Abstract
The present study aimed to investigate the role of janus kinase (JAK)1/STAT1 in interferon (IFN)-γ-induced apoptosis in human melanocytes. Following IFN-γ treatment, the viability of human melanocytes were analyzed using a Cell Counting Kit-8 assay and the apoptotic rate was determined using flow cytometry. Western blotting was also performed to analyze the phosphorylation levels of JAK1, JAK2 and the transcriptional factor STAT1, as well as the expression levels of Bcl-2, Bax, Bcl-2 homologous antagonist killer (Bak) and cleaved caspase-3. Finally, following the pretreatment with the STAT1 inhibitor fludarabine, human melanocytes were treated with IFN-γ and flow cytometry was used to detect the apoptotic rate. The results revealed that IFN-γ reduced the proliferation and induced the apoptosis of human melanocytes. In addition, IFN-γ treatment led to decreased expression levels of Bcl-2 and increased expression levels of Bax, Bak and cleaved caspase-3, alongside the activation of the JAK1/STAT1 signaling pathway. Conversely, the pretreatment with the STAT1 inhibitor fludarabine decreased the apoptotic rate of human melanocytes following IFN-γ induction. In conclusion, the findings of the present study suggested that IFN-γ may induce the apoptosis of human melanocytes by activating the JAK1/STAT1 signaling pathway, alongside increasing the expression levels of Bax, Bak and cleaved caspase-3, and decreasing the expression levels of Bcl-2.
Keywords: interferon-γ, STAT1, melanocytes, apoptosis, vitiligo
Introduction
Vitiligo is an acquired depigmenting disorder affecting 0.5% of the world population, which demonstrates no sex or ethnic differences (1). The most significant progress in the understanding of the disease etiology has been made in three research areas: Characterization of the stress responses activated by triggers of vitiligo, delineating the autoimmune components that promote the progression of the disease and identifying susceptibility genes (2). However, there are currently no existing treatments for vitiligo that can effectively promote the complete re-pigmentation with long-lasting effects, while preventing recurrence (1). Nevertheless, narrowband-UVB therapy, and the combined treatment with systemic therapies (steroids and immunosuppressants) and topical therapies (corticosteroids and calcineurin inhibitors) are commonly used to treat vitiligo (1).
Interferons (IFNs), which are widely used for antiviral and antitumor therapies, are multifunctional proteins that initiate and regulate various cellular responses, including antiviral and antiproliferative activity, controlling cell apoptosis and immune regulation (3–6). Previously, IFN-γ was discovered to inhibit T lymphocyte aggregation, which further impeded pigment loss in vitiligo model transgenic mice (7). Another study demonstrated that IFN-γ recruited T lymphocytes via the C-X-C motif ligand (CXCL)10/C-X-C chemokine receptor 3 signaling axis (8). In addition, it has been reported that the IFN-γ-induced apoptosis of melanocytes induced vitiligo (9). It was also observed that increased expression levels of IFN-γ served an important role in vitiligo-induced depigmentation via the direct induction of melanocyte apoptosis (9). Vitiligo is a disease that affects the appearance and leads to low self-esteem and social stigma (10). It is characterized by localized or generalized patches of skin depigmentation; therefore, either the reduction or absence of melanocytes in the local epidermis may be a prominent cause for the formation of vitiligo leukopluriae (11–13).
In the canonical IFN-γ signaling pathway, janus kinase 1 (JAK1) and STAT1 are the major signaling molecules that regulate the activation of downstream IFN-γ-inducible genes (14,15). For example, a previous study reported that IFN-γ was able to inhibit STAT1 signaling, which induced apoptosis and promoted pancreatic β-cell survival (16). Significant research has been conducted into the pathogenic role of increased rates of apoptosis in melanocytes during vitiligo to further understand the theoretical and experimental basis for the prevention and treatment of the disease (9); however, to the best of our knowledge, little is known in terms of its molecular mechanism and whether JAK/STAT signaling is involved in the process.
The present study aimed to demonstrate the effect of IFN-γ on the apoptosis of human melanocytes and to investigate the underlying mechanism. The results suggested that IFN-γ induction may activate the JAK1/STAT1 signaling pathway, differentially regulate the expression levels of Bcl-2, Bax, Bcl-2 homologous antagonist killer (Bak) and cleaved caspase-3, and consequently promote the apoptosis of human melanocytes.
Materials and methods
Cell culture
Human epidermal melanocytes (HEMs) from moderately pigmented skin were purchased from ScienCell Research Laboratories, Inc. HEMs were cultured at 37°C in a humidified incubator containing 5% CO2, in Medium 254 (Sigma-Aldrich; Merck KGaA), supplemented with 1% human melanocyte growth supplement (ScienCell Research Laboratories, Inc.). HEMs were treated with 100, 200 or 400 ng/ml IFN-γ at 37°C (PeproTech, Inc.).
Cell Counting Kit-8 (CCK-8) assay
A total of 2×103 HEMs/well were plated in triplicate into 96-well plates in 100 µl growth medium. Following 24, 48 and 72 h of treatment with IFN-γ, the cultured primary HEMs were collected and the viability was assessed using a CCK-8 assay (MedChemExpress), according to the manufacturer's protocol. Subsequently, the absorbance of each well was measured at 450 nm using a Dynatech MR5000 plate reader (Dynatech Laboratories).
Flow cytometric analysis of apoptosis
A total of 1×106 HEMs/well were collected and plated in triplicate into six-well plates in 2 ml growth medium. Morphological changes were observed using an inverted microscope (magnification, ×100) before (0 h) and after the treatment with IFN-γ (48 h) at 37°C. The cells were subsequently stained using the Annexin V/FITC Apoptosis Detection kit (eBioscience; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol, 48 h after the treatment with IFN-γ at 37°C. Apoptotic cells were analyzed using a flow cytometer (BD Accuri C6; BD Biosciences) and analyzed using BD CellQuest™ version 5.1 software (BD Biosciences).
Western blotting
HEMs treated with IFN-γ were collected by centrifugation (300 × g; 10 min; room temperature) and total protein was extracted using RIPA lysis buffer (10 mM NaPO4, pH 7.4, 300 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 1% deoxycholic acid and 2 mM EDTA), supplemented with protease inhibitors (Pierce; Thermo Fisher Scientific, Inc.). Total protein was quantified using a bicinchoninic acid assay kit (cat. no. BL521A; Biosharp Life Sciences) and 25 µg protein/lane was separated by 8–10% SDS-PAGE. The separated proteins were subsequently transferred onto a polyvinylidene difluoride membrane (EMD Millipore) and blocked with 5% BSA (Beyotime Institute of Biotechnology) for 1 h at room temperature. The membrane was incubated overnight at 4°C with the following specific primary antibodies: Anti-Bcl-2 (1:1,000; cat. no. BS1511; Bioworld Technology, Inc.), anti-Bax (1:1,000; cat. no. BS2538; Bioworld Technology, Inc.), anti-Bak (1:5,000; cat. no. ab32371; Abcam), anti-cleaved caspase-3 (1:500; cat. no. ab32042; Abcam), anti-phosphorylated (p)-JAK1 (1:2,000; cat. no. ab138005; Abcam), anti-p-JAK2 (1:5,000; cat. no. ab32101; Abcam), anti-p-STAT1 (1:5,000; cat. no. ab109461; Abcam), anti-GAPDH (1:5,000; cat. no. AB-P-R001; Goodhere), anti-JAK2 (1:3,000; cat. no. ab108596; Abcam), anti-JAK1 (1:5,000; cat. no. ab133666; Abcam) and anti-STAT1 (1:1,000; cat. no. ab92506; Abcam). Following the primary antibody incubation, the membranes were incubated with a horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:3,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) at room temperature in the dark for 1 h. Protein bands were visualized using an ECL reagent (Beyotime Institute of Biotechnology) and expression levels were semi-quantified using ImageJ version 1.51k software (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 software (SPSS, Inc.) and data are presented as the mean ± SD of three independent experiments. Statistical differences among multiple groups were determined using a one-way ANOVA, followed by Bonferroni's post hoc test for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
IFN-γ inhibits the proliferation of HEMs
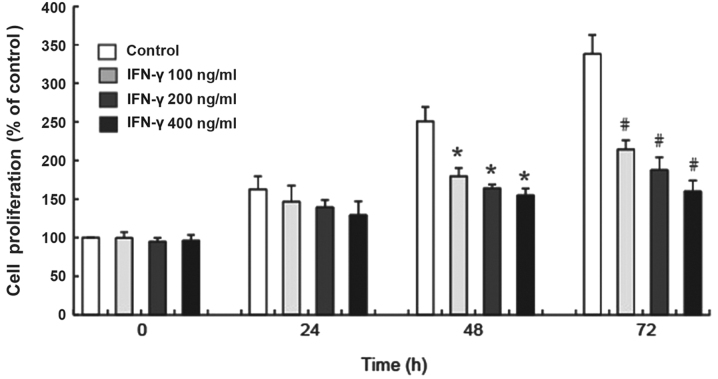
The results of the CCK-8 assay demonstrated that 100, 200 and 400 ng/ml IFN-γ significantly inhibited the proliferation of human melanocytes in vitro at 48 and 72 h compared with the control group (P<0.05; Fig. 1). Additionally, the inhibited proliferation of HEMs treated with different concentrations of IFN-γ occurred in a time-dependent manner (Fig. 1).
Figure 1.
IFN-γ decreases the proliferative rate of HEMs. HEMs were treated with 100, 200 or 400 ng/ml IFN-γ for 24, 48 and 72 h and the proliferative rate was analyzed using a Cell Counting Kit-8 assay. Data are presented as the mean ± SD from three independent HEM cultures. *P<0.05 vs. control at 48 h; #P<0.05 vs. control at 72 h. HEMs, human epidermal melanocytes; IFN, interferon.
IFN-γ promotes the apoptosis of HEMs
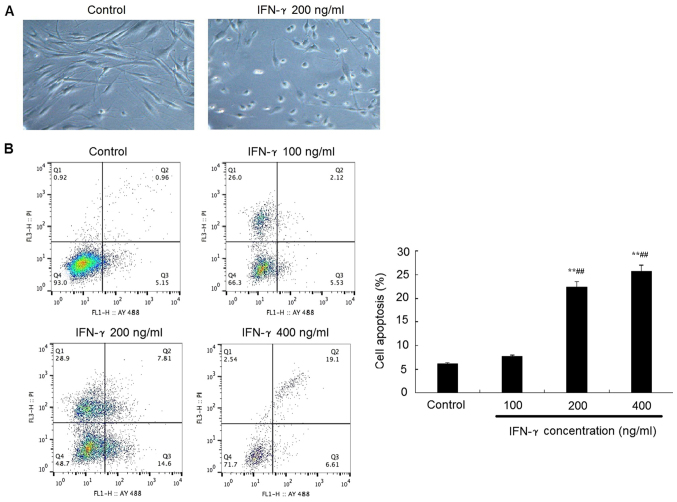
Representative morphological images revealed that the treatment with 200 ng/ml IFN-γ for 48 h promoted the HEMs to conform to a rounder morphology, with an increased number of cells in the suspension compared with the control group (Fig. 2A). In addition, flow cytometric analysis of apoptosis revealed that following the treatment with 200 or 400 ng/ml IFN-γ treatment for 48 h, the apoptotic rate of HEMs was significantly increased compared with the control group (P<0.05; Fig 2B).
Figure 2.
IFN-γ promotes apoptosis of HEMs. (A) Representative micrographs of HEMs observed under an inverted microscope (magnification, ×100) revealed a rounder morphology and cells in suspension following the treatment with 200 ng/ml IFN-γ compared with the control group. (B) Apoptotic rate of HEMs was analyzed using flow cytometry 48 h after the treatment with 100, 200 or 400 ng/ml IFN-γ. **P<0.01 vs. control; ##P<0.01 vs. IFN-γ 100 ng/ml group. HEMs, human epidermal melanocytes; IFN, interferon; PI, propidium iodide.
IFN-γ activates the JAK1/STAT1 signaling pathway
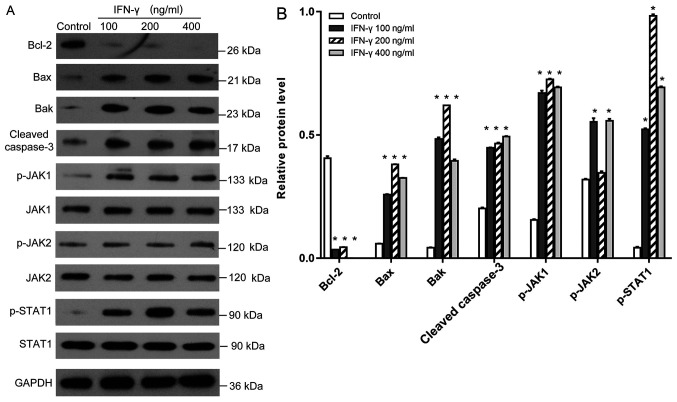
It is well established that upon IFN-γ binding to its receptor, the JAK/STAT signaling axis is activated by phosphorylation (17). Western blotting revealed that the treatment with IFN-γ at various concentrations significantly increased the phosphorylation levels of JAK1 and STAT1 (Fig. 3A and B); however, although 100 and 400 ng/ml IFN-γ demonstrated these effects on the phosphorylation status of JAK2, the 200 ng/ml group demonstrated no significant different in the phosphorylation status compared with the control group. Moreover, the difference between 100 and 400 ng/ml IFN-γ treatment was not statistically different. Meanwhile, the expression levels of the anti-apoptotic protein Bcl-2 were significantly reduced, whereas the expression levels of the proapoptotic proteins Bax, Bak and cleaved caspase-3 were significantly increased in the 100, 200 and 400 ng/ml IFN-γ treatment groups compared with the control group (P<0.05; Fig. 3A and B).
Figure 3.
Effects of IFN-γ on the expression levels of Bcl-2, Bax, Bak, cleaved caspase-3, p-JAK1, p-JAK2 and p-STAT1. (A) Western blotting was used to analyze the expression levels of the proteins in human epidermal melanocytes following the treatment with 100, 200 or 400 ng/ml IFN-γ. (B) Quantification of the western blotting bands in part A. Bcl-2, Bax, Bak, cleaved caspase-3 were normalized to GAPDH. p-JAK1, p-JAK2 and p-STAT1 were normalized to JAK-1, JAK-2 or STAT1, respectively. *P<0.05 vs. control. p-, phosphorylated; IFN, interferon; JAK, janus kinase; Bak, Bcl-2 homologous antagonist killer.
STAT1 inhibitor attenuates the proapoptotic effect of IFN-γ on melanocytes
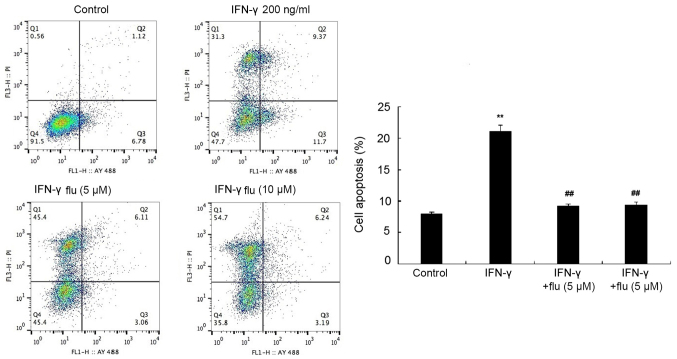
HEMs were pretreated with 5 or 10 µM fludarabine (STAT1 inhibitor) for 1 h and then treated with 200 ng/ml IFN-γ for 48 h. The apoptotic rate was analyzed by flow cytometry following Annexin V-FITC/PI double staining and it was revealed that fludarabine treatment significantly reduced the proapoptotic effects of IFN-γ on HEMs (P<0.01; Fig. 4).
Figure 4.
STAT1 inhibitor flu attenuates IFN-γ-induced apoptosis of HEMs. Flow cytometric analysis of apoptosis was performed in HEMs treated with 200 ng/ml IFN-γ without or without 5 or 10 µM flu pretreatment. **P<0.01 vs. control group; ##P<0.01 vs. IFN-γ 100 ng/ml group. IFN, interferon; HEMs, human epidermal melanocytes; flu, fludarabine.
Discussion
IFN-γ, a type III interferon, is a soluble glycoprotein with antiviral, antitumor, anti-parasite and immunoregulatory activities (2). It has been hypothesized that the IFN-γ present in the skin lesions during vitiligo is secreted by CD8+ cytotoxic T lymphocytes, which can induce the apoptosis of melanocytes (18,19); however, the mechanism of IFN-γ-induced melanocyte apoptosis remains unclear. In the present study, primary HEMs were used to investigate the toxicity of IFN-γ. The results demonstrated that 200 and 400 ng/ml IFN-γ inhibited the proliferation of HEMs in a time-dependent manner. In addition, an increased apoptotic rate of HEMs was also observed following the treatment with 200 ng/ml IFN-γ for 48 h. These results indicated that the cytotoxic effect of IFN-γ on HEMs may be predominantly achieved by inducing apoptosis. Notably, these results were consistent with previous findings (9,14). Further evidence has revealed that by binding with specific receptors, IFN-γ induced cell apoptosis as well as promoting cell proliferation. The effects of IFN-γ on cell proliferation and apoptosis have been discovered to depend on the differential expression levels of IFN-γ receptors (IFNGRs), with increased expression levels of IFNGR2 more likely to induce apoptosis (2); however, further investigations are required to determine whether IFNGR2 interferes with IFN-γ-induced melanocyte apoptosis.
The JAK/STAT signaling pathway, which widely exists in vivo and participates in multiple cellular processes, is known to serve an important role in signal transduction and the transcriptional activation of STAT1 (20–22). The present study results demonstrated an decrease in the proliferative rate in IFN-γ-treated groups following 48 and 72 h, with the difference being even more pronounced following 72 h compared with the initial treatment. However, neither the treatment for 0 nor 24 h resulted in a decrease in the proliferative rate between the IFN-γ groups and controls. These findings may be explained by the increased expression levels of p-JAK1 and p-STAT1 observed following 48 h of treatment. A previous study reported that the JAK/STAT signaling pathway served both antiproliferative and proapoptotic effects (23). Furthermore, the expression levels of p-JAK1and p-STAT1 were the highest in the 200 ng/ml IFN-γ group compared with the 100 and 400 ng/ml IFN-γ treatment groups. These findings provided evidence to suggest that the activation of the JAK1/STAT1 signaling pathway may contribute to the apoptosis of IFN-γ-induced HEMs.
Furthermore, the activation of STAT1 was previously found to be involved in the regulation of apoptosis by regulating the downstream Bcl-2 family members, Bcl-2 and Bax (24). The ratio between the anti-apoptotic protein Bcl-2 and the proapoptotic protein Bax has been revealed to be inversely correlated with the rate of apoptosis (25,26). Caspase-3 is an apoptosis-executing protein in the caspase family and functional cleaved caspase-3 is known to promote apoptosis together with Bcl-2 (27,28). The present study results discovered that melanocytes induced with IFN-γ for 48 h exhibited reduced expression levels of Bcl-2 protein and increased expression levels of Bax, Bak, cleaved caspase-3, p-JAK1 and p-STAT1; however, no significant difference was observed in the expression levels of p-JAK2 following 200 ng/ml IFN-γ treatment compared with the control. To understand whether the apoptosis was associated with the activation of the JAK/STAT1 signaling pathway in IFN-γ induced human melanocytes, the specific STAT1 inhibitor fludarabine was used in the present study; a significantly reduced apoptotic rate was discovered in HEMs co-treated with the inhibitor and IFN-γ compared with IFN-γ alone, suggesting that IFN-γ-induced human melanocyte apoptosis may be associated with the activation of the JAK/STAT1 signaling pathway, and STAT1 may regulate the expression levels of Bax, Bak and cleaved caspase-3.
IFN-γ was previously discovered to be critical for the progression of vitiligo by recruiting autoreactive CD8+ T lymphocytes to the skin through the induction of CXCL10 (28,29). The present study identified that IFN-γ could also induce the apoptosis of human melanocytes through activating the JAK1/STAT1 signaling pathway, therefore potentially serving as an initiating factor that destroys melanocytes. Future studies using animal models will be helpful in determining how important IFN-γ-induced apoptosis is compared with other mechanisms of cytotoxicity during the progression of vitiligo in vivo (29,30). These findings may have therapeutic implications, as inhibiting IFN-γ or its downstream signaling may provide a novel, effective and long-lasting therapy for vitiligo.
In conclusion, the findings of the present study suggested that IFN-γ may induce the apoptosis of human melanocytes. These proapoptotic effects of IFN-γ on HEMs may be mediated through the activation of the JAK1/STAT1 signaling pathway, increasing the expression levels of Bax, Bak and cleaved caspase-3, and decreasing the expression levels of Bcl-2. Overall, the intervention of the JAK1/STAT1 signaling pathway may be an effective method to reduce the IFN-γ-induced apoptosis of HEMs, which is important for the prevention and treatment of vitiligo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the General Subject of Medical Science and Technology Development of Nanjing Municipal Health and Family Planning Commission (grant no. YKK17275) and the Fundamental Research Funds for the Central Universities (grant no. 2242019K49253).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
QS and FW conceived and designed the study; QS and ZD conducted the experiments; MC and RC analyzed the data; QS, ZD, MC and RC drafted the manuscript; and FW revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Taieb A, Alomar A, Böhm M, Dell'anna ML, De Pase A, Eleftheriadou V, Ezzedine K, Gauthier Y, Gawkrodger DJ, Jouary T, et al. Vitiligo European Task Force (VETF); European Academy of Dermatology: Venereology (EADV); Union Europe´enne des Me´decins Spe´cialistes (UEMS) Guidelines for the management of vitiligo: The European Dermatology Forum consensus. Br J Dermatol. 2013;168:3111–19. doi: 10.1111/j.1365-2133.2012.11197.x. [DOI] [PubMed] [Google Scholar]
- 2.Manga P, Elbuluk N, Orlow SJ. Recent advances in understanding vitiligo. F1000 Res. 2016;5:5. doi: 10.12688/f1000research.8976.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arya V, Bansal M, Girard L, Arya S, Valluri A. Vitiligo at Injection Site of PEG-IFN-α 2a in Two Patients with Chronic Hepatitis C: Case Report and Literature Review. Case Rep Dermatol. 2010;2:156–164. doi: 10.1159/000320207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamadah I, Binamer Y, Sanai FM, Abdo AA, Alajlan A. Interferon-induced vitiligo in hepatitis C patients: A case series. Int J Dermatol. 2010;49:829–833. doi: 10.1111/j.1365-4632.2009.04443.x. [DOI] [PubMed] [Google Scholar]
- 5.Rozera C, Cappellini GA, D'Agostino G, Santodonato L, Castiello L, Urbani F, Macchia I, Aricò E, Casorelli I, Sestili P, et al. Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: Results from a phase I trial in advanced melanoma. J Transl Med. 2015;13:139. doi: 10.1186/s12967-015-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Liu D, Ning W, Xu A. Cytosolic dsDNA triggers apoptosis and pro-inflammatory cytokine production in normal human melanocytes. Exp Dermatol. 2015;24:298–300. doi: 10.1111/exd.12621. [DOI] [PubMed] [Google Scholar]
- 7.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashighi M, Harris JE. Interfering with the IFN-γ/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann Transl Med. 2015;3:343. doi: 10.3978/j.issn.2305-5839.2015.11.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L, Li M. Interferon-gamma Inhibits Melanogenesis and Induces Apoptosis in Melanocytes: A Pivotal Role of CD8+ Cytotoxic T lymphocytes in Vitiligo. Acta Derm Venereol. 2015;95:664–670. doi: 10.2340/00015555-2080. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Kwon HS, Jung HM, Lee H, Kim GM, Yim HW, Bae JM. Treatment Outcomes of Topical Calcineurin Inhibitor Therapy for Patients With Vitiligo: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019;155:929. doi: 10.1001/jamadermatol.2019.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahhas AF, Mohammad TF, Hamzavi IH. Vitiligo Surgery: Shuffling Melanocytes. J Investig Dermatol Symp Proc. 2017;18:S34–S37. doi: 10.1016/j.jisp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Li S, Zhu L, Guo S, Yi X, Cui T, He Y, Chang Y, Liu B, Li C, et al. Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway. Free Radic Biol Med. 2018;129:492–503. doi: 10.1016/j.freeradbiomed.2018.10.421. [DOI] [PubMed] [Google Scholar]
- 13.Yang K, Xiong X, Pallavi G, Ling Y, Ding F, Duan W, Sun W, Ding G, Gong Q, Zhu W, et al. The early repigmentation pattern of vitiligo is related to the source of melanocytes and by the choice of therapy: A retrospective cohort study. Int J Dermatol. 2018;57:324–331. doi: 10.1111/ijd.13878. [DOI] [PubMed] [Google Scholar]
- 14.Jia H, Song L, Cong Q, Wang J, Xu H, Chu Y, Li Q, Zhang Y, Zou X, Zhang C, et al. The LIM protein AJUBA promotes colorectal cancer cell survival through suppression of JAK1/STAT1/IFIT2 network. Oncogene. 2017;36:2655–2666. doi: 10.1038/onc.2016.418. [DOI] [PubMed] [Google Scholar]
- 15.Chou DH, Vetere A, Choudhary A, Scully SS, Schenone M, Tang A, Gomez R, Burns SM, Lundh M, Vital T, et al. Kinase-Independent Small-Molecule Inhibition of JAK-STAT Signaling. J Am Chem Soc. 2015;137:7929–7934. doi: 10.1021/jacs.5b04284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan DH, Greenlund AC, Tanner JW, Shaw AS, Schreiber RD. Identification of an interferon-gamma receptor alpha chain sequence required for JAK-1 binding. J Biol Chem. 1996;271:9–12. doi: 10.1074/jbc.271.1.9. [DOI] [PubMed] [Google Scholar]
- 17.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: An overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 18.Strassner JP, Harris JE. Understanding mechanisms of autoimmunity through translational research in vitiligo. Curr Opin Immunol. 2016;43:81–88. doi: 10.1016/j.coi.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richmond JM, Bangari DS, Essien KI, Currimbhoy SD, Groom JR, Pandya AG, Youd ME, Luster AD, Harris JE. Keratinocyte-Derived Chemokines Orchestrate T-Cell Positioning in the Epidermis during Vitiligo and May Serve as Biomarkers of Disease. J Invest Dermatol. 2017;137:350–358. doi: 10.1016/j.jid.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu B, Antoine DJ, Kwan K, Lundbäck P, Wähämaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci USA. 2014;111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn J, Lee J, Kim S. Interferon-gamma inhibits the neuronal differentiation of neural progenitor cells by inhibiting the expression of Neurogenin2 via the JAK/STAT1 pathway. Biochem Biophys Res Commun. 2015;466:52–59. doi: 10.1016/j.bbrc.2015.08.104. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Zheng G, Zhao L, Xu F, Qian J. Shp-2 contributes to anti-RSV activity in human pulmonary alveolar epithelial cells by interfering with the IFN-α-induced Jak/Stat1 pathway. J Cell Mol Med. 2015;19:2432–2440. doi: 10.1111/jcmm.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Dong ZR, Guo ZY, Wang CH, Tang ZY, Qu SF, Chen ZT, Li XW, Zhi XT. Aspirin enhances IFN-α-induced growth inhibition and apoptosis of hepatocellular carcinoma via JAK1/STAT1 pathway. Cancer Gene Ther. 2013;20:366–374. doi: 10.1038/cgt.2013.29. [DOI] [PubMed] [Google Scholar]
- 24.Cao ZH, Zheng QY, Li GQ, Hu XB, Feng SL, Xu GL, Zhang KQ. STAT1-mediated down-regulation of Bcl-2 expression is involved in IFN-γ/TNF-α-induced apoptosis in NIT-1 cells. PLoS One. 2015;10:e0120921. doi: 10.1371/journal.pone.0120921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Hao J, Cheng M, Zhang C, Huo C, Liu Y, Du W, Zhang X. Hyperglycemia-induced Bcl-2/Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp Cell Res. 2018;367:186–195. doi: 10.1016/j.yexcr.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Toscano ECB, Vieira ÉLM, Portela ACDC, Reis JLJ, Caliari MV, Giannetti AV, Gonçalves AP, Siqueira JM, Suemoto CK, Leite REP, et al. Bcl-2/Bax ratio increase does not prevent apoptosis of glia and granular neurons in patients with temporal lobe epilepsy. Neuropathology. 2019;39:348–357. doi: 10.1111/neup.12592. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Xia Y, Xu Z, Deng X. Propofol Suppressed Hypoxia/Reoxygenation-Induced Apoptosis in HBVSMC by Regulation of the Expression of Bcl-2, Bax, Caspase3, Kir6.1, and p-JNK. Oxid Med Cell Longev. 2016;2016:1518738. doi: 10.1155/2016/1518738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Zhu Z, Yao C, Huang Y, Zhi E, Chen H, Tian R, Li P, Yuan Q, Xue Y, et al. VEGFC/VEGFR3 Signaling Regulates Mouse Spermatogonial Cell Proliferation via the Activation of AKT/MAPK and Cyclin D1 Pathway and Mediates the Apoptosis by affecting Caspase 3/9 and Bcl-2. Cell Cycle. 2018;17:225–239. doi: 10.1080/15384101.2017.1407891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, Zhou Y, Deng A, Hunter CA, Luster AD, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi: 10.1126/scitranslmed.3007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris JE. IFN-γ in Vitiligo, Is It the Fuel or the Fire? Acta Derm Venereol. 2015;95:643–644. doi: 10.2340/00015555-2137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.