SUMMARY
KRAS G12C inhibitors have shown promise in KRAS G12C–mutant lung cancer but intrinsic and acquired resistance are common. Cotreatment with inhibitors of the protein phosphatase SHP2 can abrogate the adaptive response of cancer cells to KRAS inhibitors resulting in greater suppression of MAPK signaling and enhanced tumor growth inhibition.
In this issue of Clinical Cancer Research, Ryan and colleagues study the adaptive response of KRAS G12C–mutant cells to treatment with KRAS G12C inhibitors (1). The MAPK pathway is a key regulator of cellular proliferation, survival, and differentiation, and alterations in this pathway are present in cancers of nearly all lineages. Despite intense efforts to develop selective inhibitors of MAPK signaling as anticancer drugs, clinical success has been modest to date. Initial drug discovery efforts were focused on inhibiting downstream effectors such as MEK, but clinical efficacy was limited by the narrow therapeutic index of even highly selective allosteric inhibitors. More recent strategies have sought to leverage the biochemical mechanisms through which RAS and BRAF mutations induce pathway activation to develop mutant-specific inhibitors with less normal tissue toxicity. The greatest clinical impact to date has been observed with RAF inhibitors, such as vemurafenib, that inhibit MAPK signaling only in tumors that express codon 600 BRAF mutations. As such these drugs have no antitumor effects in tumors in which RAF signaling is driven by upstream alterations, such as those with RAS mutations.
The RAS proteins are guanosine nucleotide binding molecular switches that integrate inputs from cell surface receptors and other intracellular signaling pathways, which are then transmitted to downstream effector cascades such as the MAPK and PI3 kinase pathways. Activating RAS mutations result in the accumulation of active GTP-bound RAS. The current generation of KRAS G12C inhibitors are selective, covalent inhibitors of the G12C mutant, and as a result, these drugs do no inhibit wild-type RAS. These compounds function by trapping the KRAS G12C mutant in the GDP-bound state (2). As a result, inhibitors of upstream activators of RAS can, in a cell context–dependent manner, potentiate the activity of KRAS G12C inhibitors by increasing the pool of GDP-bound KRAS G12C. Conversely, as KRAS G12C must cycle into its inactive state for these drugs to function, cellular adaptations that increase the pool of GTP-bound KRAS can confer drug resistance.
Clinical trials of highly selective, covalent KRAS G12C inhibitors are now ongoing. Early results with one such compound, AMG 510, have been promising with 11 of the first 23 patients with KRAS G12C–mutant non–small cell lung cancer achieving a partial response (3). Unfortunately, in some patients, responses were short-lived, and minimal activity was observed in colorectal cancer where only one of the first 26 patients responded. While these results provide proof-of-concept that targeting KRAS G12C is feasible, the data suggest that mechanistically informed combinations will be required to achieve durable responses in most patients. That the clinical activity to AMG 510 has varied as a function of tumor lineage mirrors the experience with the BRAF inhibitor vemurafenib, where singleagent activity was minimal in colorectal cancer due to feedback-induced activation of EGFR signaling.
In this issue of Clinical Cancer Research, Ryan and colleagues used the covalent KRAS G12C inhibitors ARS-1620 and AMG 510 to study the adaptive response of KRAS G12C–mutant lung, colon, and pancreatic cancer cell lines to selective KRAS G12C inhibition (1). They found that KRAS G12C inhibitors downregulated MAPK pathway activation in all cell lines as demonstrated by a decrease in the expression of phosphorylated MEK, ERK, and RSK at 4 hours. However, MAPK pathway reactivation was observed in most cell lines by 24–48 hours despite continued suppression of GTP-bound KRAS. By performing isoform-specific pulldown assays, they were able to show that treatment with the KRAS G12C inhibitor resulted in a several fold increase in NRAS-GTP and HRAS-GTP levels. The results suggest that KRAS G12C–mutant cells lines adapt rapidly to selective inhibition of mutant KRAS by activating wild-type RAS and that this oncogenic bypass was sufficient to restore MAPK signaling. Further analysis revealed that the increased activation of wild-type RAS activity was the result of increased receptor tyrosine kinase (RTK) activation. Complicating matters, the specific RTK driving the rebound in MAPK signaling varied among the cell lines in the panel.
As the data suggested that cotargeting a single RTK such as EGFR would be unlikely to maximally suppress MAPK pathway activity, they analyzed whether coinhibiting the protein tyrosine phosphatase SHP2 could attenuate the activation of wild-type RAS. SHP2 activates RAS downstream of RTKs through several mechanisms, including dephosphorylation of Sprouty proteins. As predicted, cotreatment of cells with ARS-1620 and the SHP2 inhibitor, SHP099, induced more durable ERK inhibition by abrogating RTK-mediated MAPK pathway reactivation. In mice, the combination was well-tolerated and induced sustained MAPK pathway inhibition in xenograft tumors and suppression of tumor growth. On the basis of these compelling preclinical data, we agree with the authors that the KRAS G12C and SHP2 inhibitor combination warrants testing in patients.
The results reported by Ryan and colleagues are highly analogous to the adaptive response of BRAF V600E–mutated tumors to treatment with selective RAF inhibitors and suggest that the clinical development of KRAS G12C inhibitors will follow a similar path. Treatment of BRAF V600E–mutant cells with RAF inhibitors results in a rapid relief of upstream feedback inhibition of RTKs and RAS. This results in induction of BRAF dimers, which are insensitive to RAF inhibitors and reactivation of ERK signaling (4). As even a small rebound in MAPK pathway signaling can attenuate the clinical response to RAF inhibitors, combination therapies that target multiple nodes in the pathway induce more profound responses in preclinical models. Consistent with these results, the combination of RAF and MEK inhibitors is more effective than either alone in BRAF V600E–mutant melanoma.
However, MEK inhibitors, by targeting a downstream node in the MAPK pathway, can release upstream RTK and RAS from feedback inhibition which can, in some cellular contexts, result in activation of parallel signaling pathways such as the PI3 kinase pathway. Therefore, cotargeting upstream may prove to be more effective than cotargeting downstream as it would be predicted to not only induce more potent MAPK pathway inhibition, but also prevent reciprocal activation of parallel prooncogenic signaling pathways (Fig. 1). In support of this hypothesis, in colorectal cancer, where EGFR is the dominant RTK contributing to ERK reactivation, EGFR and RAF inhibitor combinations, with or without MEK inhibition, are required to durably inhibit MAPK signaling (5). The data reported by Ryan and colleagues, suggest that similar vertical cotargeting of the MAPK pathway will be required to achieve the full potential of KRAS G12C inhibitors as was required for RAF inhibitors. Furthermore, their data suggest that in BRAF V600E–mutant tumors, cotargeting of SHP2 may be preferred to RTK and RAF inhibitor combinations. It remains unknown, however, whether current SHP2 inhibitors will have an adequate therapeutic index to inhibit SHP2 and RAS activation sufficiently to induce antitumor responses in patients. It is also possible that alternative mechanisms of resistance will quickly emerge that limit the benefit of vertical pathway cotargeting, at least in some patients. Despite these concerns, we eagerly await clinical trials testing the combination of KRAS G12C (and BRAF) and SHP2 inhibitors and expect that KRAS G12C will soon join the growing list of oncogenic mutations that can be selectively targeted in patients.
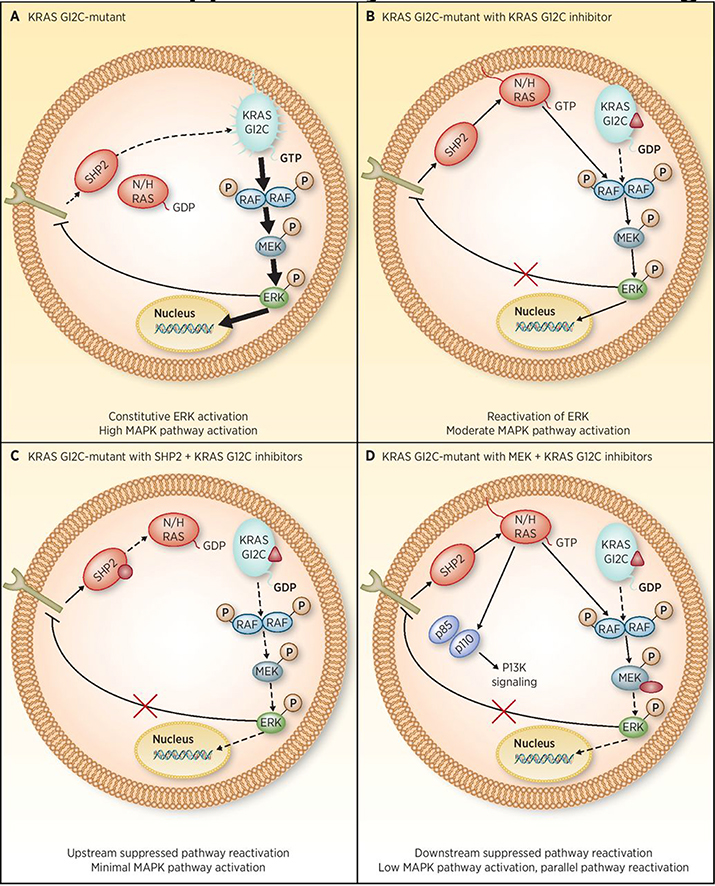
Figure 1.
KRAS G12C–mutant cancer cells restore MAPK pathway signaling following selective G12C inhibition through RTK-mediated activation of wild-type RAS. A, In KRAS G12C–mutant cancer cells, RTK activation is suppressed by ERK-mediated–negative feedback. B, Treatment of KRAS G12C–mutant cells with the covalent G12C inhibitor ARS-1620 results in a release of upstream feedback inhibition of RTKs on the cell surface, which bypass KRAS G12C inhibition by activating wild-type RAS. This results in a rebound in MAPK pathway signaling. C, Cotreatment of cells with a SHP2 inhibitor can prevent RTK-mediated activation of wild-type RAS resulting in more durable MAPK pathway inhibition. D, While cotreatment with a MEK inhibitor can also prevent rebound in MAPK pathway signaling, the resulting relief of negative feedback inhibition of RTKs and RAS can lead to activation of parallel oncogenic pathways such as the PI3K/AKT pathway.
Supplementary Material
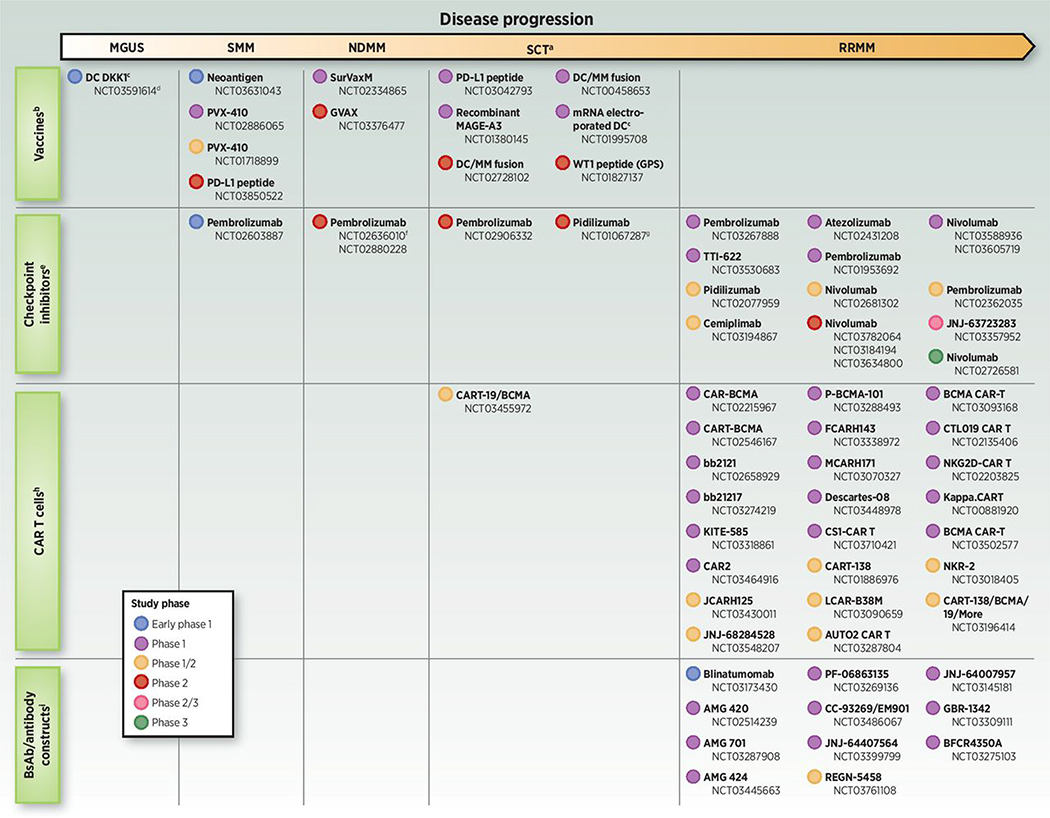
Select T-cell–dependent immuno-oncology studies across the multiple myeloma disease continuum. Studies include early stages of disease (MGUS, SMM, and NDMM), patients with multiple myeloma and low tumor burden (transplant setting), and patients in the relapsed and/or refractory multiple myeloma setting. BCMA, B-cell maturation antigen; bsAb, bispecific antibody; CAR T, chimeric antigen receptor T cell; CPI, checkpoint inhibitor; DC, dendritic cell; GPS, galinpepimut-S; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; NDMM, newly diagnosed multiple myeloma; RRMM, relapsed and/or refractory multiple myeloma; SCT, stem cell transplantation; SMM, smoldering multiple myeloma. Based on www.clinicaltrials.gov search conducted on February 20, 2019. aSCT studies may include NDMM or RRMM patients (i.e., not mutually exclusive). bStudies in RRMM are not shown, as the focus of more recent vaccine therapy trials has been for disease stages with lower tumor burden/immunosuppression. cAutologous DC vaccine. dStudy also includes SMM and multiple myeloma patients. eStudies evaluating pembrolizumab/immunomodulatory drug combinations in RRMM are not included due to clinical holds placed on some of these studies. fStudy also includes patients at first relapse. gIn conjunction with DC/multiple myeloma vaccine. hFollowing the completion of our clinicaltrials.gov search, a phase I/II study of CT053, a BCMA-targeting CAR T-cell therapy, has opened (NCT03975907). iFollowing the completion of our clinicaltrials.gov search, a phase I study of TNB-383B, a BCMA-targeting bsAb/antibody construct, has entered clinical trials (NCT03933735).
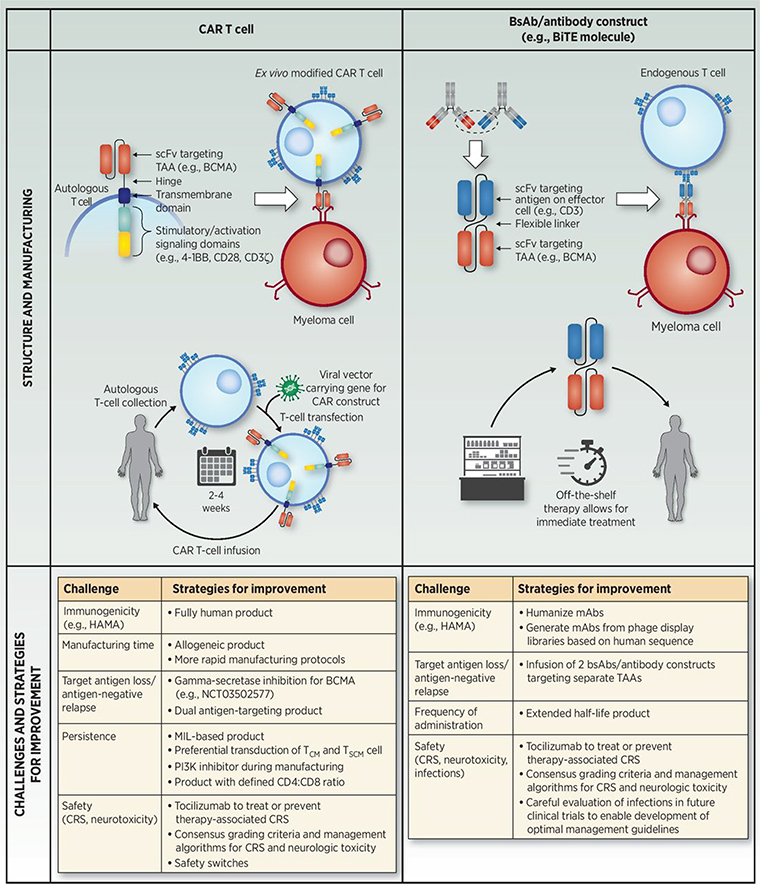
Comparison of CAR T-cell and bsAb/antibody construct immuno-oncology approaches in multiple myeloma. Similarities and differences in structure and manufacturing (43, 49, 68), as well as challenges and current strategies for improvement (75, 82, 87–92). BCMA, B-cell maturation antigen; BiTE, bispecific T-cell engager; bsAb, bispecific antibody; CAR, chimeric antigen receptor; CM, central memory; CRS, cytokine release syndrome; HAMA, human anti-mouse antibody; mAb, monoclonal antibody; MIL, marrow-infiltrating lymphocyte; scFV, single-chain variable fragment; SCM, stem cell memory; TAA, tumor-associated antigen; TCR, T-cell receptor.
| Therapy class: target(s) | Identifier (reference) | Phase |
N |
Patient population |
Immuno-oncology agent and regimen(s) | ORR | ≥CR | Safety |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligible patients enrolled | Received study treatment | Evaluable for safety/efficacy | Median (range) age, years | Prior lines of therapy | High-risk cytogenetics | Common AEs | CRS | Neurotoxicity | ||||||
| CAR T: BCMA | NCT02215967 (55) | 1 | 19 (at highest dose level) | 16 (at highest dose level) | 16 (at highest dose level) | Not reported | Median (range): 9.5 (3–19) | High-risk defined by del(17p), t(14;16), t(14;20), or t(4;14): 40% at highest dose | CAR-BCMA T cells with Cy/Flu (0.3–9 × 106/kg) | 81% (13/16) | 13% (2/16) | CRS, hypotension | Any grade: 94% (15/16); Grade ≥3: 38% (6/16) |
Occurred in the setting of severe CRS, limited to confusion or delirium; 1 patient experienced encephalopathy and muscle weakness in all extremities |
| NCT02546167 (54) | 1 | 29 | 25 | 25 | 58 (44–75) | Median (range): 7 (3–13) | High-risk defined by complex karyotype, gain 1q, del(17p), t(14;16), and/or t(4;14): 96% (24/25) | CART-BCMA cells (1–5 × 108) alone (Cohort 1) CART-BCMA cells (1–5 × 107) with Cy (Cohort 2) CART-BCMA cells (1–5 × 108) with Cy (Cohort 3) | Cohort 1: 44% (4/9); Cohort 2: 20% (1/5); Cohort 3: 64% (7/11) |
Cohort 1: 11% (1/9); Cohort 2: 0%; Cohort 3: 9% (1/11) |
Grade ≥3: • Leukopenia (44% [11/25]); • Neutropenia (44% [11/25]); • Lymphopenia (36% [9/25]) |
Any grade 88% (22/25); Grade ≥3: 32% (8/25) |
Any grade: 32% (8/25); Grade ≥3 12% (3/25) | |
| NCT02658929 (53) | 1 | 36 | 33 | 33 | 60 (37–75) | Median (range): 7 (3–23) | High risk defined by del(17p), t(4;14), or t(14;16): 45% (15/33) | bb2121 CAR T cells (50–800 × 106) with Cy/Flu | 85% (28/33); 90% (27/30) in patients receiving 150–800 × 106 CAR+ T cells | 45% (15/33) | Any grade: • Neutropenia (85% [28/33]); • CRS (76% [25/33]); • Leukopenia (61% [20/33]); • Anemia (58% [19/33]); • Thrombocytopenia (58% [19/33]) |
Any grade: 76% (25/33); Grade ≥3: 6% (2/33) |
Any grade neurologic toxic effect: 42% (14/33); Grade ≥3 neurologic toxic effect: 3% (1/33) | |
| NCT03090659 (56, 93) | 1 | 57 | 57 | 57 | At 1 of 4 clinical centers: 54 (27–72) | Median (range): 3 (1–9) | Not reported | LCAR-B38M CAR T cells (median, 0.5 × 106 cells/kg) with Cy | 88% (50/57) | 68% (39/57) | Any grade: • Pyrexia (91% [52/57]); • CRS (89% [51/57]); • Thrombocytopenia (49% [28/57]); |
Any grade: 89% (51/57); Grade ≥3: 7% (4/57) |
Any grade: 2% (1/57); Grade ≥3: 0% | |
| 1 | 17 | 17 | 17 | At 3 of 4 clinical centers: Range: 35–73 | ≥3 prior therapies: 71% | t(4;14) and del (17p): 35% (6/17) Gain(1q): 65% (11/17) Del(13q): 35% (6/17) |
LCAR-B38M CAR T cells (median, 0.6 × 106 cells/kg) with Cy or Cy/Flu | 88% (15/17) | 76% (13/17) | Any grade: • Fever: 100% (17/17); • CRS: 100% (17/17); • Cytopenia: 82% (14/17); • Liver dysfunction: 53% (9/17) |
Any grade: 100% (17/17); Grade ≥3: 41% (7/17) |
Not reported | ||
| NCT03274219 (91) | 1 | 13 | 12 | 12 | 63 (44–69) | Median (range): 7 (4–17) | Del(17p), t(4;14), t(14;16): 58% (7/12) | bb21217 CAR T cells (150 × 106) with Cy/Flu | 83% (10/12) | 25% (3/12) | Grade ≥3: • Neutropenia (83% [10/12]) • Thrombocytopenia (50% [6/12]) • Anemia (42% [5/12]) • Leukopenia (42% [5/12]) |
Any grade: 67% (8/12); Grade ≥3: 8% (1/12) |
Any grade: 25% (3/12); Grade ≥3: 8% (1/12) | |
| NCT03288493 (87) | 1 | Not reported | 12 | Safety: 12; Efficacy: 9 (3, Cohort 1; 6 Cohort 2) | Not reported | Range: 3–9 | High-risk: 64% | P-BCMA-101 CAR T cells (48–430 × 106) with Cy/Flu (Cohorts 1–3) | Cohorts 2 and 3: 83% (5/6) | Cohorts 2 and 3: 17% (1/6) | Grade ≥3: • Cytopenia • Febrile neutropenia |
Any grade: 8% (1/12); Grade ≥3: 0% | Any grade: 0% | |
| N/A (94) | N/A | Not reported | 16 | Safety: 16; Efficacy: 13 | 55 (39–67) | Median (range): 4 (2–10) | Not reported | CT053 CAR T cells with Cy/Flu | 100% (13/13) | 15% (2/13) | Grade ≥3 CAR T-related AEs: • Thrombocytopenia (19% [3/16]); • Leukopenia (19% [3/16]); • Anemia, neutropenia, fever (13% [2/16] each) |
Any grade: 19% (3/16); Grade ≥3: 6% (1/16) | Any grade: 0% | |
| NCT03338972 (88) | 1 | Not reported | 7 | Safety: 7; Efficacy: 6 | 63 (49–76) | Median (range): 8 (6–11) | Del(17p), t(4;14), and/or t(14;16): 100% (7/7) | FCARH143 CAR T cells with lymphodepletion | 4 weeks after treatment: 100% | Not reported | Not reported | CRS was limited to grade 1 or 2 in severity and reported in all patients except 1 | No events reported | |
| NCT03430011 (95) | 1/2 | 19 | 13 | 8 | 53 (36–66) | Median (range): 10 (4–15) | IMWG high-risk cytogenetics: 50% (4/8) | JCARH125 CAR T cells with Cy/Flu | 88% (7/8)a | 38% (3/8)a | Not reported | Any grade: 75% (6/8)]; Grade ≥3: 0% |
Any grade neurologic AE: 38% (3/8) Grade ≥3 neurologic AE: 13% (1/8) | |
| NCT03070327 (96) | 1 | Not repored | 11 | Safety: 10; Efficacy: 11 | Not reorted | Median (range): 6 (4–14) | High risk: 82% (9/11) | MCARH171 CAR T cells (72–818 × 106) with Cy/Flu | 64% (7/11) | Not reported | Not reported | Any grade: 60% (6/10); Grade ≥3: 20% (2/10) |
Any grade neurotoxicity: 10% (1/10) Grade ≥3 neurotoxicity: 0% | |
| NCT03093168 (97) | 1 | Not reported | 17 | 14 | Not reported | ≥3 prior regimens for study eligibility | Not reported | BCMA CAR T cells (9 × 106 cells/kg) with Cy/Flu | 79% (11/14) | 50% (7/14) | Grade ≥3 nonhematologic AEs: • Pneumonia (14% [2/14]); • Hypophosphatemia (14% [2/14]); • Hypocalcemia (14% [2/14]) |
Grade ≥3: 7% (1/14) | Grade ≥3 neurotoxicity: 7% (1/14) | |
| ChiCTR1800018137 (98) | Not reported | Not reported | 9 | 9 | Not reported | Median (range): 4 (3–5) | Not reported | CT103A CAR T cells (3 + 3 dose escalation at 1, 3, 6 × 106/kg) with Cy/Flu | 100% (9/9) | 44% (4/9) | Not reported | Grade 0–2 CRS observed in first 2 dose groups | Not reported | |
| NCT03661554 (99) | 1 | Not reported | 16 | 13 at day 28b; 7 at 10 weeks | Not reported | Average 10 prior lines | Not reported | CART-BCMA cells (2–10 × 106 CAR+ cells/kg) with Cy/Flu | 85% (11/13) at 28 d; 100% (7/7) at 10 weeks | 43% (3/7) at 10 weeks | Not reported | Grade ≥3: 2 patients; grade 0–2 CRS observed in other patients | Not reported | |
| CAR T: CD19 | NCT02135406 (100) | 1 | 12 | 10 | 10 | 61 (48–68) | Median (range): 6 (2–10) | Majority of patients had high-risk genetic or clinical characteristics | CTL019 CAR T cells with autoSCT | 80% (8/10) | 0% | Grade ≥3 AEs probably or possibly related to CTL019: autologous GVHD (gastrointestinal) and mucositis (10% [1/10] each) | Any grade: 10% (1/10); Grade ≥3: 0% |
Not reported |
| CAR T: CD19+BCMA | NCT03196414 (89) | 1/2 | Not reported | 8 | Safety: 8; Efficacy: 5 | Not reported | Not reported | Not reported | CART-19 and CART-BCMA cells as split dose (40:60) with Cy/Flu | 80% (4/5) | 0% | Any grade AE • Acute CRS (100% [8/8]) • Fatigue (100% [8/8]) • Cytopenia (100% [8/8]) • Anemia (75% [6/8]) |
Any grade: 100% (8/8) | Any grade neurotoxicity: 0% |
| ChiCTROIC-17011272 (101) | 2 | 22 | 21 | 21 | 58 years (IQR, 49.5–61) | Median (range): 6 (4–17) | Del(17p), t(14;16), t(14;20), or t(4;14): 24% (5/21) | CD19 CAR T cells (1 × 106 cells/kg) and anti-BCMA CAR T cells (1 × 106 cells/kg) with Cy/Flu | 95% (20/21) | 57% (12/21) | Any grade AE • Hypoimmunoglobulinemia (100% [21/21]) • B-cell aplasia (100% [21/21]) • Hematologic (95% [20/21]) • CRS (90% [19/21]) |
Any grade: 90% (19/21); Grade ≥3: 5% (1/21) |
Any grade: 10% (2/21) | |
| CAR T: NKG2D | NCT02203825 (102) | 1 | Not reported for MM cohort | 5c | 5c | Not reported | All patients had ≥5 prior therapies | Not reported | NKG2D-CAR T cells alone | 0% | Not reported | Not reported for MM cohort | Grade ≥3: 0% | Grade ≥3 neurotoxicity: 0% |
| CAR T: CD38+BCMA | ChiCTR1800018143 (103) | 1 | Not reported | 12 | 12 | Not reported | All enrolled patients had ≥2 prior therapies or are double-relapsed or relapse after ASCT | 67% (8/12) with genetic abnormalities | Anti-BCMA and anti-CD38 dual-target CAR T with Cy/Flu | 83% (10/12) | 42% (5/12) | Any grade AE • Hematologic toxicities in almost all patients • CRS (83% [10/12]) • Hepatotoxicity (17% [2/12]) • Nephrotoxicity (8% [1/12]) |
Any grade: 83% (10/12); Grade ≥3: 33% (4/12) |
No neurotoxicity observed |
| CAR T: CD138 | NCT01886976 (104) | 1/2 | 5 | 5 | 5 | Range, 48 to 68 years | Patients had received 5 to 18 prior chemotherapies | Not reported | CART-138 CAR T cells with conditioning | 0% | 0% | Grade ≥3: Fever (80% [4/5]) | Not reported | Not reported |
| CAR T: kappa LC | NCT00881920 (105) | 1 | Not reported | 7c | 7c | Range, 43 to 69 years | All patients had ≥1 prior therapy | Not reported | Kappa.CAR T alone or with Cy | 0% | 0% | Most common AEs reported in MM, NHL, and CLL cohorts were anemia, leukopenia, fatigue, hyper- or hypokalemia, and elevated aspartate aminotransferase | No patients had symptoms associated with severe CRS | Not reported |
| BiTE: BCMA × CD3 | NCT02514239 (74) | 1 | Not reported | 42 | 42 | 65 (39–79) | Median (range): 4 (2–13) prior lines | Standard-risk: 55%; Intermediate-risk: 40% High-risk: 2% |
AMG 420 single agent (0.2 μg/d - 800 μg/d, 4 weeks on/2 weeks off, up to 10 cycles) | 31% (13/42); 400 μg/d: 70% (7/10) | 21% (9/42); 400 μg/d: 50% (5/10) | SAEs: • Infection (31% [13/42]) • Peripheral PN (5% [2/42]) Treatment-related SAE: • Grade 3 peripheral PN (5% [2/42]) • Grade 3 edema (2% [1/42]) |
Any grade: 38% (16/42); Grade ≥3: 2% (1/42) |
2 patients reported peripheral PN as a DLT (1 at 800 μg/d, 1 at 400 μg/d dose deescalation). Both patients had grade 3 peripheral PN events, which were considered treatment-related SAEs |
| BsAb/antibody construct: BCMA × CD3 | NCT03269136, (77) | 1 | Not reported | 5 | 5 | Not reported | Not reported | Not reported | PF-06863135 (PF-3135) single agent | Not reported | Not reported | SAEs: • Grade 1 fever (not related to PF-3135) (n = 1) Treatment-emergent AE (all-causality): • The majority have been grade ≤2 • Grade 3 ALT/AST elevation (n = 1) |
No CRS events have been reported | Not reported |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; autoSCT, autologous stem cell transplantation; BCMA, B-cell maturation antigen; BiTE, bispecific T-cell engager; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CR, complete response; CRP, C-reactive protein; CRS, cytokine release syndrome; Cy, cyclophosphamide; DLT, dose-limiting toxicity; Flu, fludarabine; GVHD, graft-versus-host disease; IL6, interleukin 6; IMWG, International Myeloma Working Group; IQR, interquartile range; mPFS, median progression-free survival; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; ORR, overall response rate; PN, polyneuropathy; SAE, serious adverse events.
Includes confirmed and unconfirmed responses.
Three patients with extramedullary disease were evaluated as partial response at day 28 but were excluded from the efficacy analysis.
MM patient values only.
Table 2.
Ongoing CAR T-cell and bsAb/antibody construct trials in patients with relapsed and/or refractory multiple myeloma with unpublished data.
| Therapy class | Target | Product | Clinical trial identifier (phase) | N | Patient population | Regimen(s) |
|---|---|---|---|---|---|---|
| CAR T cella | BCMA | JNJ-68284528 | NCT03548207 (phase Ib/II) | 84 (est.) | Relapsed or refractory MM | JNJ-68284528 with lymphodepletion |
| KITE-585 | NCT03318861 (phase I) | 64 (est.) | Relapsed/refractory MM | KITE-585 CAR T cells with Cy/Flu | ||
| Descartes-08 | NCT03448978 (phase I) | 15 (est.) | Refractory MM | Descartes-08 with Cy/Flu | ||
| BCMA CAR T | NCT03502577 (phase I) | 18 (est.) | Relapsed or refractory MM | BCMA CAR T cells and LY3039478 with Cy/Flu | ||
| APRIL | AUTO2 | NCT03287804 (phase I/II) | 80 (est.) | Relapsed or refractory MM | AUTO2 (15–350 × 106 cells) with Cy/Flu | |
| CD38 | CAR2 | NCT03464916 (phase I) | 72 (est.) | Relapsed or refractory MM | CAR2 cells alone | |
| SLAMF7/CS1 | CS1-CAR T | NCT03710421 (phase I) | 30 (est.) | Relapsed or refractory MM | CS1-CAR T with Cy/Flu | |
| NKG2D | NKR-2 (CYAD-01) | NCT03018405 (phase I/II, THINK) | 146 (est.) | Relapsed or refractory MM (or AML/MDS) | NKR-2 (3 × 108–3 × 109 cells) cells alone | |
| BCMA+CD138 | CART-138/BCMA | NCT03196414 (phase I/II) | 10 (est.) | Relapsed and refractory MM | CART-138/BCMA with Cy/Flu | |
| BsAb/antibody constructb | BCMA × CD3 | AMG 701 | NCT03287908 (phase I) | 115 (est.) | Relapsed or refractory MM | Single agent |
| JNJ-64007957 | NCT03145181 (phase I) | 60 (est.) | Relapsed or refractory MM | Single agent | ||
| REGN-5458 | NCT03761108 (phase I/II) | 56 (est.) | Relapsed or refractory MM | Single agent | ||
| CC-93269 (EM901) | NCT03486067 (phase I) | 120 (est.) | Relapsed and refractory MM | Single agent | ||
| CD38 × CD3 | AMG 424 | NCT03445663 (phase I) | 120 (est.) | Relapsed or refractory MM | Single agent | |
| GBR-1342 | NCT03309111 (phase I) | 125 (est.) | Previously treated MM | Single agent | ||
| FcRH5 × CD3 | BFCR4350A | NCT03275103 (phase I) | 80 (est.) | Relapsed or refractory MM | Single agent | |
| GPRC5D × CD3 | JNJ-64407564 | NCT03399799 (phase I) | 87 (est.) | Relapsed or refractory MM | Single agent | |
| CD19 × CD3 | Blinatumomab | NCT03173430 (early phase I) | 20 (est.) | Refractory MM | Blinatumomab-autoSCT |
Abbreviations: AML, acute myeloid leukemia; autoSCT, autologous stem cell transplant; BCMA, B-cell maturation antigen; bsAb, bispecific antibody; CAR, chimeric antigen receptor; Cy, cyclophosphamide; est., estimated; Flu, fludarabine; MDS, myelodysplastic syndrome; MM, multiple myeloma.
Based on www.clinicaltrials.gov search conducted on February 20, 2019.
Following the completion of our clinicaltrials.gov search, a phase I/II study of CT053, a BCMA-targeting CAR T-cell therapy, has opened (NCT03975907).
Following the completion of our clinicaltrials.gov search, a phase I study of TNB-383B, a BCMA-targeting bsAb/antibody construct, has entered clinical trials (NCT03933735).
Acknowledgments
This work was supported by the NIH R01 CA233736 (to R. Yaeger) and Cancer Center Core Grant P30 CA008748 (to R. Yaeger and D.B. Solit).
Disclosure of Potential Conflicts of Interest
R. Yaeger is a paid consultant for Array BioPharma, reports receiving commercial research grants from Array BioPharma, Novartis Pharmaceuticals, and Boehringer Ingelheim, and is an unpaid consultant/advisory board member for ArrayBioPharma. D.B. Solit is a paid consultant for Pfizer, Loxo Oncology, Lilly Oncology, Illumina, QED Therapeutics, andVividion Therapeutics. No otherpotential conflicts ofinterest were disclosed.
References
- 1.Ryan MB, Fece de la Cruz F, Phat S, Myers DT, Wong E, Shahzade HA, et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRASG12C inhibition. Clin Cancer Res 2020;26:1633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lito P, Solomon M, Li LS, Hansen R, Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 2016;351:604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govindan R, Fakih MG, Price TJ, Falchook GS, Desai J, Kuo JC, et al. Phase 1 study of AMG 510, a novel molecule targeting KRAS G12C mutant solid tumors. Ann Oncol 2019;30:v159–v193. [Google Scholar]
- 4.Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell 2012;22:668–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discov 2018;8: 428–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.