Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the related disease (coronavirus disease 2019; COVID-19) has been notified throughout Italy since February 2020. Intensive care unit (ICU) admission rate increased following the high incidence of pneumonia-related respiratory failure [1].
Short abstract
Utility and safety of bronchoscopy during the SARS-CoV-2 outbreak https://bit.ly/3ish52k
To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the related disease (coronavirus disease 2019; COVID-19) has been notified throughout Italy since February 2020. Intensive care unit (ICU) admission rate increased following the high incidence of pneumonia-related respiratory failure [1].
The diagnosis of pneumonia relies on viral detection in respiratory samples and on the assessment of abnormal findings on chest radiography, ultrasound scanning and computed tomography (CT) [2–5].
Viral diagnosis based on naso/oropharyngeal swabs shows suboptimal accuracy (sensitivity 32–63%), owing to wrong handling of the specimen, sample collection during the late phase of the disease or low viral load [5–7].
Bronchoscopy increases the sensitivity of the molecular diagnosis in comparison with that associated with nasopharyngeal swabs [6]. Furthermore, endoscopic techniques may be useful to manage serious pulmonary disorders (e.g. obstructive atelectasis, severe haemoptysis) [8–12]. However, bronchoscopy generates aerosols and may increase the risk of SARS-CoV-2 transmission [8, 9, 12].
Limited data are available in the scientific literature on the role of bronchoscopy in cases of SARS-CoV-2 pneumonia [6–12].
The primary aim of the present study was to describe the diagnostic yield of bronchoscopy in patients with negative nasopharyngeal swab(s) and a clinical and radiological suspicion of COVID-19 pneumonia.
Indications for bronchoscopy in cases of confirmed COVID-19 patients and the assessment of the safety of bronchoscopy for healthcare workers were evaluated.
An observational, retrospective, multicentre cohort study was performed in Italy. The study protocol was approved by the ethical committees of the participating hospitals. Written informed consent was signed by recruited patients.
Adult patients who underwent bronchoscopy between March 1, and April 15, 2020 were consecutively recruited in six Italian hospitals. The indications for bronchoscopy were diagnosis of SARS-CoV-2 pneumonia in patients with previously negative nasopharyngeal swab (clinical and radiological suspicion of pneumonia) and need for undelayable procedures in COVID-19 patients (e.g. massive haemoptysis, post-obstructive atelectasis).
All bronchoscopies were performed according to the World Health Organization (WHO) guidelines: the number of persons in the room was decreased to achieve a size appropriate to the provision of adequate care and support (i.e. one physician and one nurse wearing filtering facepiece class 3 masks) [13].
The diagnosis of COVID-19 was confirmed when molecular (i.e. real-time PCR) results detected SARS-CoV-2 in any respiratory sample [4, 5]. The probability of COVID-19 was high in case of a negative PCR and COVID-19 related symptoms (i.e. fever, cough, fatigue and/or shortness of breath), CT signs (i.e. ground-glass opacity, consolidation, reticulation/thickened interlobular septa, air bronchogram), with rapid clinical changes (progression or improvement in a short time period) [4, 7].
The diagnostic yield of bronchoscopy was calculated dividing the number of patients with a molecular diagnosis of SARS-CoV-2 infection following the collection of bronchoscopic specimens by the number of patients with a suspected diagnosis of COVID-19 pneumonia.
Every healthcare worker was carefully monitored for symptoms and clinical signs suggestive for COVID-19 for ≥15 days after the procedure.
An ad hoc electronic form was adopted to collect all study variables. Qualitative and quantitative variables were summarised with absolute (relative) frequencies and mean±sd, respectively. The statistical software STATA (version 16; StataCorp, College Station, TX, USA) was used to perform all statistical computations.
109 adult patients (71% males; mean±sd age 60.0±13.6 years) were enrolled.
108 (99.1%) bronchoscopies were performed with a flexible bronchoscope and one (0.01%) with the rigid scope. 13 (11.9%) bronchoscopies were performed while patients were breathing room air; 82 (75.3%) during oxygen supplementation; three (2.7%) during noninvasive mechanical ventilation; nine (8.2%) during invasive mechanical ventilation; and two (1.8%) during extracorporeal membrane oxygenation.
In 78 (71.6%) out of 109 cases, bronchoscopy was performed to diagnose SARS-CoV-2 infection in patients with a negative nasopharyngeal swab (median of two negative swabs per patient) and a clinical and radiological suspicion of COVID-19 pneumonia. Urgent/life-saving bronchoscopies were performed in 31 (28.4%) out of 109 patients with a confirmed diagnosis of COVID-19. The clinical indications were suspected concomitant lower respiratory tract infections or pulmonary tuberculosis, obstructive atelectasis, suspected tracheal intubation-related complication (i.e. tracheal laceration), tracheostomy complications and severe haemoptysis.
The diagnostic yield of bronchoscopy to detect SARS-CoV-2 in patients with previous negative swabs and a clinical and radiological suspicion of COVID-19 pneumonia was 55.1% (43 out of 78). No differences were found between bronchoalveolar lavage (BAL) and bronchial washing (35 (57.4%) out of 61 and eight (47.1%) out of 17, respectively; p=0.45) (figure 1).
FIGURE 1.
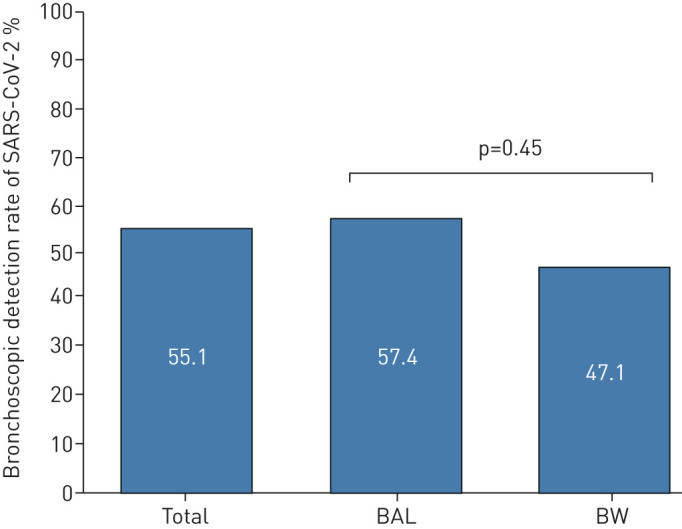
Bronchoscopic detection rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with previous negative swabs and in the presence of a clinical and radiological suspicion of coronavirus disease 2019 pneumonia, in the total cohort (78 patients) and according to bronchoalveolar lavage (BAL) and bronchial washing (BW).
Two (1.8%) out of 109 patients with previous negativity of both nasopharyngeal swabs and BAL for SARS-CoV-2 showed subsequent positive swabs. Hence, 45 (57.7%) out of 78 patients had a definite diagnosis of COVID-19 pneumonia.
The diagnosis of SARS-CoV-2 pneumonia was considered highly likely in 18 (23.1%) out of 78 patients, whereas 15 (19.2%) out of 78 were diagnosed with a lower respiratory tract infection.
One patient was co-infected with Haemophilus influenzae and SARS-CoV-2 and one patient with Aspergillus fumigatus and SARS-CoV-2. In two patients, Aspergillus spp., Candida albicans and SARS-CoV-2 were found concomitantly in the same BAL sample.
Complications related to bronchoscopy occurred in five (4.5%) out of 109 patients. Fever was recorded after BAL in two (1.8%) out of 109. Three (2.7%) out of 109 patients with a known mild respiratory failure had a transient worsening of their gas exchange after bronchoscopy performed during oxygen supplementation. No deaths were recorded.
Infections related to the endoscopic procedure did not occur in healthcare workers involved in the endoscopic activities.
To our knowledge, this is the largest study on the diagnostic yield of bronchoscopy in patients with negative nasopharyngeal swabs and a clinical/radiological suspicion of SARS-CoV-2 infection. An aetiological diagnosis is crucial to prevent viral transmission to susceptible individuals and decrease clinical complications in infected patients [1, 2]. Prompt respiratory isolation is needed to hamper viral spread, whereas an early diagnosis of COVID-19 related complications (i.e. respiratory failure) is crucial for a good prognosis [1, 2].
Our findings show that bronchoscopy might be useful in patients with suspected COVID-19 pneumonia and negative swabs, with an acceptable diagnostic performance of BAL and bronchial washing.
A recent study found a 71% detection rate of SARS-CoV-2 in 28 patients who underwent bronchoscopy in China, with 93% of positive PCR results in BAL samples [6]. Our study shows a lower diagnostic yield, but we performed a bronchoscopy only in patients with two previous negative swabs [6].
Although bronchoscopy has been relatively contraindicated during the COVID-19 pandemic, endoscopic procedures may not be postponed in some patient categories [11]. Urgent/life-saving bronchoscopies were performed in 31 patients with a confirmed COVID-19 diagnosis for obstructive atelectasis, suspected concomitant lower respiratory tract infections, severe haemoptysis, suspected tracheal lacerations in mechanically ventilated patients, tracheostomy complications and suspected concomitant pulmonary tuberculosis. Similar findings were described recently by Torrego et al. [12] in a Spanish cohort of COVID-19 patients who underwent bronchoscopy in the ICU.
Few data are available on bacterial and fungal co-infections with SARS-CoV-2 [14]. Lower respiratory tract co-infections were diagnosed with BAL in four patients. Prompt identification of co-infecting micro-organisms is associated with an early prescription of antibiotics [14].
Few bronchoscopy-related complications were recorded, with fever and mild respiratory failure being the most frequent.
The aforementioned side-effects can occur following a bronchoscopic procedure, in the line with the scientific evidence published before the pandemic [15, 16]. Neither severe complications nor deaths were described.
The WHO recommendations on airborne precautions for aerosol-generating procedures were strictly followed in the study centres; healthcare workers did not acquire any infections following the endoscopic procedures [13].
In conclusion, our study shows that bronchoscopy is a useful technique in the diagnostic pathway of COVID-19 pneumonia when nasopharyngeal swabs are negative. Urgent/life-saving procedures may be safely and successfully performed for diagnostic and therapeutic purposes in COVID-19 patients. The risk of viral transmission to healthcare workers is low when following the WHO guidelines on airborne precautions for aerosol-generating procedures.
Shareable PDF
Supplementary Material
Acknowledgements
The authors wish to acknowledge Beatrice Vigo and Simona Gentile (Respiratory Unit, ASST Santi Paolo e Carlo, San Paolo Hospital, Department of Health Sciences, Università degli Studi di Milano, Milan, Italy); Filippo Montanelli, Stefano Zucchetti, Giulia Pittalis, Laura Garzillo, Donatella Palmadessa, Luca Geroli and Giuseppe Paciocco (School of Medicine and Surgery, University of Milano Bicocca, Respiratory Unit, San Gerardo Hospital, ASST Monza, Monza, Italy); Vanina Rognoni (Microbiology Unit, ASST Lodi, Lodi, Italy); Daniela Zanoncelli, Isa Fenati, Teresa Goldaniga, Veena Arkasvipate and Saverio Bove (ASST Lodi, UOC Pneumologia, Lodi); and Giuseppe Ciaravino (Respiratory Unit, Papa Giovanni XXIII Hospital, Bergamo, Italy) for their valuable contribution to the research project.
Footnotes
Conflict of interest: M. Mondoni has nothing to disclose.
Conflict of interest: G.F. Sferrazza Papa has nothing to disclose.
Conflict of interest: R. Rinaldo has nothing to disclose.
Conflict of interest: P. Faverio has nothing to disclose.
Conflict of interest: A. Marruchella has nothing to disclose.
Conflict of interest: F. D'Arcangelo has nothing to disclose.
Conflict of interest: A. Pesci has nothing to disclose.
Conflict of interest: S. Pasini has nothing to disclose.
Conflict of interest: S. Henchi has nothing to disclose.
Conflict of interest: G. Cipolla has nothing to disclose.
Conflict of interest: F. Tarantini has nothing to disclose.
Conflict of interest: L. Giuliani has nothing to disclose.
Conflict of interest: F. Di Marco has nothing to disclose.
Conflict of interest: L. Saracino has nothing to disclose.
Conflict of interest: S. Tomaselli has nothing to disclose.
Conflict of interest: A. Corsico has nothing to disclose.
Conflict of interest: S. Gasparini has nothing to disclose.
Conflict of interest: M. Bonifazi has nothing to disclose.
Conflict of interest: L. Zuccatosta has nothing to disclose.
Conflict of interest: L. Saderi has nothing to disclose.
Conflict of interest: G. Pellegrino has nothing to disclose.
Conflict of interest: M. Davì has nothing to disclose.
Conflict of interest: P. Carlucci has nothing to disclose.
Conflict of interest: S. Centanni has nothing to disclose.
Conflict of interest: G. Sotgiu has nothing to disclose.
Support statement: This work was funded by Università degli Studi di Milano in the context of the Registry for COVID19 Emergency (RECOVER) electronic database. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Grasselli G, Zangrillo A, Zanella A, et al. . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascarella G, Strumia A, Piliego C, et al. . COVID-19 diagnosis and management: a comprehensive review. J Intern Med 2020; 288: 192–206. doi: 10.1111/joim.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol 2020; 214: 1280–1286. doi: 10.2214/AJR.20.22954 [DOI] [PubMed] [Google Scholar]
- 5.Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerg Microbes Infect 2020; 9: 747–756. doi: 10.1080/22221751.2020.1745095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Xu Y, Gao R, et al. . Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323: 1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai T, Yang Z, Hou H, et al. . Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 296: E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahidi MM, Shojaee S, Lamb CR, et al. . The use of bronchoscopy during the coronavirus disease 2019 pandemic: CHEST/AABIP guideline and expert panel report. Chest 2020; 158: 1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo F, Darwiche K, Singh S, et al. . Performing bronchoscopy in times of the COVID-19 pandemic: practice statement from an international expert panel. Respiration 2020; 99: 417–422. doi: 10.1159/000507898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondoni M, Carlucci P, Cipolla G, et al. . Bronchoscopy to assess patients with hemoptysis: which is the optimal timing? BMC Pulm Med 2019; 19: 36. doi: 10.1186/s12890-019-0795-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahidi MM, Lamb C, Murgu S, et al. . American Association for Bronchology and Interventional Pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. J Bronchology Interv Pulmonol 2020; in press [ 10.1097/LBR.0000000000000681]. 10.1097/LBR.0000000000000681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrego A, Pajares V, Fernández-Arias C, et al. . Bronchoscopy in patients with COVID-19 with invasive mechanical ventilation: a single-center experience. Am J Respir Crit Care Med 2020; 202: 284–287. doi: 10.1164/rccm.202004-0945LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO). Infection Prevention and Control During Health Care When Novel Coronavirus (nCoV) Infection is Suspected; WHO/2019-nCoV/IPC/2020.3 March 18, 2020. www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125
- 14.Rawson TM, Moore LSP, Zhu N, et al. . Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; in press [ 10.1093/cid/ciaa530]. 10.1093/cid/ciaa530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Rand IA, Blaikley J, Booton R, et al. . British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013; 68: Suppl. 1, i1–i44. doi: 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 16.Facciolongo N, Patelli M, Gasparini S, et al. . Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis 2009; 71: 8–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02767-2020.Shareable (523.2KB, pdf)


