Abstract
BACKGROUND/OBJECTIVES:
Bacterial biofilm formation within chronic wound beds, which provides an effective barrier against antibiotics, is a known cause of recalcitrant infections and a significant healthcare burden, often requiring repeated surgical debridements. Laser generated shockwaves (LGS) is a novel, minimally invasive, and nonthermal modality for biofilm mechanical debridement which utilizes compressive stress waves, generated by photonic absorption in thin titanium films to mechanically disrupt the biofilm. Prior studies have demonstrated LGS monotherapy to be selectively efficacious for biofilm disruption and safe for host tissues. In this study, we sought to determine if LGS can enhance the antimicrobial activity and biofilm disruption capability of topical antibiotic therapy.
STUDY DESIGN/MATERIALS AND METHODS:
Staphylococcus epidermidis biofilms grown in vitro on glass were treated with topical gentamicin (31, 62, and 124 μg/mL) with and without LGS (n=3–11/treatment group). Mechanical shockwaves were generated with a 1064 nm Nd:YAG laser (laser fluence 110.14 mJ/mm2, pulse duration 5 ns, spot size 3 mm). Following a 24-hour incubation period, bacterial viability was assessed by determining the number of colony-forming units (CFU) via the Miles and Misra method. Residual biofilm bioburden was analyzed using the crystal violet biofilm assay.
RESULTS:
With gentamicin monotherapy, CFU density (CFU/mm2) at 31, 62, and 124 μg/mL were (282 ± 84) × 104, (185 ± 34) × 104, and (113 ± 9) × 104, respectively. With LGS and gentamicin therapy, CFU density decreased to (170 ± 44) × 104, (89 ± 24) × 104, and (43 ± 3) × 104, respectively (p = 0.1704, 0.0302, and 0.0004 when compared with gentamicin alone). Biofilm burden as measured by the assay in the gentamicin 31, 62, and 124 μg/mL groups was reduced by 80%, 95%, and 98% when LGS was added (p = 0.0102, >0.0001, and 0.0001 for all groups when compared with gentamicin alone). Furthermore, samples treated with LGS saw an increase in susceptibility to gentamicin, in terms of reduced biofilm bioburden and CFU densities.
CONCLUSION:
LGS enhances the efficacy of topical antibiotics in an in vitro model. This has significant implications for clinical applications in the management of chronic soft tissue infections and recalcitrant chronic rhinosinusitis.
Keywords: laser generated shockwaves, biofilm, chronic wound infections, topical antibiotics
INTRODUCTION
Chronically infected wounds are those that remain open and incompletely healed for longer than 6 weeks, and can be caused by a variety of etiologies (i.e., infectious, traumatic, inflammatory, iatrogenic) (1). Due to prolonged morbidity and need for repeated therapy, such wounds impose a significant burden to the healthcare system worldwide (2,3). For instance, it is estimated that $15.6 billion was spent on wound care in 2014 (4), and $8.6 billion was spent on equipment for wound care in 2013 (5). One of the main purported contributors to persistence of chronically infected wounds is the formation of bacterial biofilms. Biofilms are comprised of complex, three-dimensional polysaccharide and protein matrices that provide a nurturing and protective microniche environment that allows for further proliferation of bacteria. Once biofilm is established, it acts as a diffusion barrier that protects the bacteria from antibiotic concentrations up to 100–1000 times the minimum inhibitory concentration (MIC), or the concentration needed to inhibit bacterial growth while suspended in solution (6,7), and up to 500–5000 times the minimum bactericidal concentration (MBC), or the concentration needed to kill bacteria while suspended in solution (7). Several pathogenic bacterial genera, including Staphylococcus and Pseudomonas, form biofilm that can contribute to their resistance against antimicrobials (8–14). In this study, Staphylococcus Epidermidis (S. epidermidis) will be used, as it is a clinically relevant microbe that readily and reliably forms biofilm. Currently, debridement remains the gold standard for mechanical gross destruction/removal or disruption of biofilms, but carries with it the risk of damaging underlying normal tissue (15). Thus, an active area of research has been in development of tissue-sparing modalities for biofilm disruption.
Laser generated shockwave (LGS) therapy is a novel, minimally invasive, and non-thermal method for the removal of bacterial biofilms (16–18). LGS utilizes compressive stress waves generated by laser ablation of a thin titanium film to mechanically disrupt the biofilm, without damaging the underlying tissue (Figure 1). Previously, it has been shown that LGS therapy can remove bacterial biofilm grown in a polystyrene dish, and the treatment resulted in an immediate 55% reduction in colony-forming unit (CFU) count of bacterial cultures grown on agar (18). Furthermore, work by Nigri et al. demonstrated that LGS therapy in combination with vancomycin therapy can reduce S. epidermidis CFU count on vascular prosthetic grafts (19). Another study by Krespi et al. showed that LGS therapy alone can be effective in removing Pseudomonas aeruginosa biofilms in vitro from commonly used prosthetic materials used in otolaryngology, such as sutures, screws, and tympanostomy tubes (20). The technology is analogous to, but disparate from extracorporeal shockwave therapy (ESWT), as it can generate peak pressures of 200 MPa with rise times of 2 ns, and without a tensile component (16). The presence of tensile in ESWT and other high intensity focused ultrasound technologies limits their translation for biofilm treatment. The tensile stress produced by these technologies is known to cause formation of cavitation bubbles, which has been shown to cause vascular damage and other soft tissue damage (21,22).
Figure 1. Mechanics of Laser Generated Shockwave.
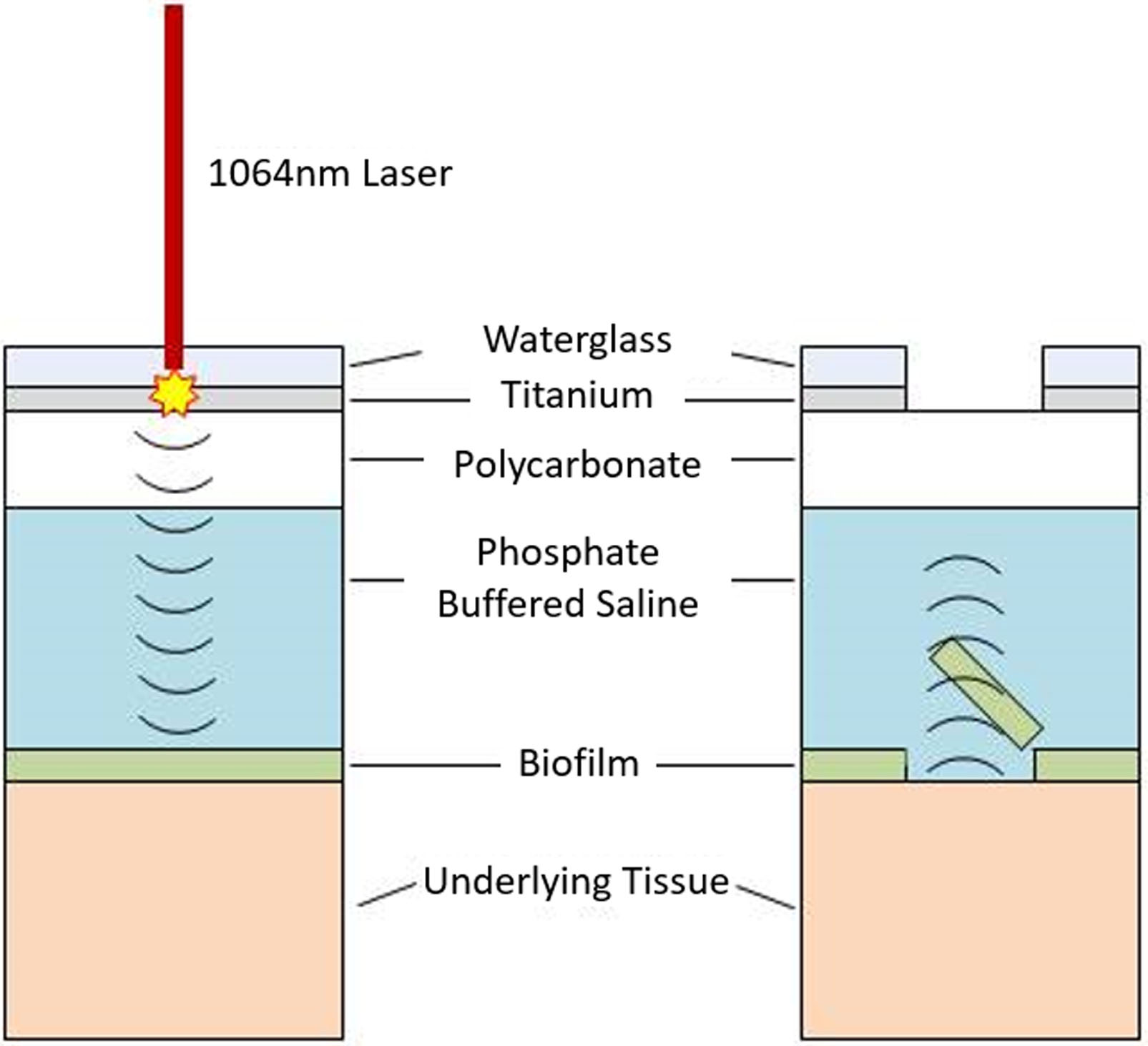
A Nd:YAG 1064nm laser is used to ablate a titanium film confined between a layer of waterglass and polycarbonate. The ablation under confinement generates a compressive shockwave that is directed to the biofilm by the waterglass. (right) The compressive wave travels through the biofilm and reflects off the biofilm and tissue interface due to the differences in the impedances. This reflection changes the compressive wave into a tensile wave which disrupts the biofilm. (left)
Currently, little work has been published to quantify the extent of biofilm removal and the long-term effects of LGS therapy on antibiotic treatment. Improved understanding of this relationship is key to clinical translation, as studies have shown that the biofilm matrix itself can inhibit the effectiveness of antibiotics (23). Disruption of the biofilm may also allow antibiotics to penetrate the biofilm and permit lower concentrations to become effective. In this study, we investigate the activity of laser generated shockwaves to enhance gentamicin activity against S. epidermidis biofilms in vitro. Gentamicin is a clinically relevant aminoglycoside class antibiotic that has good activity against a wide range of bacterial infections, including Staphylococcus species, but is limited by its systemic toxicity at higher concentrations.
MATERIALS AND METHODS
A significance level of 0.05 was used for all statistical testing.
Biofilm Preparation
Preparation of static biofilm samples on glass coverslips was adapted from Bakkiyaraj et al. (24). Twenty-four-hour Staphylococcus epidermidis ATCC 35984 cultures, incubated at 37°C on mannitol salt agar, were inoculated into 45 mL tryptic soy broth (TSB). This was then further incubated at 37°C for 18 hours. Next, the cultures were centrifuged at 3,000 rpm for 5 minutes at 4°C. The supernatant was discarded and the pellet was resuspended in 45 mL of fresh TSB. A working S. epidermidis solution was prepared by further diluting with TSB, until an optical density at 600 nm (OD600) of 0.2 was achieved, correlating to a cell density of 106 cells/mL.
22 mm glass coverslips (Schott D263M) were cleaned with deionized water, methanol, and acetone to remove residual oils and debris. The coverslips were then sterilized in an autoclave at 121°C for 15 minutes. The sterilized coverslips were individually placed in each well of a six-well culture plate, and 2 mL of the working S. epidermidis solution was added to submerge the coverslips. The culture plates were incubated at 37°C for 72 hours, with the media changed with fresh TSB every 24 hours to form mature S. epidermidis biofilms.
Mature biofilm growth is visible and manifests as an opaque layer over the entire surface of the coverslip. The mature biofilms were washed three times with 4°C phosphate buffered saline (PBS) to remove any planktonic bacteria and transferred to a new six-well plate containing 2 mL of 4°C PBS in each well to keep samples hydrated. The plates were kept on ice until ready for treatment.
Preparation of LGS substrate
A 0.5 μm layer of titanium was deposited onto a 0.1 mm thick polycarbonate plastic via RF sputtering, as described previously for plastics sample preparation (16). A 15 μm layer of waterglass was manually applied to the titanium side of the polycarbonate and allowed to dry for 20 minute. To ensure that the distance between the LGS substrate and coverslips were consistently 1 mm apart, a round 1 mm thick acrylic spacer was attached to the plastic side of the polycarbonate with double-sided tape.
LGS Treatment
The samples were divided into positive controls immediately post-treatment (0h+, n=6) and 24 hours post-treatment (24h+, n=11), gentamicin therapy alone at three different concentrations (G[31 μg/mL], n=6; G[62 μg/mL], n=6; G[124 μg/mL], n=6), LGS therapy alone immediately post-treatment (0h LGS, n=6) and 24 hours post-treatment (24h LGS, n=9), and LGS with gentamicin combination therapy (LGS + G[31 μg/mL, MIC], n=6; LGS + G[62 μg/mL, MBC], n=6; LGS + G[124 μg/mL], n=6). Following treatment, three samples from each group were used for CFU count analysis to determine viable cell concentration. The remaining samples from each group were used in the biofilm assay to determine biofilm density.
Prior to LGS treatment, 1 mL of PBS was removed from the prepared samples to lower the height of remaining solution to 1 mm. The prepared LGS substrate was placed spacer side down, thereby covering the biofilm surface of the coverslips. LGS treatment was administered across the entire sample for the following treatment groups: LGS 0h, LGS 24h, LGS + G[31 μg/mL], LGS + G[62 μg/mL], and LGS + G[124 μg/mL]. This was accomplished by focusing a 1064 nm Nd:YAG laser (Brilliant B, Quantel, France; fluence 110 mJ/mm2, pulse duration 5 ns, spot size 9 mm), to a 3 mm spot size with a long working distance lens. Using a camera attached to the scanning head, an image of the 13 cm × 10 cm scanning area was imported into an in-house controller software, programmed in Matlab (Mathworks, Natick, MA). The software actuates a pair of mirrors close to the lens to raster scanned the focused beam over the sample, based on the location of the sample in the image. This allowed for flexibility in the treatment for sample size (not needed for this study) and for sample placement. For each individual treatment, the laser ablates the thin titanium film confined between waterglass and the polycarbonate. The waterglass directs the resultant shockwave from the metal ablation down into the biofilm, while the polycarbonate provides structural integrity to the titanium film and is opaque to the laser wavelength, to prevent laser bleed-through to the sample.
After the treatment, the samples were washed three times with 4°C PBS and transferred to a new six-well plate with 2 mL of fresh 4°C PBS. LGS 0h was immediately analyzed for CFU counts and biofilm burden. PBS was removed from LGS 24h, LGS + G[31 μg/mL], LGS + G[62 μg/mL], and LGS + G[124 μg/mL] samples and 2 mL of TSB containing 0, 32, 64, 128 μg/mL gentamicin (Sigma-Aldrich, St. Louis, MO) was added to each well. The samples were then incubated at 37°C for 24 hours. Afterwards, the samples were washed three times with 4°C PBS and transferred to new wells. 2 mL of 4°C PBS was added to each well and the samples were kept on ice until ready to be analyzed via CFU counts and biofilm assay.
Non-LGS-treated samples (0h+, 24h+, G[31 μg/mL], G[62 μg/mL], G[124 μg/mL]) were treated similarly, with the exception that no prior LGS treatment was performed.
Biofilm bioburden assay
Bacterial biofilm burden was measured based a protocol previously described by Merritt et al. (25). PBS was first removed from each well of the sample. The samples were then stained with 2 mL of 0.1% crystal violet for 10 minutes. The excess staining solution was removed and the samples were washed three times with 2 mL of PBS. The plates were allowed to air dry overnight in a fume hood. 2 mL of 30% acetic acid were added to each well to resolubilize the dye. The OD600 of the solutions was then measured with a spectrophotometer (GE Healthcare BioSciences AB, Uppsala, Sweden).
A negative control (0h-) was prepared to confirm that TSB and PBS used do not adhere to the glass coverslip to produce a false positive. Three similarly cleaned and sterilized coverslips were placed in separate wells in a six-well plate. Sterile TSB was added to each well and the samples were incubated at 37°C for 72 hours. The coverslips were then washed 3 times with PBS and transferred to a new six-well plate and the biofilm assay was repeated.
The absorbance readings of the solutions were normalized by the area of the coverslip Statistical significance was determined by a two-tailed Student’s t-test.
CFU densities Analysis
CFU counts were determined based on the Miles and Mirsa method (26). Samples submerged in 2 mL of PBS from the well were broken into small pieces in solution. The sample, along with the solution, was transferred to a 14 mL culture tube. Samples were vortexed for 20 seconds to completely remove the biofilm from the glass coverslip and suspend the bacteria in solution. The glass shards from the broken coverslips acted as glass beads to scrape the biofilm off the glass. After vortexing, the originally turbid glass pieces were transparent, indicating the biofilm has been successfully suspended into solution. The solutions were then serially diluted to 1:10−7 with PBS. Using a micropipette, 3 drops (each 20 μL) of the 1:10−7 dilutions were placed on one quadrant of a mannitol salt agar plate and the droplets were allowed to be absorbed into the agar. This was repeated for the 1:10−4, 1:10−5, and 1:10−6 dilutions, and the process was repeated in triplicate. The plates were then incubated at 37°C for 24 hours or until visible colonies were observed. The colonies were counted manually and the CFU densities of the samples were calculated by first determining the total CFUs in the 2 mL of solution and then dividing by the surface area of the coverslip. Statistical significance was determined by a 2-tailed Student’s t-test, using MATLAB (Mathworks, Natick, MA).
Analysis of LGS therapy’s effect on gentamicin Therapy
To analyze how the addition of LGS therapy may potentiate gentamicin therapy, biofilm bioburden and CFU densities data sets: {24h+, G[31μg/mL], G[62μg/mL], G[124μg/mL] } and { LGS 24h, LGS + G[31μg/mL], LGS + G[31μg/mL], LGS + G[31μg/mL] } were fitted against an exponential decay without offset, y = Ae−x/t, and exponential decay with offset, y = Ae−x/t + y0 (Origin Pro 8, OriginLab, Northampton, MA), where y is the CFU density in CFU/mm2 and x is the gentamicin concentration in μg/mL. Furthermore, comparisons of A and A + y0, gives insight to the effects of LGS alone and when compared in conjunction with t, provides insight to if LGS treatment can increase the susceptibility of the S. epidermidis to gentamicin.
The two data sets’ coefficients were compared by computing a z-score. The equation for computing the z-score for y0 is given below:
The same was done for A and t. The p-value was calculated with Matlab for a two-tailed z-test:
RESULTS
Biofilm Bioburden Assay
Figure 2 illustrates the biofilm densities following different treatment parameters. When compared to 0h+, all concentrations of gentamicin-treated samples demonstrated statistically increased growth after 24 hours (p<0.05). This supports previous findings that, even at the highest concentration of gentamicin, antibiotics did not stop the growth of S epidermidis (27,28). For samples treated with LGS, when compared to LGS 24h, gentamicin prevented the regrowth of biofilm by 50.3% at 31 μg/mL (p=0.36), 87.2% at 62 μg/mL (p=0.08), and 95.3% at 124 μg/mL (p=0.06). However, when compared against their respective non-LGS-treated samples, the effects are more pronounced, with 80.8% (p=0.01), 95.3% (p<0.001), and 98.3% (p=0.0001) reductions for antibiotic concentrations of 31, 62, and 124 μg/mL, respectively.
Figure 2. Biofilm burden (OD600) as calculated by biofilm assay for S. epidermidis with LGS and gentamicin.
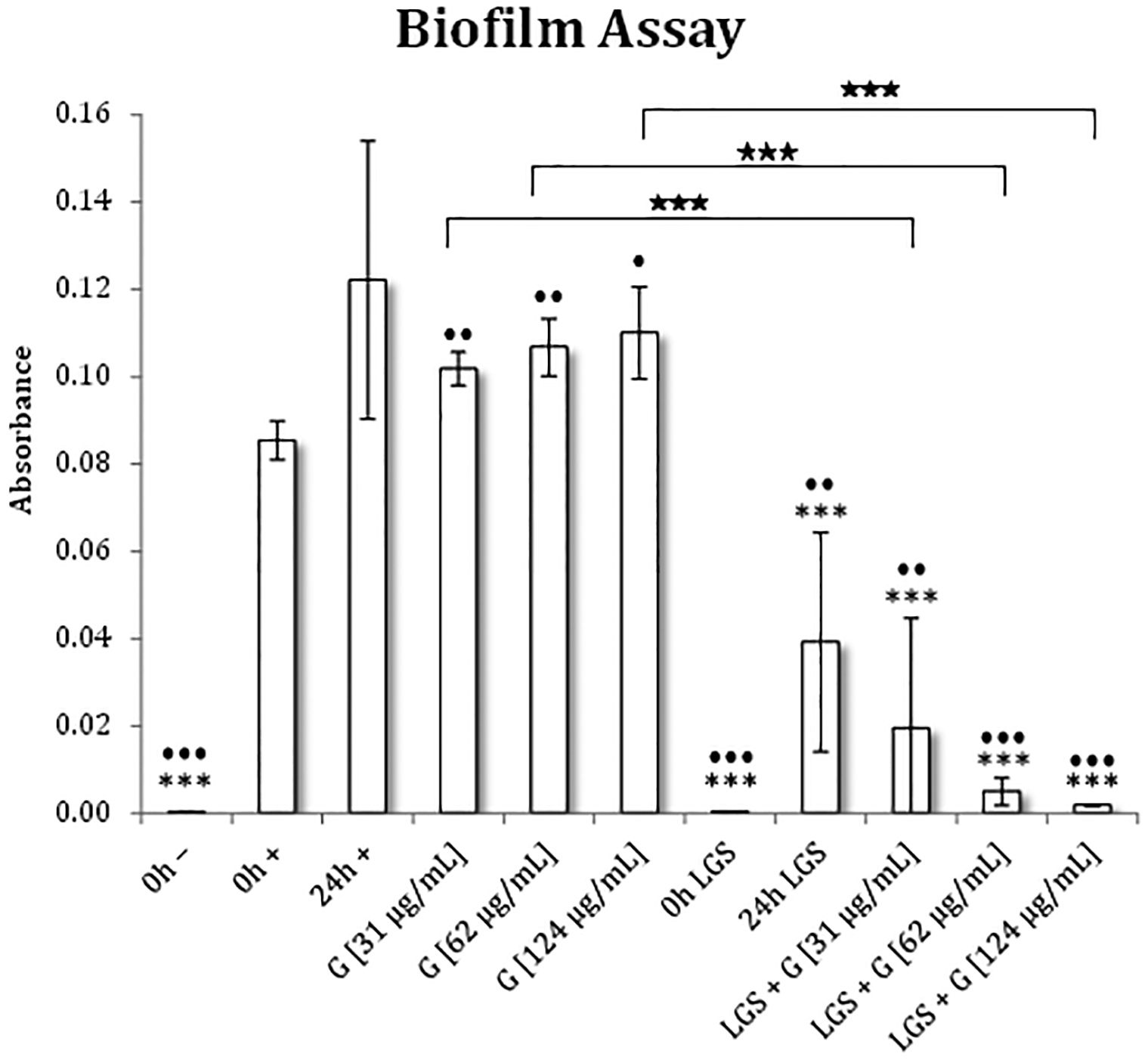
Statistical siginificance was found using a 2-tailed t-test that compared each group to 0h+ (*) and 24h+ (⦁). Pairwise comparisons were also made for gentamicin treatment with and without prior LGS treatment (★). Levels of significance are designated as follows: >5% (*), >3% (**), >1% (***).
The zero-hour negative control (0h-, n=3) had an absorbance of 0.0003 ± 0.0002/mm2. Therefore, proteins in TSB did not adhere to the glass in sufficient quantities to confound the assay.
CFU densities
Figure 3 summarizes the CFU densities of the various treatments. With gentamicin therapy only, as compared to 24h+, we found a 38.3% (p=0.17), 59.5% (p=0.037), and 75.3% (p=0.02) reduction in CFU density for gentamicin concentrations of 31, 62, 124 μg/mL, respectively. Conversely, LGS-treated samples, when compared to 24h+, saw a reduction in CFU density of 25.3% (p=0.40), 62.9% (p=0.03), 80.5% (p=0.01), and 90.5% (p=0.008), for LGS-treated samples: 24h LGS, LGS + G[31 μg/mL], LGS + G[62 μg/mL], LGS + G[124 μg/mL], respectively. Furthermore, in LGS-treated samples, when compared against their respective non-LGS treated samples, we found reductions in CFU density of 39.8% (p=0.17), 51.9% (p=0.03), and 61.6% (p=0.0004) for gentamicin concentrations of 31, 62, and 124 μg/mL, respectively.
Figure 3. CFU density for S. epidermidis with LGS and gentamicin.
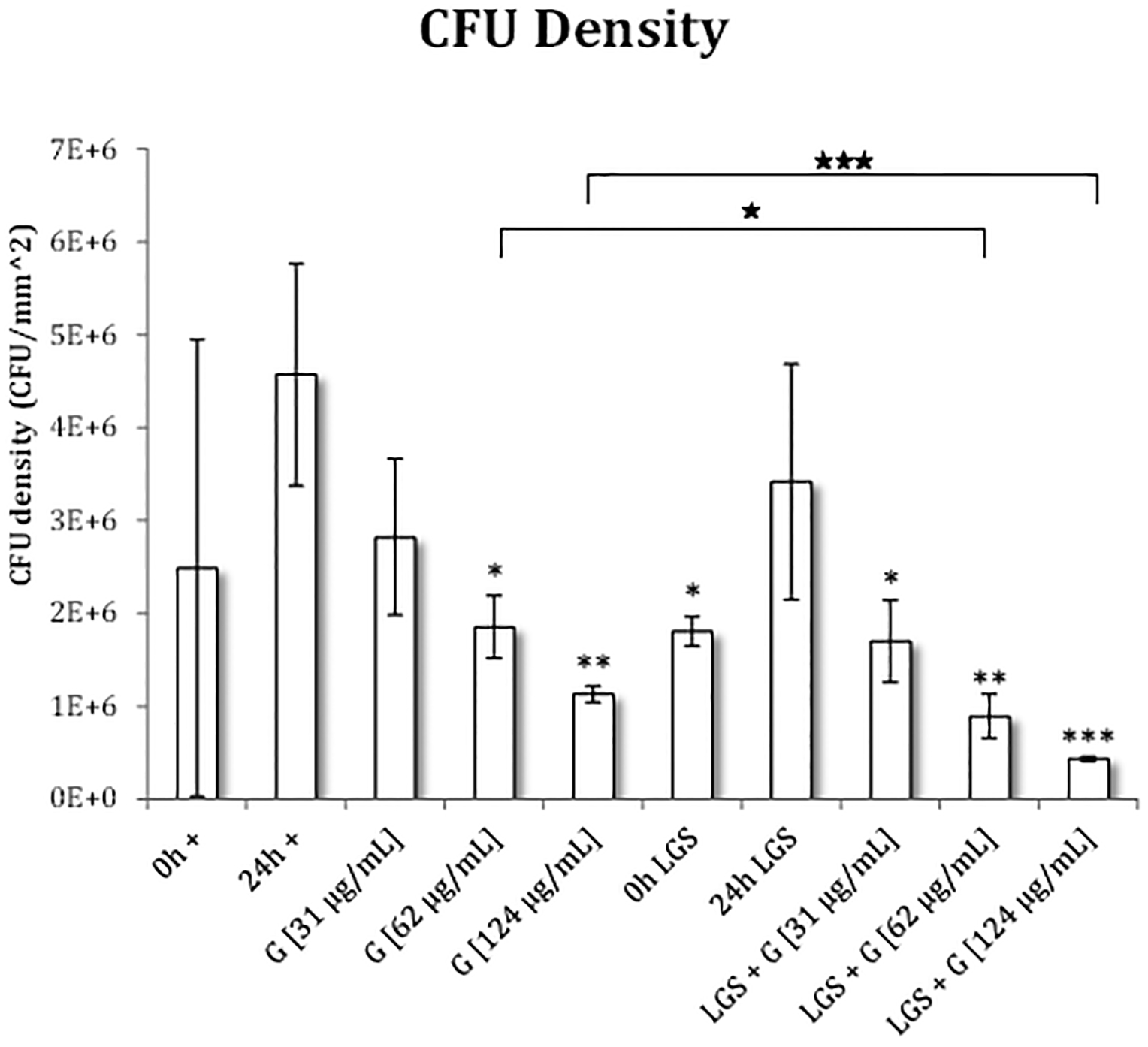
Statistical siginificance was found using a 2-tailed t-test that compared each group to 0h+ (*) and 24h+ (⦁). Pairwise comparisons were also made for gentamicin treatment with and without prior LGS treatment (★). Levels of significance are designated as follows: >5% (*), >3% (**), >1% (***).
Analysis of LGS Therapy Effect on Gentamicin Therapy
Biofilm Bioburden
When the Non-LGS treated biofilm bioburden data were fitted to an exponential decay without offset, the coefficients with their corresponding standard errors were found to be:
Similarly, the coefficients with standard error for the LGS treated biofilm bioburden data set were found to be:
When the LGS treated fit was compared to the Non-LGS treated fit, it was found that A, which represents the initial biofilm bioburden, decreased by 65.1% (p<0.0001) and t decreased by 97.9% (p>0.05), where t represents the effectiveness of increasing gentamicin concentration to lower the bioburden to a steady state, represented as 0 in this fit. The exponential fits here suggest that LGS significantly lowers the initial biofilm bioburden enough that subsequent gentamicin therapy can prevent further proliferation of the biofilm.
When the Non-LGS biofilm bioburden data were fitted to an exponential decay with offset, the coefficients were found to be:
Similarly, the coefficients for the LGS data were found to be:
When the biofilm bioburdens of LGS treated samples were compared to those of the Non-LGS treated samples, it was found that y0, which signifies the offset or the steady state of the curve, decreased by 100.8% (p > 0.0001), A increased by 238.1% (p<0.005), t increased by 592.0% (p > 0.05), where t represents the effectiveness of increasing gentamicin concentration to lower the bioburden to the steady state, represented by y0 in this fit. The initial biofilm bioburden, represented by A + y0, saw a decrease by 66.9%, which suggests that LGS is able to remove a significant portion of biofilm before gentamicin treatment. Furthermore, the large decrease in y0, to the point where, the coefficient for LGS data was very close to 0, shows that the combination of LGS with gentamicin therapy can significantly lower the bioburden, whereas the gentamicin therapy alone had little effect on the bioburden. The low t in the Non-LGS fit does not represent better antibiotic effectiveness, but rather in the context of a relatively high y0, demonstrates a poor effectiveness of gentamicin to remove the biofilm, as increasing gentamicin concentration had little effect on the biofilm.
CFU density
The coefficients with standard error when fitted to exponential decay without offset for Non-LGS treated CFU density data were found to be:
Coefficients with standard error for LGS treated set were found to be:
When the LGS treated fit was compared to the Non-LGS treated fit, it was found that A decreased by 24.3% (p<0.0001), and t decreased by 36.9% (p<0.005). As A signifies the initial concentration of bacteria, a decrease in A suggests that LGS treatment reduces the initial concentration of bacteria prior to gentamicin treatment. This can be seen in Figure 5. Furthermore, as t represents the effectiveness of gentamicin, with lower values indicating less gentamicin is needed, i.e. better effectiveness.
Figure 5. CFU density at different Gentamicin concentration with (unbold) and without (bold) prior LGS treatment.
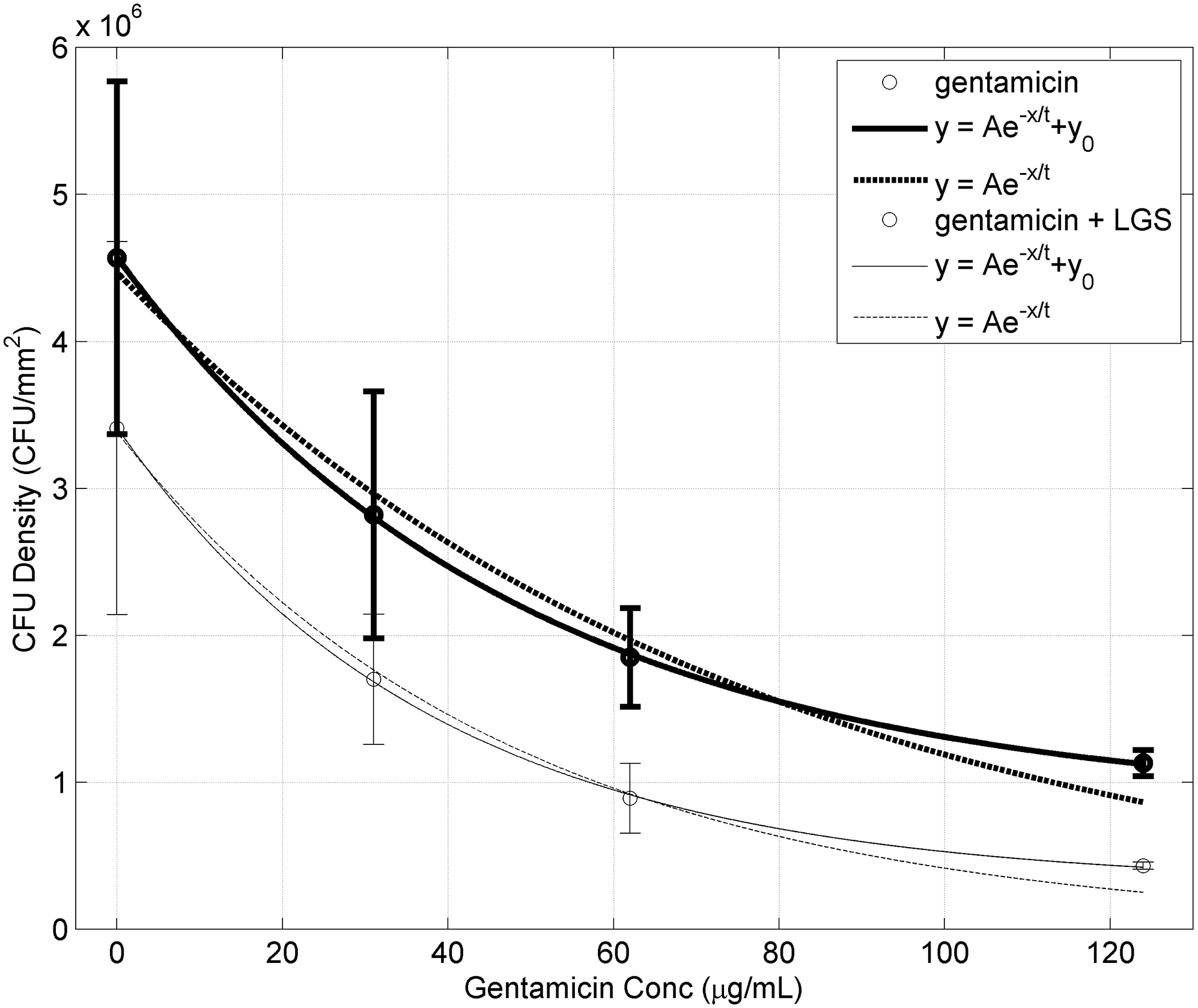
Bars represent sample standard deviation of the group. Both data sets were fitted to the exponential equation with offset: y = Ae−x/t + y0 and without offset: y = Ae−x/t.
When the Non-LGS treated set was fitted to an exponential decay with offset, the coefficients were found to be:
Similarly, the coefficients for the LGS treated set was found to be:
When the LGS treated fit was compared to the Non-LGS treated fit, it was found that y0 decreased by 63.4%, A decreased by 16.8%, t decreased by 21.4%, and A+ y0 decreased by 25.3% (all p < 0.0001). For exponential decay with offset, the initial concentration of bacteria is represented by A+ y0. Similarly the decrease in its value for the LGS treated group, suggests that LGS treatment reduces the initial concentration of bacteria prior to gentamicin treatment. This can be seen in Figure 5. As before, a decrease in t signifies an increase in the effectiveness of gentamicin. As seen in both mathematical models, there was a decrease in initial bacterial counts and an increase in susceptibility to gentamicin.
DISCUSSION
LGS is a novel, minimally invasive modality for biofilm disruption which involves generation of mechanical shockwaves from laser irradiation of a coupled substrate (16–18). Unlike standard laser technologies, there is no direct contact of thermal energy from the laser system to the tissue being treated. Early work by Krespi et al. and Nigri et al. strongly suggested the efficacy of LGS in disrupting biofilms in vitro (19,20,29), while Doukas et al. expanded on this research by showing that LGS increased transdermal drug delivery by affecting surface permeability (30,31). Our group has further expanded on LGS applications in infected wounds in in vitro models (16–18,32), and is currently underway in translating this concept to in vivo studies. However, the ability of LGS to remove biofilm has not been directly quantified. It has been suggested that sub-inhibitory concentrations of antibiotics, such as aminoglycosides, can induce biofilm formation (33), while the presence of biofilm alone can increase the MIC/MBC of glycopeptide antibiotics (23). Therefore any new treatment targeting the removal of biofilm will need to also quantify the amount of biofilm removed, especially when combined with antibiotics. This study is the first to systematically evaluate LGS and antibiotic monotherapy and combination therapy in the in vitro setting.
Presence of biofilm in wounds can present a major problem in wound healing. The resilience conferred by the biofilm makes it difficult to remove the bacteria by irrigation and antibiotics alone, and traumatic surgical debridement is frequently necessary to decrease bacterial load. Chiu et al. demonstrated that once Pseudomonas biofilm was established in the paranasal sinuses of rabbits, treatments with topical tobramycin were not able to fully eradicate the bacteria even at concentrations 400x minimum inhibitory concentration (MIC) (34). Furthermore, similar results were seen when treating Staphylococcus aureus with moxifloxacin in an in vitro setting. Concentrations of 1000x MIC were needed to see a reduction of >99% in number of viable bacteria (35).
Based on the current study results, LGS appears to potentiate the effects of gentamicin therapy when compared to antimicrobial therapy alone, as manifested by a significant decrease in CFU density and in biofilm bioburden. Analysis of the exponential fits of the biofilm bioburden and CFU densities data can provide insight into the mechanism of how LGS potentiates the effects of gentamicin therapy. The consistent decrease in the initial value, A and A + y0, in the LGS treated samples, for both biofilm and CFU fits, demonstrates that LGS therapy can remove significant portion of S. epidermidis burden. Similarly, an increase in gentamicin susceptibility was also observed, when the samples were first treated with LGS. A possible mechanism for this is that, since LGS therapy can fragment the biofilm as previously seen in scanning electron microscopy studies (32), this increased permeability may allow antibiotics to reach the bacteria more effectively. There may also be a second mechanism, where LGS can disrupt the cell wall, increasing its permeability to gentamicin. However, further research is needed to explore LGS’ direct effect on bacteria cells.
We noted that samples treated with LGS alone followed by incubation for 24 hours exhibited an increase in biofilm volume and CFU count when compared with samples treated with LGS but assessed immediately post-treatment. This suggests that LGS as a therapy alone may not actually have direct bacteriostatic or bacteriocidal effects on S. epidermidis, but rather plays an integral role in disrupting the barrier to other therapeutic agents (i.e., antibiotics). We thus interpret LGS as a means to strictly disrupt biofilm as opposed to organism killing, which lends support to its tissue-sparing nature. In addition, the mechanical nature of this treatment makes LGS therapy inherently nonspecific and broad-spectrum, and does not cross-react with inflammatory or immunologic responses.
A noticeable decrease in CFU density was seen when the samples were treated with gentamicin alone, but at each concentration a noticeable increase in biofilm density was still observed. This suggests that while gentamicin can lower CFU density of S. epidermidis in its biofilm state, it does not lower the density sufficiently to prevent further proliferation of biofilm. This may be a result of the antibiotic eliminating the less protected bacteria at the surface, while unable to prevent the underlying, biofilm-protected bacteria from continuing to produce biofilm. This is supported by previous findings that activity of antibiotics is not uniform even throughout the biofilm of one species, either due to obstacles in diffusion of the antibiotic, or the metabolic differences of the bacterial cells (7,36,37).
A study by Laverty et al. found that the MIC for planktonic S. epidermidis to be 31.25 μg/mL and 62.5 μg/mL for the MBC, while the concentration needed to completely eliminate the bacteria after the biofilm has formed to be >1000μg/mL (27). In our study, it was seen that gentamicin therapy alone hindered bacteria proliferation, but did not affect biofilm production. However, by including LGS treatment prior to the gentamicin therapy, they were able to inhibit the proliferation of the bacteria that was previously in its biofilm state and slow the production of biofilm. This suggests that LGS treatment may remove enough of the protective biofilm to cause the underlying bacteria to be susceptible to antibiotic treatment again. Also it may be important to note that the decay rate, t, for LGS for the biofilm and CFU data was between 37 and 48 mL/μg. This would suggest that a combinatory therapy of LGS and gentamicin of <200 μg/mL (4x decay rate), would be sufficient to remove >95% of the bacterial bioburden, much less than the previously reported >1000 μg/mL for gentamicin when used alone
Regarding the practicality of LGS treatment, although safety studies are currently underway, preliminary observations from our in vitro studies seem to indicate that it is appropriately safe for normal tissues. Following treatment, there was no superficial damage seen to coverslips, spacers, or film, and no evidence of thermal energy transmission. Raw materials required for the experiments were low-cost and easy to procure. Each automated treatment over a 3×5cm2 area took less than 30 seconds, making it clinically compatible.
Though biofilm disruption is naturally the first step in decreasing bacterial load within chronically infected wounds, we realize that there are variables that confound this process. Further studies, especially within an in vivo system where intact inflammatory responses, homeostatic mechanisms, and nutrition play a role, are indicated.
There are several limitations to our study. First, in the current in vitro system, we submerged the samples beneath a pool of topical antibiotics, though in practice infected wounds would likely be irrigated with topical antibiotics. Second, the treatment area in this study is rather small, whereas infected wounds that are much larger may have a significantly higher bacterial burden. Thus, it is possible that the current dosimetry overestimates the ability of LGS to disrupt biofilm, though this underscores the importance of repeated assessment and treatments in clinical application. Third, the coverslip treated is two-dimensional, whereas wounds are irregularly shaped in a three-dimensional conformation. Here a unique advantage of LGS is highlighted – the ability of the flexible substrate to conform to different wound shapes (16) – and will be evaluated in in vivo studies. Fourth, the concentrations explored here look only at the effects of LGS and gentamicin on S. epidermidis grown in culture. The effects will most likely be different in an in vivo setting, as intact, functional physiological responses would likely affect the activity of both. Finally, our current study examined only one biofilm-forming organism and one antibiotic. Though elementary in scope, the results of this study serve as a proof of concept in translational implementation and confirm that LGS itself specifically targets biofilms. Future studies will aim to examine the effects of LGS and topical antibiotics on more virulent pathogenic bacteria, such as methicillin-resistant Staphylococcus aureus, where the bacteria cells are innately resistant to a wide range of antibiotics. Furthermore, as cells deeper within the biofilm are slower growing due to lack of nutrients, these cells are often the most difficult to eradicate (38). Therefore, it may be of interest to see the effects of LGS on beta-lactam antibiotics as their activity is dependent on bacteria growth. If LGS can disrupt biofilm, then beta-lactams may have an even more potentiated effect due to faster-growing bacteria gaining access to higher levels of nutrients from membrane permeabiization.
LGS has tremendous applications in all disciplines of clinical medicine. Our immediate aim is to thoroughly evaluate this technology as an auxiliary treatment modality for cutaneous infected wounds, but there is certainly significant potential in other areas, such as applications in the treatments of sinonasal, or other mucosal diseases.
CONCLUSION
Laser generated shockwaves appear to potentiate the efficacy of topical antibiotics in an in vitro model, with confirmation of biofilm disruption. These results are the first step towards developing a minimally invasive, efficacious, and cost-effective adjunct therapy for the treatment of cutaneous and mucosal biofilms which complicate chronically infected wounds.
Figure 4. Biofilm Burden (OD600) at different Gentamicin concentration with (unbold) and without (bold) prior LGS treatment.
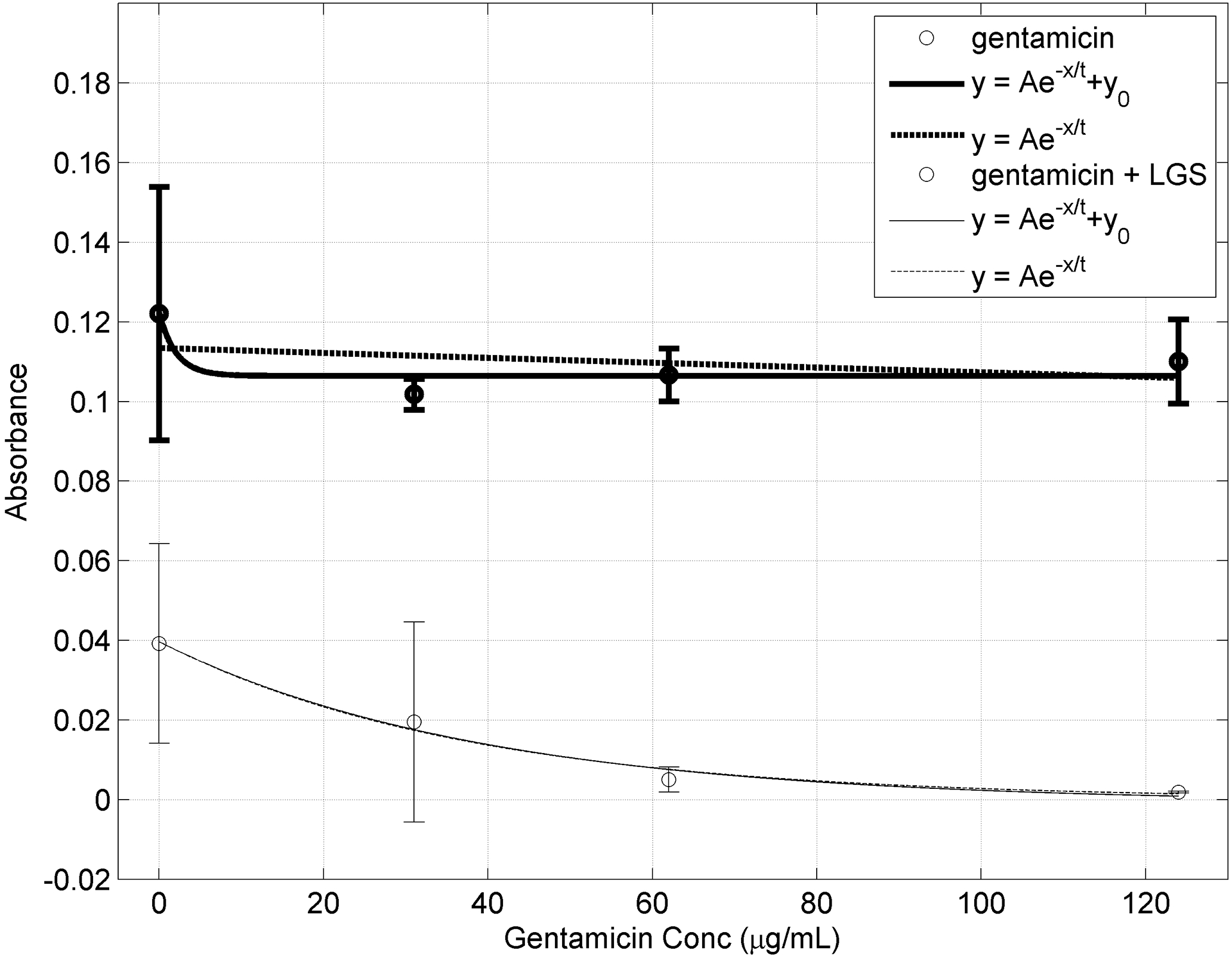
Bars represent sample standard deviation of the group. Both data sets were fitted to the exponential equation with offset: y = Ae−x/t + y0 and without offset: y = Ae−x/t.
Acknowledgement
The authors would like to thank the Clinical and Translational Science Institute (CTSI) for their statistical consultation (funded under CTSI grant UL1TR000124UCLA). The authors would also like to thank Marisol Castellanoa, Valory Banashek, Sam David Marton, and Marian Banh for their assistance in the collection of the data.
Footnotes
This study was presented at the 2016 American Society for Lasers in Surgery and Medicine Annual Conference in Boston, MA. It also received the Outstanding Early Career Abstract award.
Conflicts of Interest: None
REFERENCES
- 1.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. Journal of the American Academy of Dermatology 2008; 58(2):185–206. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006; 42 Suppl 2:S82–89. [DOI] [PubMed] [Google Scholar]
- 3.Rubinstein E, Green M, Modan M, Amit P, Bernstein L, Rubinstein A. The effects of nosocomial infections on the length and costs of hospital stay. J Antimicrob Chemother 1982; 9 Suppl A:93–100. [DOI] [PubMed] [Google Scholar]
- 4.Wound Care Market by Type (Traditional (Wound Closure, Anti infective), Basic (Films, Cleansing), Advanced (Hydrogels, Hydrocolloids, Alginate, Collagen), Active (Artificial Skin & Skin Substitutes), Pressure Relief Devices, NPWT). Pune, India: Markets and Markets; 2014. Report nr MD 2611. [Google Scholar]
- 5.Markets for Advanced Wound Management Technologies. Wellesley, MA: BCC Research LLC; 2014. [Google Scholar]
- 6.Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO J 1992; 38(3):M174–178. [DOI] [PubMed] [Google Scholar]
- 7.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet 2001; 358(9276):135–138. [DOI] [PubMed] [Google Scholar]
- 8.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009; 48(1):1–12. [DOI] [PubMed] [Google Scholar]
- 9.Jones ME, Karlowsky JA, Draghi DC, Thornsberry C, Sahm DF, Nathwani D. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int J Antimicrob Agents 2003; 22(4):406–419. [DOI] [PubMed] [Google Scholar]
- 10.Jones RN. Resistance patterns among nosocomial pathogens: trends over the past few years. Chest 2001; 119(2 Suppl):397S–404S. [DOI] [PubMed] [Google Scholar]
- 11.Livermore DM. Introduction: the challenge of multiresistance. Int J Antimicrob Agents 2007; 29 Suppl 3:S1–7. [DOI] [PubMed] [Google Scholar]
- 12.Paterson DL. Resistance in gram-negative bacteria: enterobacteriaceae. Am J Med 2006; 119(6 Suppl 1):S20–28; discussion S62–70. [DOI] [PubMed] [Google Scholar]
- 13.Rice LB. Antimicrobial resistance in gram-positive bacteria. Am J Med 2006; 119(6 Suppl 1):S11–19; discussion S62–70. [DOI] [PubMed] [Google Scholar]
- 14.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J, Jr., Infectious Diseases Society of A. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008; 46(2):155–164. [DOI] [PubMed] [Google Scholar]
- 15.Bowler PG. Wound pathophysiology, infection and therapeutic options. Ann Med 2002; 34(6):419–427. [DOI] [PubMed] [Google Scholar]
- 16.Francis NC, Kassam I, Nowroozi B, Grundfest WS, Taylor ZD. Analysis of flexible substrates for clinical translation of laser-generated shockwave therapy. Biomed Opt Express 2015; 6(3):827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramaprasad V, Navarro A, Patel S, Patel V, Nowroozi BN, Taylor ZD, Yong W, Gupta V, Grundfest WS. Effect of laser generated shockwaves 1 on ex-vivo pigskin. Lasers Surg Med 2014; 46(8):620–627. [DOI] [PubMed] [Google Scholar]
- 18.Taylor ZD, Navarro A, Kealey CP, Beenhouwer D, Haake DA, Grundfest WS, Gupta V. Bacterial biofilm disruption using laser generated shockwaves. Conf Proc IEEE Eng Med Biol Soc 2010; 2010{Taylor, 2010 #44}:1028–1032. [DOI] [PubMed] [Google Scholar]
- 19.Nigri GR, Tsai S, Kossodo S, Waterman P, Fungaloi P, Hooper DC, Doukas AG, LaMuraglia GM. Laser-induced shock waves enhance sterilization of infected vascular prosthetic grafts. Lasers in surgery and medicine 2001; 29(5):448–454. [DOI] [PubMed] [Google Scholar]
- 20.Krespi YP, Stoodley P, Hall-Stoodley L. Laser disruption of biofilm. The Laryngoscope 2008; 118(7):1168–1173. [DOI] [PubMed] [Google Scholar]
- 21.Matlaga BR, McAteer JA, Connors BA, Handa RK, Evan AP, Williams JC, Lingeman JE, Willis LR. Potential for cavitation-mediated tissue damage in shockwave lithotripsy. Journal of endourology / Endourological Society 2008; 22(1):121–126. [DOI] [PubMed] [Google Scholar]
- 22.Evan AP, Willis LR, Lingeman JE, McAteer JA. Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron 1998; 78(1):1–8. [DOI] [PubMed] [Google Scholar]
- 23.Farber BF, Kaplan MH, Clogston AG. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. The Journal of infectious diseases 1990; 161(1):37–40. [DOI] [PubMed] [Google Scholar]
- 24.Bakkiyaraj D, Pandian SK. In vitro and in vivo antibiofilm activity of a coral associated actinomycete against drug resistant Staphylococcus aureus biofilms. Biofouling 2010; 26(6):711–717. [DOI] [PubMed] [Google Scholar]
- 25.Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Current protocols in microbiology 2005; Chapter 1:Unit 1B 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. The Journal of hygiene 1938; 38(6):732–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laverty G, Alkawareek MY, Gilmore BF. The In Vitro Susceptibility of Biofilm Forming Medical Device Related Pathogens to Conventional Antibiotics. Dataset Papers in Science 2014; 2014:10. [Google Scholar]
- 28.Curtin J, Cormican M, Fleming G, Keelehan J, Colleran E. Linezolid compared with eperezolid, vancomycin, and gentamicin in an in vitro model of antimicrobial lock therapy for Staphylococcus epidermidis central venous catheter-related biofilm infections. Antimicrobial agents and chemotherapy 2003; 47(10):3145–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krespi YP, Kizhner V, Nistico L, Hall-Stoodley L, Stoodley P. Laser disruption and killing of methicillin-resistant Staphylococcus aureus biofilms. Am J Otolaryngol 2011; 32(3):198–202. [DOI] [PubMed] [Google Scholar]
- 30.Doukas AG. Laser-generated stress waves in medicine: From tissue injury to drug delivery Therapeutic Laser Applications. Orlando, FL; 1998. [Google Scholar]
- 31.Doukas AG, Kollias N. Transdermal drug delivery with a pressure wave. Advanced drug delivery reviews 2004; 56(5):559–579. [DOI] [PubMed] [Google Scholar]
- 32.Francis NC, Yao W, Grundfest WS, Taylor ZD. Laser-Generated Shockwaves as a Treatment to Reduce Bacterial Load and Disrupt Biofilm. IEEE transactions on bio-medical engineering 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman LR, D’Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005; 436(7054):1171–1175. [DOI] [PubMed] [Google Scholar]
- 34.Chiu AG, Antunes MB, Palmer JN, Cohen NA. Evaluation of the in vivo efficacy of topical tobramycin against Pseudomonas sinonasal biofilms. The Journal of antimicrobial chemotherapy 2007; 59(6):1130–1134. [DOI] [PubMed] [Google Scholar]
- 35.Desrosiers M, Bendouah Z, Barbeau J. Effectiveness of topical antibiotics on Staphylococcus aureus biofilm in vitro. American journal of rhinology 2007; 21(2):149–153. [DOI] [PubMed] [Google Scholar]
- 36.Macia MD, Rojo-Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2014; 20(10):981–990. [DOI] [PubMed] [Google Scholar]
- 37.Huang CT, Yu FP, McFeters GA, Stewart PS. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Applied and environmental microbiology 1995; 61(6):2252–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis K Riddle of biofilm resistance. Antimicrobial agents and chemotherapy 2001; 45(4):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]


