Abstract
Purpose:
Gabapentinoids are commonly prescribed for the treatment of neuropathic pain but are not recommended for the primary treatment of carpal tunnel syndrome (CTS). We sought (1) to investigate the preoperative use of gabapentinoids for the treatment of CTS and (2) to determine if preoperative exposure is associated with persistent gabapentinoid and opioid use after carpal tunnel release.
Methods:
We performed a retrospective cohort study using IBM MarketScan Research Databases (2010-2017) of patients who did not fill a gabapentinoid or opioid prescription within three months of a new CTS diagnosis undergoing surgical release. Our primary outcomes included preoperative gabapentinoid prescription fills associated with CTS and persistent prescription fills of gabapentinoids and opioids at 91-180 days postoperatively. Multivariable logistic regression models were used to evaluate the association between patient-level factors and persistent gabapentinoid and opioid use.
Results:
Of the 56,593 patients without a previous gabapentinoid or opioid prescription prior to diagnosis of CTS, 3,474 patients (6%) filled a gabapentinoid before carpal tunnel release. Overall, 835 patients (24% of the preoperative users) continued to fill gabapentinoid prescriptions at 91-180 days postoperatively. Of the preoperative gabapentinoid users, 20% (702 patients) continued to fill opioid prescriptions at 91-180 days after release. After adjusting for patient characteristics, preoperative gabapentinoid use was associated with increased odds of persistent postoperative gabapentinoid use (preoperative gabapentinoid: 22% adjusted probability (95%CI: 20.3-23.0%), no preoperative gabapentinoid use: 1% (95%CI: 1.2-1.4%)) and persistent postoperative opioid use (preoperative gabapentinoid: 18% adjusted probability (95%CI: 17-20%), no preoperative gabapentinoid: 9% (95%CI: 8.6-9.1)).
Conclusion:
Despite a lack of evidence to support the use of gabapentinoids for CTS, 6% of patients are prescribed a gabapentinoid prior to surgery, and prolonged use is common. Given the effectiveness of surgical release and the risks associated with gabapentinoids, greater attention is needed to ensure that gabapentinoids are prescribed appropriately, avoided when possible, and stopped postoperatively.
Level of Evidence:
II/Prognostic
Keywords: carpal tunnel syndrome, gabapentinoids, opioids
Introduction
The opioid epidemic has driven providers to pursue other analgesic options, including gabapentinoids. However, these new regimens also confer risks; patients who are prescribed gabapentin and opioids together have a 50% increase risk of dying from an opioid-related death as compared to patients who are prescribed opioids alone.1 Indeed, gabapentinoids (gabapentin and pregabalin) have addictive potential, specifically for patients with substance use disorders and patients who are concomitantly using opioids.2,3 In response, states have begun to regulate gabapentinoids, specifying these medications as controlled substances and included in prescription drug monitoring programs.4,5
The Food and Drug Administration (FDA) approved gabapentinoids for use in patients with post-herpetic neuralgia, diabetic neuropathy, fibromyalgia, and pain after spinal cord injury,6–8 but they are often prescribed off-label for many conditions, including for carpal tunnel syndrome (CTS).8–11 CTS is one of the most common musculoskeletal disorders and peripheral neuropathies, affecting approximately 4% of the general population and causes numbness, pain, and paresthesias of the thumb, index, and middle fingers.12,13 Prolonged compression of the median nerve because of CTS can lead to permanent sensory changes in the hand, substantial hand weakness, and atrophy of the musculature controlling thumb movement. Management of CTS generally consists of either non-surgical measures including activity modification, splinting, and steroid injections or operative intervention with open or endoscopic carpal tunnel release.14 However, gabapentinoids have a limited role in the primary treatment of CTS, randomized controlled trials have shown no benefit of gabapentinoids over placebo and guidelines recommend avoiding gabapentinoids in the treatment of CTS.14,15
There are no national data regarding how many patients are being prescribed gabapentinoids for the treatment of CTS and whether these medications are stopped after definitive surgical intervention. For CTS, where there is robust evidence supporting the recommendation to avoid gabapentinoids for treatment, the consequences of preoperative prescribing of these medications have substantial clinical and policy implications. Therefore, using a population-based analysis, we sought to examine the preoperative use of gabapentinoids for CTS. We also aimed to determine the effect of preoperative gabapentinoid use on persistent postoperative use of gabapentinoids and opioids after carpal tunnel release.
Materials and Methods
Data Source
We selected patients from the IBM MarketScan Research Databases, including the Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database, from 2010 to 2017. These databases are a national data set of employer-based health insurance claims and include over 37 billion insurance claims covering over 250 million individuals and capture data on patient-specific inpatient and outpatient clinical utilization, insurance coverage, outpatient prescription drugs, and cost data.16 This study qualified for exempt status from the Institutional Review Board at the University of Michigan because all data were de-identified.
Cohort Selection
We included patients, between the ages of 18 and 64, with a new diagnosis of CTS who underwent outpatient carpal tunnel release from January 1, 2010 until June 30, 2017. To ensure a new diagnosis of CTS and to assess for pre-diagnosis comorbidities, we used a one-year pre-diagnosis window and confirmed continuous enrollment in the database during this period. We used a new CTS diagnosis to remove patients with recurrent carpal tunnel syndrome, as they are more complex and may have other associated nerve disorders. Our cohort was comprised of patients who lacked any opioid or gabapentinoid prescriptions filled in the three months prior to the index claim linked to the diagnosis of CTS. Gabapentinoids encompassed gabapentin and pregabalin. Gabapentinoid and opioid fills were identified by National Drug codes from pharmaceutical claims. We identified patients using International Classification of Disease, Ninth Revision (ICD-9) diagnosis codes, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes (ICD-10), and Current Procedural Terminology (CPT) codes (Appendix A). We also specified that patients had continuous enrollment in the database for six-months after surgery to assess for filling of postoperative prescriptions. We excluded all patients with diagnoses of distal radius or ulna fractures, ulnar neuropathy, and nerve injuries, or patients who underwent surgical reduction of a distal radius or ulna fracture, repair of a nerve, cubital or Guyon canal release, or a fasciotomy in the year prior to CTS diagnosis. Patients were also excluded if they underwent another procedure, identified by anesthesia codes, within the six months after their index carpal tunnel release to ensure that no additional opioid or gabapentinoid prescription was filled because of another surgery. Additionally, this restriction removed any patients that were not satisfied with initial carpal tunnel release and had repeat carpal tunnel release. Appendix B demonstrates the inclusion and exclusion criteria for this study.
Outcome Variables
Our primary outcome of interest was preoperative gabapentinoid fills for the treatment of carpal tunnel syndrome. Our secondary outcomes included the persistent use of gabapentinoids and opioids following carpal tunnel release. Persistent use was defined as a prescription fill of a gabapentinoid or opioid 91-180 days after surgery, which was determined prior to analysis. This is a more conservative definition compared to the three-month postsurgical new persistent use definition by International Association for the Study of Pain.17 Like other surgical studies investigating persistent opioid use, we adopted a similar definition of persistent use of opioids and gabapentinoids because acute pain following surgical procedures is expected to resolve within 90 days postoperatively.18–20 Moreover, we purposely did not want to capture perioperative gabapentinoid use, which may be used at the time of surgery or immediately afterwards to decrease postoperative opioid use,21,22 and does not adequately reflect preoperative or persistent postoperative use of gabapentinoids. To understand the size of prescription fills, we then calculated the average number of 300mg gabapentin and 25mg pregabalin pills during the 91-180 days postoperatively. For opioids, we converted all opioid prescriptions into oral morphine equivalents and then translated these prescriptions into 5mg hydrocodone equivalents and calculated the average number of pills prescribed during the 91-180 days postoperatively.
Explanatory Variables
Our primary explanatory variable was preoperative gabapentinoid use, which was defined as a new prescription between the initial diagnosis of CTS and carpal tunnel release. We ensured that a clinical visit linked with a new diagnosis code for CTS was conducted within one week of the prescription fill. We obtained sociodemographic data, comorbidities, and preoperative gabapentinoid use. Demographic information included age, sex, household income based on metropolitan statistical area code, insurance type, and the patient’s geographic region. The presence of specific mental health disorders and pain disorders were also recorded for each patient. Mental health disorders were identified using the Agency of Healthcare Research and Quality Clinical Classification System (CCS), and pain disorders which were identified using ICD-9 and ICD-10 diagnosis codes. The Charlson Comorbidity Index was calculated and used to classify patient comorbidities as a proxy of health status.23,24
Statistical Analyses
Bivariate analyses were performed between patients with persistent gabapentinoid and opioid use and those without persistent gabapentinoid and opioid use, respectively. Additional analyses compared patients with and without preoperative gabapentinoid use. Patient characteristics, preoperative gabapentinoid use, and postoperative persistent use of gabapentinoid and opioids were examined using the Chi-square test for categorical variables and the Student t-test for continuous variables.
We then used two multivariable logistic regression models to examine the association between patient factors and postoperative persistent gabapentinoid use and postoperative persistent opioid use. We controlled for patient-level covariates including age, sex, geographic region, insurance type, household income, Charlson Comorbidity Index, mental health disorders, pain disorders, diabetes, and preoperative gabapentinoid use. Pain disorders included arthritis, back pain, neck pain, and other pain disorders. Other pain disorders were defined as temporomandibular joint pain, tension headache, fibromyalgia, chronic fatigue syndrome, migraines, and other musculoskeletal pain disorders. The postestimation marginal effects were calculated to obtain the adjusted probability of persistent gabapentinoid and opioid use. To examine the association between pain disorders and persistent postoperative gabapentinoid and opioid use, we performed sensitivity analyses using a sample restricted to patients without pain disorders. P-values were 2-tailed, and significance level at P<0.05 was set for all analyses.
Results
A total of 56,593 patients who had no record of previous gabapentinoid or opioid prescriptions within the three months prior to diagnosis were newly diagnosed with CTS and underwent carpal tunnel release between 2010 and 2017. Of these patients, 3,474 (6%) were started on a preoperative gabapentinoid (Table 1). Approximately 6% of patients undergoing CTR were started on a preoperative gabapentinoid in 2010 compared to 4% in 2017 (Table 2). The majority of patients undergoing carpal tunnel release were women (66%) and had a pain disorder (62%). The average time from diagnosis to carpal tunnel release was 284 days (standard deviation: 422 days). Patients started on a preoperative gabapentinoid were more likely to have a pain disorder (70%) compared to patients not started on a preoperative gabapentinoid (62%) (P<0.05) (Appendix C). Additionally, 25% of patients started on a preoperative gabapentinoid had a mental health disorder compared to 19% in patients not started on a preoperative gabapentinoid (P<0.05). Patients started on a preoperative gabapentinoid had a significantly longer time to surgery (average time to CTR preoperative gabapentinoid: 668 days vs. no preoperative gabapentinoid: 259 days, P<0.05).
Table 1:
Sociodemographic Characteristics of Newly Diagnosed Carpal Tunnel Syndrome Patients and Bivariate Analyses1 (2010-2017)
| No. (%) | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Overall Cohort (N=56,593) | Persistent Gabapentinoid Use (N=1,523) | No Persistent Gabapentinoid Use (N=55,070) | P-Value | Persistent Opioid Use (N=5,361) | No Persistent Opioid Use (N=51,232) | P-Value |
| Age | |||||||
| 18-29 | 1,539 (2.7) | 21 (1.4) | 1,518 (2.8) | <0.05 | 171 (3.2) | 1,368 (2.7) | <0.05 |
| 30-39 | 6,165 (10.9) | 122 (8.0) | 6,043 (11.0) | 629 (11.7) | 5,536 (10.8) | ||
| 40-49 | 13,871 (24.5) | 333 (21.9) | 13,538 (24.6) | 1,335 (24.9) | 12,536 (24.5) | ||
| 50-59 | 25,056 (44.3) | 757 (49.7) | 24,299 (44.1) | 2,357 (44.0) | 22,699 (44.3) | ||
| 60-64 | 9,962 (17.6) | 290 (19.0) | 9,672 (17.6) | 869 (16.2) | 9,093 (17.8) | ||
| Female | 37,158 (65.7) | 1,054 (69.2) | 36,104 (65.6) | <0.05 | 3,611 (67.4) | 33,547 (65.5) | <0.05 |
| Median Household Income | |||||||
| <60,000 | 10,867 (19.2) | 332 (21.8) | 10,535 (19.1) | <0.05 | 1,187 (21.8) | 9,680 (22.1) | <0.05 |
| 60,000-69,999 | 18,138 (32.1) | 465 (30.5) | 17,673 (32.1) | 1,648 (30.5) | 16,490 (30.7) | ||
| 70,000-79,999 | 9,191 (16.2) | 208 (13.7) | 8,983 (16.3) | 836 (13.7) | 8,355 (15.6) | ||
| 80,000-89,999 | 3,721 (6.6) | 76 (5.0) | 3,645 (6.6) | 352 (5.0) | 3,369 (6.6) | ||
| ≥90,000 | 14,676 (25.9) | 442 (29.0) | 14,234 (25.9) | 1,338 (29) | 13,338 (25.0) | ||
| Charlson Comorbidity Index, mean (SD) | 0.5 (1.0) | 0.8 (1.4) | 0.5 (1.0) | <0.05 | 0.6 (1.1) | 0.5 (1.0) | <0.05 |
| Diabetes | 7,845 (13.9) | 381 (25.0) | 7,464 (13.6) | <0.05 | 926 (17.3) | 6,919 (13.5) | <0.05 |
| Region | |||||||
| Northeast | 11,179 (19.8) | 241 (15.8) | 10,938 (19.9) | <0.05 | 846 (15.8) | 10,333 (20.2) | <0.05 |
| North Central | 16,067 (28.4) | 418 (27.5) | 15,649 (28.4) | 1,457 (27.2) | 14,610 (28.5) | ||
| South | 21,040 (37.2) | 680 (44.7) | 20,360 (37) | 2,248 (41.9) | 18,792 (36.7) | ||
| West | 7,870 (13.9) | 172 (11.3) | 7,698 (14) | 770 (14.4) | 7,100 (13.9) | ||
| Unknown | 437 (0.8) | 12 (0.8) | 425 (0.8) | 40 (0.8) | 397 (0.8) | ||
| Insurance | |||||||
| PPO | 33,749 (59.6) | 913 (60.0) | 32,836 (59.6) | <0.05 | 3,127 (60) | 30,622 (58.3) | <0.05 |
| Comprehensive | 2,433 (4.3) | 111 (7.3) | 2,322 (4.2) | 290 (7.3) | 2,143 (5.4) | ||
| HMO | 6,424 (11.4) | 163 (10.7) | 6,261 (11.4) | 682 (10.7) | 5,742 (12.7) | ||
| Point of Service | 4,984 (8.8) | 159 (10.4) | 4,825 (8.8) | 473 (10.4) | 4,511 (8.8) | ||
| Other | 6,110 (10.8) | 126 (8.3) | 5,984 (10.9) | 544 (8.3) | 5,566 (10.2) | ||
| Unknown | 2,893 (5.1) | 51 (3.4) | 2,842 (5.2) | 245 (3.4) | 2,648 (4.6) | ||
| Mental Health Disorders | 11,029 (19.5) | 393 (25.8) | 10,636 (19.3) | <0.05 | 1,347 (25.1) | 9,682 (18.9) | <0.05 |
| Adjustment | 1,428 (2.5) | 53 (3.5) | 1,375 (2.5) | 0.16 | 151 (2.8) | 1,277 (2.5) | 0.15 |
| Anxiety | 4,551 (8.0) | 174 (11.4) | 4,377 (8.0) | <0.05 | 564 (10.5) | 3,987 (7.8) | <0.05 |
| Disruptive | 882 (1.6) | 19 (1.3) | 863 (1.6) | 0.32 | 103 (1.9) | 779 (1.5) | <0.05 |
| Mood | 6,218 (11.0) | 232 (15.2) | 5,986 (10.9) | <0.05 | 797 (14.9) | 5,421 (10.6) | <0.05 |
| Suicide of Self-Harm | 62 (0.1) | 3 (0.2) | 59 (0.1) | 0.23 | 12 (0.2) | 50 (0.1) | <0.05 |
| Personality | 80 (0.14) | 7 (0.5) | 73 (0.1) | <0.05 | 13 (0.2) | 67 (0.1) | <0.05 |
| Psychosis | 105 (0.2) | 5 (0.3) | 100 (0.2) | 0.19 | 18 (0.3) | 87 (0.2) | <0.05 |
| Alcohol or Substance Abuse | 667 (1.2) | 33 (2.2) | 634 (1.2) | <0.05 | 106 (2.0) | 561 (1.1) | <0.05 |
| Other | 912 (1.6) | 23 (1.5) | 889 (1.6) | 0.75 | 116 (2.2) | 796 (1.6) | <0.05 |
| Pain Disorders | 35,371 (62.5) | 1,120 (73.5) | 34,251 (62.2) | <0.05 | 3,779 (70.5) | 31,592 (61.7) | <0.05 |
| Arthritis | 27,184 (48.0) | 869 (57.1) | 26,315 (47.8) | <0.05 | 2,869 (53.5) | 24,315 (47.5) | <0.05 |
| Back | 11,577 (20.5) | 470 (30.9) | 11,107 (20.2) | <0.05 | 1,413 (26.4) | 10,164 (19.8) | <0.05 |
| Neck | 7,753 (13.7) | 286 (18.8) | 7,467 (13.6) | <0.05 | 873 (16.3) | 6,880 (13.4) | <0.05 |
| Other | 12,023 (21.2) | 468 (30.7) | 11,555 (21.0) | <0.05 | 1,448 (27.0) | 10,575 (20.6) | <0.05 |
| Preoperative Gabapentinoid Use | 3,474 (6.1) | 835 (54.8) | 2,639 (4.8) | <0.05 | 702 (13.1) | 2,772 (5.4) | <0.05 |
Bivariate analyses were performed for the outcomes new persistent gabapentinoid use and new persistent opioid use.
Table 2:
Preoperative Gabapentinoid Use by Year
| Year of Diagnosis | Number of Patients with Preoperative Gabapentinoid Use (%) | Total Number of Patients Undergoing CTR |
|---|---|---|
| 2010 | 860 (6%) | 13,688 |
| 2011 | 735 (6%) | 11,449 |
| 2012 | 645 (7%) | 9,704 |
| 2013 | 493 (7%) | 7,445 |
| 2014 | 308 (5%) | 5,623 |
| 2015 | 244 (6%) | 4,207 |
| 2016 | 153 (4%) | 3,561 |
| 2017 | 36 (4%) | 916 |
Persistent Gabapentinoid Use
In the cohort of patients with no record of previous gabapentinoid prescription in the three months prior to diagnosis of CTS, 1,523 patients (3%) continued to fill gabapentinoid prescriptions 91-180 days after carpal tunnel release (Table 1). Of these postoperative gabapentinoid users, 835 patients (55%) were started on a preoperative gabapentinoid, and 688 (45%) received a new postoperative gabapentinoid prescription. Of the preoperative gabapentinoid users, 24% continued to use gabapentinoids postoperatively. Patients who were started on a preoperative gabapentinoid had larger postoperative gabapentin prescriptions (preoperative gabapentinoid users average number of 300mg gabapentin pills: 431 vs. no preoperative gabapentinoid average number of 300mg gabapentin pills: 264, P<0.05) and larger postoperative pregabalin prescriptions (preoperative gabapentinoid users average number of 25mg pregabalin pills: 871 vs. no preoperative gabapentinoid average number of 25mg pregabalin pills: 440, P<0.05). Additionally, comorbid conditions, personality disorders, arthritis, back pain, and other pain disorders increased the odds of persistent postoperative gabapentinoid use (Table 3). However, preoperative gabapentinoid use was the largest predictor for persistent postoperative gabapentinoid use (OR: 21.40, 95% CI: 19.16-23.91, P<0.05). Patients who received preoperative gabapentinoids had a 21% adjusted probability of persistent postoperative gabapentinoid use (95% CI: 20.1-22.8) compared to a 1% adjusted probability of persistent postoperative gabapentinoid use among patients who did not receive a preoperative gabapentinoid (95% CI: 1.2-1.4) (Figure 1).
Table 3:
Multivariable Logistic Regression of Persistent Postoperative Gabapentinoid Use
| Characteristic | Odds Ratio (95% CI) | P-Value |
|---|---|---|
| Age | ||
| 18-29 | 0.57 (0.36-0.90) | <0.05 |
| 30-39 | 0.73 (0.59-0.89) | <0.05 |
| 40-49 | 0.83 (0.72-0.96) | <0.05 |
| 50-59 | 1 [Reference] | - |
| 60-64 | 1.00 (0.85-1.15) | 0.85 |
| Female | 1.09 (0.97-1.23) | 0.17 |
| Median Household Income | ||
| <60,000 | 1.12 (0.96-1.31) | 0.16 |
| 60,000-69,999 | 1 [Reference] | - |
| 70,000-79,999 | 0.99 (0.83-1.18) | 0.88 |
| 80,000-89,999 | 0.88 (0.67-1.15) | 0.34 |
| ≥90,000 | 1.25 (1.08-1.44) | <0.05 |
| Charlson Comorbidity Index | 1.11 (1.06-1.17) | <0.05 |
| Diabetes | 1.38 (1.19-1.61) | <0.05 |
| Region | ||
| South | 1 [Reference] | - |
| North Central | 0.91 (0.79-1.05) | 0.18 |
| Northeast | 0.81 (0.68-0.96) | <0.05 |
| West | 0.83 (0.69-1.00) | 0.05 |
| Insurance | ||
| PPO | 1 [Reference] | - |
| Comprehensive | 1.31 (1.04-1.65) | <0.05 |
| HMO | 0.95 (0.79-1.14) | 0.59 |
| Point of Service | 1.15 (0.95-1.38) | 0.15 |
| Other | 0.84 (0.69-1.03) | 0.10 |
| Mental Health Disorders | ||
| Adjustment | 1.38 (1.02-1.88) | <0.05 |
| Anxiety | 1.24 (1.03-1.50) | <0.05 |
| Disruptive | 0.75 (0.46-1.22) | 0.25 |
| Mood | 1.09 (0.93-1.29) | 0.30 |
| Suicide of Self-Harm | 0.84 (0.23-3.03) | 0.78 |
| Personality | 3.98 (1.64-9.68) | <0.05 |
| Psychosis | 1.17 (0.42-3.26) | 0.76 |
| Alcohol or Substance Abuse | 1.20 (0.80-1.80) | 0.38 |
| Other | 0.71 (0.46-1.12) | 0.14 |
| Pain Disorders | ||
| Arthritis | 1.13 (1.01-1.27) | <0.05 |
| Back | 1.41 (1.24-1.60) | <0.05 |
| Neck | 1.03 (0.89-1.20) | 0.67 |
| Other | 1.24 (1.09-1.40) | <0.05 |
| Preoperative Gabapentinoid Use | 21.40 (19.16-23.91) | <0.05 |
Figure 1:
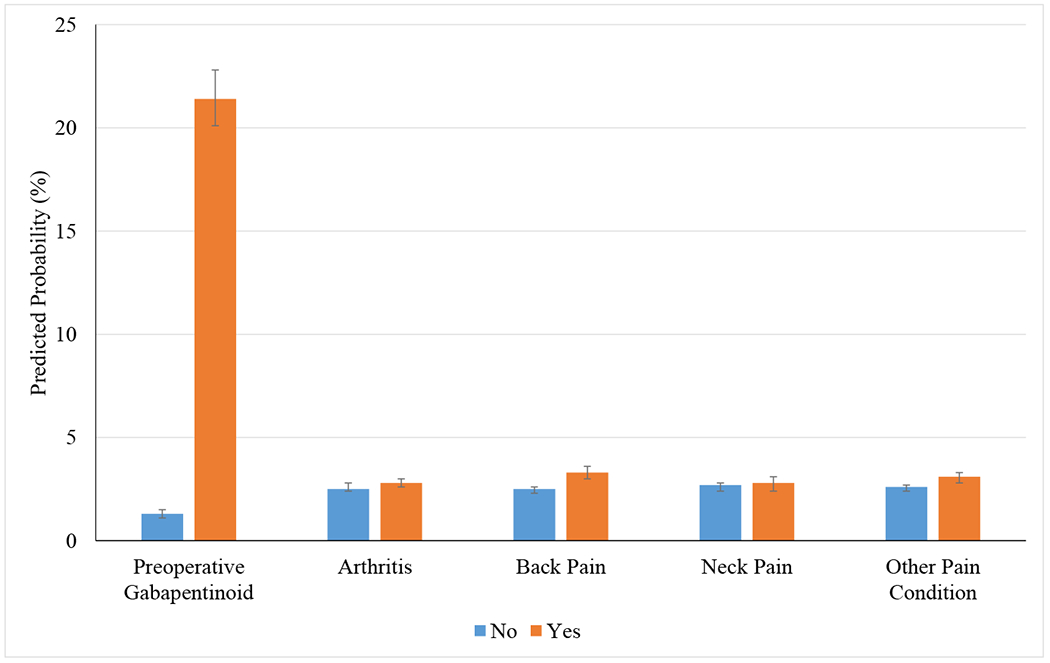
Predicted Probability of Persistent Postoperative Gabapentinoid Use
Adjusted Probability of Persistent Postoperative Gabapentinoid Use; Adjusted probability was generated using margins command for marginal effects. Error bars represent the 95% confidence intervals.
Persistent Opioid Use
Among all patients who did not receive a gabapentinoid or opioid prescription in the three months prior to CTS diagnosis, 5,361 patients (9%) filled an opioid prescription 91-180 days after carpal tunnel release (Table 1). Of the patients who received a preoperative gabapentinoid, 702 patients (20%) continued long-term opioid filling. Patients who were prescribed a preoperative gabapentinoid also had significantly larger opioid prescriptions in the 91-180 days postoperatively (preoperative gabapentinoid users average number of 5mg hydrocodone pills: 364 vs. no preoperative gabapentinoid average number of 5mg hydrocodone pills: 137, P<0.05). Comorbid conditions, mood disorders, alcohol or substance abuse disorders, arthritis, back pain, and other pain disorders increased the odds of persistent postoperative opioid use (Table 4). Furthermore, preoperative gabapentinoid use was associated with an increased odds of persistent postoperative opioid use (OR: 2.34, 95% CI: 2.14-2.56, P<0.05). Patients who received preoperative gabapentinoids had an 18% adjusted probability of persistent postoperative opioid use (95% CI: 17.1-19.6) compared to an 9% adjusted probability of persistent postoperative opioid use in patients who did not receive a preoperative gabapentinoid (95% CI: 8.6-9.1) (Figure 2).
Table 4:
Multivariable Logistic Regression of Persistent Postoperative Opioid Use
| Characteristic | Odds Ratio (95% CI) | P-Value |
|---|---|---|
| Age | ||
| 18-29 | 1.29 (1.09-1.52) | <0.05 |
| 30-39 | 1.15 (1.05-1.27) | <0.05 |
| 40-49 | 1.05 (0.98-1.13) | 0.18 |
| 50-59 | 1 [Reference] | - |
| 60-64 | 0.90 (0.83-0.98) | <0.05 |
| Female | 1.02 (0.96-1.08) | 0.63 |
| Median Household Income | ||
| <60,000 | 1.16 (1.07-1.26) | <0.05 |
| 60,000-69,999 | 1 [Reference] | - |
| 70,000-79,999 | 1.05 (0.96-1.15) | 0.26 |
| 80,000-89,999 | 1.15 (1.02-1.30) | <0.05 |
| ≥90,000 | 1.04 (0.96-1.12) | 0.36 |
| Charlson Comorbidity Index | 1.08 (1.04-1.11) | <0.05 |
| Diabetes | 1.16 (1.06-1.27) | <0.05 |
| Region | ||
| South | 1 [Reference] | - |
| North Central | 0.85 (0.79-0.92) | <0.05 |
| Northeast | 0.70 (0.64-0.76) | <0.05 |
| West | 0.93 (0.85-1.02) | 0.11 |
| Insurance | ||
| PPO | 1 [Reference] | - |
| Comprehensive | 1.37 (1.20-1.57) | <0.05 |
| HMO | 1.17 (1.07-1.28) | <0.05 |
| Point of Service | 1.04 (0.93-1.15) | 0.52 |
| Other | 0.98 (0.89-1.08) | 0.71 |
| Mental Health Disorders | ||
| Adjustment | 0.97 (0.81-1.15) | 0.69 |
| Anxiety | 1.14 (1.03-1.26) | <0.05 |
| Disruptive | 1.04 (0.84-1.28) | 0.75 |
| Mood | 1.27 (1.16-1.38) | <0.05 |
| Suicide of Self-Harm | 1.14 (0.59-2.23) | 0.69 |
| Personality | 1.22 (0.66-2.27) | 0.53 |
| Psychosis | 1.41 (0.83-2.39) | 0.21 |
| Alcohol or Substance Abuse | 1.43 (1.15-1.79) | <0.05 |
| Other | 1.13 (0.92-1.38) | 0.24 |
| Pain Disorders | ||
| Arthritis | 1.15 (1.08-1.22) | <0.05 |
| Back | 1.30 (1.21-1.39) | <0.05 |
| Neck | 1.03 (0.95-1.12) | 0.54 |
| Other | 1.22 (1.14-1.31) | <0.05 |
| Preoperative Gabapentinoid Use | 2.34 (2.14-2.56) | <0.05 |
Figure 2:

Predicted Probability of Persistent Postoperative Opioid Use
Adjusted Probability of Persistent Postoperative Opioid Use; Adjusted probability was generated using margins command for marginal effects. Error bars represent the 95% confidence intervals.
Sensitivity Analyses
After restricting the cohort to patients without preoperative pain syndrome diagnoses, preoperative gabapentinoid use was still associated with increased risk of persistent postoperative gabapentinoid use (OR: 27.45, 95% CI: 22.14-34.02, P<0.05; 20% adjusted probability of persistent postoperative gabapentinoid use among patients who had a preoperative gabapentinoid compared to 1% for patients who did not have a preoperative gabapentinoid) (Appendix D). Additionally, preoperative gabapentinoid use was associated with an increased odds of persistent postoperative opioid use (OR: 2.45, 95% CI: 2.06-2.92, P<0.05; 16% adjusted probability of persistent postoperative opioid use among patients who had a preoperative gabapentinoid compared to 7% for patients who did not have a preoperative gabapentinoid) (Appendix E).
Discussion
In this national analysis of patients who did not previously receive gabapentinoids or opioids prior to their CTS diagnosis, 6% of newly diagnosed CTS patients filled a prescription for gabapentinoids prior to surgery. Of these preoperative users, 24% continued on these medications following carpal tunnel release, and 20% continued to fill opioid medications for a prolonged period of time after surgery. Notably, patients with pain conditions and other mental health disorders had increased odds of prolonged gabapentinoid and opioid use after carpal tunnel release. It is important to note that neither gabapentinoids nor opioids play a role in the management of these conditions as well.8,25 Overall, given the limited efficacy of treatment of CTS with gabapentinoids and their associated risks, particularly when prescribed with opioids,4,26,27 these findings highlight the importance of minimizing gabapentinoid prescribing among patients with CTS.
Current evidence recommends avoiding gabapentinoids for the treatment of CTS. For patients who do not gain relief with non-surgical measures for CTS, including activity modification, splinting, and steroid injections, surgical release is indicated.14 According to the American Academy of Orthopedic Surgery clinical practice guidelines in management of CTS, moderate evidence suggests no benefit of gabapentin over placebo for treatment of CTS.14,15 Recently, there is growing interest in integrating gabapentinoids into multi-modal anesthesia protocols whereby gabapentinoids are given on the day of surgery to decrease opioid consumption during and immediately following the surgical procedure.21,22,28 A single dose of a gabapentinoid administered perioperatively is associated with decrease in opioid consumption in the 24 hours postoperatively.28–30 Many enhanced recovery after surgery pathways (ERAS) now utilize gabapentinoids to minimize opioid consumption and opioid-related side effects such as nausea, vomiting, and ileus. However, in this analysis, we specifically assessed the association of preoperative, rather than perioperative, gabapentinoid use with long-term gabapentinoid and opioid use after surgery. Though we are unable to fully discern whether preoperative gabapentinoid use was specifically for CTS, we observed that 6% of newly diagnosed patients with CTS filled a prescription for gabapentinoids prior to surgery, which may suggest that these medications are used to manage the symptoms of CTS, that patients who receive these medications have more complex pain management requirements, or may reflect the heterogeneity in provider prescribing patterns. Additionally, we found that preoperative gabapentinoid prescribing has decreased between 2010 and 2017, from 6% to 4%, which may reflect changes in prescribing behaviors as acute pain management strategies and knowledge surrounding inappropriate prescribing have changed substantially during this time period. Moreover, we found that 24% patients initiated on preoperative gabapentinoids continue to fill these prescriptions months following surgery. Finally, 20% of preoperative gabapentinoid users continued use of opioids long-term, potentially indicating a high-risk group of patients that would benefit from earlier surgical referral to possibly obviate the need for these medications or increased access to evidence-based non-surgical treatment for their initial CTS symptoms. Even after removing patients with pain syndrome diagnoses, who would be more likely to receive gabapentinoids, we continued to find persistent postoperative gabapentinoid and opioid use. However, our study does not evaluate the efficacy of gabapentinoids for severe CTS specifically in the postoperative period while the median nerve is recovering. Additional research is needed to fully understand the role of gabapentinoids during nerve recovery for severe CTS.
In the wake of the opioid epidemic, gabapentinoids are increasingly being prescribed as an alternative and in conjunction with opioids for pain management, which has led to a national increase in prescribing.4,11,31 However, the FDA has approved the use of gabapentinoids for only a few pain indications. Recently, there is growing concern of gabapentinoids’ adverse side effects, increased off-label prescribing, and possible diversion of these medications. In a study by Peckham et al., the current trends in gabapentinoid prescribing mirror those early characteristics associated with subsequent opioid abuse including high daily doses.27 Other studies have estimated that 40-65% of patients with a prescription for gabapentinoids go on to misuse, defined as consuming the medication in a way that is different than prescribed, at some point during their treatment.30 In a recent systematic review, there was a 1.6% prevalence of gabapentinoid abuse observed in the general population, which was substantial higher in patients with opioid use disorder (range: 3-68% prevalence).32 In our study, preoperative gabapentinoid use was associated with postoperative persistent use of gabapentinoids and opioids after carpal tunnel release, reflecting a concerning association of preoperative gabapentinoid use with postoperative opioid use. Moreover, in this patient population, preoperative gabapentinoid use was associated with persistent postoperative gabapentinoid and opioid use and may be a marker of patients at high-risk for persistent use. Additionally, in the sensitivity analyses with removal of patients with pain syndrome diagnoses, our findings persisted with preoperative gabapentinoid use being associated with persistent postoperative gabapentinoid and opioid use. There may be a subset of patients with differences in pain perception and tolerance that may predispose them to requiring additional medications for management.33–37 These findings underline the importance of preoperative assessment to capture risk factors for poor pain-related outcomes following surgery in order to inform the decision for surgery and to set expectations appropriately.
Given the increasing concern for the adverse effects of gabapentinoids, recent policy changes have led to the restriction of gabapentinoids. In 2005, the Federal Drug Administration deemed pregabalin a controlled substance because of its sedative properties and ability to induce tolerance and physical dependence.26,38,39 Since then, states have passed similar legislation making all gabapentinoids controlled substances and requiring registration of all gabapentinoid prescription fills in a prescription drug monitoring program (PDMP).4 The state of Kentucky has led these policy changes, first making gabapentin a restricted drug in July 2017.5 Additional states have followed suit such as Tennessee and most recently Michigan.40,41 Other states have taken a less restrictive approach and have implemented mandatory reporting to a PDMP without changing the controlled substance class of gabapentinoids.4 These policy changes attempt to reduce diversion of gabapentinoids, minimize the adverse effects of these medications, and curb the possible misuse of these drugs with opioids. Our study corroborates the concern for possible long-term combined use of gabapentinoids and opioids. Patients receiving preoperative gabapentinoids had a 21% adjusted probability of persistent postoperative gabapentinoid and 18% adjusted probability of persistent postoperative opioid use.
Our study has several limitations that are inherent to insurance claims data. The IBM MarketScan Research Databases do not contain granular clinical data including severity of CTS or the indication for prescribing medications. Nevertheless, we examined prescription fills that were in close proximity to clinical encounters for which diagnosis codes were available. However, patients may have been prescribed these medications for other diagnoses, which cannot be verified using claims data. Regardless, preoperative gabapentinoid use, either because of CTS or another diagnosis, is associated with persistent postoperative gabapentinoid and opioid use after CTR. Additionally, these findings could reflect prescriber behaviors, where some providers may be more willing to prescribe gabapentinoids and opioids, and may not reflect inherent patient characteristics associated with persistent use. There is also the possibility that patients could be misdiagnosed with CTS or postoperative complications and had continued postoperative symptoms requiring gabapentinoids and opioids. However, to account for revision surgical procedures, we excluded patients who underwent additional surgical procedures in the six-month postoperative period. Despite a one-year pre-CTS diagnosis window, we cannot fully ensure that patients had a diagnosis of primary CTS and not recurrent CTS. This is also a study of patients who were treated operatively and may have more severe disease compared to a non-operative cohort. Additionally, claims data do not capture actual use of gabapentinoids and, we cannot determine how many pills patients consumed. Moreover, the IBM MarketScan Research Databases do not contain the indications for gabapentinoid or opioid use. In order to preferentially select patients who most likely received gabapentinoids for the treatment of CTS, we ensured that all patients were gabapentinoid- and opioid-naïve prior to their diagnosis of CTS. Furthermore, we also performed sensitivity analyses restricting our cohort to patients without any pain syndrome diagnoses and obtained similar findings. However, this does not completely eliminate the possibility that these gabapentinoid prescriptions were for different conditions other than CTS. Lastly, the IBM MarketScan Research Databases only include patients with private employer-based insurance, thus these results may not be generalizable to uninsured patients or those with only Medicare, Medicaid, or disability insurance (e.g. worker’s compensation).
Given the association of preoperative gabapentinoid use with new persistent gabapentinoid and opioid use, providers treating CTS should consider preoperative gabapentinoid use as a marker for a high-risk patient. Moreover, these findings highlight the substantial number of patients that continue long-term use of gabapentinoids and understanding the reasons behind this may help limit their use, decrease potential side effects, and minimize diversion.
Supplementary Material
Acknowledgments
Financial Disclosure: Investigators were supported by National Institute on Drug Abuse (RO1 DA042859), NIAMS (P50 AR070600), the Michigan Department of Health and Human Services (E20180672-00 Michigan DHHS - MA-2018 Master Agreement Program) as well as the Substance Abuse and Mental Health Administration (SAMHSA: E20180568-00 MA-2018 Master Agreement Program) and the Centers for Disease Control and Prevention (E20182818-00 MA-2018 Master Agreement Program).
Footnotes
Publisher's Disclaimer: Disclaimer: This does not necessarily represent the views of the United States Government or Department of Veterans Affairs.
References
- 1.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case–control study. PLoS medicine. 2017;14(10):e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185–1215. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet U, Richter EL, Isbruch K, Scherbaum N. On the addictive power of gabapentinoids: a mini-review. Psychiatr Danub. 2018;30(2):142–149. [DOI] [PubMed] [Google Scholar]
- 4.Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk management and healthcare policy. 2018;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kentucky Cabinet for Health and Family Services. Important Notice: Gabapentin Becomes a Schedule 5 Controlled Substance in Kentucky. https://pharmacy.ky.gov/Documents/Gabapentin%20-%20Schedule%20V%20Controlled%20Substance.pdf. Accessed May 14, 2019.
- 6.U.S. Food and Drug Administration. Neurontin (gabapentin). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020235s036,020882s022,021129s022lbl.pdf. Accessed June 16, 2019.
- 7.U.S. Food and Drug Administration. Drug Approval Package: Lyrica (Pregabalin) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209501Orig1s000TOC.cfm. Accessed June 26, 2019. [Google Scholar]
- 8.Goodman CW, Brett AS. A Clinical Overview of Off-label Use of Gabapentinoid Drugs. JAMA Intern Med. 2019. [DOI] [PubMed] [Google Scholar]
- 9.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280(21):1831–1836. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson B Gabapentin use in neuropathic pain syndromes. Acta Neurol Scand. 2000;101(6):359–371. [DOI] [PubMed] [Google Scholar]
- 11.Goodman CW, Brett AS. Gabapentin and Pregabalin for Pain - Is Increased Prescribing a Cause for Concern? N Engl J Med. 2017;377(5):411–414. [DOI] [PubMed] [Google Scholar]
- 12.Stevens JC, Sun S, Beard CM, O’Fallon WM, Kurland LT. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38(1):134–138. [DOI] [PubMed] [Google Scholar]
- 13.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282(2):153–158. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. February 29, 2016; www.aaos.org/ctsguideline. [DOI] [PubMed] [Google Scholar]
- 15.Hui AC, Wong SM, Leung HW, Man BL, Yu E, Wong LK. Gabapentin for the treatment of carpal tunnel syndrome: a randomized controlled trial. Eur J Neurol. 2011;18(5):726–730. [DOI] [PubMed] [Google Scholar]
- 16.IBM Corporation. IBM MarketScan Research Databases for life sciences researchers. 2019; https://www.ibm.com/products/marketscan-research-databases. Accessed March 12, 2020. [Google Scholar]
- 17.International Association for the Study of Pain. IASP Task Force for the Classification of Chronic Pain in ICD-11 prepares new criteria on postsurgical and posttraumatic pain. http://www.iasp-pain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=5134&navItemNumber=643. Accessed June 11, 2019.
- 18.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. Bmj. 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson SP, Chung KC, Zhong L, et al. Risk of Prolonged Opioid Use Among Opioid-Naive Patients Following Common Hand Surgery Procedures. J Hand Surg Am. 2016;41(10):947–957 e943. [DOI] [PubMed] [Google Scholar]
- 21.Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial. JAMA surgery. 2018;153(4):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frouzanfard F, Fazel MR, Abolhasani A, Fakharian E, Mousavi G, Moravveji A. Effects of gabapentin on pain and opioid consumption after abdominal hysterectomy. Pain Research and Management. 2013;18(2):94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 25.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. Jama. 2016;315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peckham AM, Fairman KA, Sclar DA. Prevalence of Gabapentin Abuse: Comparison with Agents with Known Abuse Potential in a Commercially Insured US Population. Clin Drug Investig. 2017;37(8):763–773. [DOI] [PubMed] [Google Scholar]
- 28.Hurley RW, Cohen SP, Williams KA, Rowlingson AJ, Wu CL. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med. 2006;31(3):237–247. [DOI] [PubMed] [Google Scholar]
- 29.Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 2017;152(7):691–697. [DOI] [PubMed] [Google Scholar]
- 30.Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2015;114(1):10–31. [DOI] [PubMed] [Google Scholar]
- 31.Johansen ME. Gabapentinoid Use in the United States 2002 Through 2015. JAMA Intern Med. 2018;178(2):292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evoy KE, Morrison MD, Saklad SR. Abuse and Misuse of Pregabalin and Gabapentin. Drugs. 2017;77(4):403–426. [DOI] [PubMed] [Google Scholar]
- 33.Janda AM, As-Sanie S, Rajala B, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122(5):1103–1111. [DOI] [PubMed] [Google Scholar]
- 34.Dutta D, Brummett CM, Moser SE, et al. Heritability of the fibromyalgia phenotype varies by age. Arthritis Rheumatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brummett CM, Bakshi RR, Goesling J, et al. Preliminary validation of the Michigan Body Map. Pain. 2016;157(6):1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67(5):1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neville SJ, Clauw AD, Moser SE, et al. Association Between the 2011 Fibromyalgia Survey Criteria and Multisite Pain Sensitivity in Knee Osteoarthritis. Clin J Pain. 2018;34(10):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papazisis G, Tzachanis D. Pregabalin’s abuse potential: a mini review focusing on the pharmacological profile. Int J Clin Pharmacol Ther. 2014;52(8):709–716. [DOI] [PubMed] [Google Scholar]
- 39.Department of Justice Drug Enforcement Administration. Schedules of controlled substances: placement of pregabalin into schedule V. Final rule. Federal register. 2005;70(144):43633. [PubMed] [Google Scholar]
- 40.Tennessee General Assembly. House Bill 1832. http://wapp.capitol.tn.gov/apps/BillInfo/default.aspx?BillNumber=HB1832&GA=110. Accessed May 14, 2019.
- 41.Michigan Department of Licensing and Regulatory Affairs. Gabapentin Scheduled as Controlled Substance to Help with State’s Opioid Epidemic. https://www.michigan.gov/lara/0,4601,7-154-11472-487050--,00.html. Accessed June 11, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


