Abstract
Background
Antimicrobial resistance (AMR) changes over time and continuous monitoring provides insight on trends to inform both empirical treatment and public health action.
Aims
To survey trends in relative isolation frequency (RIF) and AMR among key bloodstream pathogens using data from the Greek Electronic System for the Surveillance of AMR (WHONET-Greece).
Methods
This observational study looked into routine susceptibility data of 50,488 blood culture isolates from hospitalised patients in 25 tertiary hospitals, participating in the WHONET-Greece for trends over time between January 2010 and December 2017. Only the first isolate per species from each patient was included. Hospital wards and intensive care units (ICUs) were analysed separately.
Results
During the study, the RIF of Acinetobacter baumannii increased in wards, as did the proportion of A. baumannii isolates, which were non-susceptibleto most antibiotics in both wards and ICUs. Coincidently, Klebsiella pneumoniae RIF declined while the respective rates of non-susceptible isolates to carbapenems and gentamicin increased. Pseudomonas aeruginosa RIF remained stable but decreasing proportions of non-susceptible isolates to all studied antibiotics, except imipenem were observed. Escherichia coli RIF increased as did the proportion of isolates non-susceptible to third-generation cephalosporins, carbapenems and fluoroquinolones. Concerning Staphylococcus aureus, a decline in the percentage of meticillin resistant isolates in ICUs was found, while the percentages of Enterococcus faecium isolates with non-susceptibility to vancomycin stayed stable.
Conclusions
Recognising these trends over time is important, since the epidemiology of AMR is complex, involving different ‘bug and drug’ combinations. This should be taken into consideration to control AMR.
Keywords: routine laboratory data, bloodstream infections, antimicrobial resistance, surveillance system, trend analysis, Greece
Introduction
Antimicrobial resistance (AMR), especially the appearance and dissemination of multiresistant bacteria, as well as the lack of alternative treatments, are a major threat to both clinical medicine and public health in Greece, elsewhere in Europe and globally. As stated in the recent World Health Organization (WHO) AMR action plans, an important cornerstone to control AMR is its surveillance [1].
Indeed, continuous monitoring of the emergence and evolution of resistance to key antimicrobials over time constitutes a crucial first step to estimate the burden of the problem, uncover trends, detect new resistance phenotypes, guide empirical antimicrobial treatment and measure the effect of interventions [2].
The routine results of the antimicrobial susceptibility tests performed daily in each hospital clinical laboratory are considered as a major resource for continuous, passive AMR surveillance since they are reliable and informative and can be collected on a daily basis without imposing any additional effort to the clinical microbiology laboratory.
Greece has been among the first countries with an electronic network based on routine susceptibility results since 1995. The Greek AMR surveillance system (WHONET-Greece) allows continuous monitoring at national level of bacterial antibiotic resistance in Greek hospitals based on the collection and processing of routine susceptibility data from the Laboratory Information System (LIS) of hospital laboratories using the WHONET software [3]. The data are publicly available (www.mednet.gr/whonet) and have been continuously submitted to the European Antimicrobial Resistance Surveillance System (EARSS) and subsequently to the European Antimicrobial Resistance Surveillance Network (EARS-net) (https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-andlaboratory-networks/ears-net) as the annual Greek AMR data.
In the present collaboration, we sought to describe the trends of the relative isolation frequency (RIF) and the AMR rates among key bloodstream pathogens and their evolution over time for the period 2010–2017 as captured by the national continuous monitoring system of routine laboratory data in order to provide information for action to both clinicians and public health authorities in Greece.
Methods
Study period and setting
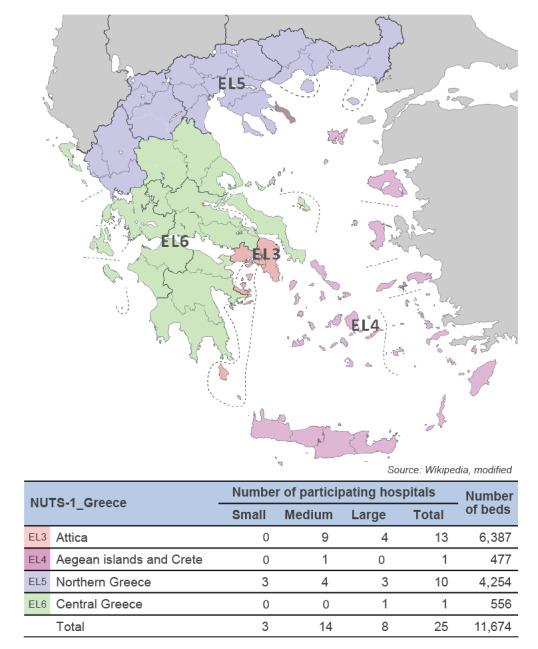
The study covered the 8-year period from January 2010 to December 2017. Twenty-five tertiary Greek hospitals, participating in the WHONET-Greece network and consistently reporting data for the entire period (a maximum of two non-consecutive semesters of non-reporting were missing in three hospitals), contributed to the study. The participating hospitals were distributed across the country and represented all four first-level nomenclature of territorial units for statistics (NUTS-1) regions of Greece. Moreover, the participating hospitals’ bed capacity ranged from 178 to 908 beds and thus small (< 200 beds), medium (200–500 beds) and large (> 500 beds) public hospitals were represented (Figure 1).
Figure 1.
Number of hospitals participating in the study and total bed capacity per first-level nomenclature of territorial units for statistics regions of Greece, WHONET-Greece AMR network, January 2010–December 2017 (n = 25 hospitals)
AMR: antimicrobial resistance; EL: Greece; NUTS1_Greece: first level of the Nomenclature of Territorial Units for Statistics for Greece; WHONET-Greece: Greek electronic surveillance system for monitoring AMR in hospitals based on routine data.
The antimicrobial susceptibility testing (AST) was performed in the hospitals’ clinical laboratories by automated systems. All participating hospital laboratories performed internal quality controls, and they took part in the annual external quality assessment provided by the United Kingdom National External Quality Assessment Service (UK-NEQAS), offered by the European Centre for Disease Prevention and Control (ECDC).
Isolate collection and relative isolation frequency
During the 8-year period, routine susceptibility data of 50,488 key Gram-negative and Gram-positive bacterial isolates from blood cultures of hospitalised patients in the participating tertiary hospitals, representing the nine most clinically important species, were gathered and studied (Table 1). From each patient, only the first isolate per species was included. The RIF of each one of the studied bacterial species was defined as its proportion among the nine bacterial species included in the study, calculated per year and ward type.
Table 1. Number of bloodstream bacterial isolates per year and species, from patients hospitalised in wards and ICUs of the 25 hospitals participating in the WHONET-Greece AMR network, 2010–2017 (n = 50,488 isolates).
| Microorganism | Year of isolation | Total | % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |||
| Number of bacteria isolated in wards | ||||||||||
| Escherichia coli | 1,048 | 1,044 | 1,074 | 1,121 | 1,124 | 1,157 | 1,337 | 1,213 | 9,118 | 26.2 |
| Klebsiella pneumoniae | 774 | 708 | 672 | 728 | 713 | 756 | 754 | 772 | 5,877 | 16.9 |
| Staphylococcus aureus | 583 | 598 | 631 | 621 | 533 | 590 | 659 | 633 | 4,848 | 13.9 |
| Pseudomonas aeruginosa | 417 | 431 | 429 | 483 | 390 | 424 | 459 | 454 | 3,487 | 10.0 |
| Acinetobacter baumannii | 317 | 346 | 390 | 348 | 384 | 456 | 416 | 471 | 3,128 | 9.0 |
| Enterococcus faecalis | 368 | 365 | 414 | 379 | 363 | 377 | 414 | 430 | 3,110 | 8.9 |
| Enterococcus faecium | 248 | 229 | 236 | 245 | 222 | 233 | 275 | 288 | 1,976 | 5.7 |
| Proteus mirabilis | 157 | 181 | 201 | 188 | 203 | 237 | 249 | 206 | 1,622 | 4.7 |
| Enterobacter spp. | 224 | 206 | 202 | 206 | 184 | 198 | 190 | 194 | 1,604 | 4.6 |
| Total in wards | 4,136 | 4,108 | 4,249 | 4,319 | 4,116 | 4,428 | 4,753 | 4,661 | 34,770 | 100.0 |
| Number of bacteria isolated in ICUs | ||||||||||
| Acinetobacter baumannii | 652 | 685 | 600 | 499 | 516 | 509 | 503 | 440 | 4,404 | 28.0 |
| Klebsiella pneumoniae | 625 | 686 | 574 | 500 | 461 | 417 | 466 | 424 | 4,153 | 26.4 |
| Pseudomonas aeruginosa | 457 | 361 | 353 | 415 | 329 | 246 | 278 | 239 | 2,678 | 17.0 |
| Enterococcus faecalis | 188 | 217 | 196 | 158 | 142 | 148 | 197 | 149 | 1,395 | 8.9 |
| Enterococcus faecium | 130 | 114 | 111 | 99 | 88 | 95 | 110 | 96 | 843 | 5.4 |
| Staphylococcus aureus | 101 | 106 | 100 | 78 | 81 | 60 | 65 | 75 | 666 | 4.2 |
| Proteus mirabilis | 107 | 115 | 103 | 54 | 60 | 75 | 69 | 72 | 655 | 4.2 |
| Enterobacter spp. | 78 | 110 | 71 | 50 | 45 | 57 | 46 | 44 | 501 | 3.2 |
| Escherichia coli | 54 | 57 | 43 | 60 | 50 | 42 | 60 | 57 | 423 | 2.7 |
| Total in ICUs | 2,392 | 2,451 | 2,151 | 1,913 | 1,772 | 1,649 | 1,794 | 1,596 | 15,718 | 100.0 |
AMR: antimicrobial resistance; ICUs: intensive care units; WHONET-Greece: Greek electronic surveillance system for monitoring AMR in hospitals based on routine data.
Classification of isolates in terms of antimicrobial susceptibility
The classification of isolates as susceptible, intermediate or resistant (including, for enterococci with acquired aminoglycoside resistance, high-level resistance (HLR) to aminoglycosides) was based on the Clinical and Laboratory Standards Institute (CLSI) (https://clsi.org) clinical breakpoints, a system which was used in Greece for routine AST interpretation during the study period. The version of WHONET software we used for the analysis was equipped with CLSI 2017–2018 breakpoints. The isolates with intermediate susceptibility were grouped with the resistant ones, forming the non-susceptible group.
Data analysis
We focused on annual trends of both RIF for the bacteria included in the study and antimicrobial non-susceptibility rates for the key antimicrobial classes traditionally used for the treatment of Gram-negative and Gram-positive bacteraemia, as well as for the presence of multidrug resistance according to the interim standard definitions for acquired resistance [4]. The data from intensive care units (ICUs) were analysed separately from medical and surgical wards. To assess trends in proportions of non-susceptible isolates over time, we used the Cochran–Armitage χ2 test for trend. A p value of ≤ 0.05 was considered significant. Statistical analysis was performed using R version 3.4.3 for Windows.
Ethical statement
For this observational study, since the confidentiality of the data was ensured by pseudo-anonymisation with unique codes for each included bacterial isolate, and data will not be identifiable back to the patient from whom they originated, an ethical approval was not needed.
Results
Relative isolation frequency and trend analysis over time
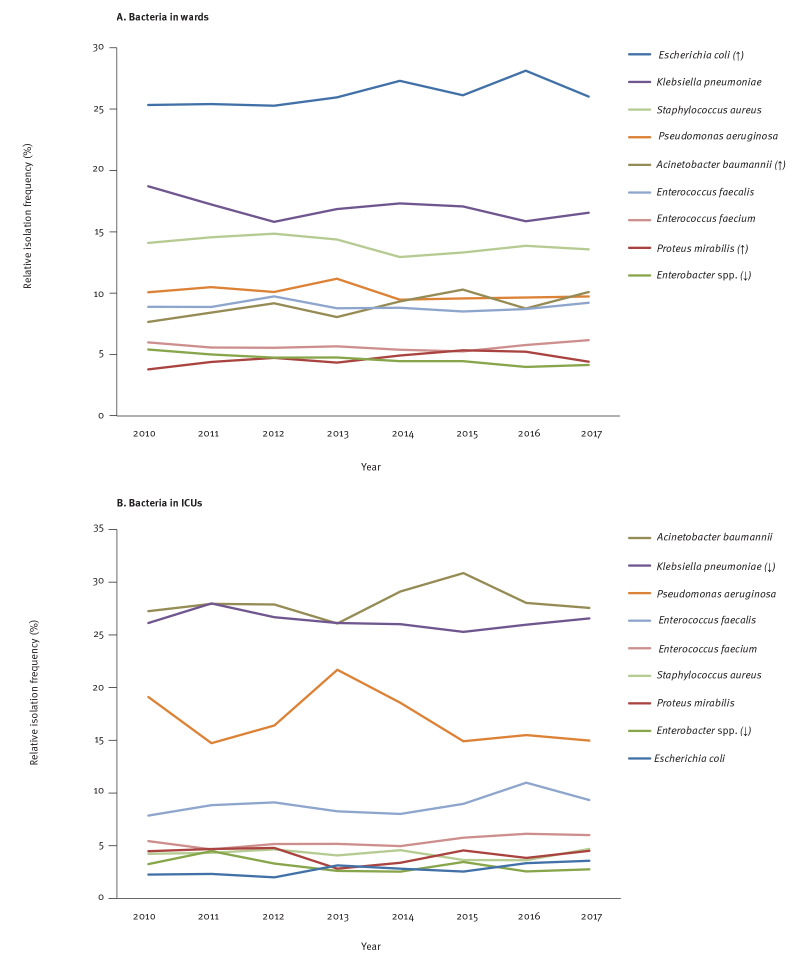
Concerning the RIF of the nine main pathogens among the bloodstream isolates from patients hospitalised in the wards (Figure 2a), Escherichia coli was the most prevalent in each year of the study period with an annual RIF ranging from 25.3% to 28.1% (median: 26.0%). This was followed by Klebsiella pneumoniae (range: 15.8–18.7%; median: 17.0%), Staphylococcus aureus (range: 12.9–14.9%; median: 14.0%), Pseudomonas aeruginosa (range: 9.5–11.2%; median: 9.9%), Enterococcus faecalis (range: 8.5–9.7%; median: 8.9%), Acinetobacter baumannii (range: 7.7–10.3%; median: 9.0%), Enterococcus faecium (range: 5.3–6.2%; median: 5.6%), Enterobacter spp. (range: 4.0–5.4%; median: 4.6%) and Proteus mirabilis (range: 3.8–5.4%; median: 4.6%).
Figure 2.
Relative isolation frequency trend analysis of nine key bloodstream pathogens from patients hospitalised in (a) the wards (n = 34,770) and (b) the ICUs (n = 15,718) of the 25 hospitals participating in the WHONET-Greece AMR network, 2010–2017
AMR: antimicrobial resistance; ICU: intensive care unit; WHONET-Greece: Greek electronic surveillance system for monitoring AMR in hospitals based on routine data.
‘↑’ and ‘↓’ indicate significant increasing and decreasing trends, respectively.
Among the studied isolates from patients hospitalised in ICUs (Figure 2b), A. baumannii was the most prevalent bloodstream isolate in each year of the study period with an annual RIF ranging from 26.1% to 30.9% (median: 27.9%) followed by K. pneumoniae (range: 25.3–28.0%; median: 26.1%), P. aeruginosa (range: 14.7–21.7%; median: 16.0%), E. faecalis (range: 7.9–11.0%; median: 8.9%), E. faecium (range: 4.7–6.1%; median: 5.3%), S. aureus (range: 3.6–4.7%; median: 4.3%), P. mirabilis (range: 2.8–4.8%; median: 4.5%), Enterobacter spp. (range: 2.5–4.5%; median: 3.0%) and E. coli (range: 2.0–3.6%; median: 2.7%).
Overall (Figure 2, 2a,b), we observed a significant increasing trend in the RIF of E. coli (p ≤ 0.001), P. mirabilis (p = 0.004) and A. baumannii (p ≤ 0.001) from patients hospitalised in the wards while their RIF remained stable over the years in ICUs. In contrast, a significant decreasing trend was observed in the RIF of K. pneumoniae from ICUs (p = 0.028) and Enterobacter spp. (p = 0.002) from patients hospitalised in both wards and ICUs. As for P. aeruginosa, no trend was observed in its RIF irrespective of the hospitalisation unit. Concerning Gram-positive bloodstream pathogens, the RIFs of S. aureus, E. faecalis and E. faecium were stable without any trend over time in both the wards and ICUs.
Trends in antimicrobial resistance rates over time
All significant trends in AMR rates over time per microorganism and hospitalisation unit are shown in Table 2 and more specifically described below.
Table 2. Significant trends in antimicrobial resistance rates over time per microorganism and hospitalisation unit (wards or ICU), WHONET-Greece AMR network, 2010–2017 (n = 48,211).
| Antibiotic | Year of isolation | p value | Trend | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |||||||||||
| NS/tested | %NS | NS/tested | %NS | NS/tested | %NS | NS/tested | %NS | NS/tested | %NS | NS/tested | %NS | NS/tested | %NS | NS/tested | %NS | |||
| Escherichia coli | ||||||||||||||||||
| Ceftazidime non-susceptible | ||||||||||||||||||
| Wards | 117/1,032 | 11.3 | 126/1,034 | 12.2 | 136/1,047 | 13.0 | 152/1,097 | 13.9 | 166/1,092 | 15.2 | 156/1,119 | 13.9 | 179/1,318 | 13.6 | 176/1,198 | 14.7 | 0.012 | ↑ |
| Meropenem non-susceptible | ||||||||||||||||||
| Wards | 2/897 | 0.2 | 5/882 | 0.6 | 9/871 | 1.0 | 10/882 | 1.1 | 10/867 | 1.2 | 9/944 | 1.0 | 12/1,046 | 1.1 | 19/939 | 2.0 | < 0.001 | ↑ |
| Ciprofloxacin non-susceptible | ||||||||||||||||||
| Wards | 265/1,037 | 25.6 | 282/1,034 | 27.3 | 311/1,045 | 29.8 | 358/1,097 | 32.6 | 369/1,095 | 33.7 | 325/1,115 | 29.1 | 433/1,331 | 32.5 | 386/1,198 | 32.2 | < 0.001 | ↑ |
| Trimethoprim/sulfamethoxazole non-susceptible | ||||||||||||||||||
| Wards | 376/1,026 | 36.6 | 365/918 | 39.8 | 362/930 | 38.9 | 413/1,034 | 39.9 | 385/1,051 | 36.6 | 335/1,059 | 31.6 | 430/1,251 | 34.4 | 383/1,154 | 33.2 | < 0.001 | ↓ |
| Enterobacter spp. | ||||||||||||||||||
| Ceftazidime non-susceptible | ||||||||||||||||||
| Wards | 98/222 | 44.1 | 66/202 | 32.7 | 69/193 | 35.8 | 61/202 | 30.2 | 67/181 | 37.0 | 65/197 | 33.0 | 42/185 | 22.7 | 61/191 | 31.9 | 0.001 | ↓ |
| Imipenem non-susceptible | ||||||||||||||||||
| Wards | 34/219 | 15.5 | 22/200 | 11.0 | 35/194 | 18.0 | 31/200 | 15.5 | 32/177 | 18.1 | 36/195 | 18.5 | 32/185 | 17.3 | 39/191 | 20.4 | 0.044 | ↑ |
| Meropenem non-susceptible | ||||||||||||||||||
| Wards | 17/196 | 8.7 | 15/181 | 8.3 | 14/165 | 8.5 | 21/176 | 11.9 | 13/126 | 10.3 | 24/156 | 15.4 | 16/154 | 10.4 | 22/166 | 13.3 | 0.042 | ↑ |
| Ciprofloxacin non-susceptible | ||||||||||||||||||
| Wards | 44/222 | 19.8 | 22/201 | 10.9 | 25/194 | 12.9 | 24/201 | 11.9 | 22/179 | 12.3 | 29/196 | 14.8 | 16/187 | 8.6 | 21/189 | 11.1 | 0.023 | ↓ |
| Tobramycin non-susceptible | ||||||||||||||||||
| Wards | 70/214 | 32.7 | 33/199 | 16.6 | 38/184 | 20.7 | 31/188 | 16.5 | 36/170 | 21.2 | 38/165 | 23.0 | 26/165 | 15.8 | 22/150 | 14.7 | 0.001 | ↓ |
| Multiresistance | ||||||||||||||||||
| Wards | 34/205 | 16.6 | 16/197 | 8.1 | 18/182 | 9.9 | 16/181 | 8.8 | 16/167 | 9.6 | 20/162 | 12.3 | 10/161 | 6.2 | 12/145 | 8.3 | 0.034 | ↓ |
| Klebsiella pneumoniae | ||||||||||||||||||
| Ceftazidime non-susceptible | ||||||||||||||||||
| Wards | 470/761 | 61.8 | 434/698 | 62.2 | 366/635 | 57.6 | 396/689 | 57.5 | 391/682 | 57.3 | 416/726 | 57.3 | 421/743 | 56.7 | 445/761 | 58.5 | 0.027 | ↓ |
| ICU | 567/612 | 92.6 | 589/652 | 90.3 | 504/556 | 90.6 | 397/455 | 87.3 | 387/442 | 87.6 | 349/403 | 86.6 | 396/453 | 87.4 | 364/411 | 88.6 | 0.001 | ↓ |
| Imipenem non-susceptible | ||||||||||||||||||
| Wards | 380/761 | 49.9 | 347/696 | 49.9 | 294/630 | 46.7 | 320/685 | 46.7 | 320/681 | 47.0 | 346/726 | 47.7 | 375/743 | 50.5 | 399/761 | 52.4 | 0.021 | ↑a |
| ICU | 536/608 | 88.2 | 567/653 | 86.8 | 461/552 | 83.5 | 366/454 | 80.6 | 369/439 | 84.1 | 341/404 | 84.4 | 399/453 | 88.1 | 351/409 | 85.8 | <0.001 | ↓b |
| Meropenem non-susceptible | ||||||||||||||||||
| Wards | 305/678 | 45.0 | 321/638 | 50.3 | 281/606 | 46.4 | 298/598 | 49.8 | 302/602 | 50.2 | 322/636 | 50.6 | 342/630 | 54.3 | 379/689 | 55.0 | < 0.001 | ↑ |
| ICU | 472/584 | 80.8 | 507/618 | 82.0 | 432/538 | 80.3 | 349/432 | 80.8 | 333/403 | 82.6 | 327/377 | 86.7 | 385/432 | 89.1 | 358/412 | 86.9 | < 0.001 | ↑ |
| Ciprofloxacin non-susceptible | ||||||||||||||||||
| ICU | 555/612 | 90.7 | 584/653 | 89.4 | 497/556 | 89.4 | 384/455 | 84.4 | 381/442 | 86.2 | 342/403 | 84.9 | 392/454 | 86.3 | 361/412 | 87.6 | 0.005 | ↓ |
| Gentamicin non-susceptible | ||||||||||||||||||
| Wards | 133/763 | 17.4 | 108/697 | 15.5 | 108/635 | 17.0 | 140/685 | 20.4 | 148/683 | 21.7 | 142/726 | 19.6 | 202/743 | 27.2 | 225/763 | 29.5 | < 0.001 | ↑ |
| ICU | 166/611 | 27.2 | 188/651 | 28.9 | 234/557 | 42.0 | 201/456 | 44.1 | 171/437 | 39.1 | 180/405 | 44.4 | 223/455 | 49.0 | 206/413 | 49.9 | < 0.001 | ↑ |
| Tobramycin non-susceptible | ||||||||||||||||||
| Wards | 458/744 | 61.6 | 427/682 | 62.6 | 304/537 | 56.6 | 335/587 | 57.1 | 354/640 | 55.3 | 293/564 | 52.0 | 319/610 | 52.3 | 313/589 | 53.1 | < 0.001 | ↓ |
| ICU | 509/570 | 89.3 | 556/634 | 87.7 | 420/472 | 89.0 | 309/370 | 83.5 | 327/408 | 80.1 | 248/288 | 86.1 | 268/327 | 82.0 | 211/256 | 82.4 | < 0.001 | ↓ |
| Cefoxitin non-susceptible | ||||||||||||||||||
| ICU | 481/529 | 90.9 | 488/551 | 88.6 | 367/483 | 76.0 | 276/364 | 75.8 | 271/364 | 74.5 | 266/360 | 73.9 | 309/425 | 72.7 | 313/395 | 79.2 | < 0.001 | ↓ |
| Multiresistance | ||||||||||||||||||
| Wards | 391/738 | 53.0 | 372/680 | 54.7 | 272/536 | 50.7 | 282/587 | 48.0 | 308/639 | 48.2 | 252/564 | 44.7 | 268/610 | 43.9 | 271/588 | 46.1 | < 0.001 | ↓ |
| ICU | 498/569 | 87.5 | 540/628 | 86.0 | 405/471 | 86.0 | 296/369 | 80.2 | 318/406 | 78.3 | 234/288 | 81.3 | 254/325 | 78.2 | 208/256 | 81.3 | < 0.001 | ↓ |
| Pseudomonas aeruginosa | ||||||||||||||||||
| Piperacillin/tazobactam non-susceptible | ||||||||||||||||||
| ICU | 179/435 | 41.1 | 88/283 | 31.1 | 62/197 | 31.5 | 73/339 | 21.5 | 98/304 | 32.2 | 54/209 | 25.8 | 65/256 | 25.4 | 47/221 | 21.3 | < 0.001 | ↓ |
| Ceftazidime non-susceptible | ||||||||||||||||||
| ICU | 226/433 | 52.2 | 173/353 | 49.0 | 182/346 | 52.6 | 201/389 | 51.7 | 153/313 | 48.9 | 102/237 | 43.0 | 118/274 | 43.1 | 97/237 | 40.9 | < 0.001 | ↓ |
| Imipenem non-susceptible | ||||||||||||||||||
| Wards | 222/404 | 55.0 | 240/425 | 56.5 | 258/410 | 62.9 | 319/455 | 70.1 | 258/377 | 68.4 | 282/409 | 68.9 | 297/446 | 66.6 | 283/448 | 63.2 | < 0.001 | ↑b |
| ICU | 315/431 | 73.1 | 252/354 | 71.2 | 255/346 | 73.7 | 290/389 | 74.6 | 250/313 | 79.9 | 192/237 | 81.0 | 214/273 | 78.4 | 179/235 | 76.2 | 0.005 | ↑ |
| Meropenem non-susceptible | ||||||||||||||||||
| Wards | 157/406 | 38.7 | 162/414 | 39.1 | 167/398 | 42.0 | 208/432 | 48.1 | 117/348 | 33.6 | 162/393 | 41.2 | 160/425 | 37.6 | 168/423 | 39.7 | 0.004 | ↑b |
| ICU | 264/435 | 60.7 | 206/325 | 63.4 | 203/335 | 60.6 | 244/385 | 63.4 | 177/301 | 58.8 | 118/228 | 51.8 | 122/254 | 48.0 | 116/230 | 50.4 | < 0.001 | ↓ |
| Ciprofloxacin non-susceptible | ||||||||||||||||||
| ICU | 244/435 | 56.1 | 174/352 | 49.4 | 185/346 | 53.5 | 205/391 | 52.4 | 148/315 | 47.0 | 106/240 | 44.2 | 98/275 | 35.6 | 96/238 | 40.3 | < 0.001 | ↓ |
| Tobramycin non-susceptible | ||||||||||||||||||
| Wards | 123/391 | 31.5 | 121/411 | 29.4 | 121/354 | 34.2 | 141/399 | 35.3 | 98/357 | 27.5 | 76/286 | 26.6 | 101/357 | 28.3 | 91/338 | 26.9 | 0.039 | ↓ |
| ICU | 214/395 | 54.2 | 165/340 | 48.5 | 144/289 | 49.8 | 169/331 | 51.1 | 125/276 | 45.3 | 60/159 | 37.7 | 68/205 | 33.2 | 60/163 | 36.8 | < 0.001 | ↓ |
| Gentamicin non-susceptible | ||||||||||||||||||
| Wards | 138/414 | 33.3 | 141/426 | 33.1 | 154/414 | 37.2 | 163/457 | 35.7 | 101/374 | 27.0 | 106/409 | 25.9 | 137/451 | 30.4 | 123/449 | 27.4 | < 0.001 | ↓ |
| ICU | 239/433 | 55.2 | 185/353 | 52.4 | 170/345 | 49.3 | 170/390 | 43.6 | 126/305 | 41.3 | 87/240 | 36.3 | 94/276 | 34.1 | 79/237 | 33.3 | < 0.001 | ↓ |
| Amikacin non-susceptible | ||||||||||||||||||
| ICU | 201/434 | 46.3 | 147/353 | 41.6 | 147/347 | 42.4 | 183/383 | 47.8 | 130/309 | 42.1 | 90/237 | 38.0 | 91/274 | 33.2 | 81/235 | 34.5 | < 0.001 | ↓ |
| Multiresistance | ||||||||||||||||||
| ICU | 156/429 | 36.4 | 116/342 | 33.9 | 127/337 | 37.7 | 155/376 | 41.2 | 118/305 | 38.7 | 72/235 | 30.6 | 74/271 | 27.3 | 73/235 | 31.1 | 0.018 | ↓ |
| Acinetobacter baumannii | ||||||||||||||||||
| Cefepime non-susceptible | ||||||||||||||||||
| Wards | 265/299 | 88.6 | 285/335 | 85.1 | 324/369 | 87.8 | 282/316 | 89.2 | 323/347 | 93.1 | 398/442 | 90.0 | 369/401 | 92.0 | 444/459 | 96.7 | < 0.001 | ↑ |
| ICU | 607/629 | 96.5 | 562/619 | 90.8 | 526/570 | 92.3 | 460/474 | 97.0 | 491/495 | 99.2 | 485/495 | 98.0 | 481/491 | 98.0 | 425/434 | 97.9 | < 0.001 | ↑ |
| Imipenem non-susceptible | ||||||||||||||||||
| Wards | 243/303 | 80.2 | 290/337 | 86.1 | 322/363 | 88.7 | 281/319 | 88.1 | 300/331 | 90.6 | 369/415 | 88.9 | 351/389 | 90.2 | 416/445 | 93.5 | < 0.001 | ↑ |
| ICU | 555/576 | 96.4 | 610/637 | 95.8 | 552/567 | 97.4 | 449/460 | 97.6 | 483/486 | 99.4 | 472/482 | 97.9 | 470/475 | 98.9 | 419/426 | 98.4 | < 0.001 | ↑ |
| Meropenem non-susceptible | ||||||||||||||||||
| Wards | 244/306 | 79.7 | 282/324 | 87.0 | 321/362 | 88.7 | 259/300 | 86.3 | 290/319 | 90.9 | 358/404 | 88.6 | 346/380 | 91.1 | 423/448 | 94.4 | < 0.001 | ↑ |
| ICU | 605/635 | 95.3 | 552/576 | 95.8 | 528/546 | 96.7 | 420/437 | 96.1 | 422/428 | 98.6 | 405/418 | 96.9 | 441/447 | 98.7 | 419/427 | 98.1 | < 0.001 | ↑ |
| Tobramycin non-susceptible | ||||||||||||||||||
| Wards | 132/284 | 46.5 | 149/330 | 45.2 | 204/325 | 62.8 | 179/270 | 66.3 | 204/322 | 63.4 | 232/316 | 73.4 | 189/276 | 68.5 | 231/271 | 85.2 | < 0.001 | ↑ |
| ICU | 339/562 | 60.3 | 412/632 | 65.2 | 341/491 | 69.5 | 311/411 | 75.7 | 352/443 | 79.5 | 317/371 | 85.4 | 290/346 | 83.8 | 227/252 | 90.1 | < 0.001 | ↑ |
| Amikacin non-susceptible | ||||||||||||||||||
| Wards | 208/291 | 71.5 | 218/301 | 72.4 | 248/344 | 72.1 | 231/304 | 76.0 | 244/316 | 77.2 | 295/390 | 75.6 | 276/343 | 80.5 | 338/408 | 82.8 | < 0.001 | ↑ |
| ICU | 484/594 | 81.5 | 451/598 | 75.4 | 405/536 | 75.6 | 379/431 | 87.9 | 396/445 | 89.0 | 388/430 | 90.2 | 370/394 | 93.9 | 299/332 | 90.1 | < 0.001 | ↑ |
| Gentamicin non-susceptible | ||||||||||||||||||
| Wards | 199/306 | 65.0 | 261/337 | 77.4 | 303/371 | 81.7 | 267/328 | 81.4 | 270/335 | 80.6 | 340/421 | 80.8 | 330/393 | 84.0 | 395/448 | 88.2 | < 0.001 | ↑ |
| ICU | 474/610 | 77.7 | 548/658 | 83.3 | 512/585 | 87.5 | 427/466 | 91.6 | 457/491 | 93.1 | 462/488 | 94.7 | 447/480 | 93.1 | 399/426 | 93.7 | < 0.001 | ↑ |
| Ciprofloxacin non-susceptible | ||||||||||||||||||
| Wards | 264/298 | 88.6 | 308/336 | 91.7 | 338/370 | 91.4 | 298/326 | 91.4 | 315/338 | 93.2 | 381/421 | 90.5 | 360/394 | 91.4 | 426/445 | 95.7 | 0.009 | ↑ |
| Multiresistance | ||||||||||||||||||
| Wards | 206/291 | 70.8 | 215/285 | 75.4 | 245/329 | 74.5 | 226/297 | 76.1 | 240/314 | 76.4 | 292/388 | 75.3 | 273/339 | 80.5 | 338/404 | 83.7 | < 0.001 | ↑ |
| ICU | 482/590 | 81.7 | 447/551 | 81.1 | 403/502 | 80.3 | 372/422 | 88.2 | 390/438 | 89.0 | 385/429 | 89.7 | 366/393 | 93.1 | 297/331 | 89.7 | < 0.001 | ↑ |
| Staphylococcus aureus | ||||||||||||||||||
| Oxacillin non-susceptiblec | ||||||||||||||||||
| ICU | 43/90 | 47.8 | 57/104 | 54.8 | 58/99 | 58.6 | 41/74 | 55.4 | 34/74 | 45.9 | 27/57 | 47.4 | 30/65 | 46.2 | 25/75 | 33.3 | 0.01 | ↓ |
| Ciprofloxacin non-susceptible | ||||||||||||||||||
| Wards | 67/268 | 25.0 | 77/328 | 23.5 | 122/463 | 26.3 | 162/513 | 31.6 | 118/469 | 25.2 | 145/523 | 27.7 | 193/595 | 32.4 | 189/556 | 34.0 | 0.001 | ↑ |
| ICU | 26/49 | 53.1 | 40/74 | 54.1 | 48/85 | 56.5 | 27/65 | 41.5 | 23/67 | 34.3 | 21/54 | 38.9 | 22/59 | 37.3 | 20/70 | 28.6 | < 0.001 | ↓ |
| Gentamicin non-susceptible | ||||||||||||||||||
| Wards | 52/573 | 9.1 | 33/570 | 5.8 | 36/623 | 5.8 | 38/601 | 6.3 | 26/512 | 5.1 | 26/575 | 4.5 | 33/653 | 5.1 | 31/630 | 4.9 | 0.003 | ↓ |
| ICU | 26/98 | 26.5 | 28/104 | 26.9 | 29/99 | 29.3 | 19/72 | 26.4 | 6/76 | 7.9 | 8/57 | 14.0 | 4/65 | 6.2 | 5/75 | 6.7 | < 0.001 | ↓ |
| Multiresistance | ||||||||||||||||||
| Wards | 40/552 | 7.2 | 24/569 | 4.2 | 27/622 | 4.3 | 32/598 | 5.4 | 18/502 | 3.6 | 21/571 | 3.7 | 18/644 | 2.8 | 20/630 | 3.2 | < 0.001 | ↓ |
| ICU | 18/88 | 20.5 | 19/104 | 18.3 | 25/99 | 25.3 | 17/72 | 23.6 | 6/74 | 8.1 | 6/57 | 10.5 | 4/65 | 6.2 | 3/75 | 4.0 | < 0.001 | ↓ |
| Enterococcus faecalis | ||||||||||||||||||
| Gentamicin HLR | ||||||||||||||||||
| Wards | 128/341 | 37.5 | 118/342 | 34.5 | 100/403 | 24.8 | 70/360 | 19.4 | 65/326 | 19.9 | 48/348 | 13.8 | 65/379 | 17.2 | 47/417 | 11.3 | < 0.001 | ↓ |
| ICU | 85/173 | 49.1 | 60/190 | 31.6 | 52/195 | 26.7 | 33/147 | 22.4 | 25/125 | 20.0 | 16/138 | 11.6 | 29/183 | 15.8 | 17/143 | 11.9 | < 0.001 | ↓b |
| Streptomycin HLR | ||||||||||||||||||
| Wards | 145/325 | 44.6 | 118/325 | 36.3 | 84/390 | 21.5 | 54/323 | 16.7 | 51/287 | 17.8 | 39/318 | 12.3 | 46/342 | 13.5 | 35/383 | 9.1 | < 0.001 | ↓ |
| ICU | 89/171 | 52.0 | 65/182 | 35.7 | 63/194 | 32.5 | 24/136 | 17.6 | 14/110 | 12.7 | 12/128 | 9.4 | 13/157 | 8.3 | 10/130 | 7.7 | < 0.001 | ↓d |
| Enterococcus faecium | ||||||||||||||||||
| Gentamicin HLR | ||||||||||||||||||
| Wards | 121/240 | 50.4 | 94/214 | 43.9 | 77/235 | 32.8 | 63/236 | 26.7 | 41/204 | 20.1 | 33/215 | 15.3 | 57/255 | 22.4 | 43/279 | 18.9 | < 0.001 | ↓b |
| ICU | 67/123 | 54.5 | 41/107 | 38.3 | 27/111 | 24.3 | 25/95 | 26.3 | 16/77 | 20.8 | 8/84 | 9.5 | 21/102 | 20.6 | 22/92 | 16.9 | < 0.001 | ↓b |
| Streptomycin HLR | ||||||||||||||||||
| Wards | 145/226 | 64.2 | 113/205 | 55.1 | 69/224 | 30.8 | 63/220 | 28.6 | 43/183 | 23.5 | 50/201 | 24.9 | 66/238 | 27.7 | 54/268 | 20.1 | < 0.001 | ↓b |
| ICU | 73/122 | 59.8 | 43/103 | 41.7 | 36/111 | 32.4 | 24/88 | 27.3 | 14/69 | 20.3 | 12/80 | 15.0 | 17/91 | 18.7 | 24/81 | 29.6 | < 0.001 | ↓b |
AMR: antimicrobial resistance; HLR: high-level resistance; ICU: intensive care unit; NS: non-susceptible; NS/tested: number of non-susceptible isolates for an antibiotic divided by the number of isolates tested for the respective antibiotic; WHONET-Greece: Greek electronic surveillance system for monitoring antimicrobial resistance in hospitals based on routine data.
‘↑’ and ‘↓’ indicate significant increasing and decreasing trends, respectively.
a A significant trend was only observed in the last 4 years of the study (2014–2017).
b A significant trend was only observed in the first 4 years of the study (2010–2013).
c Oxacillin non-susceptibility is used to test for meticillin resistance in S. aureus (MRSA).
d A significant non-linear trend.
Escherichia coli
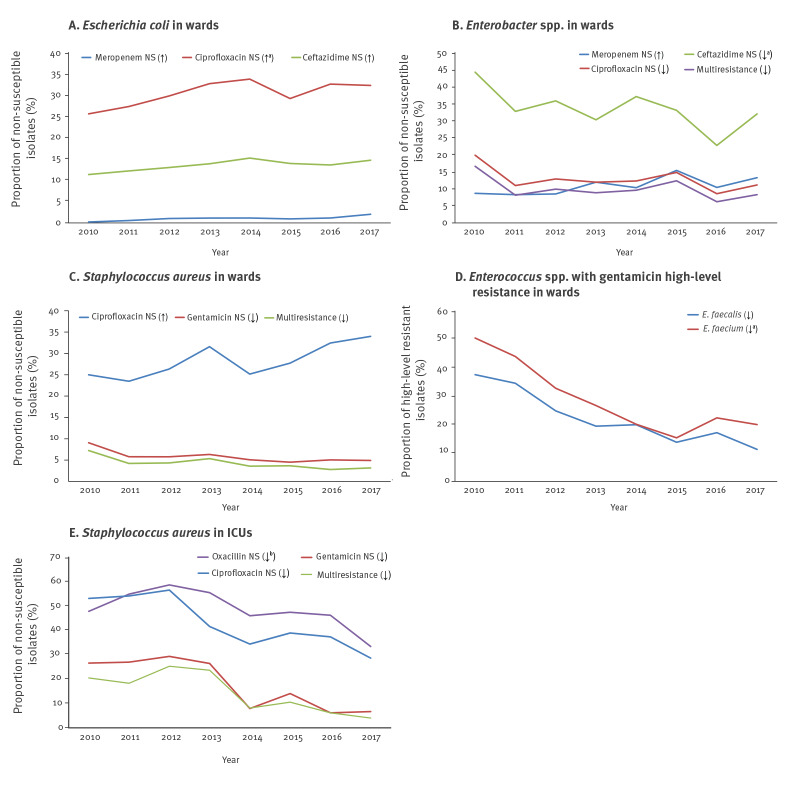
During the study period, for third-generation cephalosporins, the annual proportions of E. coli isolates non susceptible for ceftazidime from patients hospitalised in wards, significantly increased from 11.3% in 2010 to 14.7% in 2017 (p = 0.012). An apparent increase was also observed for rates of cefotaxime non-susceptible isolates, from 18.9% (190/1,005) in 2010 to 21.2% (215/1,013) in 2017. Meropenem non-susceptible isolates represented 0.2% of isolates in 2010 and their proportion increased significantly over time, reaching 2.0% in 2017 (p < 0.001). Moreover, an increasing trend in rates of isolates non-susceptible to ciprofloxacin was also observed (p < 0.001) starting at 25.6% in 2010 to reach 32.2% in 2017. On the contrary, the proportions of trimethoprim/sulfamethoxazole non-susceptible isolates decreased during the study period (p < 0.001). Regarding non-susceptibility to aminoglycosides, no trend was found for both gentamicin and tobramycin from 2010 to 2017.
Proteus mirabilis
Third-generation cephalosporins non-susceptibility among P. mirabilis isolates from patients hospitalised in the wards was 25.0% (39/156) in 2010, with an apparent increase to 34.4% (67/195) in 2013 and a decrease to 17.6% (36/204) in 2017, while in ICUs the rate of non-susceptible isolates was 52.3% (56/107) in 2010 reaching 65.3% (47/72) in 2017. No trend was observed in ciprofloxacin non-susceptibility, remaining stable at approximately 45% and above 58% in P. mirabilis isolates from patients hospitalised in wards and ICUs respectively. Regarding non-susceptibility to aminoglycosides, the proportions of gentamicin non-susceptible isolates increased from 18.6% (29/156) to 25.1% (51/203) (p = 0.03) and from 30.2% (32/106) to 54.9% (39/71) (p = 0.001) from 2010 to 2017 for patients hospitalised in wards and ICUs, respectively.
Enterobacter spp.
Rates of carbapenem non-susceptibility in Enterobacter spp. isolates from hospital wards showed increasing trends from 15.5% to 20.4% for imipenem and from 8.7% to 13.3% for meropenem in 2010 and 2017 respectively (both p = 0.04). On the contrary, decreasing trends were found for the proportions of isolates non susceptible to ceftazidime (from 44.1% to 31.9%, p = 0.001), tobramycin (from 32.7% to 14.7%, p = 0.001), ciprofloxacin (from 19.8% to 11.1%, p = 0.023) and with multiresistance (from 16.6% to 8.3%, p = 0.034) (Figure 3).
Figure 3.
Significant non-susceptibility trends concerning the main pathogen-antimicrobial combinations for (a) Escherichia coli, (b) Enterobacter spp., (c) Staphylococcus aureus, (d) Enterococcus faecalis and faecium in wards and (e) Staphylococcus aureus in ICUs, for patients hospitalised in the 25 hospitals participating in the WHONET-Greece AMR network, 2010–2017
AMR: antimicrobial resistance; ICU: intensive care unit; NS: non-susceptible; WHONET-Greece: Greek electronic surveillance system for monitoring antimicrobial resistance in hospitals based on routine data.
‘↑’ and ‘↓’ indicate significant increasing and decreasing trends, respectively.
a A significant trend was found only in the first 4 years of the study (2010–2013).
b Oxacillin non-susceptibility is used to test for meticillin resistance in S. aureus (MRSA).
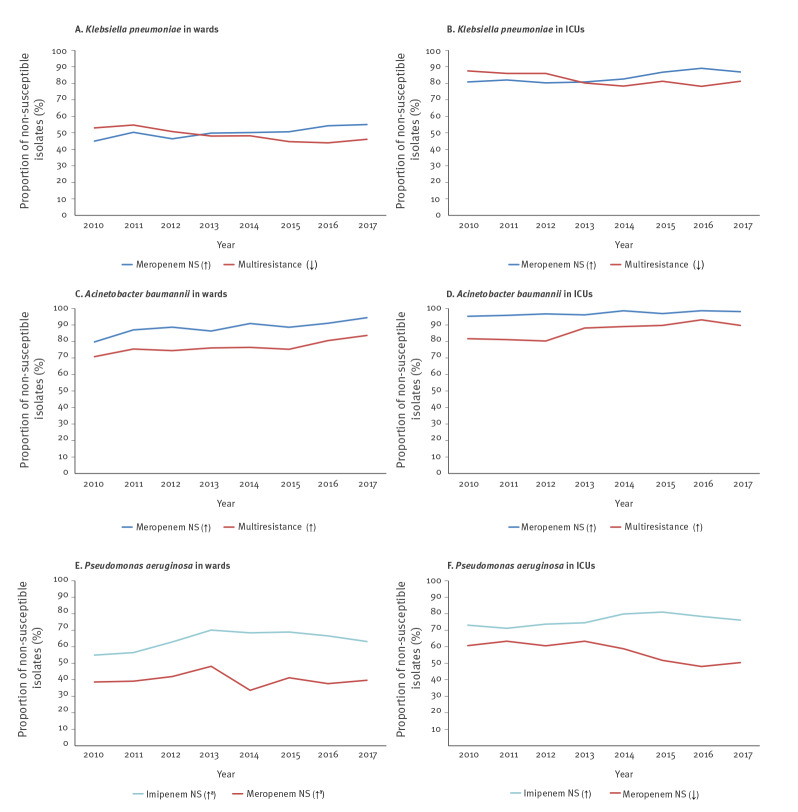
Klebsiella pneumoniae
Rates of meropenem non-susceptibility increased with proportions of isolates ranging from 45.0% to 55.0% and from 80.8% to 86.9% in patients hospitalised in wards and ICUs respectively from 2010 to 2017 (both p < 0.001). Regarding the percentages of imipenem non-susceptible isolates, we found a significant decreasing trend in ICUs during the first 4 years (from 88.2% to 80.6%, p < 0.001) and an increasing trend during the last four ones in wards (from 47.0% to 52.4%, p = 0.021). High proportions of isolates with ceftazidime non-susceptibility were observed; however, a decreasing trend in both wards (from 61.8% to 58.5%) and ICUs (from 92.6% to 88.6%) (p = 0.027 and p = 0.001, respectively) was found from 2010 to 2017. Non-susceptibility rates to fluoroquinolones did not change significantly over time for K. pneumoniae isolates from patients hospitalised in wards while a significant decrease was observed in isolates from ICU patients (p < 0.005). Regarding aminoglycosides, an increasing trend in rates of gentamicin non-susceptibility was found among isolates from both wards and ICUs (from 17.4% to 29.5% and from 27.2% to 49.9% respectively, both p < 0.001). On the contrary, a decreasing trend in rates of tobramycin non-susceptibility was observed among isolates from both wards and ICUs (both p < 0.001). Finally, multiresistance rates, meaning simultaneous non-susceptibility to ceftazidime, tobramycin and ciprofloxacin, decreased significantly over the studied years, from 53.0% to 46.1% among isolates from wards and from 87.5% to 81.3% among those from ICUs (both p < 0.001) (Figure 4).
Figure 4.
Significant non-susceptibility trends of the three main carbapenem-resistant Gram-negative pathogens from patients hospitalised in the wards and ICUs of the 25 hospitals participating in the WHONET-Greece AMR network, 2010–2017
AMR: antimicrobial resistance; ICU: intensive care unit; NS: non-susceptible; WHONET-Greece: Greek electronic surveillance system for monitoring antimicrobial resistance in hospitals based on routine data.
‘↑’ and ‘↓’ indicate significant increasing and decreasing trends, respectively.
a A significant trend was found only in the first 4 years of the study (2010–2013).
Pseudomonas aeruginosa
Decreasing trends were found in the proportions of non-susceptible P. aeruginosa isolates to almost all clinical relevant antibiotics with the exception of imipenem. In terms of carbapenems, the percentage of imipenem non-susceptible isolates significantly increased among isolates from patients hospitalised in wards (p < 0.001) and ICUs (p = 0.005), while proportions of non-susceptible meropenem isolates remained stable during the study period or even showed a decreasing trend in ICUs (from 60.7% to 50.4%, p < 0.001). The proportions of isolates with ceftazidime non-susceptibility also presented a decreasing trend in ICUs from 52.2% to 40.9% (p < 0.001).
Finally, the rates of isolates non-susceptible to fluoroquinolones and aminoglycosides were found to significantly decrease during the study period (both p < 0.001) (Figure 4).
Acinetobacter baumannii
From 2010 to 2017, the proportion of non-susceptible isolates to cefepime, imipenem, meropenem, aminoglycosides, as well as those with multiresistance increased significantly (all p < 0.001) in both wards and ICUs of the participating hospitals. Of note, the proportion of carbapenem resistant isolates was consistently very high in both wards and ICUs during the whole study period, ranging for meropenem from 79.7% and 95.3% in 2010 to 94.4% and 98.1% in 2017 in wards and ICUs respectively (Figure 4).
Staphylococcus aureus
A significant decreasing trend was observed in the proportion of meticillin-resistant S. aureus (MRSA) from ICU patients, ranging from 47.8% in 2010 to 33.3% in 2017 (p = 0.01) as well as for ciprofloxacin, ranging from 53.1% in 2010 to 28.6% in 2017 (p < 0.001) (Figure 3e). In contrast, an increasing trend was found for the rates of non-susceptibility to ciprofloxacin in S. aureus isolates from patients hospitalised in wards, ranging from 25.0% in 2010 to 34.0% in 2017 (p = 0.001). Finally, the proportions of isolates with non-susceptibility to gentamicin as well as with a combined non-susceptibility to meticillin and gentamicin were significantly decreased in both wards and ICUs (all p < 0.004), (Figure 3c,e).
Enterococcus faecalis and Enterococcus faecium
Regarding rates of isolates with HLR to gentamicin and streptomycin, decreasing trends were observed among both E. faecalis and E. faecium isolates (Figure 3d). The proportions of isolates non-susceptible to vancomycin among E. faecalis bloodstream isolates from patients in ICUs decreased from 7.3% (13/179) to 1.4% (2/148) from 2010 to 2017. The rates of isolates non-susceptible to vancomycin from 2010 to 2017 among E. faecium isolates was found at similar levels in wards (21.6% (53/245) to 28.4% (76/268)) and ICUs (22.7% (29/128) to 28.4% (31/109)), however, no linear increasing trend was found in either hospital departments.
Discussion
We analysed the RIF and the routine susceptibility data for 50,488 key bloodstream isolates recovered from patients hospitalised in wards and ICUs of 25 hospitals participating in the WHONET-Greece AMR surveillance network during an 8-year period and report several major findings regarding the observed trends over time.
E. coli was the most common cause of bloodstream infection (BSI) in patients hospitalised in wards. The high proportion of isolates with non-susceptibility to third-generation cephalosporins with an increasing trend over the years, coupled with similar trends for proportions of isolates with non-susceptibility to fluoroquinolones is a serious concern since prompt administration of an effective empirical antimicrobial treatment is essential at both patient and public health level. Moreover, over the years, an increasing trend was observed for the proportion of ward-patient E. coli isolates, which were non-susceptible to carbapenems. According to the results of the European Survey on Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) published in 2017 [5] carbapenemase-producing E. coli are becoming more widespread in Europe, thus requiring close surveillance. Acquisition of carbapenemase genes by E. coli [5-12] is concerning, since E. coli spreads in the community more readily than K. pneumoniae. Moreover E. coli from the digestive tract of asymptomatic carriers are common vectors of promiscuous plasmids, which could also accelerate spread of resistance [5].
The observed significant increasing trend of non-susceptible ciprofloxacin isolates is possibly consistent with further dissemination of sequence type (ST)131 strains which Mavroidi et al. found [13] responsible for the increasing fluoroquinolone resistance during 2011 in Central Greece. E. coli ST131 is associated with human urinary tract infections and BSIs and was first described in 2008 as a major clone linked to the spread of extended-spectrum beta-lactamase CTX-M-15. It has since disseminated worldwide and strains resistant to fluoroquinolones, aminoglycosides, trimethoprim-sulfamethoxazole and carbapenems have been reported, limiting treatment options for this pathogen [13,14].
Regarding K. pneumoniae, the major finding was the increasing trend in the proportion of isolates with meropenem non-susceptibility, not only in the ICUs but also in wards of the participating hospitals. This finding may reflect the evolving molecular epidemiology of carbapenemase-producing K. pneumoniae. It is well documented that Greece has been facing high rates of carbapenem resistance among clinical K. pneumoniae since 2002. This was initially due to a multiclonal plasmid-mediated epidemic of Verona Integron-encoded Metallo-beta-lactamase (VIM)-producers during the period 2002–2007 [15]. From 2007, the introduction and rapid clonal dissemination of mainly ST258 K. pneumoniae carbapenemase (KPC)-producers occurred [16,17]. KPC has since been the predominant carbapenemase among the hospital K. pneumoniae population. However, starting 2011, K. pneumoniae ST11 producing New Delhi Metallo-beta-lactamase (NDM)-1 emerged in the country and was identified retrospectively as the cause of a prolonged outbreak of healthcare-associated infections in a University hospital at North-Western Greece [18]. Two years later, NDM-1 producers belonging to ST11 were reported in Athens [19]. Of note, soon after the introduction of NDM in the country, the EuSCAPE project conducted between November 2013 and April 2014 in 10 hospitals all over Greece found it to rank as the second most frequent carbapenemase [5]. This was also reported by a subsequent nationwide multicentre study during the period 2014–2016 [20], indicating further dissemination. Moreover, according to a report published in 2015 [21], while KPC-2 production provides Enterobacteriaceae with intermediate resistance or even decreased susceptibility to meropenem in many cases, NDM-1 production allows HLR to meropenem. Regarding Oxacillinase (OXA)-48-carbapenemase, in 2012 an outbreak of OXA-48 carbapenemase-producing K. pneumoniae ST11 was recorded for the first time in the country [22]. Nevertheless, no major epidemics of OXA-48 producing K. pneumoniae were recorded since their introduction in Greece and OXA-48 remained rare during the period 2014–2016 [20]. The ongoing carbapenem and/or colistin-resistant Enterobacteriaceae (CCRE) project as part of the European Antimicrobial Resistance Genes Surveillance Network (EURGen-Net) will provide updated and more detailed information on the distribution of carbapenemase-producing K. pneumoniae in Greece as well as in Europe.
The observed increasing trend in the proportion of K. pneumoniae isolates non susceptible to gentamicin over the study period in both wards and ICUs, reaching respectively up to 30% and 50%, is of concern, since gentamicin was found to be the most active in vitro aminoglycoside in clinical use, among the four available (amikacin, gentamicin, tobramycin and netilmicin) in a nationwide multicentre study between 2014 and 2016 of 300 carbapenem-resistant K. pneumoniae isolates from Greek hospitals [23].
For P. aeruginosa, the decreasing trends in rates of isolates with non-susceptibility for all of the studied antibiotics except imipenem, for which an increasing non-susceptibility trend was found, could reflect the decrease in isolation of VIM-producing P. aeruginosa, in the last 20 years [24,25] and the increasing role of non-enzymatic mechanisms of carbapenem resistance among this bacterial population. Specifically, the inactivation, downregulation, or even loss of OprD porin is well documented to confer resistance only to imipenem [26,27]. The possible relative decreasing proportion of VIM producers among the P. aeruginosa carbapenem-resistant population could affect also other antibiotic classes like aminoglycosides and fluoroquinolones since VIM producers commonly exhibit multidrug resistant phenotypes and thus could explain the overall decreasing trend we found for aminoglycosides and quinolones.
With respect to A. baumannii, the observed significant increasing trend in its RIF in the wards and its predominance in the ICUs of the participating hospitals, coupled with increasing trends in the proportion of isolates non-susceptible to almost all antimicrobials and especially to carbapenems is of outstanding importance. However, more concerning are reports of colistin-resistant/carbapenem-resistant A. baumannii isolates which constitute a great challenge for both clinical practice and public health [28]. Molecular epidemiology studies at the national level have revealed that since 2010, OXA-23 producing A. baumannii appeared and further disseminated in Greece replacing the former endemic OXA-58 producers and displaying higher minimum inhibitory concentrations (MIC)s to carbapenems due to their higher hydrolytic activity [29]. The latter characteristic has been considered as a comparative advantage to survive and predominate in the hospital setting [30]. A nationwide study from 2015 confirmed that carbapenem-resistant A. baumannii isolates in Greek hospitals produce almost exclusively the OXA-23 carbapenemase and they belong mainly to the international clone (IC) 2 and to a lesser extent, IC1 [29].
For S. aureus, the decline during the study period in the percentage of MRSA is in alignment with the situation in a majority of European Union (EU)/European Economic Area (EEA) countries where MRSA percentages seem to be stabilising or even decreasing [31]. The decline has been reported for an even larger time period (2000–2015) from a study of MRSA BSI cases in a big University hospital in Greece, where the authors reported a decreasing trend in the incidence of MRSA BSIs from 1.69 per 10,000 patient days in 2000 to 1.39 per 10,000 patient days in 2015 (p = 0.038) and in prevalence from 64.7% to 36.4% (p = 0.008), respectively. The observed decline in MRSA BSI rates was associated with changes in the population structure of the organism; the pandemic healthcare-acquired (HA)-MRSA clone ST239-III progressively declined, parallel to the increased isolation frequency of two clonal complexes (CCs): HA-MRSA CC5 and CA-MRSA CC80 [32].
Regarding enterococci, Greece is among the European countries with the lowest percentages in both E. faecalis and E. faecium of gentamicin HLR, at least since 2015 (https://atlas.ecdc.europa.eu). Furthermore, the observed significant decreasing trend during our study period is in accordance with that of the EU/EEA between 2014 and 2017 as well as the trend from almost one fourth of the countries that report national AMR data in EARS-net [31]. On the other hand, the observed level of E. faecium non-susceptibility to vancomycin, even without a significant trend over time, is a cause of concern and highlights the need for close monitoring. Contrary to many other bacterium–antimicrobial group combinations under surveillance by EARS-Net, no distinct geographical pattern could be seen for vancomycin-resistant E. faecium, as high resistance levels were reported from countries in southern, eastern and northern Europe [31].
As it is clearly indicated by the aforementioned data, AMR is a dynamic phenomenon with new resistance mechanisms or combination of resistance mechanisms that can emerge or be introduced and be disseminated rapidly in different bacterial species. In this respect, the Greek continuous electronic AMR surveillance system, focusing on the overall epidemiology of susceptible and resistant bacteria and assisted by an early warning system for new or important resistance phenotypes has developed into a successful, cost-effective tool for the surveillance of AMR in the country.
In more details, during the 2000s, in order to trace the emergence and spread of new resistance mechanisms and to further characterise them, each hospital laboratory had to report immediately through the early warning system certain new or important resistant phenotypes to both the Hospital Infection Control Committee and the Greek Centre for Diseases Control and Prevention and also to send the isolates for further testing in the relevant reference laboratories. It was this surveillance process that enabled Greek Public Health authorities to timely identify the emergence of carbapenem resistant Gram-negative pathogens and especially VIM-producing P. aeruginosa [25], VIM- [33] and KPC-producing K. pneumoniae [16,17] in the hospital setting. During the 2010s, through the National Action Plan for the prevention and control of nosocomial infections caused by carbapenem resistant Gram-negative pathogens in healthcare settings (the PROKROUSTIS project) and subsequently the relevant national legislation for the surveillance of healthcare-associated infections, protocols for sending important isolates to the national surveillance centres for confirmation and characterisation have been implemented. This process enabled us to timely identify the new entry of NDM and OXA-48 carbapenemases in the country [18,19,22] as well as OXA-23 in A. baumannii [28-30]. Overall, since 1995, WHONET-Greece has been providing valuable background information on the evolution of the complex epidemiology of AMR to be used in developing the strategy to combat this problem at the hospital, regional and national level.
This study has some limitations. The first may result from the potential impact of biases in sampling practices of routine clinical diagnostic data, since, in general, routine clinical data would often overestimate resistance because of the tendency to culture specimens from patients with treatment failures and/or complicated medical histories. This problem could be further enhanced by selective culturing and antimicrobial susceptibility testing due to the ongoing financial crisis in Greece. However, the biases are consistent from year to year. Moreover, the level of financial crisis, in terms of hospital budget cuts, is not as extensive as to affect critical diagnostic procedures, such as blood culturing (data not shown). Therefore one may still identify important temporal trends using routine AMR data from bacteraemias. Another possible limitation is that, while colistin has been increasingly used since 2010 in Greece as a fundamental companion drug for the treatment of the carbapenem-resistant Enterobacteriaceae P. aeruginosa and A. baumannii, we could neither study colistin non-susceptibility prevalence nor its evolution over time in the period 2010–2017. This was mainly due to technical challenges of testing colistin susceptibility with the gradient tests or semiautomatic instruments used in the hospitals’ laboratories during the study. However, based on the recently issued recommendations from CLSI and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [34], the Greek National Antibiogram Committee (NAC) now advises hospitals to use broth microdilution as the gold-standard method for colistin MIC determination, and the increasing compliance of hospital laboratories will likely enable the WHONET-Greece system to study colistin non-susceptibility trends prospectively in the forthcoming years. A third limitation is that we could not take some important data under consideration in our analysis, such as dates of hospital admission and/or discharge, details on the treatment received by patients as well as clinical outcomes. Indeed, at present, Greek hospital clinical laboratories do not have direct access to patients’ data even if basic patient demographic data are available. This limitation could be solved by linking routine microbiology data with other existing relevant datasets in the hospital, as part of the overall national strategy to upgrade health information systems.
To achieve this, as well as a comprehensive AMR surveillance system in the country, the WHONET software could be used as the basic tool for all microbiology laboratories to input their data. As all laboratories enter or transfer their reports into WHONET, the resulting files will be inter-compatible and will enable compiling to inform both national and international multicentre surveillance networks [35] without any additional microbiology staff’s dedicated time.
Monitoring AMR trends is an indiscernible means of assessing and possibly modifying the implemented national initiatives and interventions according to the recent national legislation. Moreover, timely and targeted dissemination of national surveillance data to all major stakeholders at European level should become an essential component of efforts to control the threat of AMR in European Union and European Economic Area countries and reduce its burden. In this context, we have found the use of routine hospital laboratory data from the Greek continuous electronic AMR surveillance system, to be important not only at hospital and national level but European level as well.
Acknowledgements
The authors acknowledge the work performed by the staff of the participating clinical microbiology laboratories.
WHONET-Greece study group affiliations of authors
“Agios Pavlos” General Hospital, Thessaloniki, A Koteli, P Fitas; “Asclepeion Voulas” General Hospital, Attiki, M Economou, C Konsolakis; “Evangelismos” General Hospital, Athens, Κ Fountoulis, E Perivolioti; “G Gennimatas” General Hospital, Athens, H Vagiakou, G Ganteris; “G. Papanikolaou” General Hospital, Thessaloniki, E Tsorlini; “Ippokrateio” General Hospital of Thessaloniki, Thessaloniki, E Vagdatli, A Karantani; “KAT” General Hospital, Athens, S Tsiplakou, V Papaioannou; “Laikon” General Hospital, Athens, G Maropoulos, I Deliolanis; “P & A Kyriakou” Children's Hospital, Athens, E Lebessi, A Doudoulakakis; “Red Cross” General Hospital, Athens, V Baka, A Platania; “Saint Savas” Anticancer Hospital, Athens, E Chinou; “Sismanoglion” General Hospital, Athens, M Martsoukou, K Velentza; “Sotiria” General Hospital of Chest Diseases, Athens, S Karabela, ES Moraitou; “ Theageneio” Anticancer Hospital, Thessaloniki, P Kazila; “Tzaneio” General Hospital, Piraeus, K Digalaki, O Zarkotou; General Hospital of Chania, Crete (Hania), M Papadogianni, K Zervaki; General Hospital of Kavala “Agios Silas”, Kavala, I Karatzoglou; General Hospital of N Ionia “Konstantopouleio-Patision”, Athens, E Platsouka, Z Roussou; General Hospital of Serres, Serres, F Markou; General Hospital of Veria, Veria, V Thomoglou; General Hospital of Xanthi, Xanthi, G Stamatopoulou; Naval and Veterans Hospital of Athens, Athens, E Mournianakis, I Theodorakos; University Hospital “AHEPA”, Thessaloniki, L Skoura, E Protonotariou; University Hospital of Alexandroupolis, Alexandroupoli, M Panopoulou, A Koutsidou; University Hospital of Larissa, Larissa, E Petinaki, A Vasdeki.
Conflict of interest: None declared.
Authors’ contributions: M. Polemis & K. Tryfinopoulou contributed equally in this paper.
MP managed the database, performed all data analyses and reviewed the manuscript.
KT contributed in the interpretation of the data, wrote the manuscript’s first draft and revisions.
PG reviewed the manuscript.
AV designed and coordinated the analysis, and reviewed the manuscript.
The WHONET-Greece study group provided routine susceptibility data.
All authors discussed the results and approved the final manuscript.
References
- 1.World Health Organization (WHO). Global action plan on antimicrobial resistance. Geneva: WHO; 2015. Available from: http://www.who.int/antimicrobial-resistance/publications/http://www.who.int/antimicrobial-resistance/publications/ [DOI] [PubMed]
- 2. Simonsen GS. Antimicrobial resistance surveillance in Europe and beyond. Euro Surveill. 2018;23(42):1800560. 10.2807/1560-7917.ES.2018.23.42.1800560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vatopoulos AC, Kalapothaki V, Legakis NJ, The Greek Network for the Surveillance of Antimicrobial Resistance An electronic network for the surveillance of antimicrobial resistance in bacterial nosocomial isolates in Greece. Bull World Health Organ. 1999;77(7):595-601. [PMC free article] [PubMed] [Google Scholar]
- 4. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 5. Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, et al. European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017;17(2):153-63. 10.1016/S1473-3099(16)30257-2 [DOI] [PubMed] [Google Scholar]
- 6. Miriagou V, Tzelepi E, Gianneli D, Tzouvelekis LS. Escherichia coli with a self-transferable, multiresistant plasmid coding for metallo-beta-lactamase VIM-1. Antimicrob Agents Chemother. 2003;47(1):395-7. 10.1128/AAC.47.1.395-397.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scoulica EV, Neonakis IK, Gikas AI, Tselentis YJ. Spread of bla(VIM-1)-producing E. coli in a university hospital in Greece. Genetic analysis of the integron carrying the bla(VIM-1) metallo-beta-lactamase gene. Diagn Microbiol Infect Dis. 2004;48(3):167-72. 10.1016/j.diagmicrobio.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 8. Galani I, Souli M, Chryssouli Z, Katsala D, Giamarellou H. First identification of an Escherichia coli clinical isolate producing both metallo-beta-lactamase VIM-2 and extended-spectrum beta-lactamase IBC-1. Clin Microbiol Infect. 2004;10(8):757-60. 10.1111/j.1469-0691.2004.00913.x [DOI] [PubMed] [Google Scholar]
- 9. Tsakris A, Poulou A, Themeli-Digalaki K, Voulgari E, Pittaras T, Sofianou D, et al. Use of boronic acid disk tests to detect extended- spectrum beta-lactamases in clinical isolates of KPC carbapenemase-possessing enterobacteriaceae. J Clin Microbiol. 2009;47(11):3420-6. 10.1128/JCM.01314-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mavroidi A, Miriagou V, Malli E, Stefos A, Dalekos GN, Tzouvelekis LS, et al. Emergence of Escherichia coli sequence type 410 (ST410) with KPC-2 β-lactamase. Int J Antimicrob Agents. 2012;39(3):247-50. 10.1016/j.ijantimicag.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 11. Papagiannitsis CC, Bitar I, Malli E, Tsilipounidaki K, Hrabak J, Petinaki E. IncC blaKPC-2-positive plasmid characterised from ST648 Escherichia coli. J Glob Antimicrob Resist. 2019;19:73-7. 10.1016/j.jgar.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 12. Tsakris A, Poulou A, Bogaerts P, Dimitroulia E, Pournaras S, Glupczynski Y. Evaluation of a new phenotypic OXA-48 disk test for differentiation of OXA-48 carbapenemase-producing Enterobacteriaceae clinical isolates. J Clin Microbiol. 2015;53(4):1245-51. 10.1128/JCM.03318-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mavroidi A, Miriagou V, Liakopoulos A, Tzelepi Ε, Stefos A, Dalekos GN, et al. Ciprofloxacin-resistant Escherichia coli in Central Greece: mechanisms of resistance and molecular identification. BMC Infect Dis. 2012;12(1):371. 10.1186/1471-2334-12-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27(3):543-74. 10.1128/CMR.00125-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vatopoulos A. High rates of metallo-beta-lactamase-producing Klebsiella pneumoniae in Greece--a review of the current evidence. Euro Surveill. 2008;13(4):8023. [PubMed] [Google Scholar]
- 16. Maltezou HC, Giakkoupi P, Maragos A, Bolikas M, Raftopoulos V, Papahatzaki H, et al. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece). J Infect. 2009;58(3):213-9. 10.1016/j.jinf.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 17. Giakkoupi P, Maltezou H, Polemis M, Pappa O, Saroglou G, Vatopoulos A, Greek System for the Surveillance of Antimicrobial Resistance KPC-2-producing Klebsiella pneumoniae infections in Greek hospitals are mainly due to a hyperepidemic clone. Euro Surveill. 2009;14(21):19218. 10.2807/ese.14.21.19218-en [DOI] [PubMed] [Google Scholar]
- 18. Voulgari E, Gartzonika C, Vrioni G, Politi L, Priavali E, Levidiotou-Stefanou S, et al. The Balkan region: NDM-1-producing Klebsiella pneumoniae ST11 clonal strain causing outbreaks in Greece. J Antimicrob Chemother. 2014;69(8):2091-7. 10.1093/jac/dku105 [DOI] [PubMed] [Google Scholar]
- 19. Giakkoupi P, Tryfinopoulou K, Kontopidou F, Tsonou P, Golegou T, Souki H, et al. Emergence of NDM-producing Klebsiella pneumoniae in Greece. Diagn Microbiol Infect Dis. 2013;77(4):382-4. 10.1016/j.diagmicrobio.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 20. Galani I, Karaiskos I, Karantani I, Papoutsaki V, Maraki S, Papaioannou V, et al. On Behalf Of The Study Collaborators Epidemiology and resistance phenotypes of carbapenemase-producing Klebsiella pneumoniae in Greece, 2014 to 2016. Euro Surveill. 2018;23(31):1700775. 10.2807/1560-7917.ES.2018.23.30.1700775 [DOI] [PubMed] [Google Scholar]
- 21. Fattouh R, Tijet N, McGeer A, Poutanen SM, Melano RG, Patel SN. What Is the Appropriate Meropenem MIC for Screening of Carbapenemase-Producing Enterobacteriaceae in Low-Prevalence Settings? Antimicrob Agents Chemother. 2016;60(3):1556-9. 10.1128/AAC.02304-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voulgari E, Zarkotou O, Ranellou K, Karageorgopoulos DE, Vrioni G, Mamali V, et al. Outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in Greece involving an ST11 clone. J Antimicrob Chemother. 2013;68(1):84-8. 10.1093/jac/dks356 [DOI] [PubMed] [Google Scholar]
- 23. Galani I, Nafplioti K, Adamou P, Karaiskos I, Giamarellou H, Souli M, Study Collaborators Nationwide epidemiology of carbapenem resistant Klebsiella pneumoniae isolates from Greek hospitals, with regards to plazomicin and aminoglycoside resistance. BMC Infect Dis. 2019;19(1):167. 10.1186/s12879-019-3801-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsakris A, Pournaras S, Woodford N, Palepou MF, Babini GS, Douboyas J, et al. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J Clin Microbiol. 2000;38(3):1290-2. 10.1128/JCM.38.3.1290-1292.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giakkoupi P, Petrikkos G, Tzouvelekis LS, Tsonas S, Legakis NJ, Vatopoulos AC, WHONET Greece Study Group Spread of integron-associated VIM-type metallo-beta-lactamase genes among imipenem-nonsusceptible Pseudomonas aeruginosa strains in Greek hospitals. J Clin Microbiol. 2003;41(2):822-5. 10.1128/JCM.41.2.822-825.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meletis G, Vavatsi N, Exindari M, Protonotariou E, Sianou E, Haitoglou C, et al. Accumulation of carbapenem resistance mechanisms in VIM-2-producing Pseudomonas aeruginosa under selective pressure. Eur J Clin Microbiol Infect Dis. 2014;33(2):253-8. 10.1007/s10096-013-1952-3 [DOI] [PubMed] [Google Scholar]
- 27. Liakopoulos A, Mavroidi A, Katsifas EA, Theodosiou A, Karagouni AD, Miriagou V, et al. Carbapenemase-producing Pseudomonas aeruginosa from central Greece: molecular epidemiology and genetic analysis of class I integrons. BMC Infect Dis. 2013;13(1):505. 10.1186/1471-2334-13-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oikonomou O, Sarrou S, Papagiannitsis CC, Georgiadou S, Mantzarlis K, Zakynthinos E, et al. Rapid dissemination of colistin and carbapenem resistant Acinetobacter baumannii in Central Greece: mechanisms of resistance, molecular identification and epidemiological data. BMC Infect Dis. 2015;15(1):559. 10.1186/s12879-015-1297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pournaras S, Dafopoulou K, Del Franco M, Zarkotou O, Dimitroulia E, Protonotariou E, et al. Greek Study Group on Acinetobacter Antimicrobial Resistance Predominance of international clone 2 OXA-23-producing-Acinetobacter baumannii clinical isolates in Greece, 2015: results of a nationwide study. Int J Antimicrob Agents. 2017;49(6):749-53. 10.1016/j.ijantimicag.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 30. Liakopoulos A, Miriagou V, Katsifas EA, Karagouni AD, Daikos GL, Tzouvelekis LS, et al. Identification of OXA-23-producing Acinetobacter baumannii in Greece, 2010 to 2011. Euro Surveill. 2012;17(11):20117. [PubMed] [Google Scholar]
- 31.European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe – Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017. Stockholm: ECDC; 2018. [Google Scholar]
- 32. Nikolaras GP, Papaparaskevas J, Samarkos M, Tzouvelekis LS, Psychogiou M, Pavlopoulou I, et al. Changes in the rates and population structure of methicillin-resistant Staphylococcus aureus (MRSA) from bloodstream infections: A single-centre experience (2000-2015). J Glob Antimicrob Resist. 2019;17:117-22. 10.1016/j.jgar.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 33. Giakkoupi P, Xanthaki A, Kanelopoulou M, Vlahaki A, Miriagou V, Kontou S, et al. VIM-1 Metallo-β-lactamase-producing Klebsiella pneumoniae strains in Greek hospitals. J Clin Microbiol. 2003;41(8):3893-6. 10.1128/JCM.41.8.3893-3896.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group. EUCAST; 2016. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf
- 35. O’Brien TF, Stelling J. The world’s microbiology laboratories can be a global microbial sensor network. Biomedica. 2014;34(0) Suppl 1;9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]