Abstract
Background
Balamuthia mandrillaris is a free-living ameba that causes rare, nearly always fatal disease in humans and animals worldwide. B. mandrillaris has been isolated from soil, dust, and water. Initial entry of Balamuthia into the body is likely via the skin or lungs. To date, only individual case reports and small case series have been published.
Methods
The Centers for Disease Control and Prevention (CDC) maintains a free-living ameba (FLA) registry and laboratory. To be entered into the registry, a Balamuthia case must be laboratory-confirmed. Several sources were used to complete entries in the registry, including case report forms, CDC laboratory results, published case reports, and media information. SAS© version 9.3 software was used to calculate descriptive statistics and frequencies.
Results
We identified 109 case reports of Balamuthia disease between 1974 and 2016. Most (99%) had encephalitis. The median age was 36 years (range 4 months to 91 years). Males accounted for 68% of the case patients. California had the highest number of case reports followed by Texas and Arizona. Hispanics constituted 55% for those with documented ethnicity. Exposure to soil was commonly reported. Among those with a known outcome, 90% of patients died.
Conclusions
Balamuthia disease in the United States is characterized by a highly fatal encephalitis that affects patients of all ages. Hispanics were disproportionately affected. The southwest region of the U.S. reported the most cases. Clinician awareness of Balamuthia as a cause of encephalitis might lead to earlier diagnosis and initiation of treatment, resulting in better outcomes.
Summary
We describe 109 case reports of Balamuthia disease in the United States between 1974 and 2016. Most were male with encephalitis, had a median age of 36 years, and were reported from southwestern states. Ninety percent of patients died.
Keywords: Balamuthia mandrillaris, free-living ameba, granulomatous amebic encephalitis
INTRODUCTION
Balamuthia mandrillaris is a free-living ameba found in the environment that causes rare cases of human disease, including cutaneous and central nervous system (CNS) disease called granulomatous amebic encephalitis (GAE). Initially thought to be a nonpathogenic soil organism, it was first reported to cause disease in a pregnant mandrill that died of meningoencephalitis at the San Diego Zoo [1]. Since then, Balamuthia has also been reported to cause infection in other animals including dogs, horses, and primates [2–5]. Before the case in the mandrill, other GAE cases that appeared to be caused by Acanthamoeba, a similar free-living ameba, had been reported. However, the brain tissue from these cases did not react with Acanthamoeba-specific immunohistochemical tests [6, 7]. Previously known as a leptomyxid ameba, because of some similar morphologic characteristics, the organism causing the Acanthamoeba-like infections was established as a new genus and species, Balamuthia mandrillaris, based on fundamental differences in morphology, physiology, and antigens [8]. Ribosomal RNA sequencing and phylogenetic analyses showed it to be closely related to Acanthamoeba, while confirming the distinct genus and species [9, 10].
Although Balamuthia and Acanthamoeba are distinct organisms, they are generally unable to be distinguished in tissue sections by light microscopy only. The life cycle of Balamuthia mandrillaris has two stages: a trophozoite and cyst. The trophozoite ranges in size from 12–60 μm, is usually uninucleate (with occasional binucleate forms seen), and pleomorphic while the cyst is 12–30 μm, uninucleate, and spherical [11]. Balamuthia is also distinguished from Acanthamoeba in that it cannot be cultured on bacteria-coated agar plates but must have mammalian cell culture for cultivation in the laboratory.
While Balamuthia was thought to be an environmental organism like Acanthamoeba, at the time of its initial description, it had not been isolated from the environment [8, 12]. Since that time, there have been a few reports of isolation from soil, dust, and water [13–16]. Despite these findings, the exact ecologic niche of Balamuthia mandrillaris remains unknown.
Balamuthia is thought to enter the human body via the lungs by inhalation of cysts or direct contamination of a break in the skin[17]. Although animal-model evidence exists for infection of the brain via the olfactory nerve pathway, similar to Naegleria fowleri [18], this route is not considered relevant for human Balamuthia infection because histopathologic findings from cases do not show olfactory lobe involvement of the type seen in Naegleria fowleri cases. In further support of hematogenous spread, the amebae frequently cluster around blood vessels and autopsies have shown multiple infected tissues [19, 20]. Balamuthia mandrillaris primarily affects two organ systems in humans: the skin and the CNS. Infection occurs in both immunocompetent and immunocompromised patients. Onset is described as subacute to chronic with nonspecific neurologic symptoms in GAE cases [11]. When the skin is involved, lesions are often singular and located on the central face but occasionally on the extremities or trunk. They have been described as painless plaques that may progress to central ulceration [21]. Balamuthia usually presents with manifestations in a single organ system, as with GAE or cutaneous balamuthiasis. However, autopsy specimens from patients with GAE have demonstrated amebae in other organs, such as the kidneys, lungs, and adrenal glands [6, 20]. Mortality is high for Balamuthia infections; few survivors have been reported [22–24]. Treatment of Balamuthia disease has been based on in vitro drug activity and the small number of survivor case reports. Drugs with known activity include pentamidine, fluconazole, flucytosine, sulfadiazine, azithromycin, clarithromycin, and miltefosine [25, 26].
What little is known about the epidemiology of Balamuthia infections has been derived from case reports and small case series. In the United States, these originated with the California Encephalitis Project (CEP), an enhanced surveillance program to test encephalitis cases for both common and uncommon pathogens that operated in California from 1998 to 2010; CEP reported 10 cases of Balamuthia from 1999–2007 [27]. The CEP’s case series and others, and additional case series from Peru, show widely ranging patient ages and male predominance [27, 28]. There is also evidence that a high proportion of Balamuthia patients are of Hispanic ethnicity [29]. Soil exposures are reported frequently in Balamuthia cases [27]. In 2009, a new mode of transmission was documented when two kidney transplant recipients became ill simultaneously with encephalitis. It was later determined that the donor of the kidneys had died of Balamuthia GAE and had transmitted the infection to the kidney recipients via the transplanted organs [30]. Subsequently, two additional Balamuthia transplant-transmitted outbreaks were reported in 2010 and 2012 [30, 31]. As Balamuthia is an environmental organism, exposure is likely common; a serosurvey of healthy landscapers and blood donors reported seropositivity prevalence of 2.5–3.6% [32]. Another serosurvey showed high levels of seropositivity among a group of West Africans living in rural areas. The same study also showed the antibody assay to be specific for Balamuthia with little cross-reactivity with Acanthamoeba spp [33].
As one of the few centers in the United States with the ability to confirm Balamuthia infection in clinical specimens, the Centers for Disease Control and Prevention (CDC) has been in a unique position to collect data on a large number of Balamuthia infections. To shed light on this little known but devastating infection, we report a case series of Balamuthia infections confirmed at CDC.
METHODS
The CDC free-living ameba (FLA) registry was used as the primary data source for this study. Cases were classified on the basis of the state of exposure, if known, or by the state of residence or diagnosis, if state of exposure was not known. SAS© version 9.3 software and Excel were used to calculate descriptive statistics and frequencies.
RESULTS
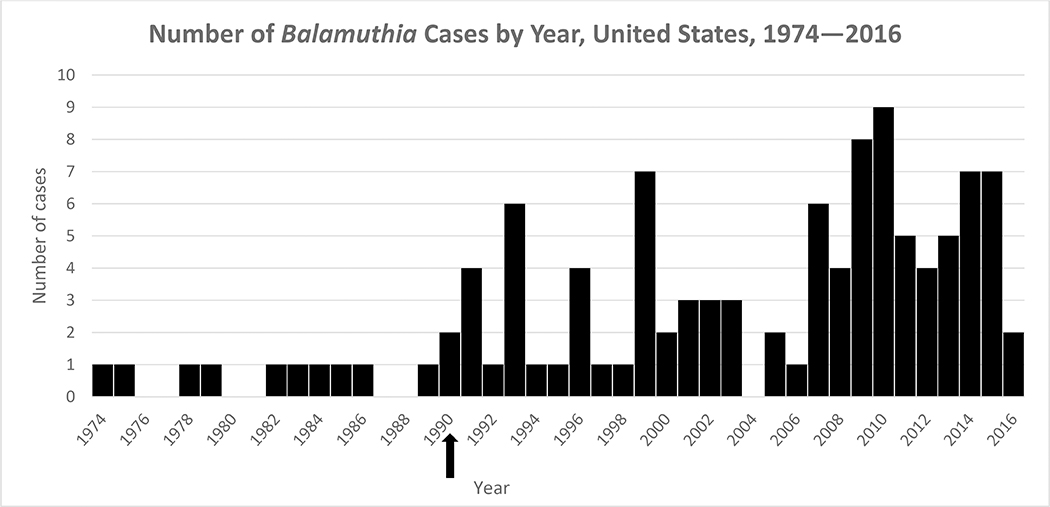
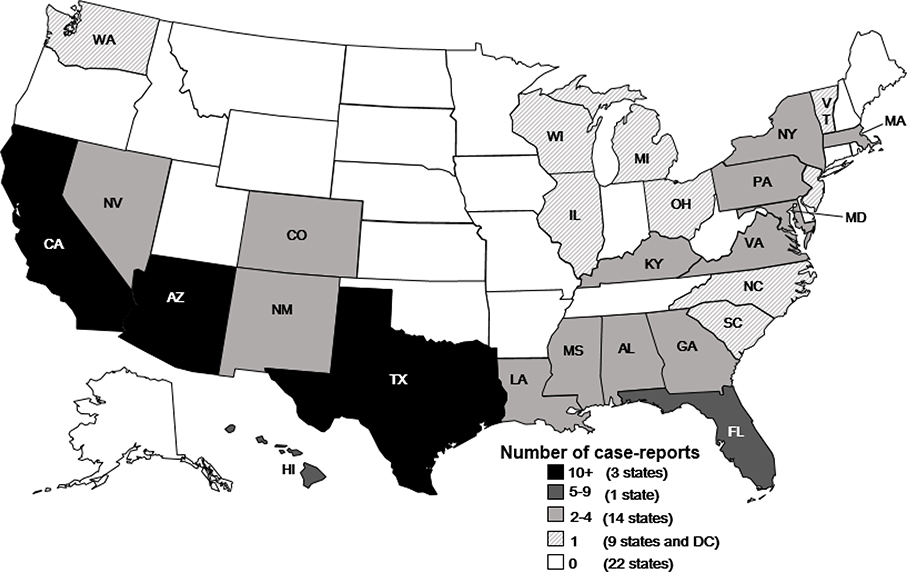
During 1974–2016, 109 case reports of Balamuthia disease (range 0–9 cases per year) were reported in the United States (Figure 1). The median age of patients was 36 years (n=109; range 4 months–91 years); 19% (n=20) were age less than 5 years). Of the 108 case patients with sex reported, 68% were male. For the 59 case patients where race was reported, 68% were white, 22% were black, 10% were Asian/Pacific Islander, and 3% were American Indian. Of the 58 case patients whose ethnicity was reported, 55% were Hispanic (Table 1). Balamuthia cases were reported from 27 states and the District of Columbia (Figure 2). State of exposure was documented in 30 cases and included California (12 cases), Arizona (4 cases), Texas (3 cases), Mississippi (3 cases),Hawaii (2 cases), and 1 case each from Georgia, Florida, New Mexico, Illinois, Virginia, and Washington. Of 41 case patients with a documented soil exposure history, 35 (85%) reported soil-related exposures, including those categorized as gardening/landscaping/yard work or play and farming/ranching/agricultural. A history of swimming or recreational water exposures was reported in 66% (21/32) of case patients with a documented water exposure history. Thirteen cases patients reported both water and soil exposure. Symptom onset occurred in all months of the year in those patients with a documented symptom onset date (n=65) with no apparent seasonality.
Figure 1.
Arrow denotes the year Balamuthia mandrillaris was first described as a human pathogen. Cases reported prior to 1990 were retrospectively diagnosed.
Table 1.
Demographic Characteristics of Balamuthia Disease Patients, United States, 1974–2016
| Median age (range) (N=106) | 36 years (0.33–91 years) |
| Age less than 5 years (n, %) | 20 (19) |
| Sex (n, %) (N=108) | |
| Male | 75 (68) |
| Female | 33 (32) |
| Race (n, %) (N=59) | |
| White | 40 (68)a |
| Black | 13 (22) |
| Asian/Pacific Islander | 6 (10) |
| American Indian | 2 (3) |
| Ethnicity (n, %) (N=58) | |
| Hispanic | 32 (55) |
| Non-Hispanic | 26 (45) |
Percents sum to >100% due to some cases reporting more than one race.
Figure 2.
Balamuthia Cases by State, United States, 1974–2016*
*Cases were classified on the basis of the state of exposure, if known, or by the state of residence or diagnosis, if state of exposure was not known.
The median length of time from symptom onset until death was 24 days (range 4–450 days, n=43) and the median hospital stay was 21 days (range 4–372 days, n=52). Balamuthia disease manifested as encephalitis in 99% of patients. Seven of 109 case patients (6%) had a combination of GAE and cutaneous disease and one patient had cutaneous disease only. Five case patients who had GAE had evidence of amebae in other organs on post-mortem exam including lung, liver, kidney, pancreas, and adrenal glands. Among 82 case patients ≥10 years of age, 18% reported alcohol misuse and 22% reported illegal drug use. Among 94 patients with information on comorbid conditions, 39% had an immunocompromised condition, including diabetes (9), HIV/AIDS (9), solid organ transplant (6), and use of immunosuppressive drugs (5) (Table 2). Ten (10%) patients of 101 with a known outcome survived their Balamuthia infection (Table 2).
Table 2.
Clinical Characteristics of Balamuthia Disease Patients, United States, 1974–2016
| Median time from symptom onset–death (range), in days (N=43) | 24 (4–450) |
| Median hospital stay (range), in days (N=52) | 21 (4–372) |
| Type of Balamuthia disease, n (%) (N=109) | |
| Encephalitis only | 101 (93) |
| Cutaneous only | 1 (<1) |
| Encephalitis + cutaneous | 7 (6) |
| Co-morbid conditions | |
| Alcohol misuse (% among cases ≥ 10 yrs) (N=82) | 15 (18) |
| Illegal drug use (% among cases ≥ 10 yrs) (N=82) | 18 (22) |
| Immunocompromised (N=94) | 37 (39) |
| Diabetes | 9 (10) |
| HIV/AIDS | 9 (10) |
| Solid organ transplant | 6 (6) |
| Immunosuppressive drug | 5 (5) |
| Liver cirrhosis | 3 (3) |
| Renal failure | 3 (3) |
| Other hematologic condition | 2 (2) |
| Other autoimmune condition | 2 (2) |
| Cancer | 1(1) |
| Glucose-6-phosphate dehydrogenase deficiency | 1(1) |
| Survived (N=101) | 10 (10) |
| General clinical features on presentation, n ( % ) (N= 101) | |
| Fever | 39 (39) |
| Headache | 39 (39) |
| Vomiting | 30 (30) |
| Lethargy | 28 (28) |
| Nausea | 19 (19) |
| Stiff neck | 7 (7) |
| Anorexia | 7 (7) |
| Weight loss | 3 (3) |
| Cough | 2 (2) |
| Diarrhea | 2 (2) |
| Neurologic clinical features on presentation, n (%) (N=101) | |
| Mental status changes | 30 (30) |
| Seizures | 21 (21) |
| Weakness | 19 (19) |
| Confusion | 15 (15) |
| Ataxia | 14 (14) |
| Hemiparesis | 13 (13) |
| Behavior changes | 10 (10) |
| Aphasia | 7 (7) |
| Cranial nerve VI palsy | 5 (5) |
| Cranial nerve XII palsy | 4 (4) |
| Admitting diagnosis (N=52) | |
| Neoplasm/metastases | 12 (23) |
| Tuberculous meningitis | 5 (10) |
| Brain abscess | 4 (8) |
| Toxoplasmosis | 4 (8) |
| Acute disseminated encephalomyelitis | 3 (6) |
| Neurocysticercosis | 3 (6) |
| Stroke | 2 (4) |
| Septic emboli | 2 (4) |
| Viral encephalitis | 2 (4) |
Data was available on 101 patients regarding clinical features at initial presentation with Balamuthia disease. The most common general clinical features on presentation were fever (39%), headache (39%), vomiting (30%), and lethargy (28%). The most common neurologic features on presentation were altered mental status (30%), seizures (21%), and weakness (19%).
When CSF data were available, the median white blood cell count was 106 cells/μL with a median of 57% lymphocytes, median protein of 105 mg/dL, and median glucose of 46 mg/dL (Table 3). One patient was reported to have had amebae visualized on a wet mount of the CSF. Sixty-six percent of patients had Balamuthia disease diagnosed by indirect immunofluorescence, followed by 47% by polymerase chain reaction (PCR), 45% by histopathology, and 3% by culture. Fifty-nine percent were diagnosed by a combination of tests. This testing was primarily performed on brain tissue (96 had a brain biopsy) followed by skin tissue (10 had a skin biopsy). Six patients had Balamuthia detected in the CSF by PCR or culture. Eighty-eight percent of Balamuthia case patients required a brain biopsy to aid in making the diagnosis (Table 3).
Table 3.
Laboratory and Radiologic Findings in Balamuthia Disease Patients, United States, 1974–2016
| Cerebrospinal fluid values | Median (Range) |
|---|---|
| WBC (cells/μL) (N=64) | 106 (0–4100) |
| % Neutrophil (N=38) | 0 (0–90) |
| % Lymphocyte (N=38) | 57 (0–100) |
| % Monocyte (N=38) | 9.5 (0–100) |
| RBC (cells/ μL) (N=54) | 2 (0–44,500) |
| Protein (mg/dL) (N=62) | 105 (0–1042) |
| Glucose (mg/dL) (N=62) | 46 (0–240) |
| Balamuthia diagnostic testing | N (%) |
| Indirect Immunofluorescence | 61/92 (66) |
| PCR | 43/92 (47) |
| Histopathology | 41/92 (45) |
| Culture | 3/92 (3) |
| Combination | 54/92 (59) |
| Brain biopsy performed | 96/109 (88) |
Brain imaging data were available for 92 patients, all demonstrating abnormal findings. Typical Balamuthia GAE findings on brain imaging (computed tomography [CT] and magnetic resonance imaging [MRI]) included enhancing lesions (29%), multifocal lesions (23%), and edema (27%). Lesions were located throughout the brain with no predilection for a particular region.
The most common medications given initially to Balamuthia case patients were acyclovir, amphotericin B, ceftriaxone, isoniazid, metronidazole, and rifampin. Overall, 9 Balamuthia survivors had treatment data reported and received at least one of the following drugs: azithromycin (7/9) or clarithromycin (6/9), fluconazole (7/9), flucytosine (7/9), and sulfadiazine (8/9). Over half of survivors received pentamidine (6/9) as part of their treatment. Three of 9 survivors received miltefosine (Table 4) [22, 23, 34–38].
Table 4.
Drug Combinations Received by Balamuthia Disease Patients with an Antemortem Diagnosis by Outcome, United States, 1974–2016
| Year | Age (yrs) | Treatment | Outcome | Comment |
|---|---|---|---|---|
| 1996 | 64 | Amphotericin B, azithromycin, ceftriaxone, clarithromycin, ethambutol, fluconazole, flucytosine, isoniazid, pentamidine, rifampin, steroid (prednisone and dexamethasone), sulfadizine, pyrazinamide, doxycycline, trifluoperazine | Survived | Case described in Deetz et al. |
| 2000 | 5 | Acyclovir, azithromycin, clarithromycin, fluconazole, flucytosine, ketoconazole, metronidazole, pentamidine, thioridizine | Survived | Case described in Deetz et al. |
| 2002 | 72 | Clarithromycin, fluconazole, pentamidine, sulfadiazine | Survived | Case described in Jung et al. |
| 2007 | 43 | Albendazole, ciprofloxacin, clarithromycin, fluconazole, pentamidine, sulfadiazine, vancomycin, meropenem, posaconazole | Died | |
| 2007 | 2 | Clarithromycin, fluconazole, flucytosine, pentamidine, sulfadiazine, thioridizine | Survived | Case described in Cary et al. |
| 2009 | 31 | Azithromycin, fluconazole, flucytosine, miltefosine, pentamidine, sulfadiazine | Died | Case described in MMWR 2010 |
| 2009 | 27 | Azithromycin, fluconazole, flucytosine, miltefosine, pentamidine, sulfadiazine, | Survived | Case described in MMWR 2010 |
| 2010 | 24 | Albendazole, amphotericin B, azithromycin, miltefosine, sulfadiazine, linezolid | Died | Received only a few days of treatment prior to death |
| 2010 | 11 | Amphotericin B liposomal, azithromycin, fluconazole, flucytosine, metronidazole, miltefosine, pentamidine, dexamethasone, thioridazine, meropenem | Died | Treated with varying combinations of these drugs for 1–2 months |
| 2010 | 84 | Azithromycin, flucytosine, sulfadiazine, minocycline, clobetasol propionate spray | Survived | Had cutaneous disease + encephalitis |
| 2010 | 27 | Albendazole, amphotericin B liposomal, azithromycin, clarithromycin, fluconazole, flucytosine, metronidazole, miltefosine, pentamidine, dexamethasone, sulfadiazine, trimethoprim/sulfa, voriconazole | Survived | |
| 2011 | 67 | Metronidazole, miltefosine, pentamidine, steroid (unspecified) | Died | |
| 2012 | 82 | Acyclovir, amphotericin B liposomal, azithromycin, ceftriaxone, flucytosine, pentamidine, pyrimethamine, hydrocortisone, sulfadiazine, trimethoprim/sulfa, voriconazole, doxycycline, vancomycin | Died | Case described in Schafer et al. |
| 2012 | 16 | Acyclovir, ceftriaxone, clarithromycin, fluconazole, flucytosine, miltefosine, pentamidine, rifampin, sulfadiazine, meropenem | Died | Received ~2 weeks of treatment prior to death |
| 2012 | 39 | Albendazole, azithromycin, clarithromycin, fluconazole, flucytosine, miltefosine, pentamidine, sulfadiazine | Died | Received ~ 1 month of treatment prior to death |
| 2013 | 13 | Amphotericin B liposomal, azithromycin, fluconazole, rifampin, dexamethasone, sulfadiazine, caspofungin, meropenem | Died | Received only a few days of treatment prior to death |
| 2014 | 63 | Albendazole, amphotericin B liposomal, azithromycin, clarithromycin, fluconazole, flucytosine, sulfadiazine, miltefosine | Survived | |
| 2014 | 20 | Azithromycin, fluconazole, flucytosine, miltefosine, pentamidine, sulfadiazine | Died | Received ~ 10 days of treatment prior to death |
| 2014 | 91 | Azithromycin, sulfadiazine | Survived | Cutaneous disease only; case described in Chang et al. |
| 2014 | 32 | Albendazole, azithromycin, fluconazole, flucytosine, miltefosine, pentamidine, sulfadiazine | Died | Received ~ 6 weeks of treatment prior to death |
| 2014 | 50 | Azithromycin, fluconazole, flucytosine, miltefosine, pentamidine, sulfadiazine | Died | Received ~ 1 week of treatment prior to death |
| 2015 | 15 | Acyclovir, amphotericin B liposomal, azithromycin, flucytosine, metronidazole, pentamidine, methylprednisolone, sulfadiazine, voriconazole, cefotaxime, vancomycin | Died | Received only a few days of treatment prior to death |
| 2015 | 6 | Azithromycin, fluconazole, flucytosine, miltefosine, pentamidine, sulfadiazine | Died | Only received 3 days of treatment prior to death; case described in Joo et al. |
| 2015 | 63 | Acyclovir, amphotericin B, azithromycin, ceftriaxone, fluconazole, flucytosine, miltefosine, pentamidine, methylprednisolone, vancomycin | Died | Received ~ 2 weeks of treatment prior to death |
Among 52 patients with a recorded initial admitting diagnosis, the most common diagnoses were neoplasm/metastases (12), tuberculous meningitis (5), brain abscess (4), toxoplasmosis (4), acute disseminated encephalomyelitis (3), and neurocysticercosis (3). Other initial diagnoses included stroke, septic emboli, and viral encephalitis.
Twenty-seven patients (including the 10 known survivors) received an antemortem diagnosis of Balamuthia disease, allowing for treatment initiation. The median time from symptom onset to initiation of treatment in this sub-group was 30 days (range 6–557 days). All but four of these antemortem-diagnosed cases occurred in the last 10 years.
DISCUSSION
As a rare and highly fatal infection, the large case series of Balamuthia disease presented here represents a unique opportunity to describe this infection and examine patterns that might help better diagnose, treat, and possibly prevent this devastating infection. This case series confirms that Balamuthia disease is highly fatal, with fewer than 10% of patients surviving.
In this case series, some epidemiologic patterns can be discerned. As seen in Figure 1, there were an increasing number of cases reported in the last decade, which likely reflects increasing recognition of Balamuthia disease in more recent years once it became known as a human pathogen and not necessarily a true increase in cases. While patient age ranged from 4 months to 91 years, it is notable that the median age was 36 years. This median age differs from that reported for encephalitis hospitalization and death, which are highest at the extremes of life: < 1 year and >65 years of age [39, 40]. The predominance of male patients in this case series also differs from the female predominance reported for encephalitis hospitalization and death of all causes [39]. The reasons for these differences are not clear but might be a result of different data sources and more recent years of data that were used for the referenced analyses. It might also represent how Balamuthia has the ability to infect people of all ages and immune status, rather than just the extremes of age. Another notable demographic pattern is that of those with a reported ethnicity, more than 50% were Hispanic, which has been previously reported [29]. The findings of male predominance as well as Hispanic ethnicity might reflect higher exposure to occupations with heavy soil exposure such as agriculture and landscaping [41].
The western and southern regions of the United States reported the most Balamuthia cases. While the western and southern regions of the United States might provide an environment that is conducive to the growth of Balamuthia, reporting bias might have contributed to this finding. The California Encephalitis Project that operated in California during the time period of this study represents a form of active surveillance that likely promoted the identification of Balamuthia cases that might not have otherwise been identified. As other states did not have this kind of surveillance and relied on passive identification of cases, the western region might appear to have more cases than the rest of the country. However, it is worth exploring the possibility that the hot, dry environment of the U.S. Southwest might be the type of environment in which Balamuthia thrives. This geographic predilection for the southwest United States might also explain why we found a higher number of cases with Hispanic ethnicity, since people of Hispanic ethnicity comprise a higher percentage of the southwest U.S. population [42]. Not surprisingly, given what is known about where Balamuthia is found in the environment, soil exposures were reported in 85% of patients with a documented soil exposure history while water exposures were only reported in 66% of patients with a documented water exposure history. Unfortunately, given how common soil exposure is and the lack of knowledge about what the incubation period is for Balamuthia infection, for most cases it is difficult to pinpoint exactly what exposure led to infection.
Unlike patients with disease caused by Acanthamoeba, a closely-related free-living ameba that causes CNS, cutaneous, and disseminated disease in primarily immunocompromised people [11], fewer than 40% of the patients with Balamuthia disease in this case series were immunocompromised. However, alcohol or drug use was reported as a co-morbidity in 18% and 22% of Balamuthia patients, which is higher than reported in the general population (10% and 6.5%) in the National Survey on Drug Use and Health https://www.cdc.gov/nchs/data/hus/hus16.pdf#050).
As a rarely reported infection, Balamuthia disease is known to few clinicians. Although the infection has been reported from most parts of the world, only one other group in Peru has reported on multiple cases of Balamuthia disease [24, 28]. The Peruvian experience is largely similar to ours, with one notable exception: the Peruvian case series reported that a majority of their patients presented initially with a cutaneous lesion, whereas in this series only 5% of patients were reported to have a cutaneous form of the disease [28]. This is an important distinction as the finding of a skin lesion presents an opportunity for an easier and earlier diagnosis and treatment to prevent progression to neurologic disease. Although it seems to be a rare finding in U.S. Balamuthia patients, when Balamuthia is suspected, a careful skin exam should be conducted, as detection of a lesion consistent with cutaneous balamuthiasis might allow for early diagnosis on easily accessible tissue. Additionally, Balamuthia should be considered as an infectious etiology for one or more chronic skin lesions and as an etiology for meningitis/encephalitis in the presence of a skin lesion, particularly in the presence of other epidemiologic clues, such as Hispanic ethnicity, soil or water exposure, residence in or travel to the Southwest United States, and male sex although such factors need not be present for Balamuthia to be in the differential diagnosis.
The presenting clinical features in this series were not specific to Balamuthia disease. Neuroimaging can be helpful in that almost all patients have abnormal brain imaging, often with parenchymal lesions. The CSF profile does not distinguish Balamuthia disease from other more common causes of encephalitis [43]. It shows that most Balamuthia patients have a mildly elevated white blood cell (WBC) count with a lymphocytic predominance, elevated protein, and low normal glucose. By comparison, the CSF profile that is generally seen in patients with primary amebic meningoencephalitis caused by Naegleria fowleri has a greatly elevated WBC count with a neutrophilic predominance, elevated protein, and low glucose [44]. The nonspecific presenting clinical features and CSF profile of Balamuthia GAE are likely a factor in the delayed diagnosis in most patients. Only 27 patients in this case series received an antemortem diagnosis of Balamuthia disease. Among this group, the median time from onset of symptoms to initiation of Balamuthia-specific treatment was 30 days.
Further complicating the diagnosis of Balamuthia disease is the frequent need for brain tissue on which to perform confirmatory testing. Unlike Naegleria fowleri, Balamuthia is rarely detected in the CSF of infected patients, either visually by microscope or by PCR. In this case series, 88% (96/109) of patients had a brain biopsy performed and only 6% (6/109) had Balamuthia detected in CSF. While CDC is usually willing to perform Balamuthia PCR on CSF since a positive result would be helpful and facilitate earlier initiation of treatment, clinicians should be aware that a negative result on CSF does not rule out the diagnosis of Balamuthia disease and often brain tissue must be obtained.
The initial treatment received by many Balamuthia case patients included acyclovir, ceftriaxone, isoniazid, and rifampin. These medications are not effective against Balamuthia and reflect empiric treatment that is used for more common causes of meningoencephalitis such as herpes simplex virus, Neisseria meningitides, Streptococcus pneumoniae, and tuberculosis. The drugs used to treat survivors are drugs that are commonly available in U.S. hospital inpatient pharmacies and have been shown to have activity against Balamuthia. Based on their in vitro activity and successful use in survivor cases, the following drug combination is recommended for treatment of Balamuthia infection: pentamidine, sulfadiazine, flucytosine, fluconazole, azithromycin or clarithromycin, and miltefosine (which is now available commercially in the United States).
Encephalitis of any cause remains a significant cause of morbidity and mortality in the United States and fewer than half of cases have an etiology identified [39, 40]. While Balamuthia is likely one of the rarer causes of encephalitis, it should be considered in the differential diagnosis, early in the patient’s evaluation if possible, but certainly if other more common causes have been ruled out or if there are epidemiologic and clinical suggestions in favor of GAE. Clinician awareness of Balamuthia as a cause of encephalitis (as well as chronic skin lesions) might lead to more antemortem diagnoses and earlier initiation of treatment, possibly resulting in better outcomes for these patients.
Experts at CDC are available 24/7 for diagnostic and clinical assistance for clinicians who suspect Balamuthia disease in a patient they are evaluating. Clinicians should call the CDC Emergency Operations Center at 770–488-7100 to obtain a consultation.
Acknowledgements
The authors wish to dedicate this manuscript to Dr. Govinda Visvesvara who established the free-living ameba laboratory at CDC and first identified and described Balamuthia mandrillaris as a free-living ameba with pathogenic potential. This work would not have been possible without his expertise and dedication.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts of interest.
REFERENCES
- 1.Visvesvara GS, Martinez AJ, Schuster FL, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol 1990; 28(12): 2750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rideout BA, Gardiner CH, Stalis IH, Zuba JR, Hadfield T, Visvesvara GS. Fatal infections with Balamuthia mandrillaris (a free-living amoeba) in gorillas and other Old World primates. Vet Pathol 1997; 34(1): 15–22. [DOI] [PubMed] [Google Scholar]
- 3.Finnin PJ, Visvesvara GS, Campbell BE, Fry DR, Gasser RB. Multifocal Balamuthia mandrillaris infection in a dog in Australia. Parasitol Res 2007; 100(2): 423–6. [DOI] [PubMed] [Google Scholar]
- 4.Hodge PJ, Kelers K, Gasser RB, Visvesvara GS, Martig S, Long SN. Another case of canine amoebic meningoencephalitis--the challenges of reaching a rapid diagnosis. Parasitol Res 2011; 108(4): 1069–73. [DOI] [PubMed] [Google Scholar]
- 5.Kinde H, Visvesvara GS, Barr BC, Nordhausen RW, Chiu PH. Amebic meningoencephalitis caused by Balamuthia mandrillaris (leptomyxid ameba) in a horse. J Vet Diagn Invest 1998; 10(4): 378–81. [DOI] [PubMed] [Google Scholar]
- 6.Anzil AP, Rao C, Wrzolek MA, Visvesvara GS, Sher JH, Kozlowski PB. Amebic meningoencephalitis in a patient with AIDS caused by a newly recognized opportunistic pathogen. Leptomyxid ameba. Arch Pathol Lab Med 1991; 115(1): 21–5. [PubMed] [Google Scholar]
- 7.Gordon SM, Steinberg JP, DuPuis MH, Kozarsky PE, Nickerson JF, Visvesvara GS. Culture isolation of Acanthamoeba species and leptomyxid amebas from patients with amebic meningoencephalitis, including two patients with AIDS. Clin Infect Dis 1992; 15(6): 1024–30. [DOI] [PubMed] [Google Scholar]
- 8.Visvesvara GS, Schuster FL, Martinez AJ. Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephalitis in humans and other animals. J Eukaryot Microbiol 1993; 40(4): 504–14. [DOI] [PubMed] [Google Scholar]
- 9.Stothard DR, Schroeder-Diedrich JM, Awwad MH, et al. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J Eukaryot Microbiol 1998; 45(1): 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg 2003; 68(1): 65–9. [PubMed] [Google Scholar]
- 11.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 2007; 50(1): 1–26. [DOI] [PubMed] [Google Scholar]
- 12.Schuster FL, Dunnebacke TH, Booton GC, et al. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol 2003; 41(7): 3175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Isolation of Balamuthia amebas from the environment. J Eukaryot Microbiol 2003; 50 Suppl: 510–1. [DOI] [PubMed] [Google Scholar]
- 14.Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology 2004; 150(Pt 9): 2837–42. [DOI] [PubMed] [Google Scholar]
- 15.Niyyati M, Lorenzo-Morales J, Rezaeian M, et al. Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res 2009; 106(1): 279–81. [DOI] [PubMed] [Google Scholar]
- 16.Baquero RA, Reyes-Batlle M, Nicola GG, et al. Presence of potentially pathogenic free-living amoebae strains from well water samples in Guinea-Bissau. Pathog Glob Health 2014; 108(4): 206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 2004; 34(9): 1001–27. [DOI] [PubMed] [Google Scholar]
- 18.Kiderlen AF, Laube U. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol Res 2004; 94(1): 49–52. [DOI] [PubMed] [Google Scholar]
- 19.Matin A, Siddiqui R, Jayasekera S, Khan NA. Increasing importance of Balamuthia mandrillaris. Clin Microbiol Rev 2008; 21(3): 435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recavarren-Arce S VC, Gotuzzo E, Cabrera J. Amoeba angeitic lesions of the central nervous system in Balamuthia madrillaris amoebiasis. Human Pathology 1999; 30: 269–73. [DOI] [PubMed] [Google Scholar]
- 21.Bravo F, Cabrera J, Gotuzzo E, Visvesvara GS. Cutaneous manifestations of infection by free-living amebas In: Hengge Tyring L. Tropical Dermatology. 2nd ed: Elsevier Churchill Livingstone, 2006. [Google Scholar]
- 22.Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis 2003; 37(10): 1304–12. [DOI] [PubMed] [Google Scholar]
- 23.Cary LC, Maul E, Potter C, et al. Balamuthia mandrillaris meningoencephalitis: survival of a pediatric patient. Pediatrics 2010; 125(3): e699–703. [DOI] [PubMed] [Google Scholar]
- 24.Martinez DY, Seas C, Bravo F, et al. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis 2010; 51(2): e7–11. [DOI] [PubMed] [Google Scholar]
- 25.Schuster FL, Visvesvara GS. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat 2004; 7(1): 41–51. [DOI] [PubMed] [Google Scholar]
- 26.Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol 2006; 53(2): 121–6. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Balamuthia amebic encephalitis -- California, 1999−−2007. Morbidity and Mortality Weekly Report 2008; 57(28): 768–71. [PubMed] [Google Scholar]
- 28.Bravo FG, Seas C. Balamuthia mandrillaris amoebic encephalitis: an emerging parasitic infection. Curr Infect Dis Rep 2012; 14(4): 391–6. [DOI] [PubMed] [Google Scholar]
- 29.Schuster FL, Glaser C, Honarmand S, Maguire JH, Visvesvara GS. Balamuthia amebic encephalitis risk, Hispanic Americans. Emerg Infect Dis 2004; 10(8): 1510–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farnon EC, Kokko KE, Budge PJ, et al. Transmission of Balamuthia mandrillaris by organ transplantation. Clin Infect Dis 2016; 63(7): 878–88. [DOI] [PubMed] [Google Scholar]
- 31.Gupte AA, Hocevar SN, Lea AS, et al. Transmission of Balamuthia mandrillaris through solid organ transplantation: utility of organ recipient serology to guide clinical management. Am J Transplant 2014; 14(6): 1417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson BR, Kucerova Z, Roy SL, et al. Serologic survey for exposure following fatal Balamuthia mandrillaris infection. Parasitol Res 2014; 113(4): 1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiderlen AF, Radam E, Tata PS. Assessment of Balamuthia mandrillaris-specific serum antibody concentrations by flow cytometry. Parasitol Res 2009; 104(3): 663–70. [DOI] [PubMed] [Google Scholar]
- 34.Jung S, Schelper RL, Visvesvara GS, Chang HT. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med 2004; 128(4): 466–8. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Balamuthia mandrillaris transmitted through organ transplantation --- Mississippi, 2009. MMWR Morb Mortal Wkly Rep 2010; 59(36): 1165–70. [PubMed] [Google Scholar]
- 36.Schafer KR, Shah N, Almira-Suarez MI, et al. Disseminated Balamuthia mandrillaris Infection. J Clin Microbiol 2015; 53(9): 3072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang OH, Liu F, Knopp E, et al. Centrofacial balamuthiasis: case report of a rare cutaneous amebic infection. J Cutan Pathol 2016; 43(10): 892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joo SJ, Thompson AB, Philipsborn R, et al. An unusual cause of fever and jeadache in a school-aged male. Clin Pediatr 2018: 9922818772056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George BP, Schneider EB, Venkatesan A. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000–2010. PLoS One 2014; 9(9): e104169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tack DM, Holman RC, Folkema AM, Mehal JM, Blanton JD, Sejvar JJ. Trends in encephalitis-associated deaths in the United States, 1999–2008. Neuroepidemiology 2014; 43(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 41.United States Department of Labor Bureau of Labor Statistics. Employed persons by detailed occupation, sex, race, and Hispanic or Latino ethnicity Available at: https://www.bls.gov/cps/cpsaat11.htm.
- 42.United State Census Bureau. The Hispanic Population: 2010. 2010 Census Briefs 2011. [Google Scholar]
- 43.Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis 2013; 57(8): 1114–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capewell LG, Harris AM, Yoder JS, et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937–2013. J Pediatric Infect Dis Soc 2015; 4(4): e68–75. [DOI] [PubMed] [Google Scholar]