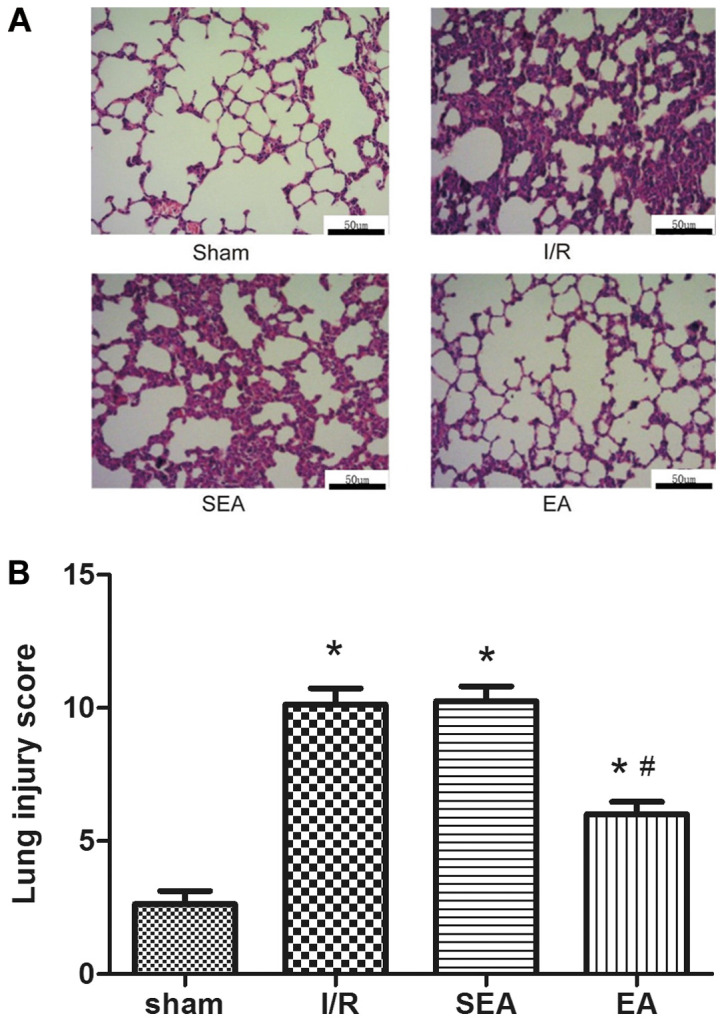
H&E staining and lung injury. (A) Representative photomicrographs of lung sections stained with H&E. Scale bar, 50 µm. (B) Lung injury scores. Data are presented as the mean ± SD. N=8 in each group. *P<0.05 vs. sham; #P<0.05 vs. I/R. EA, electroacupuncture; I/R, ischemia/reperfusion; SEA, sham electroacupunture; H&E, hematoxylin and eosin.
EA pre-treatment regulates secretion of inflammatory cytokines in limb I/R-injured rats
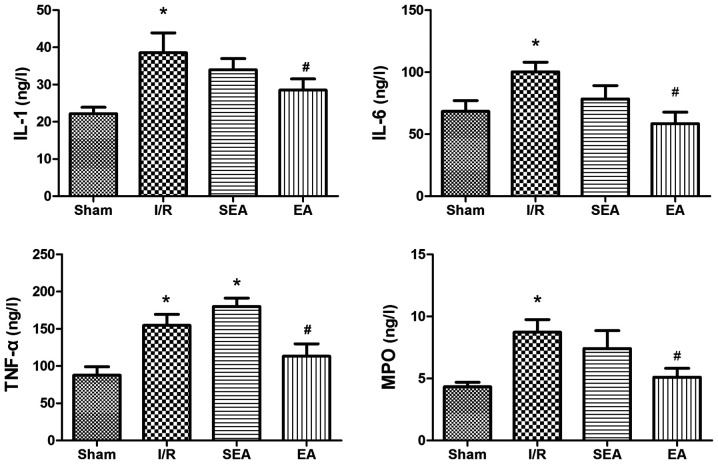
To examine the effects of EA pre-treatment on lung inflammation, the levels of inflammatory factors IL-1, IL-6, TNF-α and MPO were measured in pulmonary tissue using ELISA (Fig. 5). The secretion of these factors was significantly increased in the I/R and SEA groups compared with the sham group. However, EA pre-treatment effectively inhibited limb I/R-induced secretion of inflammatory cytokines IL-1, IL-6, TNF-α and MPO.
Figure 5.
Inflammatory cytokines and MPO. IL-1, IL-6, TNF-α and MPO levels were measured in the lung. Data are presented as the mean ± SD. N=8 in each group. *P<0.05 vs. sham; #P<0.05 vs. I/R. EA, electroacupuncture; I/R, ischemia/reperfusion; SEA, sham electroacupunture; IL, interleukin; TNF-α, tumor necrosis factor-α; MPO, myeloperoxidase.
EA pre-treatment suppresses activation of the TLR4/NF-κB pathway in limb I/R- injured rats
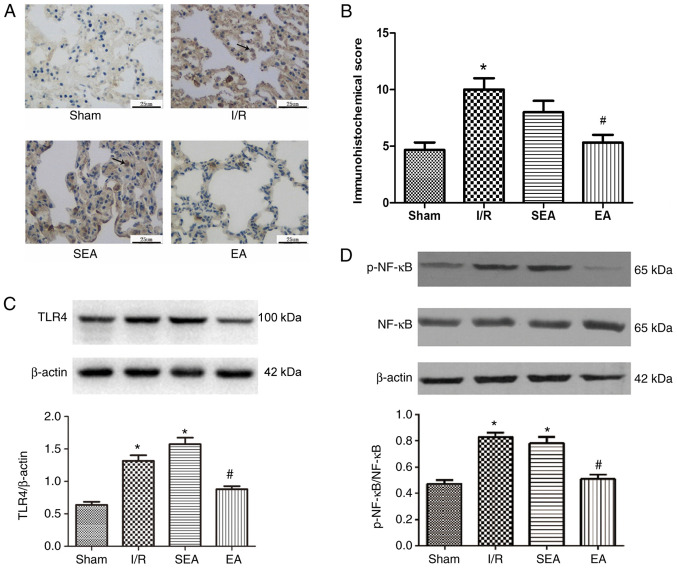
TLR4 plays a major role in the development of lung inflammatory injury. Activated TLR4 stimulates downstream NF-κB, resulting in increased release of inflammatory cytokines (18). To determine the mechanism underlying the anti-inflammatory property of EA, the effect of EA pre-treatment on the TLR4/NF-κB signaling pathway was examined in the injured lung (Fig. 6). Immunohistochemistry demonstrated that TLR4 expression was increased in the lung tissue of rats in the I/R and SEA groups, compared with the sham group. By contrast, the IHS was significantly reduced in the EA group, compared with the I/R group. Moreover, TLR4 and p-NF-κB expression levels were significantly increased in the I/R and SEA groups compared with the sham group. However, compared with the I/R group, the expression levels of TLR4 and p-NF-κB were significantly reduced in the EA group. These findings suggested that pre-treatment with EA at the ST36 and SP6 acupoints could suppress the activation of the TLR4/NF-κB signaling pathway, thus attenuating pulmonary inflammatory injury induced by limb I/R in rats.
Figure 6.
TLR4, p-NF-κB and NF-κB expression. (A) TLR4 expression in the lung was evaluated using (B) immunohistochemistry. Scale bar, 25 µm. Representative western blotting images and statistical analysis of (C) TLR4, (D) p-NF-κB and NF-κB expression levels in the lung. Data are presented as the mean ± SD. N=8 in each group. *P<0.05 vs. sham; #P<0.05 vs. I/R. EA, electroacupuncture; I/R, ischemia/reperfusion; SEA, sham electroacupunture, p, phosphorylated; TLR4, Toll-like receptor 4.
Discussion
In the present study, 30-min EA pre-conditioning significantly reduced the inflammatory response and release of inflammatory cytokines in the lungs of rats with limb I/R-induced injury. Moreover, the mechanism underlying this protective effect may be related to the TLR4/NF-κB signaling pathway.
Limb I/R can cause significant damage to distant organs, including lung tissue. Limb I/R-induced lung injury can occur following orthopedic surgery, major vascular injury, limb thrombosis and other surgical procedures (19). Previous studies have reported that I/R-induced lung injury resulted from the release of inflammatory cytokines, enhancement of oxidative stress and increased apoptosis during reperfusion (20). Furthermore, limb I/R could induce the activation of neutrophils, monocytes and macrophages, which can promote inflammation and release a large number of inflammatory mediators, including cytokines and chemokines (21). Inflammatory factors in the ischemic area and the extensive release of oxygen free radicals cause damage to distal organs and the lung is particularly susceptible to acute injury (22). Previous studies on animal models have demonstrated that the expression levels of inflammatory cytokines, such as TNF-α, IL-1, IL-6 and IL-8, are significantly increased after limb I/R (23–25). The occurrence of acute lung injury is closely related to the activation of various inflammatory factors; the extensive release of inflammatory cytokines is one of the most important factors that ultimately leads to the onset of systemic inflammatory response syndrome (24).
TNF-α is a key mediator of acute lung injury, acute respiratory distress syndrome and early lung tissue injury (26). During the lung injury process, TNF-α is primarily produced by alveolar macrophages, which can mobilize polymorphonuclear leukocytes in the blood to accumulate at lung injury sites, thus activating inflammatory cells and endothelial cells, and supporting the formation of factors such as endothelial cells and neutrophils that increase the expression levels of IL-1, IL-6 and IL-8 (27). TNF-α can also promote polymorphonuclear leukocyte degranulation and the release of lysosomes, mediate alveolar-capillary membrane damage and increase the function of the inflammatory cascade (28). IL-1 has a strong chemotactic effect, inducing the expression of vascular endothelial cell adhesion molecules, thus promoting leukocyte adhesion (29). Moreover, IL-1 primarily serves a role in chemoattraction, macrophage stimulation and increase of downstream cell and chemical factors. IL-6 is also involved in systemic immune responses and inflammatory cascades, and can activate neutrophils to exacerbate the production of inflammatory mediators after trauma, effectively enhancing the severity of tissue injury (30). MPO is produced by polymorphonuclear leukocytes, and the levels of active MPO reflect the activation of polymorphonuclear leukocytes in the lungs (31).
In the present study, the expression levels of proinflammatory cytokines, such as IL-1, IL-6 and TNF-α, were significantly elevated following I/R. This was accompanied by a reduction in intrapulmonary exchange function in the I/R group. The incidence of lung injury was also significantly increased, together with TLR4 and NF-κB expression levels. However, EA pre-treatment at the ST36 and SP6 points could significantly reduce the extent of lung injury, as well as the expression levels of inflammatory cytokines, TLR4 and NF-κB, while significantly improving lung exchange function. Therefore, these results suggested that EA pre-treatment had an anti-inflammatory effect, as well as a potential protective effect on the lungs.
EA, as a minimally invasive intervention, has been reported to have a significant anti-inflammatory effect in numerous diseases (32). ST36 and SP6 are two main anti-inflammatory EA points. Our previous clinical studies demonstrated that transcutaneous electrical acupoint stimulation at the ST36 and SP6 acupoints could inhibit the inflammatory response in the patients undergoing limb ischemia-reperfusion (33). Torres-Rosas et al (34) also revealed that EA pre-treatment at these acupoints could inhibit inflammation in a murine model of sepsis established by cecal ligation and puncture or lipopolysaccharide.
The TLR4/NF-κB pathway is one of the pathways of EA anti-inflammatory. Lan et al (35) suggested that EA at ST36 and Quchi points decreased the levels of inflammatory factors in the brain after reperfusion injury in a middle cerebral artery occlusion rat model, by inhibiting the TLR4/NF-κB pathway. Chen et al (36) also reported that EA pre-conditioning could reduce the occurrence of cognitive dysfunction caused by limb I/R, via the inhibition of microglial activity. Previous animal and clinical studies on inflammation-induced lung injury indicated that EA had an anti-inflammatory effect that contribute to lung protection (37,38). Similarly, the present study suggested that EA pre-treatment at the ST36 and SP6 points had a significant anti-inflammatory effect. Moreover, compared with the control group, lung function and lung injury indexes were signficantly improved, as were TLR4 and NF-κB expression levels. Collectively, the present findings suggested that the anti-inflammatory effect of EA could prevent lung injury and the underlying mechanism may be related to the regulation of the TLR4/NF-κB pathway in the lungs.
Previous studies have reported that the TLR4/NF-κB pathway plays a key role in lung injury leading to several inflammatory conditions, including septic, mechanical and I/R lung injury (39,40). Furthermore, inhibition of the TLR4/NF-κB pathway activity can greatly lower the incidence of lung injury (41). In the present study, limb I/R increased TLR4 and NF-κB expression levels in the lungs and induced the release of inflammatory cytokines, indicating that TLR4/NFκB was involved in the development of lung injury after limb I/R. A previous study reported that EA could significantly reduce TLR4 expression in cerebral I/R injury, thus inhibiting the occurrence of an inflammatory response in the brain (35). In the present study, it was also demonstrated that EA attenuated the expression levels of TLR4 and NF-κB in the lungs. Therefore, activation of the TLR4/NF-κB signaling pathway may be the primary mediator of lung injury after limb I/R. Moreover, the anti-inflammatory effect of EA may be mediated via the inhibition of the TLR4 signaling pathway, leading to a protective effect on the lung.
However, the present study had a number of limitations. TLR4 agonits were not included in the present study, as these reagents could potentially cause lung damage. Therefore, whether a TLR4 agonist could reverse the protective effects exerted by EA pre-treatment was not evaluated. In addition, the experiments described in the present study focused on TLR4 expression in the lungs and did not locate TLR4 receptors in other tissues.
In conclusion, the present study demonstrated that EA pre-treatment at the ST36 and SP6 acupoints improved lung oxygenation and exerted an anti-inflammatory effect on lung injury induced by limb I/R, which was via the inhibition of the TLR4/NF-κB pathway. Thus, the present findings suggested that EA may be used as a potentially effective therapy for lung injury induced by limb I/R.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Natural Science Foundation of China (grant nos. 81603685, 81573742, 81704180 and 81774109), The Zhejiang Province Natural Science Foundation (grans nos. LY19H290008 and LY15H290006) and The Wenzhou Municipal Science and Technology Bureau (grant no. 2018ZY003).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YM, JW and YL conceived and designed the study. QY, KX, MFB, YT, GL and CZ performed the experiments, analyzed the data, interpreted the experimental results, prepared the figures and drafted the manuscript. QD, WG and YM were involved in analyzing the data and revising the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental procedures and animal care were approved by The Animal Experimentation Ethics Committee of Wenzhou Medical University and were conducted in accordance with the Guidelines of The National Institutes of Health on the Care and Use of Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Xu YL, Zhang MH, Guo W, Xue Y, Du X, Zhang T, Wu N, Wu Y. MicroRNA-19 restores vascular endothelial cell function in lower limb ischemia-reperfusion injury through the KLF10-dependent TGF-β1/Smad signaling pathway in rats. J Cell Biochem. 2018;119:3225–9315. doi: 10.1002/jcb.27207. [DOI] [PubMed] [Google Scholar]
- 2.Tang B, Ma L, Yao X, Tan G, Han P, Yu T, Liu B, Sun X. Hydrogen sulfide ameliorates acute lung injury induced by infrarenal aortic cross-clamping by inhibiting inflammation and angiopoietin 2 release. J Vasc Surg. 2017;65:501–508.e1. doi: 10.1016/j.jvs.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Campanholle G, Landgraf RG, Gonçalves GM, Paiva VN, Martins JO, Wang PH, Monteiro RM, Silva RC, Cenedeze MA, Teixeira VP, et al. Lung inflammation is induced by renal ischemia and reperfusion injury as part of the systemic inflammatory syndrome. Inflamm Res. 2010;59:861–869. doi: 10.1007/s00011-010-0198-0. [DOI] [PubMed] [Google Scholar]
- 4.Liao WI, Wu SY, Wu GC, Pao HP, Tang SE, Huang KL, Chu SJ. Ac2-26, an Annexin A1 Peptide, Attenuates Ischemia-Reperfusion-Induced Acute Lung Injury. Int J Mol Sci. 2017;18:18. doi: 10.3390/ijms18081771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu MJ, Liu BJ, Wang CL, Wang GH, Tian Y, Wang SH, Li J, Li PY, Zhang RH, Wei D, et al. Epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor and effectively alleviates acute lung injury induced by H9N2 swine influenza virus. Int Immunopharmacol. 2017;52:24–33. doi: 10.1016/j.intimp.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Areal H, Abrantes J, Esteves PJ. Signatures of positive selection in Toll-like receptor (TLR) genes in mammals. BMC Evol Biol. 2011;11:368. doi: 10.1186/1471-2148-11-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherif IO, Al-Shaalan NH. Vildagliptin Attenuates Hepatic Ischemia/Reperfusion Injury via the TLR4/NF-κB Signaling Pathway. Oxid Med Cell Longev. 2018;2018:3509091. doi: 10.1155/2018/3509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Zhu X, Chen J, Yang S, Sun R, Yang G. The interaction between Toll-like receptor 4 signaling pathway and hypoxia-inducible factor 1α in lung ischemia-reperfusion injury. J Surg Res. 2014;188:290–297. doi: 10.1016/j.jss.2013.11.1086. [DOI] [PubMed] [Google Scholar]
- 9.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 10.Yunhe F, Bo L, Xiaosheng F, Fengyang L, Dejie L, Zhicheng L, Depeng L, Yongguo C, Xichen Z, Naisheng Z, et al. The effect of magnolol on the Toll-like receptor 4/nuclear factor κB signaling pathway in lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2012;689:255–261. doi: 10.1016/j.ejphar.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 11.Zijlstra FJ, van den Berg-de Lange I, Huygen FJ, Klein J. Anti-inflammatory actions of acupuncture. Mediators Inflamm. 2003;12:59–69. doi: 10.1080/0962935031000114943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong LR, Kan YX, Lian Y, Dong SA, Zhao DH, Shi J, Yu JB. Electroacupuncture Attenuates Limb Ischemia- Reperfusion-Induced Lung Injury Via p38 Mitogen-Activated Protein Kinase-Nuclear Factor Erythroid-2-Related Factor-2/Heme Oxygenase Pathway. J Surg Res. 2020;246:170–181. doi: 10.1016/j.jss.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Carbone L. Pain management standards in the eighth edition of the Guide for the Care and Use of Laboratory Animals. J Am Assoc Lab Anim Sci. 2012;51:322–328. [PMC free article] [PubMed] [Google Scholar]
- 14.Yassin MM, Harkin DW, Barros D'Sa AA, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002;26:115–121. doi: 10.1007/s00268-001-0169-2. [DOI] [PubMed] [Google Scholar]
- 15.Santos EL, Dias BH, Andrade AC, Pascoal AM, Vasconcelos Filho FE, Medeiros F, Guimarães SB. Effects of acupuncture and electroacupuncture on estradiol-induced inflammation and oxidative stress in health rodents. Acta Cir Bras. 2013;28:582–588. doi: 10.1590/S0102-86502013000800005. [DOI] [PubMed] [Google Scholar]
- 16.Chen CM, Wang LF, Su B, Hsu HH. Methylprednisolone effects on oxygenation and histology in a rat model of acute lung injury. Pulm Pharmacol Ther. 2003;16:215–220. doi: 10.1016/S1094-5539(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 17.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::AID-CNCR17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Peng LY, Shi HT, Yuan M, Li JH, Song K, Huang JN, Yi PF, Shen HQ, Fu BD. Madecassoside Protects Against LPS-Induced Acute Lung Injury via Inhibiting TLR4/NF-κB Activation and Blood-Air Barrier Permeability. Front Pharmacol. 2020;11:807. doi: 10.3389/fphar.2020.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao YR, Wang D, Liu Y, Shan L, Zhou JL. The PI3K/Akt, p38MAPK, and JAK2/STAT3 signaling pathways mediate the protection of SO2 against acute lung injury induced by limb ischemia/reperfusion in rats. J Physiol Sci. 2016;66:229–239. doi: 10.1007/s12576-015-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Men X, Han S, Gao J, Cao G, Zhang L, Yu H, Lu H, Pu J. Taurine protects against lung damage following limb ischemia reperfusion in the rat by attenuating endoplasmic reticulum stress-induced apoptosis. Acta Orthop. 2010;81:263–267. doi: 10.3109/17453671003587085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou WC, Kao MC, Yue CT, Tsai PS, Huang CJ. Caffeine Mitigates Lung Inflammation Induced by Ischemia-Reperfusion of Lower Limbs in Rats. Mediators Inflamm. 2015;2015:361638. doi: 10.1155/2015/361638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou H, Sun X. Post-treatment curcumin reduced ischemia-reperfusion-induced pulmonary injury via the Notch2/Hes-1 pathway. J Int Med Res. 2019;2019:300060519892432. doi: 10.1177/0300060519892432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240–245. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 24.Zhao YR, Lv WR, Zhou JL. Role of carbonyl sulfide in acute lung injury following limb ischemia/reperfusion in rats. Eur J Med Res. 2017;22:12. doi: 10.1186/s40001-017-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Zhou C, Jiang J, Su Q, Ding X. Blockade of HMGB1 preserves vascular homeostasis and improves blood perfusion in rats of acute limb ischemia/reperfusion. Microvasc Res. 2017;112:37–40. doi: 10.1016/j.mvr.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Yu S, Xie J, Xiang Y, Dai S, Yu D, Sun H, Chen B, Zhou M. Downregulation of TNF-α/TNF-R1 Signals by AT-Lipoxin A4 May Be a Significant Mechanism of Attenuation in SAP-Associated Lung Injury. Mediators Inflamm. 2019;2019:9019404. doi: 10.1155/2019/9019404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Campos T, Deree J, Coimbra R. From acute pancreatitis to end-organ injury: Mechanisms of acute lung injury. Surg Infect (Larchmt) 2007;8:107–120. doi: 10.1089/sur.2006.011. [DOI] [PubMed] [Google Scholar]
- 28.Yang WS, Lee JM, Han NJ, Kim YJ, Chang JW, Park SK. Mycophenolic acid attenuates tumor necrosis factor-alpha-induced endothelin-1 production in human aortic endothelial cells. Atherosclerosis. 2010;211:48–54. doi: 10.1016/j.atherosclerosis.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Tan J, Liu Y, Song L, Li D, Cui X. Amelioration of lung ischemia reperfusion injury by JNK and p38 small interfering RNAs in rat pulmonary microvascular endothelial cells in an ischemia reperfusion injury lung transplantation model. Mol Med Rep. 2018;17:1228–1234. doi: 10.3892/mmr.2017.7985. [DOI] [PubMed] [Google Scholar]
- 30.Pimenta MB, Aguilar-Nascimento JE, Martins DC, Silva DR, Bacelo KL, Bocchese IC, Zaffani S, Zaffani E, Silveira EA, Carmo AV, Ferreira SS. The intestinal tract as the major source of interleukin 6 production during abdominal aortic clamping and hind limb ischaemia-reperfusion injury. Acta Cir Bras. 2007;22(Suppl 1):34–39. doi: 10.1590/S0102-86502007000700008. [DOI] [PubMed] [Google Scholar]
- 31.Schmekel B, Seveus L, Xu SY, Venge P. Human neutrophil lipocalin (HNL) and myeloperoxidase (MPO). Studies of lung lavage fluid and lung tissue. Respir Med. 2000;94:564–568. doi: 10.1053/rmed.2000.0776. [DOI] [PubMed] [Google Scholar]
- 32.Jin H, Guo J, Liu J, Lyu B, Foreman RD, Yin J, Shi Z, Chen JDZ. Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2017;313:G192–G202. doi: 10.1152/ajpgi.00254.2016. [DOI] [PubMed] [Google Scholar]
- 33.Mo Y, Chen S, Yang L, Huang L, Jin D, Yu Z, Wang L, Wang L, Luo S, Wang J. The Effect of Transcutaneous Electrical Acupoint Stimulation on Inflammatory Response in Patients Undergoing Limb Ischemia-Reperfusion. Mediators Inflamm. 2017;2017:8369737. doi: 10.1155/2017/8369737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan L, Tao J, Chen A, Xie G, Huang J, Lin J, Peng J, Chen L. Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int J Mol Med. 2013;31:75–80. doi: 10.3892/ijmm.2012.1184. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Zhou J, Li J, Yang SB, Mo LQ, Hu JH, Yuan WL. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012;1432:36–45. doi: 10.1016/j.brainres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Geng WY, Liu ZB, Song NN, Geng WY, Zhang GH, Jin WZ, Li L, Cao YX, Zhu DN, Shen LL. Effects of electroacupuncture at Zusanli (ST36) on inflammatory cytokines in a rat model of smoke-induced chronic obstructive pulmonary disease. J Integr Med. 2013;11:213–219. doi: 10.3736/jintegrmed2013024. [DOI] [PubMed] [Google Scholar]
- 38.Yu JB, Dong SA, Luo XQ, Gong LR, Zhang Y, Wang M, Cao XS, Liu DQ. Role of HO-1 in protective effect of electro-acupuncture against endotoxin shock-induced acute lung injury in rabbits. Exp Biol Med (Maywood) 2013;238:705–712. doi: 10.1177/1535370213489487. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Q, Wu J, Lin Z, Hua Q, Zhang W, Ye L, Wu G, Du J, Xia J, Chu M, et al. Resolvin D1 Alleviates the Lung Ischemia Reperfusion Injury via Complement, Immunoglobulin, TLR4, and Inflammatory Factors in Rats. Inflammation. 2016;39:1319–1333. doi: 10.1007/s10753-016-0364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramani V, Madhusoodhanan R, Kosanke S, Awasthi S. A TLR4-interacting SPA4 peptide inhibits LPS-induced lung inflammation. Innate Immun. 2013;19:596–610. doi: 10.1177/1753425912474851. [DOI] [PubMed] [Google Scholar]
- 41.Standiford LR, Standiford TJ, Newstead MJ, Zeng X, Ballinger MN, Kovach MA, Reka AK, Bhan U. TLR4-dependent GM-CSF protects against lung injury in Gram-negative bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L447–L454. doi: 10.1152/ajplung.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.