Abstract
Purpose of Review
The supplementation of dietary additives into processed foods has exponentially increased in the past few decades. Similarly, the incidence rates of various diseases, including metabolic syndrome, gut dysbiosis and hepatocarcinogenesis, have been elevating. Current research reveals that there is a positive association between food additives and these pathophysiological diseases. This review highlights the research published within the past 5 years that elucidate and update the effects of dietary supplements on liver and intestinal health.
Recent Findings
Some of the key findings include: enterocyte dysfunction of fructose clearance causes non-alcoholic fatty liver disease (NAFLD); non-caloric sweeteners are hepatotoxic; dietary emulsifiers instigate gut dysbiosis and hepatocarcinogenesis; and certain prebiotics can induce cholestatic hepatocellular carcinoma (HCC) in gut dysbiotic mice. Overall, multiple reports suggest that the administration of purified, dietary supplements could cause functional damage to both the liver and gut.
Summary
The extraction of bioactive components from natural resources was considered a brilliant method to modulate human health. However, current research highlights that such purified components may negatively affect individuals with microbiotal dysbiosis, resulting in a deeper break of the symbiotic relationship between the host and gut microbiota, which can lead to repercussions on gut and liver health. Therefore, ingestion of these dietary additives should not go without some caution!
Keywords: Gut Microbiome, Hepatocellular Carcinoma, High Fructose Corn Syrup, Artificial Sweeteners, Emulsifiers, Probiotics and Prebiotics
I. Introduction
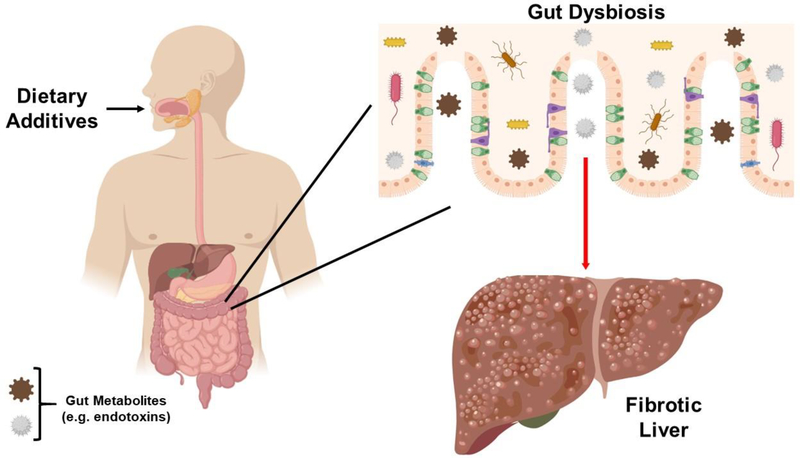
Since ancient times, natural antimicrobial food additives have been used to extend the shelf life of foods and to reduce the risk of infection and microbial spoilage (1). Nowadays, synthetic additives are utilized to preserve the conditions of modern food processing (1). Since the passing of the Food Additives Amendment (1958), the Food and Drug Administration (FDA) has assessed the safety of various food additives, which has resulted in the Generally Regarded as Safe (GRAS) labelling of high fructose corn syrup (HFCS) (2), artificial sweeteners (3), emulsifiers (4–6), and some probiotics (i.e. L. acidophilus) (7). Interestingly, the FDA permits food manufacturers to self-affirm GRAS status for prebiotics (8). While dietary additives have had their merits for food industries, current research suggests that many of them can be harmful for the gut microbiome and the liver (as summarized in Figure 1).
Figure 1: Ingestion of food additives can cause gut dysbiosis and liver dysfunction.
Our diet is one of the greatest influencers on the gut microbiota. An imbalance in intestinal microbiota composition (alias gut dysbiosis) can cause systemic effects, including impacting liver health. In this case, the consumption of dietary additives (i.e. HFCS, emulsifiers, flavor enhancers, prebiotics) can cause gut dysbiosis, resulting in the generation of gut endotoxins (i.e. LPS), which travel through the portal vein towards the liver. These endotoxins can initiate hepatic inflammation, which can progress to fibrosis and hepatocarcinogenesis.
The gut microbiota consists of a variety of microorganisms, including bacteria, archaea and eukarya (9). Over thousands of years, the host and gut microbiota have developed a mutualistic relationship; yet, various environmental factors are still able to either enhance or destroy this symbiotic friendship. When there is a microbial imbalance within the gut (alias dysbiosis), this causes alterations in metabolic and immune responses, which can, in turn, begin a domino effect that increases our risk for disease. These include gut-microbiota associated steatosis and non-alcoholic fatty liver disease (NAFLD), which can progress to non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC; most common liver malignancy). The domino effect starts with a compromise in intestinal integrity, which allows gut bacterial- derived microbial-associated molecular patterns (MAMPs) to escape into the portal vein and travel to the liver. These endotoxins then activate toll-like receptors found on hepatic stellate and Kupffer cells, which instigates pro-inflammatory signaling pathways (10–12). This hepatic inflammation, in turn, greatly increases the risk of developing HCC.
Our diet has a strong influence on the gut microbiota and overall host physiology. Much research has gone into understanding how our diet impacts the gut microbiota and its associated diseases. For example, it has been extensively demonstrated that an obesogenic diet can cause spontaneous development of steatosis, fibrosis and HCC (13, 14). Comparatively, the influence of dietary additives on the gut microbiota-HCC axis is relatively underexplored. This review highlights the current research (within the past five years) on what is known about the food additive – gut microbiota – HCC axis. However, considering the lack of current evidence on the relationship between dietary additives and HCC, most studies presented herein will focus on the high risk precursors for hepatocarcinogenesis.
II. High Fructose Corn Syrup: A Manufactured Sugar
Background
Since the 1970s, the American diet has exhibited a monumental increase in sugar consumption (15). This elevation is mostly attributed to the introduction of high fructose corn syrup (HFCS) in many processed foods and beverages. HFCS is produced by an enzymatic process that leads to the partial isomerization of glucose, resulting in fructose formation (16). Specifically, HFCS is principally found in two forms, HFCS-42 and HFCS-55, with the fructose to glucose ratio being 42/58 and 55/45, respectively (16, 17). While the association between HFCS consumption and health risks was relatively under-explored, current research has linked fructose to being a major risk factor for a variety of diseases, including obesity [as reviewed in (18)], non-alcoholic fatty liver disease (NAFLD) [as reviewed in (17)], hypertension (19, 20), gut dysbiosis (21, 22), and hepatocellular carcinoma (HCC) (23, 24). In line, several studies have reported that an isocaloric-HFCS restricted diet implemented on both lean and obese children reduced hepatic de novo lipogenesis (25), steatosis (26), and other indices of metabolic syndrome (27) that could progress to NAFLD. Considering that NAFLD patients have a greater risk in developing hepatocarcinogenesis (28), this may indicate HFCS as a potential liver carcinogen, analogous to the recent report that HFCS enhances intestinal tumor growth (29).
The HFCS-NAFLD Axis
This interrelationship between HFCS and various health consequences has been attributed to the unique hepatic metabolism of fructose [as reviewed in (30)]. While the liver was assumed to be the primary site for fructose clearance, Jang et al. recently demonstrate that, through oral administration of 13C-fructose, the small intestine is actually the major location for fructose clearance; yet, this function is impaired under high fructose consumption as it saturates the intestinal fructose clearance capacity, leading to the spillover of fructose into the portal vein, which then accumulates into the liver (31). This overabundance of hepatic fructose can lead to an increase in energy metabolism since fructose is capable of bypassing the phosphofructokinase regulatory step in glycolysis (32). When the energy storages become full, this results in the accumulation of the Krebs cycle byproduct, citrate, which allosterically activates cytoplasmic acetyl-CoA carboxylase (ACC) and thus, initiates hepatic de novo lipogenesis (33). Additionally, HFCS consumption results in the downregulation of hepatic peroxisome proliferator-activated receptor α (PPARα), which is associated with reduced mitochondrial β-oxidation (34). Moreover, hepatic ketohexokinase (KHK; rate limiting enzyme for fructose metabolism) metabolizes fructose without a negative feedback system, resulting in a dramatic decrease in ATP and phosphate levels, which results in hepatic production of uric acid (35). Considering the strong linkage between HFCS and hepatic de novo lipogenesis, high fructose-induced enterocyte dysfunction might explain, in part, the role of fructose in promoting NAFLD. Moreover, this might explain the hyperuricemia that is exhibited in adolescent patients with NAFLD (36) and NASH (37). Along with accumulating hepatic fructose, the retention of uric acid may be due to fructose suppressing intestinal uric acid excretion through the activation of NADPH oxidase (38). The detrimental outcomes of fructose and uric acid has led researchers to target KHK and xanthine oxidase, respectively, for therapeutic approaches. Limiting activity of either enzyme is shown to be protective against steatosis, NAFLD, and NASH (39, 40). Whether these therapeutics can further inhibit against fructose-induced HCC should be explored.
The HFCS-Microbiome Axis
In addition to entering enterohepatic circulation, fructose can travel down to the colon and interact with the gut microbiota. This results in alterations of the fecal bacterial composition, including an 88% increase in the Firmicutes (F) to Bacteroidetes (B) ratio (22), where an increase in this F/B ratio indicates gut dysbiosis. Similarly, maternal consumption of fructose leads to a significant reduction of the ‘beneficial’ bacteria (i.e. Lactobacillus) within the fecal microbiome (21). Considering the positive correlation between butyrate-producing Firmicutes and adiposity (41), this could explain, at least in part, as to why maternal consumption of HFCS increases lipogenesis and adiposity in the offspring for both rats (42) and humans (43). Additionally, elevated KHK expression negatively affects tight junctions (44), which increases gut permeability and endotoxin release into the portal vein (45). These endotoxins (i.e. LPS) can travel and activate hepatic toll-like receptor 4 (TLR4), leading to fibrosis and hepatocarcinogenesis (46). Interestingly, citrulline may be a therapeutic option to revert the negative effects of fructose, as it has been reported that citrulline supplementation increases Bacteroidetes and Prevotella (47) and attenuates liver fat accumulation (48). Moreover, the probiotic strain, L. brevis DM9218, can enhance intestinal barrier function, which is associated with reduced hepatic lipopolysaccharide (LPS) levels, retardation of hyperuricemia and amelioration of fructose-induced liver damage (49). It would be interesting to observe whether citrulline or L. brevis DM9218 could be utilized as therapeutic options for patients with Hereditary Fructose Intolerance who are prone to develop obesity-independent hepatic steatosis (50).
III. Artificial Sweeteners: A Bitter Sweet Alternative to Sucrose
Background
Sweet taste receptors are ubiquitously found throughout the body, including the gastrointestinal tract. The binding of sucrose and artificial sweeteners to the heterodimeric G-coupled proteins, T1R2 and T1R3, activates both peripheral gustatory and, in turn, brain gustatory nerves, which regulate metabolic responses to maintain energy balance [as reviewed in (51)]. Compared to sucrose, the advantage of non-caloric sweeteners is their limited disruption on energy homeostasis due to the fact that artificial sweeteners are a hundred fold sweeter (52). Currently, there are six non-caloric artificial sweeteners (NAS) on the market: aspartame, saccharin, sucralose, acesulfame potassium, cyclamate and neotame. This section of the review will delve into further detail of each artificial sweetener and their impacts on gut and liver health.
Saccharin
Saccharin (1,2-benzisothiazol-3-one-1,1-dioxide) is the first synthetic artificial sweetener with a 300-fold increase in sweetness, making this NAS one of the most popular substitutes for sucrose. Yet, there have been alarming reports that saccharin presents hepatotoxic properties (53), where short-term exposure causes transaminitis (i.e. ALT, AST and ALP) (54). Moreover, six month exposure of saccharin promotes hepatic inflammation, as indicated by elevations in iNOS and TNFα (55). While no studies have directly linked saccharin to the progression of HCC, the conformational changes that saccharin causes to the promoter of the potent tumor suppressor, p53 (56), could implicate a CD44-independent mechanism of diminished tumor surveillance and promotion of HCC progenitors (57). Similarly, Wistar rats fed saccharin have diminished expression of p27 (a tumor suppressor), while having overexpression of the key oncogene, H-ras (58). Along with influencing liver health, saccharin perturbs and alters the gut microbiota, which includes promoting Bacteroidetes, Turicibacter and Clostridales, while reducing Firmicutes (55, 59). Despite the beneficial effects of lowering the F/B ratio, the elevation of pro-inflammatory bacteria (i.e. Turicibacter) still indicates a potential negative effect of saccharin ingestion. While there is limited information on how saccharin effects intestinal health, the unequivocal hepatotoxicity of saccharin should instigate a reevaluation on its current US acceptable daily intake (ADI) of 5mg/kg body weight.
Aspartame
The N-L-α-aspartyl-L-phenylalanine1-methyl ester, aspartame, is a synthetic sweetener fortified in foods and beverages. Aspartame is around 200 times sweeter than sucrose (60), which has made this NAS another alternative for sucrose. The ADI of aspartame established by the European Food Safety Authority and FDA are 40 and 50 mg/kg/day, respectively (61). However, recent studies demonstrate the negative impacts of aspartame on gut and liver health, which may cause for an ADI update. For example, multiple studies confirm that long-term intake of aspartame induces liver degeneration, mononuclear cell infiltration, necrosis and fibrosis, which may be mediated through the dysregulation of adipocytokines and an imbalance in redox homeostasis (58, 62–64). Additionally, aspartame in combination with potassium sorbate induces a mitochondrial-mediated apoptosis pathway, which is associated with the loss of the mitochondria membrane potential (65). While aspartame presents hepatotoxic effects, since this NAS is readily metabolized to phenylalanine, aspartic acid and methanol (60, 63), it is difficult to determine whether these byproducts are the true culprits in prompting hepatic damage. In order to address this concern, a recent study demonstrates that folate deficiency aggravates aspartame-induced liver injury. Normally, folate protects against the aspartame byproducts, methanol and formate; therefore, by eliminating folate through the immunosuppressive drug, methotrexate (MTX), it was established that the metabolites may play a part in aspartame-mediated hepatic damage (66). In regards to HCC, there is limited understanding on potential tumorigenic properties for aspartame, except for the analogous effects of saccharin on H-ras and p27 (58). Along with hepatic damage and potential carcinogenesis, low-dose aspartame influences the gut microbiota composition through increasing the total fecal bacteria load and the relative abundance of Enterobacteriaceae (67). Intriguingly, Martinson et al. recently demonstrate that resident Enterobacteriaceae clonal populations, including pathogenic E. coli, have little stability within the ‘healthy’ human gut (68). In fact, E. coli populations within the gut can have a turnover of over months to a year (68). This low ‘stability’ may explain the strong relationship between the expansion of Enterobacteriaceae and inflammatory diseases of the gastrointestinal tract (69). Alongside, the overgrowth of Enterobacteriaeceae is associated with the severity of cirrhosis (70–72). This current evidence suggests that aspartame could be linked to gut dysbiotic-associated maladies, including liver disease; therefore, future studies are warranted to elucidate this important gap in the field.
Sucralose
The substituted disaccharide, sucralose (1,6-dichloro-1,6-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside), also exhibits potent effects on both intestinal and hepatic health. Sucralose can cause gut dysbiosis as indicated through altered Proteobacteria (73, 74) and Clostridium cluster XIVa (75) compositions within the fecal microbiome. Moreover, sucralose increases the abundance of other pro-inflammatory bacteria (i.e. Turicibacter), which is associated with hepatic inflammation (76). Additionally, the administration of sucralose induces various hepatic features, including degeneration of hepatocytes, lymphocyte infiltration and fibrosis (77). When considering liver proteomics, the effect of sucralose may be due to ribosomal inactivation, which enhances gut microbiota-mediated hepatic inflammation (78). Whether this could progress to hepatocarcinogenesis is unclear; yet, a few reviews state that the administration of sucralose presents no carcinogenetic properties (79, 80). In contrast to these reports, a recent study demonstrated that a sucralose diet fed to Swiss mice from prenatal to natural life-span death resulted in hematopoietic neoplasias (81), which indicates a potential tumorigenesis property of sucralose. While this is a striking result, it has been reviewed that sucralose may have greater health effects on humans than on rodents (82); therefore, more research is needed to determine the differential effects between this NAS on separate species.
Acesulfame Potassium, Neotame, and Cyclamate
The current information regarding acesulfame potassium (alias Ace-K) on hepatic and gut health is limited. In terms of the gut microbiota, the consumption of Ace-K can affect the bacterial composition in a sex-dependent manner (75). Moreover, Ace-K dramatically decreases the relative abundance of multiple genera, including Lactobacillus and Clostridium, whereas Bacteroides is highly expressed (83). Likewise, a recent finding indicates that four-week administration of neotame reduces the α-diversity and alters the β-diversity of the fecal microbiome (84). Analogous to these results, the streptozotocin-high fat diet (STZ-HFD) induced NASH-HCC mouse model is found to be associated with an elevation in Bacteroides and a reduction in α-diversity (85). This could implicate Ace-K as a potential promoter of liver disease, including HCC, through alterations in the gut microbiome; however, long-term studies are necessary to elucidate this possibility. Similar to Ace-K, not much information is known about the impact of neotame or cyclamate on liver and gut health; however, considering that cyclamate is converted to cyclohexylamine by the gut microbiota (86), it would be an interesting avenue to see how this gut metabolite could affect human health. Overall, ongoing research is needed to further explore the utilization of these NAS in both rodent and human studies.
IV. Emulsifiers and Flavor Enhancers in Processed Foods
Background
To optimize food appearance, texture and mouthfeel, emulsifiers and flavor enhancers are the key agents. Emulsifiers are comprised of proteins, phospholipids and carbohydrates, where their water-oil suspension are utilized to extend shelf-life and encapsulate unpleasant aroma and/or bioactive compounds [as reviewed in (87)], whereas flavor enhancers intensify and amplify the savor within foods. While these ingredients do possess important merits in terms of food storage and taste, their impact on the gut-liver axis can lead to undesired consequences, including mucosal inflammation and hepatic dysfunction.
Carboxymethylcellulose and Polysorbate 80
The two most popular dietary emulsifiers, carboxymethylcellulose (CMC) and polysorbate 80 (P80), are ubiquitous components of processed foods that enhance texture and extend shelf life (88). Alongside these properties, emulsifiers can alter the murine (89, 90) and human (88) microbiome in a sex-dependent manner, which further promotes metabolic syndrome (91) and colitis (90). Moreover, CMC and P80 promote microbiota encroachment, which is associated with reduced mucus thickness (90, 92). Interestingly, these negative effects of emulsifiers are ablated in germ-free mice and in the highly restricted microbiota, gnotobiotic mouse model termed Altered Schaedler Flora (88). It would be compelling to determine whether certain antibiotics could also protect against the detrimental effects of emulsifiers on the gut microbiota. Additionally, this would elucidate which bacterial species are leading to the low-grade inflammation induced by emulsifiers. This could further provide, at least in part, a therapeutic approach to reduce P80-mediated fatty liver, steatosis, and hepatocyte ballooning, along with diminishing oxidative stress (91). While these emulsifiers are not reported to cause HCC, the fact that P80 can induce pre-HCC risk factors should warrant for future studies to determine whether long-term administration of this emulsifier could reveal potential carcinogenic properties.
Lecithin
Another popular emulsifier, lecithin, is a food additive and the main component of phosphatidylcholine. Interestingly, while lecithin does not directly impact host physiology, its metabolites may cause concern for hepatic and intestinal health. When lecithin or its byproduct choline interacts with the gut microbiota they are metabolized into trimethylamine (TMA), which is further oxidized by hepatic flavin monooxygenases to form trimethylamine N-oxide (TMAO) (93). Besides its endogenous generation, TMA and TMAO can originate from natural food products, like fish. Interestingly, TMAO can get converted back to TMA, predominantly by Enterobacteriaceae, which leads to a continuous cycle known as retroconversion (94). While lecithin itself may not be regarded as harmful, TMAO has been indicated to be an independent risk marker and factor for NAFLD (95). Additionally, elevated serum TMAO and diminished serum choline (96), along with diminished urinary TMAO (97), levels are associated with primary liver cancer, including HCC. This overproduction of TMAO may indicate a gut flourish of Enterobacteriaceae and a compromised intestinal barrier. Interestingly, it has been reported that the consumption of soy lecithin, as a phospholipid source for infant formula, skews the gut bacterial community towards elevated Enterococcaceae and Enterobacteriaceae (98), which are two bacterial strains that are significantly associated with cirrhosis disease progression (99). It is plausible that, analogous to HCC, the relationship between these bacteria and cirrhosis could be related to TMAO production. Besides TMAO generation, there is another metabolic pathway that lecithin can pursue. After its breakdown to choline, this byproduct could be irreversibly oxidized to betaine, leading to the pathway of betaine ➔ homocysteine ➔ methionine ➔ S-adenosylmethionine (SAM) (100). Interestingly, high intakes of choline and betaine is associated with reduced primary liver cancer incidence (100, 101) whereas methionine and SAM is highly associated to HCC risk (101). This one-carbon metabolism from choline to methionine and SAM is linked to DNA methylation (102), which is usually altered during cancer development. Therefore, while lecithin and choline may not directly impact liver cancer development, its metabolism could provide the key components to promote SAM-mediated DNA methylation and thus, promote tumorigenesis.
Monosodium glutamate
Monosodium glutamate (MSG) is a widely, frequently used flavor enhancer and stabilizer in ready-made or packaged foods. It has been recently demonstrated that MSG causes inflammatory infiltration and disorganized hepatic architecture (103). Moreover, MSG-treated rats have elevated serum enzymes for liver dysfunction, which may be due to the over accumulation of glutamine generated from the glutamate counterpart of MSG (103). Additionally, MSG initiates oxidative stress on the liver, as it can dissociate into free radicals (103). Along with inducing liver injury, MSG-treatment promotes the transition from NAFLD to NASH (104) to pre-neoplastic lesions, including HCC (105). The specific mechanisms of MSG-induced HCC needs further clarification. Regarding the gut microbiota, there is limited information as to how MSG alters bacterial diversity. Therefore, future studies are required to elucidate the influence of MSG on gut dysbiosis and HCC.
VI. Probiotics
Background
According to the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), a probiotic contains “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (106). Some of the probiotics incorporated as food supplements include various strains of Lactobacillus and Bifidobacteria, along with the E. coli strain Nissle 1917 (107). These probiotics have been highly advertised as potent dietary agents against NAFLD [as reviewed in (108)]. This section will go into further details on how probiotics affect the gut microbiome-liver axis.
Probiotics – Microbiota – Liver Axis
One of the popular uses of probiotics is supplementing them into yogurt to alleviate constipation through improved gut motility, which is further associated with a balanced gut microbiota (109). Recently, the utilization of probiotics has expanded towards other health targets. For example, maternal consumption of HFCS during gestation and lactation induces hypertension in rat offspring; yet, this is counteracted when the fructose-fed mothers are also administered L. casei (20). This protection may be due to this probiotic boosting propionate-producing Prevotella (20), where propionate is associated with attenuation in hypertension and overall better cardiovascular health (110). Along with directly affecting intestinal bacterial communities, probiotics can influence the enteric nervous system (ENS); for example, L. rhamnosus (1 × 1010 CFU/ml) administration induces ROS in a formyl-peptide receptor 1 (FPR1)-dependent manner (111). Moreover, L. plantarum RYPR1 contains bile salt hydrolase (BSH) activity, which increases the longevity of this probiotic through the deconjugation of primary bile acids (112). At the same time, however, elevated BSH activity promotes the generation of the even more toxic, secondary bile acids; therefore, this indicates that RYPR1 must contain an unknown protection mechanism against these gut metabolites.
Along with modulating intestinal health, various probiotics have hepatoprotective properties. L. plantarum, for example, alleviates aflatoxin-induced hepatic injury (113), while L. rhamnosus ameliorates high fructose-induced NAFLD (114). Comparatively, L. plantarum promotes antioxidants and the excretion of aflatoxins (113), whereas L. rhamnosus boosts the Bacteroidetes to Firmicutes ratio and heightens tight junction expressions (114). Additionally, L. rhamnosus limits the spillover of endotoxins into the portal vein and normalizes hepatic lipid metabolism, which further reduces hepatic inflammation and fat accumulation, respectively (114). Moreover, the butyrate-producing probiotic, C. butryicum MIYAIRI 588, prevents the progression from steatosis to hepatocarcinogenesis through Nrf2-mediated upregulation of anti-oxidative enzymes (115). Additionally, L. johnsonii BS15 can effectively prevent NAFLD through upregulating antioxidants, suppressing insulin resistance, improving the gut barrier and modulating the microbiota (116). Along with the mentioned Bifidobacteria and Lactobacillus-derived probiotics, one very popular probiotic in the alleviation of chronic inflammatory diseases is E. coli Nissle 1917 [as reviewed in (117)]. Interestingly, the potent effects of Nissle 1917 may be, in part, to their responses in natural selection and competitive fitness, including self-generated mutations to better modulate carbohydrate utilization, stress response and adherence (118).
The previously mentioned examples are single strain probiotics; however, there are many probiotics that contain multiple bacterial strains. The most popular combination for probiotics are members of Lactobacilli and Bifidobacterium groups, which are fortified in many foods and dietary supplements (119). For example, probiotic yogurt containing L. acidophilus La5 and B. lactis Bb12 can improve NAFLD markers (120). Likewise, a probiotic capsule containing two strains of Lactobacilli and two strains of Bifidobacterium is able to alleviate pediatric NAFLD (121). Another combination for a probiotic includes a 1:1:1 ratio of L. acidopilus, B. infantis and Bacillus cereus. Administration of this probiotic flourishes and diminishes anaerobic and aerobic gut bacteria, respectively, during the progression of NAFLD; moreover, the probiotic mixture upregulates tight junctions, which is associated with lessened endotoxin-activation of TLR4 signaling and amelioration of liver pathology (119). VSL#3 (commercialized as Visbiome®) is another popular probiotic that is comprised of eight Gram-positive strains: one Streptococcus, four Lactobacilli, and three Bifidobacterium (122). When treating VSL#3 to aged Wistar rats, this probiotic causes a positive, robust change to the intestinal microbiota through the decrease in the F/B ratio (122). Alongside, VSL#3 administration increased the abundance of anti-inflammatory bacteria (i.e. Prevotella) along with their metabolites (i.e. propionate), promoted IL-10 signaling and inhibited pro-inflammatory helper T cell secretion from the gut to the liver (122). These beneficial effects of VSL#3 foreshadow that this probiotic could alleviate the bountiful levels of liver injury markers (i.e. ALT) during hepatic diseases, but future study is required to confirm this prediction.
Considering that HCC is highly associated with the gut microbiota profile and inflammation in NAFLD (72), these striking findings on probiotic alleviation of NAFLD through modulation of the gut microbiome insinuates that these live microorganisms may be able to prevent hepatocarcinogenesis. Many combinations of probiotic strains (i.e. L. rhamnosus LC705 and Propionibacterium freudenreichii subsp. Shermani) have been utilized as a dietary approach to reduce the risk of HCC development [as reviewed in (123)]. Likewise, VSL#3 has been proposed as a probiotic to reduce HCC risk (123). Along with being utilized as an independent probiotic, heat-inactivated VSL#3 in combination with L. rhamnosus GG (LGG) and viable E. coli Nissle 1917 (EcN) generates a novel prebiotic mixture known as Prehop (122). This multi-component probiotic can alleviate gut microbiota-associated HCC development through inhibiting angiogenesis, shifting the bacteria community to Bacteroidetes, Prevotella and Oscillibacter, along with promoting the differentiation of intestinal Treg cells and reducing Th17-mediated inflammation (122). While all of these mentioned probiotics have been reported to provide beneficial effects, there are conflicting reports as to the full effectiveness of these microorganisms. Zmora et al. demonstrates that probiotics have a marked resistance to mucosal colonization; yet, these varied between murine and humans, where in the human gut microbiome, probiotics had region and strain-specified mucosal localization patterns (124). Likewise, Suez et al. observed that probiotics actually delay the reconstitution of the gut microbiome after antibiotic treatment compared to spontaneous/regular recovery (125). Hence, the true ‘beneficial’ effects of probiotics on the intestinal microbiome needs further investigation.
VII. Prebiotics: Nutrient Extraction a Good Health Compromise?
The current definition of a prebiotic is ‘a substrate that is selectively utilized by host microorganisms conferring a health benefit’ (126). Generally, these are non-viable substrates that provide essential nutrients for probiotic bacteria, including Bifidobacterium and Lactobacilli (126). Yet, there is also a chance of cross-feeding, where the fermented product(s) generated from the ‘good’ bacteria could promote the ‘bad’ bacteria (127). Examples of prebiotics include fructans, fructo-oligosaccharides, and galacto-oligosaccharides. While prebiotics have been highly advertised to alleviate and prevent various metabolic diseases through the modulation of the gut microbiota [as reviewed in (128)], current controversial research indicates that there are too many variabilities in the results and more studies are required to understand the impact of prebiotics on metabolic, hepatic and intestinal health. This final section of the review will explore the multiple prebiotics that are on the market and the recent updates on how they impact overall health.
Inulin
Originating from chicory roots and Jerusalem artichokes, inulin (β 2→1 linkages) is the most widely studied and utilized plant fructan. It is estimated that U.S daily consumption of this oligosaccharide ranges from 1.3–3.5g, which is less than half of the recommended amounts (129). To increase the availability of this polysaccharide, inulin is extracted from its natural source then purified as a commercial product for processed foods (129). Along with providing a great source of fiber, inulin fortification has been utilized as a texture modifier and a fat and sugar replacer [reviewed in (129)]. With the recent FDA GRAS status of inulin-containing foods (130), there is no doubt that the consumption of inulin-supplemented foods will positively progress. Likewise, the multiple reports on the health benefits of inulin, including protection against hypertension (20) and high fat diet (HFD)-induced metabolic syndrome (131), will further boost the incorporation of this fructan into people’s diets. Interestingly, these positive effects are attributed to inulin modulating the gut microbiome, including preserving the gut barrier integrity (132) and limiting gut-microbiota mediated proteolysis (133). Additionally, this non-digestible carbohydrate promotes the ‘good’, probiotic gut bacteria, including Bifidobacteria (134, 135) and Lactobacilli (136). While these are the two prime phyla that are modulated by inulin, heterogeneous reports have made it difficult to elucidate how this fructan can affect other bacteria in the microbiome (131, 135–139). Interestingly, Chassaing and Gewirtz reveal that inulin generates profound differences between the mucosal and fecal microbiome at both the phyla and species levels (137). This suggests that the fecal and intestinal microbiome may have distinct, complex microbial ecosystems.
Inulin has antioxidant properties (140), which has made it a candidate agent to protect against hepatotoxicity. For example, inulin protects against drug- (141) and chemical-induced (142, 143) liver injury through the scavenging of ROS and promoting levels of glutathione in its reduced state. Moreover, in alcoholic-induced liver damage (ALD), inulin promotes better intestinal health and barrier integrity, which lessens the release of endotoxins (i.e. LPS) and thus, reduces the activation of the pro-inflammatory TLR4-macrophage axis (136). Alleviation of hepatic injury is further promoted when inulin is paired with the flavenol, catechin (143). Interestingly, catechin alone has greater hepatoprotective effects than inulin alone or in combination with catechin (143). While it seems that inulin has positive effects on liver health, what must be acknowledged is that these rodent studies administered inulin for short time periods, ranging from less than 2 weeks (141), 3 weeks (143), and 6 weeks (136). Likewise, in a recent human study, the association of inulin with NAFLD was only a 3-month study (144), which limits observing the long-term effects of inulin on hepatic health. Alarmingly, our group discovered that prolonged inulin feeding for 24 weeks can result with cholestatic liver cancer in gut dysbiotic mice (145). Specifically, we observed that a subset (40%) of toll-like receptor 5 deficient (Tlr5KO) mice developed hyperbilirubinemia and cholemia within 10 days of inulin feeding and then icteric HCC by 6 months. This cholestatic phenotype is associated with a reduction in intestinal intraluminal bile acids, resulting in limited FXR signaling, which would result in the overabundance of these hepatotoxic detergents inside the liver. Moreover, we observed elevations of Clostridia in the fecal microbiome, which indicates elevated generation of toxic secondary bile acids (i.e. deoxycholate). When considering that deoxycholate provokes the senescence-associated secretory phenotype (SASP) in hepatic stellate cells, this could explain, in part, the promotion of hepatic pro-inflammatory and tumorigenetic factors that could progress to HCC (146). In general, more research is certainly required to further understand the mechanism(s) for inulin-induced cholestatic HCC.
β-glucan
Oats and barley are great resources for obtaining β-glucan, a prebiotic that is distinct from inulin due to differences in molecular weight, solubility and glycosidic linkages [as reviewed in (147)]. The popularity of β-glucan is due to is cholesterol lowering properties through increased bile acid synthesis (148), which is further associated with an increase in bile excretion (149). Due to this property, β-glucan is highly fortified in many foods, including cereal, which is usually in combination with phytate to increase the stabilization of this polysaccharide (150). Interestingly, the structure of β-glucan can vary based on its source (i.e. oats vs. barley), which might explain why the polysaccharide originating from oats is more effective in promoting probiotic gut bacteria than barley [as reviewed in (151)]. Similarly, the molecular weight of β-glucan can determine its effectiveness in being a prebiotic, where the difference between 100 and 530 kDa can stimulate and demote probiotic bacteria, respectively (152). Moreover, it has been reported that an intermediate molecular weight of 28 kDa is the most promising candidate to be developed as novel prebiotic (147). Yet, similar to inulin, there are various reports (153–157) as to how β-glucan impacts the gut bacterial composition. What is more consistent is the interaction between β-glucan and the pattern recognition receptor (PRR), dectin-1 (alias Clecl7a). β-glucan activation of dectin-1 can initiate various immune responses in the gut mucosa, including upregulating IL-10 and retinol dehydrogenases (153). Along with promoting intestinal health, β-glucan has demonstrated beneficial effects towards the liver, including protecting against carbon tetrachloride-induced liver injury (158), alleviating hepatic steatosis (154), ameliorating NASH through anti-fibrotic and anti-oxidative properties (159, 160), and suppressing HCC (161, 162). The anti-tumor properties of β-glucan could be derived from its ability to upregulate CD4 T cell modulation and neutrophil infiltration into tumor cells (163).
FOS, GOS and Pectin
Fructo-oligosaccharides (FOS) and galactose-oligosaccharides (GOS) are two important groups of non-digestible carbohydrates. These prebiotics in natural foods usually exist in low quantities (127), which is why FOS and GOS are heavily fortified in foods. Likewise, pectin (a methylated ester of polygalactouronic acid) is commercially extracted from citrus peels, apple pomace, sugar beet pulp and potato pulp (164). Comparatively, pectin is fortified in foods like yogurt, whereas FOS and GOS are popular supplements in infant formula as a means to mimic the microbiome of breast-fed infants (165). Interestingly, FOS supplementation to suckling rats can sway the adult microbiota, including promoting Bifidobacteria and attenuating Firmicutes (166), while GOS-containing infant formula can promote Bifidobacterium during the first year of life (167). Yet, FOS and GOS can not 100% mimic human milk oliogsaccharides, where breast-fed infants have higher Bifidobacterium numbers and a lower diversity in comparison to formula-fed infants (165). These differences could arise from a couple of factors, including dosage (168) and whether the polysaccharide originates from a semi-purified or non-purified source (169). Similarly, the physiological effects of pectin can vary based from its natural source; for example, artichoke-derived pectin can stimulate the growth of Bifidobacterium, Lactobacillus, Bacteroidetes and Prevotella more efficiently than sunflower-derived pectin (170). Likewise, apple-derived pectin supplemented to a HFD rebalances the F/B ratio and increases claudin expression, which results in less endotoxemia and TLR4 signaling (171). Moreover, apple pectin (4% wt/wt in drinking water) significantly attenuates the thickness of submucosa and collagen in a radiation-induced intestinal fibrosis rodent model (172). Along with alleviating intestinal fibrosis, citrus pectin can stop the progression of carbon tetrachloride-induced hepatic fibrosis through the inhibition of galectin-3 and induction of apoptosis in stellate cells (173). Similarly, pectin, FOS and GOS can ameliorate liver injury, steatosis, NAFLD, and NASH (174–179). Yet, similar to inulin, these previous studies involved short-term administration, which has limited understanding how these other prebiotics can affect hepatic health long-term. Shockingly, our group demonstrates that FOS and pectin can induce cholestatic HCC and gut dysbiosis (145), but not to the same degree as inulin. This indicates that long-term administration of these prebiotics can cause detrimental effects on both liver and intestinal health.
Future thoughts
Mammals and their gut microbiome have developed a mutualistic ‘give and take’ relationship. Specifically, we provide a nutrient-rich environment for bacteria to thrive, where their microbial colonization and hydrolytic gut metabolites heavily impact our innate immune responses and thus, impact host pathophysiology. We have already mentioned about how we attempt to maintain this gut symbiosis through the utilization of pro- and prebiotics. To take it a few steps further, much research has explored the therapeutic potential of symbiotics, which consists of differential combinations between pro- and prebiotics. Various reports have demonstrated that these symbiotics can alleviate metabolic syndrome (180), promote intestinal health (181–183), and ameliorate steatosis, fibrosis, NAFLD and NASH (184, 185). Hence, these symbiotics might provide a better avenue to therapies on gut and liver health; yet, more research is required the effects of various symbiotic combinations.
VIII. Conclusions
The ever growing population throughout the globe demands exploitation of natural resources, including extraction of various dietary ingredients from multiple foods. Through the advancement in technology, food scientists and industries can isolate and exploit such precious bioactive components from natural resources for human health. At first, this was considered appreciable and commendable as positive results have been reported; however, such bioactive components may not work in isolation or within a certain group of individuals, including microbiotal dysbiotic patients. In fact, the introduction of these purified ingredients could further break the holobiont relationship between the host and microbiota, which can lead to repercussions on hepatic health (as summarized in Figure 1). While more research is warranted to further determine how these dietary additives effect human health, this review provides a profound leap and in-depth understanding of the food supplements that we ingest on a daily basis.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent: All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Hrncirova L, Hudcovic T, Sukova E, Machova V, Trckova E, Krejsek J, et al. Human gut microbes are susceptible to antimicrobial food additives in vitro. Folia Microbiol (Praha). 2019. [DOI] [PubMed] [Google Scholar]
- 2.Administration USFaD. CFR - Code of Federal Regulations Title 21, Sec. 184.1866 High fructose corn syrup 2018 [Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1866.
- 3.Administration USFaD. Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States 2018 [Available from: https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm.
- 4.Administration USFaD. CFR - Code of Federal Regulations Title 21, Sec. 182.1745 Sodium carboxymethylcellulose 2018 [Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.1745.
- 5.Administration USFaD. CFR - Code of Federal Regulations Title 21, Sec. 172.840 Polysorbate 80. 2018 [Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.840.
- 6.Administration USFaD. CFR - Code of Federal Regulations Title 21, Sec. 184.1400 Lecithin 2018 [Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1400.
- 7.Administration USFaD. Microorganisms & Microbial-Derived Ingredients Used in Food (Partial List) 2018 [Available from: https://www.fda.gov/food/ingredientspackaginglabeling/gras/microorganismsmicrobialderivedingredients/default.htm.
- 8.Kumar H, Salminen S, Verhagen H, Rowland I, Heimbach J, Banares S, et al. Novel probiotics and prebiotics: road to the market. Curr Opin Biotechnol. 2015;32:99–103. [DOI] [PubMed] [Google Scholar]
- 9.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang JW, Chen XH, Ren Z, Zheng SS. Gut microbial dysbiosis associates hepatocellular carcinoma via the gut-liver axis. Hepatobiliary Pancreat Dis Int. 2019;18(1):19–27. [DOI] [PubMed] [Google Scholar]
- 11.Roderburg C, Luedde T. The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes. 2014;5(4):441–5. [DOI] [PubMed] [Google Scholar]
- 12.Tao X, Wang N, Qin W. Gut Microbiota and Hepatocellular Carcinoma. Gastrointest Tumors. 2015;2(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65(3):579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara E Relationship between Obesity, Gut Microbiome and Hepatocellular Carcinoma Development. Dig Dis. 2015;33(3):346–50. [DOI] [PubMed] [Google Scholar]
- 15.Newens KJ, Walton J. A review of sugar consumption from nationally representative dietary surveys across the world. J Hum Nutr Diet. 2016;29(2):225–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White JS, Hobbs LJ, Fernandez S. Fructose content and composition of commercial HFCS- sweetened carbonated beverages. Int J Obes (Lond). 2015;39(1):176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira RM, Botezelli JD, da Cruz Rodrigues KC, Mekary RA, Cintra DE, Pauli JR, et al. Fructose Consumption in the Development of Obesity and the Effects of Different Protocols of Physical Exercise on the Hepatic Metabolism. Nutrients. 2017;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komnenov D, Levanovich PE, Rossi NF. Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms. Nutrients. 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu CN, Lin YJ, Hou CY, Tain YL. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients. 2018;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Astbury S, Song A, Zhou M, Nielsen B, Hoedl A, Willing BP, et al. High Fructose Intake During Pregnancy in Rats Influences the Maternal Microbiome and Gut Development in the Offspring. Front Genet. 2018;9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volynets V, Louis S, Pretz D, Lang L, Ostaff MJ, Wehkamp J, et al. Intestinal Barrier Function and the Gut Microbiome Are Differentially Affected in Mice Fed a Western-Style Diet or Drinking Water Supplemented with Fructose. J Nutr. 2017;147(5):770–80. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa T, Maehara N, Kai T, Arai S, Miyazaki T. Dietary fructose-induced hepatocellular carcinoma development manifested in mice lacking apoptosis inhibitor of macrophage (AIM). Genes Cells. 2016;21(12):1320–32. [DOI] [PubMed] [Google Scholar]
- 24.Dowman JK, Hopkins LJ, Reynolds GM, Nikolaou N, Armstrong MJ, Shaw JC, et al. Development of hepatocellular carcinoma in a murine model of nonalcoholic steatohepatitis induced by use of a high-fat/fructose diet and sedentary lifestyle. Am J Pathol. 2014;184(5):1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz JM, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW, et al. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children With Obesity. Gastroenterology. 2017;153(3):743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibarra-Reynoso LDR, Lopez-Lemus HL, Garay-Sevilla ME, Malacara JM. Effect of Restriction of Foods with High Fructose Corn Syrup Content on Metabolic Indices and Fatty Liver in Obese Children. Obes Facts. 2017;10(4):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustig RH, Mulligan K, Noworolski SM, Tai VW, Wen MJ, Erkin-Cakmak A, et al. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity (Silver Spring). 2016;24(2):453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155(6):1828–37 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang S-K, Murphy CJ, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science. 2019;363(6433):1345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman MA, Samuel VT. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol Metab. 2016;27(10):719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, et al. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 2018;27(2):351–61 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Softic S, Cohen DE, Kahn CR. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig Dis Sci. 2016;61(5):1282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poolsri WA, Phokrai P, Suwankulanan S, Phakdeeto N, Phunsomboon P, Pekthong D, et al. Combination of Mitochondrial and Plasma Membrane Citrate Transporter Inhibitors Inhibits De Novo Lipogenesis Pathway and Triggers Apoptosis in Hepatocellular Carcinoma Cells. Biomed Res Int. 2018;2018:3683026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mock K, Lateef S, Benedito VA, Tou JC. High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J Nutr Biochem. 2017;39:32–9. [DOI] [PubMed] [Google Scholar]
- 35.Bawden SJ, Stephenson MC, Ciampi E, Hunter K, Marciani L, Macdonald IA, et al. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: A (31)P MRS study. Clin Nutr. 2016;35(3):645–9. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan JS, Le MT, Pan Z, Rivard C, Love-Osborne K, Robbins K, et al. Oral fructose absorption in obese children with non-alcoholic fatty liver disease. Pediatr Obes. 2015;10(3):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017;66(5):1031–6. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko C, Ogura J, Sasaki S, Okamoto K, Kobayashi M, Kuwayama K, et al. Fructose suppresses uric acid excretion to the intestinal lumen as a result of the induction of oxidative stress by NADPH oxidase activation. Biochim Biophys Acta Gen Subj. 2017;1861(3):559–66. [DOI] [PubMed] [Google Scholar]
- 39.Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017;127(11):4059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakatsu Y, Seno Y, Kushiyama A, Sakoda H, Fujishiro M, Katasako A, et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2015;309(1):G42–51. [DOI] [PubMed] [Google Scholar]
- 41.Goffredo M, Mass K, Parks EJ, Wagner DA, McClure EA, Graf J, et al. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J Clin Endocrinol Metab. 2016;101(11):4367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toop CR, Muhlhausler BS, O’Dea K, Gentili S. Impact of perinatal exposure to sucrose or high fructose corn syrup (HFCS-55) on adiposity and hepatic lipid composition in rat offspring. J Physiol. 2017;595(13):4379–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuruk AA, Nergiz-Unal R. Maternal dietary free or bound fructose diversely influence developmental programming of lipogenesis. Lipids Health Dis. 2017;16(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sellmann C, Priebs J, Landmann M, Degen C, Engstler AJ, Jin CJ, et al. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J Nutr Biochem. 2015;26(11):1183–92. [DOI] [PubMed] [Google Scholar]
- 45.Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO, et al. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int J Hepatol. 2014;2014:560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seki K, Kitade M, Nishimura N, Kaji K, Asada K, Namisaki T, et al. Oral administration of fructose exacerbates liver fibrosis and hepatocarcinogenesis via increased intestinal permeability in a rat steatohepatitis model. Oncotarget. 2018;9(47):28638–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jegatheesan P, Beutheu S, Freese K, Waligora-Dupriet AJ, Nubret E, Butel MJ, et al. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Br J Nutr. 2016;116(2):191–203. [DOI] [PubMed] [Google Scholar]
- 48.Jegatheesan P, Beutheu S, Ventura G, Sarfati G, Nubret E, Kapel N, et al. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clin Nutr. 2016;35(1):175–82. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Mei L, Deng Y, Liu Y, Wei X, Liu M, et al. Lactobacillus brevis DM9218 ameliorates fructose-induced hyperuricemia through inosine degradation and manipulation of intestinal dysbiosis. Nutrition. 2018;62:63–73. [DOI] [PubMed] [Google Scholar]
- 50.Aldamiz-Echevarria L, de Las Heras J, Couce ML, Alcalde C, Vitoria I, Bueno M, et al. Non-alcoholic fatty liver in hereditary fructose intolerance. Clin Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 51.Lee AA, Owyang C. Sugars, Sweet Taste Receptors, and Brain Responses. Nutrients. 2017;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suez J, Korem T, Zilberman-Schapira G, Segal E, Elinav E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes. 2015;6(2):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrejic BM, Mijatovic VM, Samojlik IN, Horvat OJ, Calasan JD, Dolai MA. The influence of chronic intake of saccharin on rat hepatic and pancreatic function and morphology: gender differences. Bosn J Basic Med Sci. 2013;13(2):94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amin KA, AlMuzafar HM. Alterations in lipid profile, oxidative stress and hepatic function in rat fed with saccharin and methyl-salicylates. Int J Clin Exp Med. 2015;8(4):6133–44. [PMC free article] [PubMed] [Google Scholar]
- 55.Bian X, Tu P, Chi L, Gao B, Ru H, Lu K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem Toxicol. 2017;107(Pt B):530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansourian M, Mahnam K, Rajabi HR, Roushani M, Doustimotlagh AH. Exploring the binding mechanism of saccharin and sodium saccharin to promoter of human p53 gene by theoretical and experimental methods. J Biomol Struct Dyn. 2019:1–17. [DOI] [PubMed] [Google Scholar]
- 57.Dhar D, Antonucci L, Nakagawa H, Kim JY, Glitzner E, Caruso S, et al. Liver Cancer Initiation Requires p53 Inhibition by CD44-Enhanced Growth Factor Signaling. Cancer Cell. 2018;33(6):1061–77 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alkafafy Mel S, Ibrahim ZS, Ahmed MM, El-Shazly SA. Impact of aspartame and saccharin on the rat liver: Biochemical, molecular, and histological approach. Int J Immunopathol Pharmacol. 2015;28(2):247–55. [DOI] [PubMed] [Google Scholar]
- 59.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. [DOI] [PubMed] [Google Scholar]
- 60.Haighton L, Roberts A, Jonaitis T, Lynch B. Evaluation of aspartame cancer epidemiology studies based on quality appraisal criteria. Regul Toxicol Pharmacol. 2019;103:352–62. [DOI] [PubMed] [Google Scholar]
- 61.FDA 101: Dietary Supplements: US Food and Drug Administration; 2017. [updated 11/06/2017 Available from: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm050803.htm.
- 62.Lebda MA, Tohamy HG, El-Sayed YS. Long-term soft drink and aspartame intake induces hepatic damage via dysregulation of adipocytokines and alteration of the lipid profile and antioxidant status. Nutr Res. 2017;41:47–55. [DOI] [PubMed] [Google Scholar]
- 63.Finamor I, Perez S, Bressan CA, Brenner CE, Rius-Perez S, Brittes PC, et al. Chronic aspartame intake causes changes in the trans-sulphuration pathway, glutathione depletion and liver damage in mice. Redox Biol. 2017;11:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adaramoye OA, Akanni OO. Effects of long-term administration of aspartame on biochemical indices, lipid profile and redox status of cellular system of male rats. J Basic Clin Physiol Pharmacol. 2016;27(1):29–37. [DOI] [PubMed] [Google Scholar]
- 65.Qu D, Jiang M, Huang D, Zhang H, Feng L, Chen Y, et al. Synergistic Effects of The Enhancements to Mitochondrial ROS, p53 Activation and Apoptosis Generated by Aspartame and Potassium Sorbate in HepG2 Cells. Molecules. 2019;24(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashok I, Sheeladevi R. Oxidant stress evoked damage in rat hepatocyte leading to triggered nitric oxide synthase (NOS) levels on long term consumption of aspartame. J Food Drug Anal. 2015;23(4):679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palmnas MS, Cowan TE, Bomhof MR, Su J, Reimer RA, Vogel HJ, et al. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS One. 2014;9(10):e109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinson JNV, Pinkham NV, Peters GW, Cho H, Heng J, Rauch M, et al. Rethinking gut microbiome residency and the Enterobacteriaceae in healthy human adults. ISME J. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu W, Winter MG, Byndloss MX, Spiga L, Duerkop BA, Hughes ER, et al. Precision editing of the gut microbiota ameliorates colitis. Nature. 2018;553(7687):208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanduzzi Zamparelli M, Rocco A, Compare D, Nardone G. The gut microbiota: A new potential driving force in liver cirrhosis and hepatocellular carcinoma. United European Gastroenterol J. 2017;5(7):944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58(5):949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69(1):107–20. [DOI] [PubMed] [Google Scholar]
- 73.Chassaing B, Gewirtz AT. Not so Splendid for the Gut Microbiota. Inflamm Bowel Dis. 2018;24(5):1055–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez-Palacios A, Harding A, Menghini P, Himmelman C, Retuerto M, Nickerson KP, et al. The Artificial Sweetener Splenda Promotes Gut Proteobacteria, Dysbiosis, and Myeloperoxidase Reactivity in Crohn’s Disease-Like Ileitis. Inflamm Bowel Dis. 2018;24(5):1005–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uebanso T, Ohnishi A, Kitayama R, Yoshimoto A, Nakahashi M, Shimohata T, et al. Effects of Low-Dose Non-Caloric Sweetener Consumption on Gut Microbiota in Mice. Nutrients. 2017;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bian X, Chi L, Gao B, Tu P, Ru H, Lu K. Gut Microbiome Response to Sucralose and Its Potential Role in Inducing Liver Inflammation in Mice. Front Physiol. 2017;8:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhurandhar D, Bharihoke V, Kalra S. A histological assessment of effects of sucralose on liver of albino rats. Morphologie. 2018;102(338):197–204. [DOI] [PubMed] [Google Scholar]
- 78.Liu CW, Chi L, Tu P, Xue J, Ru H, Lu K. Quantitative proteomics reveals systematic dysregulations of liver protein metabolism in sucralose-treated mice. J Proteomics. 2019;196:1–10. [DOI] [PubMed] [Google Scholar]
- 79.Magnuson BA, Roberts A, Nestmann ER. Critical review of the current literature on the safety of sucralose. Food Chem Toxicol. 2017;106(Pt A):324–55. [DOI] [PubMed] [Google Scholar]
- 80.Berry C, Brusick D, Cohen SM, Hardisty JF, Grotz VL, Williams GM. Sucralose NonCarcinogenicity: A Review of the Scientific and Regulatory Rationale. Nutr Cancer. 2016;68(8):1247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.M S, M P, E T, L F, F M, M L, et al. Sucralose administered in feed, beginning prenatally through lifespan, induces hematopoietic neoplasias in male swiss mice. Int J Occup Environ Health. 2016;22(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin X The Effect of Splenda on Gut Microbiota of Humans Could be Much More Detrimental Than in Animals and Deserves More Extensive Research. Inflamm Bowel Dis. 2019;25(2):e7. [DOI] [PubMed] [Google Scholar]
- 83.Bian X, Chi L, Gao B, Tu P, Ru H, Lu K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS One. 2017;12(6):e0178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chi L, Bian X, Gao B, Tu P, Lai Y, Ru H, et al. Effects of the Artificial Sweetener Neotame on the Gut Microbiome and Fecal Metabolites in Mice. Molecules. 2018;23(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie G, Wang X, Liu P, Wei R, Chen W, Rajani C, et al. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget. 2016;7(15):19355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drasar BS, Renwick AG, Williams RT. The conversion of cyclamate into cyclohexylamine by gut bacteria. Biochem J. 1971;123(4):26P–7P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Halmos EP, Mack A, Gibson PR. Review article: emulsifiers in the food supply and implications for gastrointestinal disease. Aliment Pharmacol Ther. 2019;49(1):41–50. [DOI] [PubMed] [Google Scholar]
- 88.Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66(8):1414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holder MK, Peters NV, Whylings J, Fields CT, Gewirtz AT, Chassaing B, et al. Dietary emulsifiers consumption alters anxiety-like and social-related behaviors in mice in a sex-dependent manner. Sci Rep. 2019;9(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh RK, Wheildon N, Ishikawa S. Food Additive P-80 Impacts Mouse Gut Microbiota Promoting Intestinal Inflammation, Obesity and Liver Dysfunction. SOJ Microbiol Infect Dis. 2016;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lock JY, Carlson TL, Wang C-M, Chen A, Carrier RL. Acute Exposure to Commonly Ingested Emulsifiers Alters Intestinal Mucus Structure and Transport Properties. Scientific Reports. 2018;8(1):10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janeiro MH, Ramirez MJ, Milagro FI, Martinez JA, Solas M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients. 2018;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoyles L, Jimenez-Pranteda ML, Chilloux J, Brial F, Myridakis A, Aranias T, et al. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome. 2018;6(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu ZY, Tan XY, Li QJ, Liao GC, Fang AP, Zhang DM, et al. Trimethylamine N-oxide, a gut microbiota-dependent metabolite of choline, is positively associated with the risk of primary liver cancer: a case-control study. Nutr Metab (Lond). 2018;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cox IJ, Aliev AE, Crossey MM, Dawood M, Al-Mahtab M, Akbar SM, et al. Urinary nuclear magnetic resonance spectroscopy of a Bangladeshi cohort with hepatitis-B hepatocellular carcinoma: A biomarker corroboration study. World J Gastroenterol. 2016;22(16):4191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nejrup RG, Licht TR, Hellgren LI. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ-free mice. Sci Rep. 2017;7(1):3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou RF, Chen XL, Zhou ZG, Zhang YJ, Lan QY, Liao GC, et al. Higher dietary intakes of choline and betaine are associated with a lower risk of primary liver cancer: a case-control study. Sci Rep. 2017;7(1):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Butler LM, Arning E, Wang R, Bottiglieri T, Govindarajan S, Gao YT, et al. Prediagnostic levels of serum one-carbon metabolites and risk of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Newman AC, Maddocks ODK. One-carbon metabolism in cancer. Br J Cancer. 2017;116(12):1499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elbassuoni EA, Ragy MM, Ahmed SM. Evidence of the protective effect of l-arginine and vitamin D against monosodium glutamate-induced liver and kidney dysfunction in rats. Biomed Pharmacother. 2018;108:799–808. [DOI] [PubMed] [Google Scholar]
- 104.Coelho CFF, Franca LM, Nascimento JR, Dos Santos AM, Azevedo-Santos APS, Nascimento FRF, et al. Early onset and progression of non-alcoholic fatty liver disease in young monosodium l-glutamate-induced obese mice. J Dev Orig Health Dis. 2018:1–8. [DOI] [PubMed] [Google Scholar]
- 105.Nakanishi Y, Tsuneyama K, Fujimoto M, Salunga TL, Nomoto K, An JL, et al. Monosodium glutamate (MSG): a villain and promoter of liver inflammation and dysplasia. J Autoimmun. 2008;30(1–2):42–50. [DOI] [PubMed] [Google Scholar]
- 106.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 107.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52(12):7577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meng X, Li S, Li Y, Gan RY, Li HB. Gut Microbiota’s Relationship with Liver Disease and Role in Hepatoprotection by Dietary Natural Products and Probiotics. Nutrients. 2018;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dimidi E, Christodoulides S, Scott SM, Whelan K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv Nutr. 2017;8(3):484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Marko L, Hoges S, et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019;139(11):1407–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chandrasekharan B, Saeedi BJ, Alam A, Houser M, Srinivasan S, Tansey M, et al. Interactions Between Commensal Bacteria and Enteric Neurons, via FPR1 Induction of ROS, Increase Gastrointestinal Motility in Mice. Gastroenterology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yadav R, Singh PK, Puniya AK, Shukla P. Catalytic Interactions and Molecular Docking of Bile Salt Hydrolase (BSH) from L. plantarum RYPR1 and Its Prebiotic Utilization. Front Microbiol. 2016;7:2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang L, Duan C, Zhao Y, Gao L, Niu C, Xu J, et al. Reduction of Aflatoxin B1 Toxicity by Lactobacillus plantarum C88: A Potential Probiotic Strain Isolated from Chinese Traditional Fermented Food “Tofu”. PLoS One. 2017;12(1):e0170109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ritze Y, Bardos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, et al. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9(1):e80169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8(5):e63388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xin J, Zeng D, Wang H, Ni X, Yi D, Pan K, et al. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol. 2014;98(15):6817–29. [DOI] [PubMed] [Google Scholar]
- 117.Scaldaferri F, Gerardi V, Mangiola F, Lopetuso LR, Pizzoferrato M, Petito V, et al. Role and mechanisms of action of Escherichia coli Nissle 1917 in the maintenance of remission in ulcerative colitis patients: An update. World J Gastroenterol. 2016;22(24):5505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crook N, Ferreiro A, Gasparrini AJ, Pesesky MW, Gibson MK, Wang B, et al. Adaptive Strategies of the Candidate Probiotic E. coli Nissle in the Mammalian Gut. Cell Host Microbe. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xue L, He J, Gao N, Lu X, Li M, Wu X, et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Scientific Reports. 2017;7:45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97(12):7386–93. [DOI] [PubMed] [Google Scholar]
- 121.Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J Pediatr Gastroenterol Nutr. 2017;64(3):413–7. [DOI] [PubMed] [Google Scholar]
- 122.Li J, Sung CY, Lee N, Ni Y, Pihlajamaki J, Panagiotou G, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113(9):E1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wan MLY, El-Nezami H. Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy. Hepatobiliary Surg Nutr. 2018;7(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell. 2018;174(6):1388–405 e21. [DOI] [PubMed] [Google Scholar]
- 125.Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell. 2018;174(6):1406–23 e16. [DOI] [PubMed] [Google Scholar]
- 126.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. [DOI] [PubMed] [Google Scholar]
- 127.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Choque Delgado GT, Tamashiro W. Role of prebiotics in regulation of microbiota and prevention of obesity. Food Res Int. 2018;113:183–8. [DOI] [PubMed] [Google Scholar]
- 129.Mensink MA, Frijlink HW, van der Voort Maarschalk K, Hinrichs WL. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr Polym. 2015;130:405–19. [DOI] [PubMed] [Google Scholar]
- 130.Administration USFaD. GRAS Notices 2002. [Available from: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=grasnotices&id=118.
- 131.Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, et al. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe. 2018;23(1):41–53 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pham VT, Seifert N, Richard N, Raederstorff D, Steinert R, Prudence K, et al. The effects of fermentation products of prebiotic fibres on gut barrier and immune functions in vitro. PeerJ. 2018;6:e5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X, Gibson GR, Costabile A, Sailer M, Theis S, Rastall RA. Prebiotic supplementation of in vitro faecal fermentations inhibits proteolysis by gut bacteria and host diet shapes gut bacterial metabolism and response to intervention. Appl Environ Microbiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. MBio. 2019;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vandeputte D, Falony G, Vieira-Silva S, Wang J, Sailer M, Theis S, et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66(11):1968–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang X, He F, Zhang Y, Xue J, Li K, Zhang X, et al. Inulin Ameliorates Alcoholic Liver Disease via Suppressing LPS-TLR4-Mpsi Axis and Modulating Gut Microbiota in Mice. Alcohol Clin Exp Res. 2019;43(3):411–24. [DOI] [PubMed] [Google Scholar]
- 137.Chassaing B, Gewirtz AT. Identification of Inner Mucus-Associated Bacteria by Laser Capture Microdissection. Cell Mol Gastroenterol Hepatol. 2019;7(1):157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Healey G, Murphy R, Butts C, Brough L, Whelan K, Coad J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br J Nutr. 2018;119(2):176–89. [DOI] [PubMed] [Google Scholar]
- 139.Li K, Zhang L, Xue J, Yang X, Dong X, Sha L, et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019. [DOI] [PubMed] [Google Scholar]
- 140.Shang HM, Zhou HZ, Yang JY, Li R, Song H, Wu HX. In vitro and in vivo antioxidant activities of inulin. PLoS One. 2018;13(2):e0192273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kalantari H, Asadmasjedi N, Abyaz MR, Mahdavinia M, Mohammadtaghvaei N. Protective effect of inulin on methotrexate- induced liver toxicity in mice. Biomed Pharmacother. 2019;110:943–50. [DOI] [PubMed] [Google Scholar]
- 142.Correa-Ferreira ML, Verdan MH, Dos Reis Livero FA, Galuppo LF, Telles JE, Alves Stefanello ME, et al. Inulin-type fructan and infusion of Artemisia vulgaris protect the liver against carbon tetrachloride-induced liver injury. Phytomedicine. 2017;24:68–76. [DOI] [PubMed] [Google Scholar]
- 143.Liu J, Lu JF, Wen XY, Kan J, Jin CH. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. Int J Biol Macromol. 2015;72:1479–84. [DOI] [PubMed] [Google Scholar]
- 144.Javadi L, Khoshbaten M, Safaiyan A, Ghavami M, Abbasi MM, Gargari BP. Pro- and prebiotic effects on oxidative stress and inflammatory markers in non-alcoholic fatty liver disease. Asia Pac J Clin Nutr. 2018;27(5):1031–9. [DOI] [PubMed] [Google Scholar]
- 145.Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell. 2018;175(3):679–94 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. [DOI] [PubMed] [Google Scholar]
- 147.Lam KL, Ko KC, Li X, Ke X, Cheng WY, Chen T, et al. In Vitro Infant Faecal Fermentation of Low Viscosity Barley beta-Glucan and Its Acid Hydrolyzed Derivatives: Evaluation of Their Potential as Novel Prebiotics. Molecules. 2019;24(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Y, Harding SV, Thandapilly SJ, Tosh SM, Jones PJH, Ames NP. Barley beta-glucan reduces blood cholesterol levels via interrupting bile acid metabolism. Br J Nutr. 2017;118(10):822–9. [DOI] [PubMed] [Google Scholar]
- 149.Thandapilly SJ, Ndou SP, Wang Y, Nyachoti CM, Ames NP. Barley beta-glucan increases fecal bile acid excretion and short chain fatty acid levels in mildly hypercholesterolemic individuals. Food Funct. 2018;9(6):3092–6. [DOI] [PubMed] [Google Scholar]
- 150.Wang YJ, Zhan R, Sontag-Strohm T, Maina NH. The protective role of phytate in the oxidative degradation of cereal beta-glucans. Carbohydr Polym. 2017;169:220–6. [DOI] [PubMed] [Google Scholar]
- 151.Jayachandran M, Chen J, Chung SSM, Xu B. A critical review on the impacts of beta-glucans on gut microbiota and human health. J Nutr Biochem. 2018;61:101–10. [DOI] [PubMed] [Google Scholar]
- 152.Mikkelsen MS, Jensen MG, Nielsen TS. Barley beta-glucans varying in molecular mass and oligomer structure affect cecal fermentation and microbial composition but not blood lipid profiles in hypercholesterolemic rats. Food Funct. 2017;8(12):4723–32. [DOI] [PubMed] [Google Scholar]
- 153.Gudi R, Perez N, Johnson BM, Sofi MH, Brown R, Quan S, et al. Complex dietary polysaccharide modulates gut immune function and microbiota, and promotes protection from autoimmune diabetes. Immunology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun SS, Wang K, Ma K, Bao L, Liu HW. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin J Nat Med. 2019;17(1):3–14. [DOI] [PubMed] [Google Scholar]
- 155.Teixeira C, Prykhodko O, Alminger M, Fak Hallenius F, Nyman M. Barley Products of Different Fiber Composition Selectively Change Microbiota Composition in Rats. Mol Nutr Food Res. 2018;62(19):e1701023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luo Y, Zhang L, Li H, Smidt H, Wright AG, Zhang K, et al. Different Types of Dietary Fibers Trigger Specific Alterations in Composition and Predicted Functions of Colonic Bacterial Communities in BALB/c Mice. Front Microbiol. 2017;8:966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Angelis M, Montemurno E, Vannini L, Cosola C, Cavallo N, Gozzi G, et al. Effect of Whole-Grain Barley on the Human Fecal Microbiota and Metabolome. Appl Environ Microbiol. 2015;81(22):7945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vetvicka V, Garcia-Mina JM, Proctor M, Yvin JC. Humic acid and glucan: protection against liver injury induced by carbon tetrachloride. J Med Food. 2015;18(5):572–7. [DOI] [PubMed] [Google Scholar]
- 159.Nakashima A, Sugimoto R, Suzuki K, Shirakata Y, Hashiguchi T, Yoshida C, et al. Anti-fibrotic activity of Euglena gracilis and paramylon in a mouse model of non-alcoholic steatohepatitis. Food Sci Nutr. 2019;7(1):139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suchecka D, Harasym J, Wilczak J, Gromadzka-Ostrowska J. Hepato- and gastro- protective activity of purified oat 1–3, 1–4-beta-d-glucans of different molecular weight. Int J Biol Macromol. 2016;91:1177–85. [DOI] [PubMed] [Google Scholar]
- 161.Siddiqui S, Ahmad R, Khan MA, Upadhyay S, Husain I, Srivastava AN. Cytostatic and Anti-tumor Potential of Ajwa Date Pulp against Human Hepatocellular Carcinoma HepG2 Cells. Sci Rep. 2019;9(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Elsonbaty SM, Zahran WE, Moawed FS. Gamma-irradiated beta-glucan modulates signaling molecular targets of hepatocellular carcinoma in rats. Tumour Biol. 2017;39(8):1010428317708703. [DOI] [PubMed] [Google Scholar]
- 163.Zou S, Duan B, Xu X. Inhibition of tumor growth by beta-glucans through promoting CD4(+) T cell immunomodulation and neutrophil-killing in mice. Carbohydr Polym. 2019;213:370–81. [DOI] [PubMed] [Google Scholar]
- 164.Allwyn Sundar Raj A* RS, Jayabalan R and Ranganathan TV A Review on Pectin: Chemistry due to General Properties of Pectin and its Pharmaceutical Uses: Open Access Scientific Reports; 2012. [Available from: https://www.omicsonline.org/scientific-reports/srep550.php.
- 165.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017;81(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morel FB, Oozeer R, Piloquet H, Moyon T, Pagniez A, Knol J, et al. Preweaning modulation of intestinal microbiota by oligosaccharides or amoxicillin can contribute to programming of adult microbiota in rats. Nutrition. 2015;31(3):515–22. [DOI] [PubMed] [Google Scholar]
- 167.Sierra C, Bernal MJ, Blasco J, Martinez R, Dalmau J, Ortuno I, et al. Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: a multicentre, randomised, double-blind and placebo-controlled trial. Eur J Nutr. 2015;54(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mao B, Li D, Zhao J, Liu X, Gu Z, Chen YQ, et al. Metagenomic insights into the effects of fructo-oligosaccharides (FOS) on the composition of fecal microbiota in mice. J Agric Food Chem. 2015;63(3):856–63. [DOI] [PubMed] [Google Scholar]
- 169.Genda T, Kondo T, Hino S, Sugiura S, Nishimura N, Morita T. The Impact of Fructo-Oligosaccharides on Gut Permeability and Inflammatory Responses in the Cecal Mucosa Quite Differs between Rats Fed Semi-Purified and Non-Purified Diets. J Nutr Sci Vitaminol (Tokyo). 2018;64(5):357–66. [DOI] [PubMed] [Google Scholar]
- 170.Ferreira-Lazarte A, Kachrimanidou V, Villamiel M, Rastall RA, Moreno FJ. In vitro fermentation properties of pectins and enzymatic-modified pectins obtained from different renewable bioresources. Carbohydr Polym. 2018;199:482–91. [DOI] [PubMed] [Google Scholar]
- 171.Jiang T, Gao X, Wu C, Tian F, Lei Q, Bi J, et al. Apple-Derived Pectin Modulates Gut Microbiota, Improves Gut Barrier Function, and Attenuates Metabolic Endotoxemia in Rats with Diet-Induced Obesity. Nutrients. 2016;8(3):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang J, Ding C, Dai X, Lv T, Xie T, Zhang T, et al. Soluble Dietary Fiber Ameliorates Radiation-Induced Intestinal Epithelial-to-Mesenchymal Transition and Fibrosis. JPEN J Parenter Enteral Nutr. 2017;41(8):1399–410. [DOI] [PubMed] [Google Scholar]
- 173.Abu-Elsaad NM, Elkashef WF. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can J Physiol Pharmacol. 2016;94(5):554–62. [DOI] [PubMed] [Google Scholar]
- 174.Borges Haubert NJ, Marchini JS, Carvalho Cunha SF, Suen VM, Padovan GJ, Jordao AAJ, et al. Choline and Fructooligosaccharide: Non-alcoholic Fatty Liver Disease, Cardiac Fat Deposition, and Oxidative Stress Markers. Nutr Metab Insights. 2015;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chappuis E, Morel-Depeisse F, Bariohay B, Roux J. Alpha-Galacto-Oligosaccharides at Low Dose Improve Liver Steatosis in a High-Fat Diet Mouse Model. Molecules. 2017;22(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66(4):806–15. [DOI] [PubMed] [Google Scholar]
- 177.Li W, Zhang K, Yang H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J Agric Food Chem. 2018;66(30):8015–25. [DOI] [PubMed] [Google Scholar]
- 178.Matsumoto K, Ichimura M, Tsuneyama K, Moritoki Y, Tsunashima H, Omagari K, et al. Fructo-oligosaccharides and intestinal barrier function in a methionine-choline-deficient mouse model of nonalcoholic steatohepatitis. PLoS One. 2017;12(6):e0175406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shtriker MG, Peri I, Taieb E, Nyska A, Tirosh O, Madar Z. Galactomannan More than Pectin Exacerbates Liver Injury in Mice Fed with High-Fat, High-Cholesterol Diet. Mol Nutr Food Res. 2018;62(20):e1800331. [DOI] [PubMed] [Google Scholar]
- 180.Ke X, Walker A, Haange SB, Lagkouvardos I, Liu Y, Schmitt-Kopplin P, et al. Synbiotic-driven improvement of metabolic disturbances is associated with changes in the gut microbiome in diet-induced obese mice. Mol Metab. 2019;22:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abrahamse-Berkeveld M, Alles M, Franke-Beckmann E, Helm K, Knecht R, Kollges R, et al. Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study. J Nutr Sci. 2016;5:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hibberd AA, Yde CC, Ziegler ML, Honore AH, Saarinen MT, Lahtinen S, et al. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef Microbes. 2019;10(2):121–35. [DOI] [PubMed] [Google Scholar]
- 183.Rajkumar H, Kumar M, Das N, Kumar SN, Challa HR, Nagpal R. Effect of Probiotic Lactobacillus salivarius UBL S22 and Prebiotic Fructo-oligosaccharide on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. J Cardiovasc Pharmacol Ther. 2015;20(3):289–98. [DOI] [PubMed] [Google Scholar]
- 184.Asemi Z, Aarabi MH, Hajijafari M, Alizadeh SA, Razzaghi R, Mazoochi M, et al. Effects of Synbiotic Food Consumption on Serum Minerals, Liver Enzymes, and Blood Pressure in Patients with Type 2 Diabetes: A Double-blind Randomized Cross-over Controlled Clinical Trial. Int J Prev Med. 2017;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. [DOI] [PubMed] [Google Scholar]