Introduction
Margin-negative gastrectomy is the cornerstone of management in early gastric cancer; however, a subset of patients with early gastric cancer experience early recurrence and poor survival despite optimal margin-negative resection1. Although previous studies have identified clinicopathological factors associated with this phenomenon1,2, its genomic underpinnings have not been investigated.
This study explored differences in functional oncogenic molecular alterations in a cohort of patients with gastric cancer treated at Memorial Sloan Kettering Cancer Center (MSK) demonstrating extremes of survivorship, as well as a comparator cohort from The Cancer Genome Atlas (TCGA)3, to identify a distinct genomic profile associated with unexpected poor survival in early gastric cancer.
Methods
After obtaining Institutional Review Board approval (protocol number 16-091), ten patients with pT1–2N0 early gastric cancer who underwent D1+/D2 gastrectomy with curative intent at MSK from 1996 to 2003, and who died from disease within 5 years (MSK-EGC1 cohort), and 19 patients from the TCGA (TCGA-EGC cohort) who were alive without recurrence at 5 years after resection (Fig. S1a, supporting information) were identified, and their molecular profiles were compared.
Relevant genomic findings from this comparison were then validated by comparing with genomic data from three clinically annotated cohorts of patients with gastric cancer who had upfront D1+/D2 gastrectomy with curative intent at MSK (10 patients in each group) demonstrating extremes of survivorship: T1–2N0 early gastric cancer without recurrence (MSK-EGC2 cohort), locally advanced gastric cancer (T3–4N+) without recurrence (MSK-LAGC1 cohort) and T3–4N+ gastric cancer with recurrence (MSK-LAGC2 cohort), all by 5 years (Fig. 1a and Table 1). Results were validated in a previously described cohort of 204 patients with metastatic gastric cancer; clinical and genomic data for this cohort are available publicly4.
Fig. 1. Discovery and validation of the molecular profile of patients with resected early gastric cancer and unexpectedly poor survival.

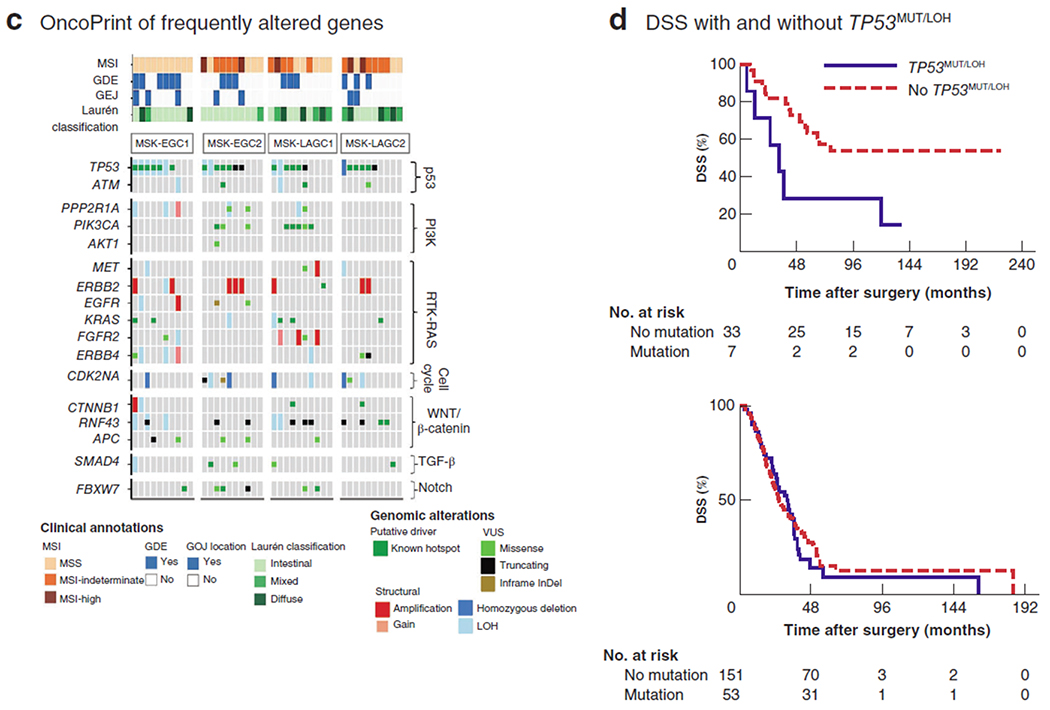
a Study design illustrating exploratory and validation analyses. MSK-EGC1, patients with early gastric cancer (EGC) who had gastrectomy with curative intent at Memorial Sloan Kettering Cancer Center (MSK) with disease-specific survival (DSS) of less than 5 years; TCGA-ECG, patients with early gastric cancer from The Cancer Genome Atlas with overall survival (OS) of 5 years or more after resection; MSK-EGC2, as MSK-EGC1 but with no recurrence at 5 years; MSK-LAGC1, patients with locally advanced gastric cancer without recurrence at 5 years; MSK-LAGC2, patients with locally advanced gastric cancer with recurrence at 5 years. b Comparison of molecular profiles of the MSK-EGC1 and TCGA-EGC cohorts. The box-and-whisker plots compare tumour mutation burden (P=0·161, t test) and somatic copy number alteration (CNA) frequency (P=0·534, t test) between the two cohorts. Below, OncoPrint representation of the 23 most frequently altered genes in MSK-EGC1 and TCGA-EGC cohorts. Types of genomic alteration are grouped as putative driver mutations, variants of undetermined significance (VUS) or structural alterations. LOH, loss of heterozygosity. Relative somatic alteration frequencies in recurrently altered genes in the MSK-EGC1 (black) and TCGA-EGC (grey) cohorts are shown alongside. c OncoPrint representation of the 17 most frequently altered genes in clinically annotated extremes-of-survivorship cohorts undergoing gastrectomy with curative intent. Genes are grouped by their respective signalling pathways. Types of genomic alterations are grouped as putative driver mutations, structural alterations or VUS. Relevant clinical annotations, including microsatellite instability (MSI) status, genome doubling events (GDE), gastro-oesophageal junction (GOJ) location and Laurén histological classification, are indicated for each sequenced sample. d Kaplan–Meier analysis of DSS, stratified by presence or absence of co-occurring TP53MUT/LOH in the pooled extremes-of-survivorship MSK cohort (top) (P=0·018, log rank test) and in patients with metastatic gastric tumours (below) (P=0·713, log rank test).
Table 1.
Demographic and clinicopathological characteristics of patients with resected gastric adenocarcinoma used for MSK-IMPACT™ sequencing, stratified by stage-matched extremes-of-survivorship cohorts
| MSK-EGC1 (n = 10) | MSK-EGC2 (n = 10) | MSK-LAGC1 (n = 10) | MSK-LAGC2 (n = 10) | |
|---|---|---|---|---|
| Age at diagnosis (years)* | 70 | 68 | 63 | 63 |
| Sex ratio (M : F) | 6:4 | 7:3 | 7:3 | 7:3 |
| Caucasian | 9 | 7 | 7 | 9 |
| Tumour location | ||||
| GOJ/proximal third | 4 | 2 | 0 | 3 |
| Body/middle third | 4 | 3 | 7 | 5 |
| Antrum/distal third | 2 | 5 | 3 | 2 |
| Type of gastrectomy | ||||
| Distal/proximal (subtotal) | 8 | 9 | 6 | 5 |
| Total/combined with partial oesophagectomy | 2 | 1 | 4 | 5 |
| Resection margin | ||||
| Negative (R0) | 10 | 10 | 10 | 10 |
| Positive (R1–2) | 0 | 0 | 0 | 0 |
| Extent of lymphadenectomy | ||||
| D1+ | 1 | 2 | 1 | 1 |
| D2 | 9 | 8 | 9 | 9 |
| pT category | ||||
| pT1–2 | 10 | 10 | 0 | 0 |
| pT3–4 | 0 | 0 | 10 | 10 |
| pN status | ||||
| pN0 | 10 | 10 | 0 | 0 |
| pN+ | 0 | 0 | 10 | 10 |
| Laurén classification | ||||
| Intestinal | 7 | 9 | 4 | 3 |
| Diffuse | 2 | 0 | 3 | 3 |
| Mixed | 1 | 1 | 3 | 4 |
| Adjuvant therapy | ||||
| None | 10 | 10 | 4 | 2 |
| Chemotherapy alone | 0 | 0 | 2 | 1 |
| Chemoradiotherapy | 0 | 0 | 4 | 7 |
| Disease recurrence | 10 | 0 | 0 | 10 |
| Recurrence pattern | – | – | ||
| Locoregional only | 0 | 0 | ||
| Distant only | 7 | 8 | ||
| Locoregional and distant | 3 | 2 | ||
| Salvage chemotherapy at recurrence | 8 | – | – | 9 |
Values are median. MSK-EGC1, patients with early gastric cancer (EGC) who had gastrectomy with curative intent at Memorial Sloan Kettering Cancer Center (MSK) with disease-specific survival of less than 5 years; MSK-EGC2, as MSK-EGC1 but with no recurrence at 5 years; MSK-LAGC1, patients with locally advanced gastric cancer without recurrence at 5 years; MSK-LAGC2, patients with locally advanced gastric cancer with recurrence at 5 years; GOJ, gastro-oesophageal junction.
Genomic sequencing and analysis
DNA from tumour and matched normal tissue was sequenced using MSK-IMPACT™ (Integrated Mutation Profiling of Actionable Cancer Targets; Department of Pathology, MSK), a targeted exome capture-based, US Food and Drug Administration-approved, next-generation, sequencing assay. Sequencing output was processed as described previously4,5 and in Appendix S1 (supporting information).
Tumour mutation burden (TMB) was calculated as the total number of non-synonymous mutations divided by the actual number of bases analysed. MSIsensor scores represent the unstable proportion of all tested microsatellites. Cases were classified as microsatellite stable (MSIsensor score below 3), microsatellite instability–indeterminant (score 3–10) or microsatellite instability–high (MSI-H) (score 10 or more)6. Allele-specific DNA copy number, including loss of heterozygosity (LOH) and shallow gains, of key mutant tumour suppressors was inferred using the Fraction and Allele-Specific Copy Number Estimates from Tumor Sequencing (FACETS) bioinformatic pipeline (see Appendix S1, supporting information).
Comparison of genomic alterations
The most commonly altered genes implicated in the TCGA stomach adenocarcinoma analysis were identified and used for genomic comparison between cohorts of interest. For further details, see Appendix S1 (supporting information).
Statistical analysis
Continuous variables were compared with two-tailed t tests, and categorical variables with Fisher’s exact test. Disease-specific survival (DSS) was used for all survival analyses, and examined from date of gastrectomy to date of death from disease or last available follow-up. Death from unrelated causes was censored. Kaplan–Meier survival curves were generated and compared with the log rank test. Statistical analyses were performed using SAS® v9.4 (SAS Institute, Cary, North Carolina, USA) and GraphPad Prism® v8.0 (GraphPad Software, La Jolla, California, USA).
Results
Distinct genomic profile associated with poor survival
In the MSK-EGC1 cohort, all of whom had T1–T2 N0 early gastric cancer, underwent gastrectomy with curative intent and died from disease within 5 years, the median times to recurrence and DSS were 11·7 months and 2·3 years respectively. Patients selected in the TCGA-EGC cohort survived for at least 5 years after gastrectomy. Comparison of clinicopathological factors in the MSK-EGC1 and TCGA-EGC cohorts is presented in Table S1 (supporting information).
In these two early gastric cancer cohorts, somatic gene alterations were most frequent in TP53 (67 per cent), ERBB2 (37 per cent), APC (30 per cent), and PPP2R1A (30 per cent). Although TMB and MSIsensor scores did not differ significantly between the two cohorts (Fig. S1b, supporting information), TP53 hotspot mutations were significantly more frequent in the MSK-EGC1 cohort with poor prognosis (6 of 10 versus 4 of 19 in the TCGA-EGC cohort; P=0·050). Somatic copy number alteration frequency, including LOH at TP53 (6 of 10 versus 6 of 19 respectively; P=0·236) and genome-doubling events (6 of 10 versus 9 of 19; P=0·699), did not differ significantly between the MSK-EGC1 and TCGA-EGC cohorts. TP53 alterations predicted to result in protein stabilization (TP53 hotspots co-occurring with LOH (TP53MUT/LOH)7) were significantly more frequent in the MSK-EGC1 cohort than in the TCGA-EGC cohort (5 of 10 versus 2 of 19; P=0·030) (Fig. 1b).
Validation of genomic profile in extremes-of-survivorship cohorts
To validate the association of TP53MUT/LOH with poor survival in early gastric cancer, the molecular profiles of tumours in the MSK-EGC1 cohort were compared with those of two cohorts demonstrating DSS of at least 5 years: patients with T1–2N0 (MSK-EGC2) or T3–4N+ (MSK-LAGC1) disease. The median follow-up of surviving patients was 136·2 (range 72·8–221·4) months (Fig. S2a, supporting information). The prevalence of diffuse Laurén histology, location of tumour at the gastro-oesophageal junction (GOJ), MSIsensor scores and TMB did not differ significantly between these three cohorts (Fig. 1c). Although the frequency of TP53 hotspots and TP53 LOH did not vary statistically significantly across cohorts, co-altered TP53MUT/LOH remained more prevalent in the MSK-EGC1 cohort than in the MSK-EGC2 or MSK-LAGC1 cohorts (5 of 10 versus 1 of 10 versus 1 of 10 respectively; P = 0·151) (Fig. 1c).
PIK3CA hotspot mutations were enriched in MSK-LAGC1 compared with MSK-EGC1 and MSK-EGC2 (4 of 10 versus 0 of 10 versus 1 of 10 respectively; P = 0·094) (Fig. 1c). Given the co-association of PIK3CA mutations and the TCGA consensus Epstein–Barr virus (EBV) subtype3, immunohistochemistry and in situ hybridization for EBV were performed (Appendix S1, supporting information) for the four PIK3CA mutant tumours in MSK-LAGC1, revealing focal EBV positivity in three (Fig. S2b, supporting information).
To ascertain whether TP53MUT/LOH was broadly informative of poor outcomes or whether its prognostic significance was specific to patients with early disease, its frequency in MSK-EGC1 was compared with that in the MSK-LAGC2 cohort (T3–4N+ gastric cancer and DSS less than 5 years). Although the two cohorts were well matched for diffuse, GOJ and MSI-H tumours, TP53MUT/LOH was not observed in MSK-LAGC2 (Fig. 1c).
Comparison of alterations in cancer signalling pathways
No statistically significant differences in the frequency of alterations in p53, PI3K, RTK-Ras, cell cycle, WNT/β-catenin, Notch, TGF-β, or Myc signalling pathways were observed across the four extremes-of-survivorship MSK cohorts (Fig. S3, supporting information).
Adverse prognostic role of TP53MUT/LOH in early gastric cancer
When pooling the extremes-of-survivorship cohorts (40 patients), those with TP53MUT/LOH tumours demonstrated significantly worse DSS than patients with tumours without TP53MUT/LOH (median 32·5 months versus not reached respectively; P=0·018) (Fig. 1d). Conversely, DSS did not differ significantly when cohorts were stratified by other molecular events related to chromosomal instability, such as TP53 LOH alone, genome-doubling events, or p53 and RTK-Ras pathway-level alterations (data not shown). Notably, TP53MUT/LOH was not associated with worse overall survival in patients with metastatic gastric cancer (P = 0·712) (Fig. 1d), further confirming the unique prognostic significance of TP53MUT/LOH in early disease states.
Discussion
Current clinical models and stage subgroupings cannot yet reliably identify which patients with early gastric cancer are at risk of premature recurrence or death. This study reports a novel genomic profile, evaluable at baseline, associated with poor survival specifically in patients with early gastric cancer who are candidates for complete resection. Co-occurring hotspot mutations and loss of heterozygosity in TP53 (TP53MUT/LOH) were enriched in patients with T1–2N0 gastric cancer experiencing recurrence and death within 5 years of complete resection. TP53MUT/LOH was neither enriched nor prognostic in patients with locally advanced or metastatic gastric cancer. Moreover, TP53MUT/LOH, but not other markers of chromosomal instability, was prognostic of survival in patients with resected gastric cancer demonstrating extremes of survivorship. Although hypothesis-generating, these data suggest that TP53MUT/LOH may be a novel biomarker of poor survival in patients with early gastric cancer.
The ability to examine biological correlates of high-risk clinical phenotypes in the present study was facilitated by the extremes-of-survivorship approach used here. This strategy alleviates the considerable problem of deciphering high-risk subsets in unselected populations, a strategy that is often underpowered and its effects diluted by the frequent inclusion of patients yet to reach important outcome milestones8. As such, an extreme outlier approach is decidedly advantageous in disease settings where the outcome of interest is observed infrequently, such as disease-related death in patients with completely resected T1–2 N0 gastric cancer.
TP53MUT/LOH was not observed in all patients in the MSK-EGC1 cohort with poor prognosis. Therefore, this finding must be validated in a larger cohort of patients with early gastric cancer who demonstrate unexpectedly poor survival. One explanation may be the contribution of alternative putative drivers of metastatic progression in these patients, such as EGFR or ERBB2/HER-2 amplification9, FBXW7 hotspot mutations10 or B2M LOH11. Alternatively, functional TP53 LOH may have been underestimated due to ‘copy-neutral’ LOH, as seen with epigenetic silencing or reduced p53 mRNA expression with intact genomic copy number12. Indeed, the relative contributions of these molecular alterations to prognosis in early gastric cancer warrant further investigation.
This study uncovered a novel association between TP53MUT/LOH and poor prognosis in patients with resected early gastric cancer. Interestingly, emerging evidence suggests that TP53MUT/LOH is a prerequisite for mutant p53 stabilization and oncogenic gain-of-function, which manifests as accelerated metastasis, increased drug resistance and worse survival in certain cancer types (such as breast cancer and sarcoma)7. Thus, it is possible that acquisition of oncogenic gain-of-function is an incipient tumorigenic event in this high-risk patient subgroup with early gastric cancer. Further investigation is warranted into the consequences of stabilized p53 hotspots, such as associated epigenetic, RNA and protein-level changes, as well as immune repercussions in the tumour microenvironment. From a clinical perspective, the present findings, if corroborated in larger data sets, may call for heightened surveillance or additional standard/investigational therapies in TP53MUT/LOH tumour-bearing patients with early gastric cancer.
Supplementary Material
Acknowledgements
The genomic data used in this study are available publicly at www.cbioportal.org. A de-identified clinical data set is available from the corresponding author on reasonable request.
Footnotes
Presented in part to the Society of Surgical Oncology Annual Meeting, Seattle, Washington, USA, March 2017
Disclosure: The authors declare no conflict of interest.
Supporting information
Additional supporting information can be found online in the Supporting Information section at the end of the article.
References
- 1.Cao L, Selby LV, Hu X, Zhang Y, Janjigian YY, Tang L et al. Risk factors for recurrence in T1–2N0 gastric cancer in the United States and China. J Surg Oncol 2016; 113: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai JF, Kim S, Kim K, Li C, Oh SJ, Hyung WJ et al. Prediction of recurrence of early gastric cancer after curative resection. Ann Surg Oncol 2009; 16: 1896–1902. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janjigian YY, Sanchez-Vega F, Jonsson P, Chatila WK, Hechtman JF, Ku GY et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 2018; 8: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT). a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015; 17: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu B, Ye K, Zhang Q, Lu C, Xie M, McLellan MD et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics 2014; 30: 1015–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandrova EM, Mirza SA, Xu S, Schulz-Heddergott R, Marchenko ND, Moll UM. p53 loss-of-heterozygosity is a necessary prerequisite for mutant p53 stabilization and gain-of-function in vivo. Cell Death Dis 2017; 8: e2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLuca GC, Ramagopalan SV, Herrera BM, Dyment DA, Lincoln MR, Montpetit A et al. An extremes of outcome strategy provides evidence that multiple sclerosis severity is determined by alleles at the HLA-DRB1 locus. Proc Natl Acad Sci U S A 2007; 104: 20 896–20 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemoine NR, Jain S, Silvestre F, Lopes C, Hughes CM, McLelland E et al. Amplification and overexpression of the EGF receptor and c-erbB-2 proto-oncogenes in human stomach cancer. Br J Cancer 1991; 64: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korphaisarn K, Morris VK, Overman MJ, Fogelman DR, Kee BK, Raghav KPS et al. FBXW7 missense mutation: a novel negative prognostic factor in metastatic colorectal adenocarcinoma. Oncotarget 2017; 8: 39 268–39 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung JT, Hamilton RL, Ohnishi K, Ikeura M, Potter DM, Nikiforova MN et al. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin Cancer Res 2013; 19: 1816–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh N, Hilsenbeck S, Creighton CJ, Dayaram T, Shuck R, Shinbrot E et al. Effects of TP53 mutational status on gene expression patterns across 10 human cancer types. J Pathol 2014; 232: 522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


