Abstract
Single-atom catalysts (SACs) have attracted widespread interest for many catalytic applications because of their distinguishing properties. However, general and scalable synthesis of efficient SACs remains significantly challenging, which limits their applications. Here we report an efficient and universal approach to fabricating a series of high-content metal atoms anchored into hollow nitrogen-doped graphene frameworks (M-N-Grs; M represents Fe, Co, Ni, Cu, etc.) at gram-scale. The highly compatible doped ZnO templates, acting as the dispersants of targeted metal heteroatoms, can react with the incoming gaseous organic ligands to form doped metal–organic framework thin shells, whose composition determines the heteroatom species and contents in M-N-Grs. We achieved over 1.2 atom % (5.85 wt %) metal loading content, superior oxygen reduction activity over commercial Pt/C catalyst, and a very high diffusion-limiting current (6.82 mA cm–2). Both experimental analyses and theoretical calculations reveal the oxygen reduction activity sequence of M-N-Grs. Additionally, the superior performance in Fe-N-Gr is mainly attributed to its unique electron structure, rich exposed active sites, and robust hollow framework. This synthesis strategy will stimulate the rapid development of SACs for diverse energy-related fields.
Short abstract
An efficient and universal approach to fabricating a series of high-content metal atoms anchored into hollow nitrogen-doped graphene frameworks is developed via a nicely designed process.
Introduction
Single atom catalysts (SACs) featuring the maximum atom utilization efficiency, high electron conductivity, and high activity of metal centers have gained significant attention in the field of heterogeneous electrocatalysis, with particular interest in atomic metal–nitrogen moieties supported on carbon substrates (M-N-Cs).1−5 The well-designed nanostructured SACs with the hope to boost the electrochemical reaction process on active metal sites can provide fast mass transport/electron transfer needed for the desired reactions.6−11 Achieving a series of stable SACs with a high metal loading, however, presents a great challenge due to their intrinsically high surface free energy and ease of aggregation.12−14 The design principle toward high-performance SACs is therefore aiming to simultaneously increase the number and reactivity of the exposed active sites.15−17
Intensive efforts have been made in the exploration and development of synthetic methodologies for M-N-Cs in the past.18−21 A series of M-N-Cs were synthesized through a conventional impregnation approach, where the metal atoms are stabilized on the carbon substrate with thermal annealing treatment after the impregnation of metal ions.7,22,23 This impregnation method endowed the resulting SACs with tunable compositions, high electron conductivity, fast mass transport, and exposed active site. Yet, the low metal loading in M-N-Cs from this process led to limited numbers of active sites, deteriorating their electrocatalytic activity. In an attempt to increase the mass loading of SACs, direct pyrolysis of metal ions-doped Zn-containing metal–organic framework (MOF) crystals has been proposed. The volatilization of low boiling point Zn in the pyrolysis leads to the formation of SACs located in bulk nitrogen-doped carbon frameworks.24−27 Although the MOF-derived approach provided a relatively unique direction toward preparing SACs, the obtained SACs usually exhibit sluggish mass transport and low electrocatalytic activity because most of their active sites are buried inside the carbon framework arising from the solution synthesis of the precursors as well as the limited species and concentrations of doped metal ions in MOFs. Although photochemical,28,29 high-temperature atom trapping,30,31 and atomic layer deposition32,33 with a rather sophisticated and specialized procedure have also been reported for creating SAC, their large-scale production toward practical application is severely limited due to the low yield and limited metal species together with the high cost and complicated process. Therefore, it is highly desired to develop a facile, yet universal and low-cost strategy to endow the prepared M-N-Cs with rich exposed active sites.
Herein, employing highly compatible doped ZnO solid solutions as new precursors, we develop a facile and universal method to successfully construct a series of single metal atoms (Fe, Co, Ni, and Cu, for example) anchored in hollow nitrogen-doped graphene frameworks (M-N-Grs) with high metal loadings over 1.2 atom % for ORR application (Figure S1). During the whole formation process, the metal heteroatoms in doped ZnO solid solutions are well dispersed into thin doped metal–organic framework (MOF) shells via an interfacial coordination reaction with gaseous organic ligands and are stabilized into nitrogen-doped graphene lattices by controlled pyrolysis and template removal. The spherical aberration correction electron microscopy and extended X-ray absorption fine structure measurements have both confirmed the atomic dispersion and local atomic coordination configuration of M-N-Grs. The resultant M-N-Grs with similar morphologies and metal loadings displayed the ORR activity sequence of Fe > Co > Cu > Ni. Furthermore, theoretical calculations on their already known atomic configurations reveal that the catalytic activities toward the whole ORR pathways are highly determined by the electron structures and atomic coordination configurations of single metals in SACs, which are in accordance with the experimental results. These understandings give in-depth insights into the structure–property correlation and shed light on designing advanced SACs for specific reactions.
Results and Discussion
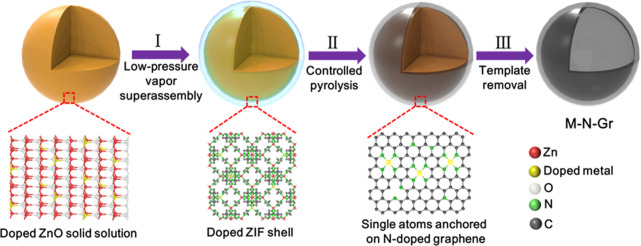
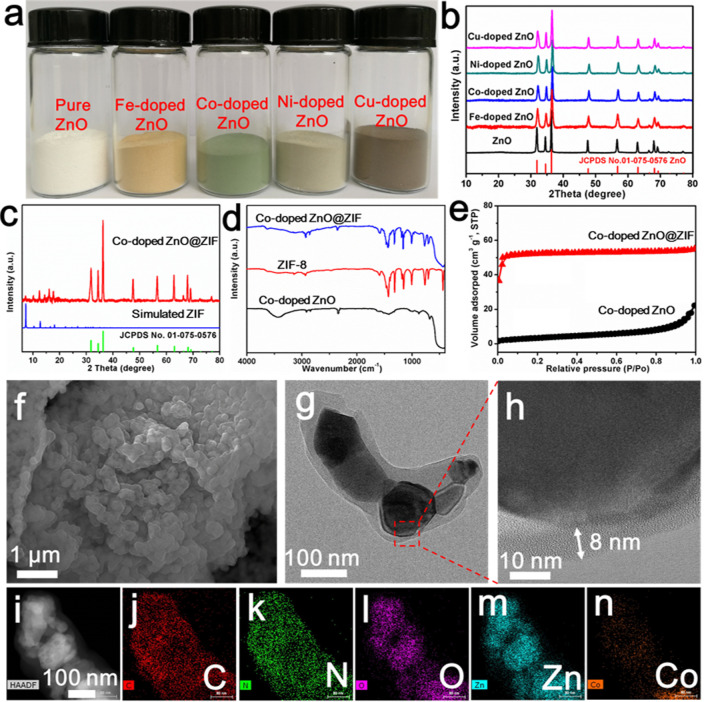
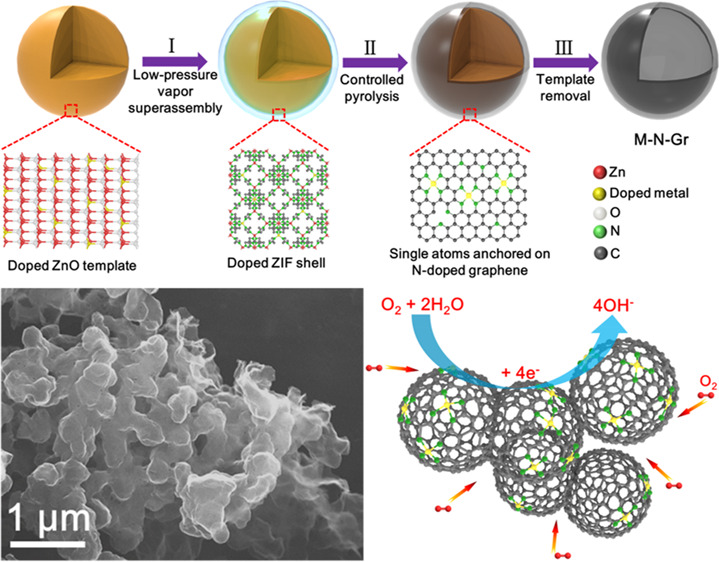
The overall formation process of M-N-Grs is illustrated in Figure 1, involving a well-designed three-step process. First, highly compatible doped ZnO solid solutions were used as new precursors.34,35 For demonstration, a series of Fe-, Co-, Ni-, Cu-doped ZnO nanoparticles were synthesized by a high-yield sol–gel method.36 As shown in the optical image, each doped ZnO sample shows one specific color, which is attributed to the variation of their doped metal ion-induced bandgaps (Figure 2a and Figure S2). X-ray diffraction (XRD) patterns of doped ZnO samples show all diffraction peaks of pure ZnO without any impurity, indicating the formation of well-defined substitutional solid solutions (Figure 2b). These doped metal ions possessed a similar radius with Zn ions, guaranteeing the relatively high doping concentration of the resulting doped ZnO solid solutions (Table S1). For example, the Co-doped ZnO nanoparticles exhibit an average diameter around 200 nm and a good dispersity (Figure S3a–f). The scanning transmission electron microscopy (STEM) and energy-dispersive X-ray spectroscopy (EDS) mapping images further confirmed the uniform distribution of Zn, Co, and O elements in single nanoparticles (Figure S3g–j). Then, the as-prepared doped ZnO samples were treated by a controllable low-pressure vapor superassembly.37,38 Under low pressure (∼50 Pa) and high temperature (140 °C), the gaseous organic ligands (2-methylimidazole, 2-MIM) fill the whole reaction system and react with the doped ZnO nanoparticles, resulting in the in situ formation of thin doped zeolitic imidazolate framework (ZIF) shells. The proposed reaction process is described as follows: doped ZnO (s) + 2-MIM (g) → doped ZnO@ZIF (s) + H2O (g). During this process, the doped atoms in ZnO experience the breaking of previous metal–oxygen bonds and form new coordination bonds with organic ligands, resulting in their further dispersion due to the formation of expanded open frameworks. Because of the interfacial (solid–gas) reaction mechanism, these doped ions are well dispersed into the newly formed MOFs without selectivity, which is superior to traditional solution methods of doped MOFs. As shown in the EDS spectrum, the existence of a Co signal on the outer shell further indicates the formation of a Co-doped ZIF shell on Co-doped ZnO template (Figure S4). Subsequently, after controlled pyrolysis, the doped ZIF shells are converted into uniform single doped atoms anchored on thin N-doped graphene shells (M-N-Grs) because of the volatilization of low-boiling-point zinc, resulting in the formation of the core–shell structure (doped ZnO@M-N-Grs). Eventually, the templates are removed by hydrochloric acid and heat treatment. Morphology-preserved, relatively high yield, thin, and robust M-N-Grs are obtained (Figure S5). In brief, this template implantation method experiences the oriented growth of doped MOFs on doped ZnO templates by low-pressure vapor superassembly, in situ formation of M-N-Grs on them via controlled pyrolysis, and subsequent template removal.
Figure 1.
Schematic illustration of the formation process of M-N-Grs from doped ZnO solid solutions.
Figure 2.
Characterizations of doped ZnO solid solutions and the formation of the ZIF shell. The optical image (a) and XRD patterns (b) of pure ZnO and various doped ZnO samples. (c) XRD pattern of Co-doped ZnO@ZIF nanoparticles. (d) FTIR spectra of Co-doped ZnO nanoparticles, Co-doped ZnO@ZIF nanoparticles, and ZIF-8 nanocrystals. (e) N2 adsorption–desorption isotherms of Co-doped ZnO nanoparticles and Co-doped ZnO@ZIF nanoparticles. SEM image (f), TEM image (g), and HRTEM image (h) of Co-doped ZnO@ZIF nanoparticles. (i–n) HAADF-STEM image and the corresponding EDS elemental maps of Co-doped ZnO@ZIF nanoparticles for C, N, O, Zn, and Co.
In this strategy, the oriented growth of doped ZIF shells determines the formation of the M-N-Grs. Taking Co-doped ZnO (Co0.03Zn0.97O) nanoparticles as an example, a series of characterizations were performed to confirm the formation of doped ZIF shells. XRD patterns displayed all diffraction peaks from ZnO and simulated ZIF, indicating the existence of ZIF (Figure 2c). Fourier-transform infrared (FTIR) spectra further showed the typical vibrations of ZIF in those ZnO@ZIF nanoparticles (Figure 2d). The specific surface area of the obtained Co-doped ZnO@ZIF nanoparticles was around 165.6 m2 g–1, much larger than that of the initial Co-doped ZnO nanoparticles (12.2 m2 g–1) (Figure 2e). The variation of their corresponding Langmuir isotherms from type II to I indicated the formation of the microporous ZIF shells (Figure S6). SEM and TEM images showed inner template nanoparticles and outer integrated ZIF shells with an 8 nm thickness (Figure 2f–h). Furthermore, a high-angle annular dark-field STEM (HAADF-STEM) image and the corresponding EDS mapping images revealed the core–shell structure (Figure 2i–n).
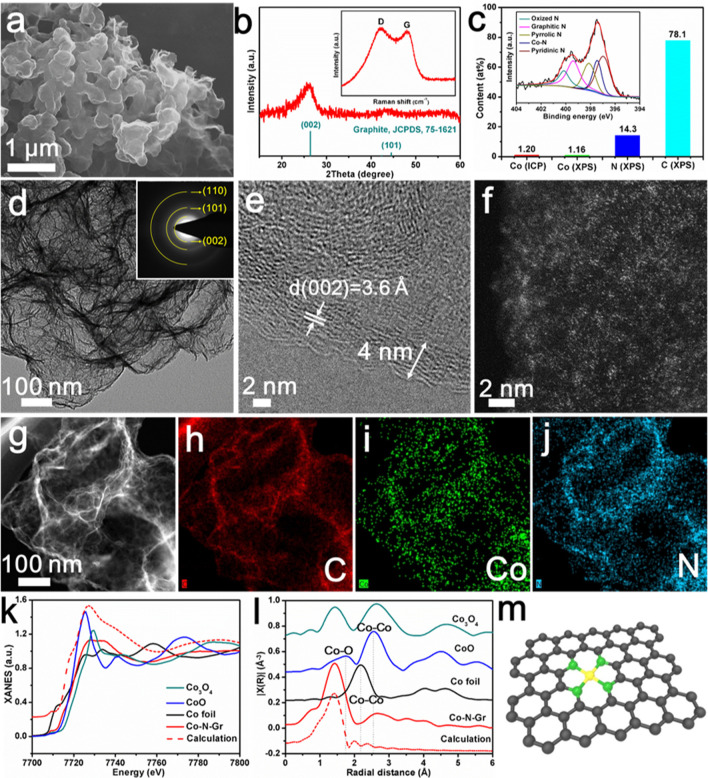
Figure 3 shows the structural characterizations of the resulting Co-N-Gr sample. SEM images displayed the interconnected graphene framework (Figure 3a and Figure S7). Some broken framework revealed the interior hollow characteristics. The XRD pattern of Co-N-Gr showed only two diffraction peaks located at around 26.0° and 44.0°, corresponding to the (020) and (101) planes from graphite, respectively (Figure 3b). No signals from Co nanoparticles were detected, suggesting good dispersion of Co atoms in the product. The Raman spectrum of Co-N-Gr showed two characteristic bands located at approximately 1350 and 1600 cm–1, corresponding to the D band (disoriented carbon) and G band (graphitic carbon), respectively (inset of Figure 3b). A high ID/IG band intensity ratio further demonstrated the defect-rich structure of Co-N-Gr. The nitrogen adsorption–desorption analysis showed a high specific surface area around 794.3 m2 g–1 (Figure S7e,f). Furthermore, the atomic concentration of Co in Co-N-Gr obtained by X-ray photoelectron spectroscopy (XPS) was 1.16 atom %, slightly lower than that from inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis (1.2 atom %, 5.85 wt %) due to the surface detection property of XPS (Figure 3c). The nitrogen content from XPS analysis was up to 14.3 atom %, beneficial for high metal loading. TEM images showed the thin shell was composed of small discontinuous graphitic layers with a shell thickness of ∼4 nm and interlayer spacings of ∼3.6 Å (Figure 3d,e). Furthermore, a “Z-contrast” HAADF-STEM image displayed well-dispersed Co atoms as brighter dots on the darker carbon-based substrate (Figure 3f). The EDS mapping results (Figure 3g–j) demonstrate the uniform distribution of Co and N elements on the selected hollow graphene framework in Co-N-Gr. In addition, the X-ray absorption near-edge structure (XANES) spectra displayed that the peak position of Co-N-Gr was located between Co foil and CoO, indicating that the valence state of Co atoms lay between Co0 and CoII (Figure 3k). The corresponding Fourier transforms (FT) obtained from the extended X-ray absorption fine structure (EXAFS) showed that the main peak of Co-N-Gr was located at approximately 1.4 Å (Figure 3l), shorter than the Co–O peak at 1.72 Å in standard CoO, suggesting the formation of Co–N bonds in the Co-N-Gr sample. In contrast, no Co–Co peak or other high-shell peaks were observed in Co-N-Gr, confirming the isolated Co atoms. According to the fitting parameters, the average coordination number of Co-N in Co-N-Gr was about 4.0, demonstrating its Co-N4 structure (Figure 3m).
Figure 3.
Structural characterizations of the Co-N-Gr sample. (a) SEM image. (b) XRD pattern and Raman spectrum (inset). (c) Elemental content of Co-N-Gr obtained from XPS and ICP-AES measurements, and high-resolution N 1s XPS spectrum (inset) divided into five Voigt-type line-shaped peaks. (d) TEM image and SAED pattern (inset). (e) HRTEM image. (f) High-solution HAADF-STEM image. (g–j) HAADF-STEM image and the corresponding EDS elemental maps: C (red), Co (green), and N (cyan). Co K-edge XANES spectra (k) and Fourier transform (FT) of the EXAFS (l) of Co-N-Gr, Co, CoO, Co3O4, and calculated Co-N-Gr. (m) Schematic model of Co-N-Gr, including C (black), N (green), and Co (yellow) atoms.
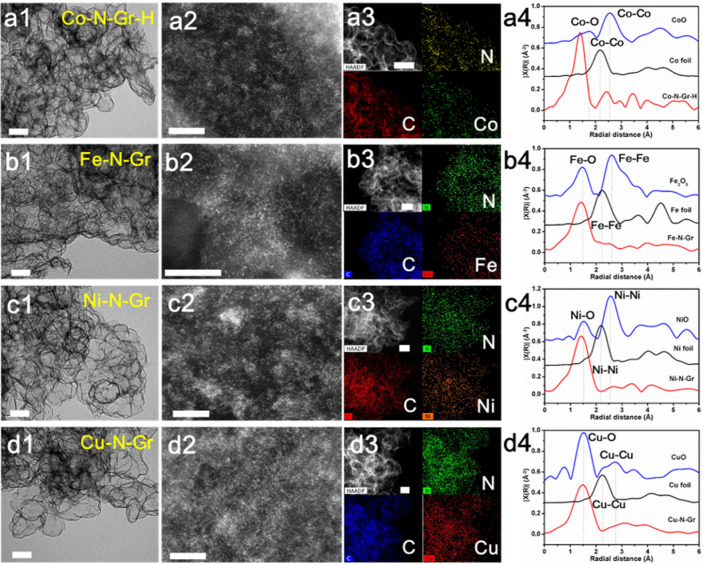
To confirm the generality of the template transformation strategy, a series of single metal atoms anchored in hollow graphene frameworks from various doped ZnO solid solutions were obtained according to the aforementioned process, including high-concentration Co-N-Gr (Co-N-Gr-H), Fe-N-Gr, Ni-N-Gr, and Cu-N-Gr. SEM and TEM images displayed similar interconnected graphene frameworks (Figure 4 and Figures S8–S15). HAADF-STEM images and FT-EXAFS spectra both showed the atomically dispersed metal atoms in M-N-Grs. The average coordination numbers of metal-N in Fe-N-Gr, Ni-N-Gr, and Cu-N-Gr were fitted to be 4.1, 3.8, and 3.1, respectively (Figure S16 and Table S2). Because their coordination numbers were close to 4.0, both Fe-N-Gr and Ni-N-Gr possessed an M-N4 structure and the same atomic configuration with Co-N-Gr, consistent with many previous works.22,39 The low coordination number of Cu-N in Cu-N-Gr could be attributed to its complex atomic structures, including not only main Cu-N4 but also partial Cu-N3 and Cu-N2 structures.40,41 Furthermore, the high-resolution Cu 2p XPS spectrum of Cu-N-Gr exhibits two distinct split peaks, which is different from those in other SACs (Figure S17). This phenomenon demonstrates the complex atomic structures in Cu-N-Gr. In addition, the weak zinc signals in these catalysts indicated very low zinc concentration. Although the zinc atoms cannot be fully removed, a similar and very low zinc concentration in these catalysts has a negligible influence on their electrocatalytic performance. These already known well-defined atomic configurations of M-N-Grs offered ideal platforms to establish the relationships between the atomic structures and the catalytic properties in both experimental characterizations and theoretical calculations.
Figure 4.
The broad extension of the template transformation method. (a1–a4) TEM image, HR-HAADF-STEM image, STEM image and corresponding EDS elemental maps, and FT-EXAFS spectra of Co-N-Gr-H. (b1–b4) TEM image, HR-HAADF-STEM image, STEM image and corresponding EDS elemental maps, and FT-EXAFS spectra of Fe-N-Gr. (c1–c4) TEM image, HR-HAADF-STEM image, STEM image and corresponding EDS elemental maps, and FT-EXAFS spectra of Ni-N-Gr. (d1–d4) TEM image, HR-HAADF-STEM image, STEM image and corresponding EDS elemental maps, and FT-EXAFS spectra of Cu-N-Gr. Scale bars: 100 nm (a1, b1, cl, d1), 5 nm (a2, b2, c2, d2), 100 nm (a3, b3, c3, d3).
In addition, extortionate doping concentration of isolated metal atoms on nitrogen-doped carbon-based supports led to the instability of the obtained SACs. When Co0.1Zn0.9O nanoparticles with higher doping concentration were synthesized and selected as templates, the resulting product displayed the formation of some nanoparticles (Figure S18). Even after the acid treatment, the existence of some nanoparticles in the derived product is attributed to the protection of confined carbon coatings and further aggregation of metal atoms in SACs during the high-temperature process. Therefore, for the formation of well-defined and stable SACs, the selected doped ZnO nanocrystals should fulfill two prerequisites as ideal templates: (1) the well-defined solid solution formation of doped metal ions in ZnO lattices, and (2) appropriate doping concentrations. For our strategy, the whole transformation efficiency of doped metal atoms from doped ZnO solid solutions to single-atom catalysts is dependent on the content of doped ZIF shells during the low-pressure vapor superassembly process. Although the transformation efficiency is relatively low due to the formation of thin doped ZIF shells, a hollow graphene framework can be efficiently obtained. Therefore, for our synthesis strategy, it is a trade-off between a thin hollow framework and transformation efficiency. Compared with other previously reported methods for SACs, this strategy exhibits wide adaptability, relatively high yield, and high metal loading content, showing great potential in the development of SACs in frontier fields (Table S3).22,26,27,29,32,42,43 In addition, compared with previously reported MOF-derived SACs, higher metal concentration SACs using this template transformation strategy are mainly attributed to the high-concentration doped ZIF shell and the stabilization effect and atom implantation from doped ZnO cores.
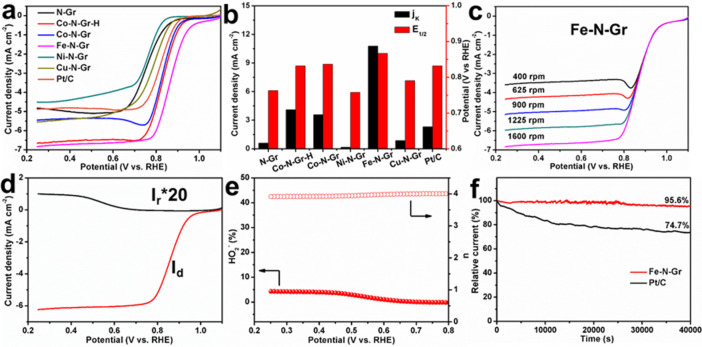
The metal centers, doping concentrations, and coordination configurations in SACs play significant roles in their ORR performances. The Co-N-Gr, Fe-N-Gr, Ni-N-Gr, and Cu-N-Gr showed similar metal loadings, confirming the effects of different metal centers on their ORR performances. Moreover, compared with Co-N-Gr (1.2 atom %), the Co-N-Gr-H sample with a higher metal concentration of 2.03 atom % was used to reveal the importance of doping concentrations. To evaluate the ORR catalytic activity of the obtained M-N-Grs, cyclic voltammograms (CVs) were first measured in O2-saturated 0.1 M KOH solution (Figures S19 and S20). The Fe-N-Gr sample displayed a remarkable oxygen reduction peak at 0.878 V versus a reversible hydrogen electrode (RHE). The ORR activity was also investigated by steady-state linear sweep voltammetry (LSV) on a rotating disk electrode (RDE) (Figure 5a). The Fe-N-Gr sample exhibited the best ORR activity in terms of the most positive onset potential (Eonset) and half-wave potential (E1/2). The E1/2 values of Fe-N-Gr, N-Gr, Co-N-Gr-H, Co-N-Gr, Ni-N-Gr, Cu-N-Gr, and commercial Pt/C were 0.87, 0.76, 0.83, 0.84, 0.76, 0.79, and 0.83 V, respectively. The Co-N-Gr-H catalyst possessed a higher diffusion-limiting current than Co-N-Gr, indicating that a higher metal loading resulted in more active sites and thus higher current density. Further, the corresponding kinetic current densities (Jk) were calculated using the Koutecky–Levich (K-L) equation.44 The Fe-N-Gr sample displayed a superior Jk of 10.8 mA cm–2 at 0.85 V, much higher than that of commercial Pt/C (2.3 mA cm–2) and N-Gr (0.6 mA cm–2) (Figure 5b). The average electron transfer number (n) was around 4.01, suggesting an obvious 4e– ORR pathway (Figure 5c).45 In terms of on-set potential, half-wave potential, and kinetic current density, the poor catalytic activity of N-Gr strongly demonstrated the critical role of isolated doping iron atoms in the Fe-N-Gr catalyst for superior ORR performance. On the basis of the rotational ring-disk electrode (RRDE) measurement, the H2O2 yield of Fe-N-Gr catalyst was calculated to be below 5% within the 0.2–0.8 V range, and the corresponding calculated n ranged from 3.9 to 4.0 (Figure 5d,e). When tested by chronoamperometry after 40 000 s at 0.7 V and 1600 rpm, the Fe-N-Gr catalyst exhibited a higher current retention (95.6%) than that of Pt/C catalyst (74.7%) (Figure 5f). The LSV curves before and after long-term durability test almost overlapped, indicating its outstanding stability (Figure S19c).
Figure 5.
ORR electrocatalytic performances of various metal atoms anchored on hollow graphene frameworks. (a) LSV curves of N-Gr, Co-N-Gr-H, Co-N-Gr, Fe-N-Gr, Ni-N-Gr, Cu-N-Gr, and commercial Pt/C in O2-saturated 0.1 M KOH at a scan rate of 5 mV·s–1 and 1600 rpm. (b) The corresponding Jk at 0.85 V and E1/2. (c) LSV curves of Fe-N-Gr in O2-saturated 0.1 M KOH at a scan rate of 5 mV·s–1 and different rotation rates. (d) RRDE voltammograms recorded with Fe-N-Gr in O2-saturated 0.1 M KOH at 1600 rpm. (e) Peroxide yields and electron numbers of Fe-N-Gr at various potentials based on RRDE data. (f) Chronoamperometric curves of Fe-N-Gr and Pt/C in O2-saturated 0.1 M KOH at 1600 rpm and 0.7 V versus RHE.
To examine the crossover effect of methanol, the Pt/C catalyst showed a sharp decrease in the reduction current after injecting methanol in KOH solution during the chronoamperometry, while the Fe-N-Gr catalyst displayed no reduction current change, indicating excellent tolerance against methanol (Figure S21). Moreover, after the durability test, the Fe-N-Gr sample retained robust hollow graphene frameworks and well-isolated Fe atoms (Figure S22). The above-mentioned experimental results suggest our Fe-N-Gr catalyst is a promising catalyst among the obtained SACs toward ORR in alkaline media and also surpasses most of the reported nonprecious metal catalysts (Table S4). Furthermore, electron paramagnetic resonance (EPR) spectroscopy was performed to identify its electron structure (Figure S23a). The obvious peaks from the EPR spectra at 77 and 2 K were assigned to the unpaired electrons in Fe atoms, which were regarded as the active sites for many potential electrocatalytic reactions.46,47 In addition, from ultraviolet photoelectron spectroscopy (UPS) analysis, the Fe-N-Gr catalyst exhibited a much lower work function of 4.1 eV than that of N-Gr (4.7 eV), greatly facilitating the movement of the donated electrons from the catalyst to the reaction molecules and thus accelerating the whole ORR process (Figure S23b). In brief, the catalytic activity and reaction pathway for the whole ORR process are highly determined by the metal centers of M-N-Grs.
Density functional theory (DFT) calculation of a four-electron ORR process (details in Supporting Information) revealed the difference among the dopant atoms (Fe, Co, Ni, and Cu) with similar metal-N4 structures.48 The different free energy diagrams of the reaction pathway are illustrated (Figure 6a and Tables S5 and S6). It seemed that step (ii) became the rate-determining step with the highest energy uphill with the maximum 1.27 eV for Cu-N-Gr and 1.20 eV for Ni-N-Gr, respectively, followed by Co-N-Gr (0.96 eV) and the minimal 0.75 eV for Fe doped one. It is worth noting the energy barrier of Fe-N-Gr at step (v) as slightly higher than that at step (ii) with 0.78 versus 0.75 eV, which was still much lower than the highest energy barriers for other dopant atoms. Thus, the Fe dopant endowed the graphene sheet with the best ORR catalytic performance among different doping atoms, consistent with experiments.48 Finally, we should note that the calculated maximum energy barrier of 1.27 V at step (ii) suggested the slightly worse electrocatalytic performance of Cu-N-Gr than that of Ni-N-Gr. Our DFT result agrees with a previous DFT study (Fe > Co > Ni > Cu).49 However, the discrepancy of Cu-N-Gr between calculations and experiments might be attributed to its multiple possible structures. It has been reported that not only the present 4-pyridine-N structure but also other forms of Cu-anchored graphene sheet can be synthesized, which may have a different catalytic performance than the former.41
Figure 6.
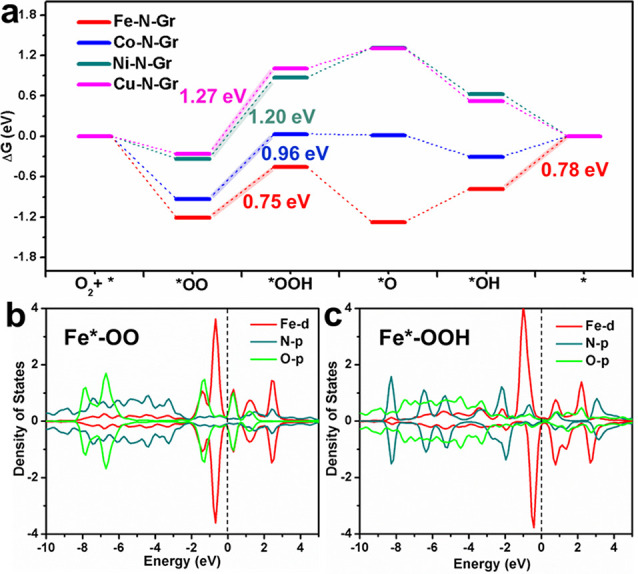
DFT simulations on ORR. (a) Free energy diagram on the M-N-Gr surface at equilibrium potential (U = 1.23 V). Blue, green, black, and red lines represent the doping atoms of Fe, Co, Ni, and Cu, respectively. Partial density of states (PDOS) of Fe doped intermediate stages for Fe*-OO (b) and Fe-*OOH (c). The red, blue, and green curves illustrated the PDOS of metal atoms’ d-orbitals and oxygen and nitrogen atoms’ p-orbitals. The Fermi level located at E = 0 eV.
Partial density of state (PDOS) results (Figure 6b,c and Figure S24) were plotted to reveal the reaction mechanism. The strong overlap of the metal’s d-orbitals with the oxygens’ p-orbital in the *-OO intermediate indicated the binding strength of O2 molecules onto the M-N-Gr substance. Hence, a great energy downhill happens at step (i) for Fe and Co dopant (ΔG = −1.20 or −0.93 eV) in contrast with the Ni-doped or Cu-doped ones with a lower extent of orbitals overlap. For the *-OO intermediate, the orbital overlap of Co-N-Gr was found to be stronger than that of Fe-N-Gr (Figure 6c and Figure S24b), suggesting a lower formation free energy. However, the barrier was still lower for the Fe doped one at step (ii), indicating an exceptional electrocatalytic performance in experiments. It may be attributed to the resulting peak shift of Fe’s d-orbitals in the *-OO intermediate (Figure 6c). Moreover, the equilibrium metal–oxygen bond distances for different intermediates are summarized in Table S7. Compared with other M-N-Grs, the intermediates of Fe-N-Gr exhibited shorter equilibrium metal–oxygen binding distances, which might be the origin of its highest ORR activity (Figure S25).50,51 It revealed that the highest energy uphill of Ni-N-Gr at step (ii) may originate from the largest bonding distance (2.71 Å), compared to 1.75 Å for the Fe doped one. Then, with the assistance of proton adsorption (*-OOH intermediate), the bond distance in Ni-N-Gr recovered to about 2.13 Å, still larger than that in Fe-N-Gr (1.78 Å). Thus, the extra free energy was required for the forward movement of oxygen atoms, while it essentially elevated the free energy of Ni*-OOH above the horizontal line (ΔG = 0 eV in Figure 6a), indicating the most inferior electrocatalytic performance. A similar phenomenon was also observed for the Cu-N-Gr substance. Therefore, the large metal–oxygen overlaps and short equilibrium metal–oxygen distances of intermediates in Fe-N-Gr may simultaneously promote the reaction kinetics for oxygen reduction.
Conclusion
In summary, a facile and universal method, which can be potentially applied to obtain various SACs with tunable metal centers and concentrations from specific doped ZnO solid solution precursors, was developed for the first time. The template transformation mechanism was clearly revealed. Four representative catalysis atoms, including Fe, Co, Ni, and Cu, were chosen and anchored in hollow nitrogen-doped graphene frameworks, whose coordination configurations were evidenced by X-ray absorption fine structure analysis. Systematic electrochemical experiments and theoretical calculations uncovered that the catalytic activities toward the whole ORR pathways are highly determined by the electron structures and atomic coordination configurations of single metal centers. Our work paves the way toward the rational design and synthesis of highly efficient SACs for broad frontier applications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51832004 and 51521001), the National Key Research and Development Program of China (2016YFA0202603), the Natural Science Foundation of Hubei Province (2019CFA001), the Programme of Introducing Talents of Discipline to Universities (B17034), and the Yellow Crane Talent (Science & Technology) Program of Wuhan City. Use of the Advanced Photon Source (9-BM), an Office of Science user facilities, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c00458.
The experimental details on the synthesis and characterization of M-N-Gr; computation details; schematic illustration of M-N-Gr for ORR; characterizations of doped ZnO solid solutions and doped ZnO@ZIF nanoparticles; characterizations of M-N-Gr samples; yield evaluation of the Co-N-Gr product; ORR electrocatalytic performances of M-N-Gr samples; PDOS results of M-N-Gr samples; atomic configurations and structure details for ORR intermediates; comparison of synthesis methods and ORR catalytic performances (PDF)
Author Contributions
# J.M., J. Li, and J. Liu contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Jiao Y.; Zheng Y.; Jaroniec M.; Qiao S. Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. 10.1039/C4CS00470A. [DOI] [PubMed] [Google Scholar]
- Dai L.; Xue Y.; Qu L.; Choi H. J.; Baek J. B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. 10.1021/cr5003563. [DOI] [PubMed] [Google Scholar]
- Yang X. F.; Wang A. Q.; Qiao B. T.; Li J.; Liu J. Y.; Zhang T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. 10.1021/ar300361m. [DOI] [PubMed] [Google Scholar]
- Jin H.; Liu X.; Chen S.; Vasileff A.; Li L.; Jiao Y.; Song L.; Zheng Y.; Qiao S.-Z. Heteroatom-Doped Transition Metal Electrocatalysts for Hydrogen Evolution Reaction. ACS Energy Lett. 2019, 4, 805–810. 10.1021/acsenergylett.9b00348. [DOI] [Google Scholar]
- Liu J.; Zhu D.; Zheng Y.; Vasileff A.; Qiao S.-Z. Self-supported earth-abundant nanoarrays as efficient and robust electrocatalysts for energy-related reactions. ACS Catal. 2018, 8, 6707–6732. 10.1021/acscatal.8b01715. [DOI] [Google Scholar]
- Zhang M.; Wang Y. G.; Chen W.; Dong J.; Zheng L.; Luo J.; Wan J.; Tian S.; Cheong W. C.; Wang D.; Li Y. Metal (hydr)oxides@polymer core-shell strategy to metal single-atom materials. J. Am. Chem. Soc. 2017, 139, 10976–10979. 10.1021/jacs.7b05372. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Jia Y.; Gao G.; Yan X.; Chen N.; Chen J.; Soo M. T.; Wood B.; Yang D.; Du A.; Yao X. Graphene defects trap atomic Ni species for hydrogen and oxygen evolution reactions. Chem. 2018, 4, 285–297. 10.1016/j.chempr.2017.12.005. [DOI] [Google Scholar]
- Zhang J.; Zhao Z.; Xia Z.; Dai L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. 10.1038/nnano.2015.48. [DOI] [PubMed] [Google Scholar]
- Zhu C.; Shi Q.; Xu B. Z.; Fu S.; Wan G.; Yang C.; Yao S.; Song J.; Zhou H.; Du D.; Beckman S. P.; Su D.; Lin Y. Hierarchically porous M-N-C (M = Co and Fe) single-atom electrocatalysts with robust MNx active moieties enable enhanced ORR performance. Adv. Energy Mater. 2018, 8, 1801956. 10.1002/aenm.201801956. [DOI] [Google Scholar]
- Zhao Y.; Ling T.; Chen S.; Jin B.; Vasileff A.; Jiao Y.; Song L.; Luo J.; Qiao S.-Z. Non-metal single-iodine-atom electrocatalysts for the hydrogen evolution reaction. Angew. Chem., Int. Ed. 2019, 58, 12252–12257. 10.1002/anie.201905554. [DOI] [PubMed] [Google Scholar]
- Tang C.; Jiao Y.; Shi B.; Liu J.-N.; Xie Z.; Chen X.; Zhang Q.; Qiao S.-Z. Coordination tunes selectivity: Two-electron oxygen reduction on high-loading molybdenum single-atom catalysts. Angew. Chem., Int. Ed. 2020, 59, 9171–9176. 10.1002/anie.202003842. [DOI] [PubMed] [Google Scholar]
- Wang A. Q.; Li J.; Zhang T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. 10.1038/s41570-018-0010-1. [DOI] [Google Scholar]
- Liu J. Y. Catalysis by supported single metal atoms. ACS Catal. 2017, 7, 34–59. 10.1021/acscatal.6b01534. [DOI] [Google Scholar]
- Liu L.; Corma A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. 10.1021/acs.chemrev.7b00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.; Fu S.; Shi Q.; Du D.; Lin Y. Single-atom electrocatalysts. Angew. Chem., Int. Ed. 2017, 56, 13944–13960. 10.1002/anie.201703864. [DOI] [PubMed] [Google Scholar]
- Kirk C.; Chen L. D.; Siahrostami S.; Karamad M.; Bajdich M.; Voss J.; Nørskov J. K.; Chan K. Theoretical investigations of the electrochemical reduction of CO on single metal atoms embedded in graphene. ACS Cent. Sci. 2017, 3, 1286–1293. 10.1021/acscentsci.7b00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D.; Zhuang Z.; Cao X.; Zhang C.; Peng Q.; Chen C.; Li Y. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem. Soc. Rev. 2020, 49, 2215–2264. 10.1039/C9CS00869A. [DOI] [PubMed] [Google Scholar]
- Hulsey M. J.; Zhang J.; Yan N. Harnessing the wisdom in colloidal chemistry to make stable single-atom catalysts. Adv. Mater. 2018, 30, 1802304. 10.1002/adma.201802304. [DOI] [PubMed] [Google Scholar]
- Qin R.; Liu P.; Fu G.; Zheng N. Strategies for stabilizing atomically dispersed metal catalysts. Small Methods 2018, 2, 1700286. 10.1002/smtd.201700286. [DOI] [Google Scholar]
- Yang H. B.; Hung S.-F.; Liu S.; Yuan K.; Miao S.; Zhang L.; Huang X.; Wang H.-Y.; Cai W.; Chen R.; Gao J.; Yang X.; Chen W.; Huang Y.; Chen H. M.; Li C. M.; Zhang T.; Liu B. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147. 10.1038/s41560-017-0078-8. [DOI] [Google Scholar]
- Wang B.; Wang X.; Zou J.; Yan Y.; Xie S.; Hu G.; Li Y.; Dong A. Simple-cubic carbon frameworks with atomically dispersed iron dopants toward high-efficiency oxygen reduction. Nano Lett. 2017, 17, 2003–2009. 10.1021/acs.nanolett.7b00004. [DOI] [PubMed] [Google Scholar]
- Fei H.; Dong J.; Feng Y.; Allen C. S.; Wan C.; Volosskiy B.; Li M.; Zhao Z.; Wang Y.; Sun H.; An P.; Chen W.; Guo Z.; Lee C.; Chen D.; Shakir I.; Liu M.; Hu T.; Li Y.; Kirkland A. I.; Duan X.; Huang Y. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72. 10.1038/s41929-017-0008-y. [DOI] [Google Scholar]
- Fei H.; Dong J.; Arellano-Jimenez M. J.; Ye G.; Dong Kim N.; Samuel E. L.; Peng Z.; Zhu Z.; Qin F.; Bao J.; Yacaman M. J.; Ajayan P. M.; Chen D.; Tour J. M. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668. 10.1038/ncomms9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. X.; Pei J. J.; He C. T.; Wan J. W.; Ren H. L.; Zhu Y. Q.; Wang Y.; Dong J. C.; Tian S. B.; Cheong W. C.; Lu S. Q.; Zheng L. R.; Zheng X. S.; Yan W. S.; Zhuang Z. B.; Chen C.; Peng Q.; Wang D. S.; Li Y. D. Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction. Angew. Chem., Int. Ed. 2017, 56, 16086–16090. 10.1002/anie.201710599. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Hwang S.; Wang M.; Feng Z.; Karakalos S.; Luo L.; Qiao Z.; Xie X.; Wang C.; Su D.; Shao Y.; Wu G. Single atomic iron catalysts for oxygen reduction in acidic media: Particle size control and thermal activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. 10.1021/jacs.7b06514. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Ji S.; Wang Y.; Dong J.; Chen W.; Li Z.; Shen R.; Zheng L.; Zhuang Z.; Wang D.; Li Y. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2017, 56, 6937–6941. 10.1002/anie.201702473. [DOI] [PubMed] [Google Scholar]
- Yin P.; Yao T.; Wu Y.; Zheng L.; Lin Y.; Liu W.; Ju H.; Zhu J.; Hong X.; Deng Z.; Zhou G.; Wei S.; Li Y. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem., Int. Ed. 2016, 55, 10800–10805. 10.1002/anie.201604802. [DOI] [PubMed] [Google Scholar]
- Liu P. X.; Zhao Y.; Qin R. X.; Mo S. G.; Chen G. X.; Gu L.; Chevrier D. M.; Zhang P.; Guo Q.; Zang D. D.; Wu B. H.; Fu G.; Zheng N. F. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 2016, 352, 797–801. 10.1126/science.aaf5251. [DOI] [PubMed] [Google Scholar]
- Wei H.; Huang K.; Wang D.; Zhang R.; Ge B.; Ma J.; Wen B.; Zhang S.; Li Q.; Lei M.; Zhang C.; Irawan J.; Liu L. M.; Wu H. Iced photochemical reduction to synthesize atomically dispersed metals by suppressing nanocrystal growth. Nat. Commun. 2017, 8, 1490. 10.1038/s41467-017-01521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.; Li A.; Liu J. C.; Li Z.; Chen W.; Gong Y.; Zhang Q.; Cheong W. C.; Wang Y.; Zheng L.; Xiao H.; Chen C.; Wang D.; Peng Q.; Gu L.; Han X.; Li J.; Li Y. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 2018, 13, 856–861. 10.1038/s41565-018-0197-9. [DOI] [PubMed] [Google Scholar]
- Jones J.; Xiong H. F.; Delariva A. T.; Peterson E. J.; Pham H.; Challa S. R.; Qi G. S.; Oh S.; Wiebenga M. H.; Hernandez X. I. P.; Wang Y.; Datye A. K. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. 10.1126/scienceaaf8800. [DOI] [PubMed] [Google Scholar]
- Cheng N.; Stambula S.; Wang D.; Banis M. N.; Liu J.; Riese A.; Xiao B.; Li R.; Sham T. K.; Liu L. M.; Botton G. A.; Sun X. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. 10.1038/ncomms13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H.; Cheng H.; Yi H.; Lin Y.; Yao T.; Wang C.; Li J.; Wei S.; Lu J. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 2015, 137, 10484–10487. 10.1021/jacs.5b06485. [DOI] [PubMed] [Google Scholar]
- Pan F.; Song C.; Liu X.; Yang Y.; Zeng F. Ferromagnetism and possible application in spintronics of transition-metal-doped ZnO films. Mater. Sci. Eng., R 2008, 62, 1–35. 10.1016/j.mser.2008.04.002. [DOI] [Google Scholar]
- Park S. Y.; Kim B. J.; Kim K.; Kang M. S.; Lim K. H.; Lee T. I.; Myoung J. M.; Baik H. K.; Cho J. H.; Kim Y. S. Low-temperature, solution-processed and alkali metal doped ZnO for high-performance thin-film transistors. Adv. Mater. 2012, 24, 834–838. 10.1002/adma.201103173. [DOI] [PubMed] [Google Scholar]
- Ba-Abbad M. M.; Kadhum A. A. H.; Bakar Mohamad A.; Takriff M. S.; Sopian K. The effect of process parameters on the size of ZnO nanoparticles synthesized via the sol-gel technique. J. Alloys Compd. 2013, 550, 63–70. 10.1016/j.jallcom.2012.09.076. [DOI] [Google Scholar]
- Meng J.; Liu X.; Li J.; Li Q.; Zhao C.; Xu L.; Wang X.; Liu F.; Yang W.; Xu X.; Liu Z.; Niu C.; Mai L. General oriented synthesis of precise carbon-confined nanostructures by low-pressure vapor superassembly and controlled pyrolysis. Nano Lett. 2017, 17, 7773–7781. 10.1021/acs.nanolett.7b03982. [DOI] [PubMed] [Google Scholar]
- Meng J.; Liu X.; Niu C.; Pang Q.; Li J.; Liu F.; Liu Z.; Mai L. Advances in metal-organic framework coatings: Versatile synthesis and broad applications. Chem. Soc. Rev. 2020, 49, 3142–3186. 10.1039/C9CS00806C. [DOI] [PubMed] [Google Scholar]
- Deng D. H.; Chen X. Q.; Yu L.; Wu X.; Liu Q. F.; Liu Y.; Yang H. X.; Tian H. F.; Hu Y. F.; Du P. P.; Si R.; Wang J. H.; Cui X. J.; Li H. B.; Xiao J. P.; Xu T.; Deng J.; Yang F.; Duchesne P. N.; Zhang P.; Zhou J. G.; Sun L. T.; Li J. Q.; Pan X. L.; Bao X. H. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 2015, 1, e1500462. 10.1126/sciadv.1500462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.; Han G.-F.; Noh H.-J.; Kim S.-J.; Lu Y.; Jeong H. Y.; Fu Z.; Baek J.-B. Boosting oxygen reduction catalysis with abundant copper single atom active sites. Energy Environ. Sci. 2018, 11, 2263–2269. 10.1039/C8EE01169A. [DOI] [Google Scholar]
- Wu H.; Li H.; Zhao X.; Liu Q.; Wang J.; Xiao J.; Xie S.; Si R.; Yang F.; Miao S.; Guo X.; Wang G.; Bao X. Highly doped and exposed Cu(I)–N active sites within graphene towards efficient oxygen reduction for zinc–air batteries. Energy Environ. Sci. 2016, 9, 3736–3745. 10.1039/C6EE01867J. [DOI] [Google Scholar]
- Qu Y.; Li Z.; Chen W.; Lin Y.; Yuan T.; Yang Z.; Zhao C.; Wang J.; Zhao C.; Wang X.; Zhou F.; Zhuang Z.; Wu Y.; Li Y. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat. Catal. 2018, 1, 781–786. 10.1038/s41929-018-0146-x. [DOI] [Google Scholar]
- Fei H.; Dong J.; Wan C.; Zhao Z.; Xu X.; Lin Z.; Wang Y.; Liu H.; Zang K.; Luo J.; Zhao S.; Hu W.; Yan W.; Shakir I.; Huang Y.; Duan X. Microwave-assisted rapid synthesis of graphene-supported single atomic metals. Adv. Mater. 2018, 30, 1802146. 10.1002/adma.201802146. [DOI] [PubMed] [Google Scholar]
- Li M. F.; Zhao Z. P.; Cheng T.; Fortunelli A.; Chen C. Y.; Yu R.; Zhang Q. H.; Gu L.; Merinov B. V.; Lin Z. Y.; Zhu E. B.; Yu T.; Jia Q. Y.; Guo J. H.; Zhang L.; Goddard W. A.; Huang Y.; Duan X. F. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. 10.1126/science.aaf9050. [DOI] [PubMed] [Google Scholar]
- Wang Z. L.; Xu D.; Zhong H. X.; Wang J.; Meng F. L.; Zhang X. B. Gelatin-derived sustainable carbon-based functional materials for energy conversion and storage with controllability of structure and component. Sci. Adv. 2015, 1, e1400035. 10.1126/sciadv.1400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H. T.; Cullen D. A.; Higgins D.; Sneed B. T.; Holby E. F.; More K. L.; Zelenay P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–483. 10.1126/science.aan2255. [DOI] [PubMed] [Google Scholar]
- Zitolo A.; Goellner V.; Armel V.; Sougrati M. T.; Mineva T.; Stievano L.; Fonda E.; Jaouen F. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942. 10.1038/nmat4367. [DOI] [PubMed] [Google Scholar]
- Li F.; Shu H.; Hu C.; Shi Z.; Liu X.; Liang P.; Chen X. Atomic mechanism of electrocatalytically active Co-N complexes in graphene basal plane for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 27405–27413. 10.1021/acsami.5b09169. [DOI] [PubMed] [Google Scholar]
- Xu H.; Cheng D.; Cao D.; Zeng X. C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 2018, 1, 339–348. 10.1038/s41929-018-0063-z. [DOI] [Google Scholar]
- Seh Z. W.; Kibsgaard J.; Dickens C. F.; Chorkendorff I.; Norskov J. K.; Jaramillo T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- Suntivich J.; May K. J.; Gasteiger H. A.; Goodenough J. B.; Shao-Horn Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. 10.1126/science.1212858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.