Abstract
BACKGROUND
Bone tissue engineering is an area of continued interest within orthopaedic surgery, as it promises to create implantable bone substitute materials that obviate the need for autologous bone graft. Recently, oxysterols – oxygenated derivatives of cholesterol – have been proposed as a novel class of osteoinductive small molecules for bone tissue engineering. Here, we present the first systematic review of the in vivo evidence describing the potential therapeutic utility of oxysterols for bone tissue engineering.
AIM
To systematically review the available literature examining the effect of oxysterols on in vivo bone formation.
METHODS
We conducted a systematic review of the literature following PRISMA guidelines. Using the PubMed/MEDLINE, Embase, and Web of Science databases, we queried all publications in the English-language literature investigating the effect of oxysterols on in vivo bone formation. Articles were screened for eligibility using PICOS criteria and assessed for potential bias using an expanded version of the SYRCLE Risk of Bias assessment tool. All full-text articles examining the effect of oxysterols on in vivo bone formation were included. Extracted data included: Animal species, surgical/defect model, description of therapeutic and control treatments, and method for assessing bone growth. Primary outcome was fusion rate for spinal fusion models and percent bone regeneration for critical-sized defect models. Data were tabulated and described by both surgical/defect model and oxysterol employed. Additionally, data from all included studies were aggregated to posit the mechanism by which oxysterols may mediate in vivo bone formation.
RESULTS
Our search identified 267 unique articles, of which 27 underwent full-text review. Thirteen studies (all preclinical) met our inclusion/exclusion criteria. Of the 13 included studies, 5 employed spinal fusion models, 2 employed critical-sized alveolar defect models, and 6 employed critical-sized calvarial defect models. Based upon SYRCLE criteria, the included studies were found to possess an overall “unclear risk of bias”; 54% of studies reported treatment randomization and 38% reported blinding at any level. Overall, seven unique oxysterols were evaluated: 20(S)-hydroxycholesterol, 22(R)-hydroxycholesterol, 22(S)-hydroxycholesterol, Oxy4/Oxy34, Oxy18, Oxy21/Oxy133, and Oxy49. All had statistically significant in vivo osteoinductive properties, with Oxy4/Oxy34, Oxy21/Oxy133, and Oxy49 showing a dose-dependent effect in some cases. In the eight studies that directly compared oxysterols to rhBMP-2-treated animals, similar rates of bone growth occurred in the two groups. Biochemical investigation of these effects suggests that they may be primarily mediated by direct activation of Smoothened in the Hedgehog signaling pathway.
CONCLUSION
Present preclinical evidence suggests oxysterols significantly augment in vivo bone formation. However, clinical trials are necessary to determine which have the greatest therapeutic potential for orthopaedic surgery patients.
Keywords: Oxysterol, Bone tissue engineering, Critical-sized defect, Biomaterial, Orthopaedic surgery, Systematic review
Core tip: Here we present the first systematic review evaluating the utility of oxysterols for bone tissue engineering. Thirteen preclinical studies examining seven unique oxysterols were evaluated; all examined compounds were found to have statistically significant in vivo osteoinductive properties, with some showing dose-dependent effects. Importantly, eight studies found oxysterols to have similar osteoinductive properties to rhBMP-2 in treated animals. These effects are thought to occur through direct activation of Smoothened in the Hedgehog signaling pathway. Future clinical work is necessary to determine the exact therapeutic utility of these molecules in orthopaedic surgery patients.
INTRODUCTION
Each year, millions of patients undergo bone grafting as part of orthopaedic, neurosurgical, or maxillofacial procedures[1,2]. The present “gold standard” bone graft material is autologous bone, which possesses the three classic properties of an ideal graft: Osteoconduction, osteoinduction, and osteogenesis[2-4]. However, local autologous bone supply is often insufficient for adequate grafting, and orthotopic grafting requires an additional surgery for graft harvest, which may result in donor-site morbidity[4,5]. Furthermore, donor material may fail to engraft, leading to nonunion in a nontrivial proportion of treated patients[6-8].
To address these issues, extensive work has been performed over the past several decades, creating the field of bone tissue engineering, which comprises all efforts aimed at creating implantable bone substitutes[9]. In their most basic form, bone tissue engineering products constitute a biological scaffold to facilitate new bone growth often combined with bioactive molecules and/or cells that induce new bone formation[9-11]. One prime example is the Infuse® Bone Graft (Medtronic Spinal & Biologics; Memphis, TN) – a collagen sponge with adsorbed recombinant human bone morphogenetic protein-2 (rhBMP-2)[12] that has been used in more than a million neurosurgical and orthopaedic patients since receiving Food and Drug Administration (FDA) approval in 2002[12,13]. Despite this widespread use, the Infuse® product can add significantly to surgical costs, has a concerning side effect profile, and does not ensure bony union, creating the opportunity for other, superior bone graft materials to be developed[14-16].
Recently, a class of steroid derivatives called oxysterols (or hydroxycholesterols) have been studied for their potential osteoinductive effects[17,18]. Common nomenclature of these molecules has yet to be standardized, with multiple “Oxy” names existing for some identical species[19,20]. At their core, oxysterols are simply cholesterol oxidation products, which occur naturally in the human body[18,21]. These compounds, such as 27-hydroxycholesterol (the most abundant circulating oxysterol in humans), have been described in a number of clinical contexts, though are perhaps best known for their role in cholesterol homeostasis, inflammation, and apoptosis[22,23]. Yet it was not until 2004 that the first description of the osteogenic activity of oxysterols was made, when Kha et al[24] demonstrated that specific oxysterols could induce osteogenic differentiation and matrix mineralization in in vitro preparations of murine mesenchymal stem cells (MSCs).
In vivo support for the osteogenic potential of oxysterols was subsequently reported in a rat model of critical-sized calvarial defects[17]. Investigation into these molecules has subsequently increased, including several studies exploring oxysterols as an adjuvant to or replacement for rhBMP-2, the current “gold standard” osteoinductive factor employed for bone tissue engineering. Given the newness of these investigations, there has yet to be a review examining the effect of oxysterols on in vivo bone formation. To address this, here we provide the first systematic review of the effect of oxysterols on in vivo bone formation as a means of evaluating the potential therapeutic utility of oxysterols for bone tissue engineering.
MATERIALS AND METHODS
Electronic literature search
Based upon the PRISMA guidelines[25] we queried the PubMed/MEDLINE, Embase, and Web of Science databases for all English-language literature examining the effect of oxysterols on in vivo bone formation. An example search query employed for the PubMed/MEDLINE database was: ("oxysterol" OR "hydroxycholesterol") AND "bone". We also queried the bibliographies of the included studies for additional sources. Studies identified from the databases were screened for eligibility using the PICOS criteria (Population, Intervention, Comparators, Outcomes, and Study Type)[26], as follows: Population – laboratory animals; intervention – use of oxysterol-containing biomaterials to promote in vivo bone formation; comparators – biomaterials with no bioactive agents or non-oxysterol osteoinductive agents; outcomes – overall fusion rate (spinal fusion studies) and percent bone regeneration (critical-sized defect studies); study type – studies presenting primary in vivo results. Studies were excluded if they presented only in vitro data, did not present primary data, or did not have full-text availability. Eligible studies were screened against these criteria by two reviewers (Cottrill E and Lazzari J) with a third reviewer (Pennington Z) resolving any discrepancies between the first two reviewers.
Quality assessment
Study methodological quality was assayed using an adapted version of the SYRCLE Risk of Bias tool[27,28]. Additionally, we included two items from the consensus statement of “Good laboratory practice: Preventing introduction of bias at the bench”[29] to evaluate overall methodological quality: (1) Was a sample-size calculation provided? and (2) Was a statement on potential conflicts of interest provided? All included studies were screened against these fourteen items by two reviewers (Cottrill E and Lazzari J) with a third reviewer (Ehresman J) resolving any discrepancies.
Data extraction
From full texts we extracted details regarding animal species, surgical/defect model, oxysterol/graft materials used, control/comparative treatment (if available), primary method of assessing bony growth, and primary outcome. For studies employing a spinal fusion model, the primary outcome was fusion rate at last follow-up and for alveolar and calvarial defect studies the primary outcome was percent bone regeneration. These outcomes were chosen a priori as objective, study-appropriate metrics of in vivo bone formation to standardize data collection. In cases where these primary outcomes were not available, other objective metrics of in vivo bone formation (e.g., bone volumes) were recorded.
Data were tabulated and described by both surgical/defect model and oxysterol employed. Additionally, data from all included studies were aggregated to posit the mechanism by which oxysterols may mediate in vivo bone formation.
RESULTS
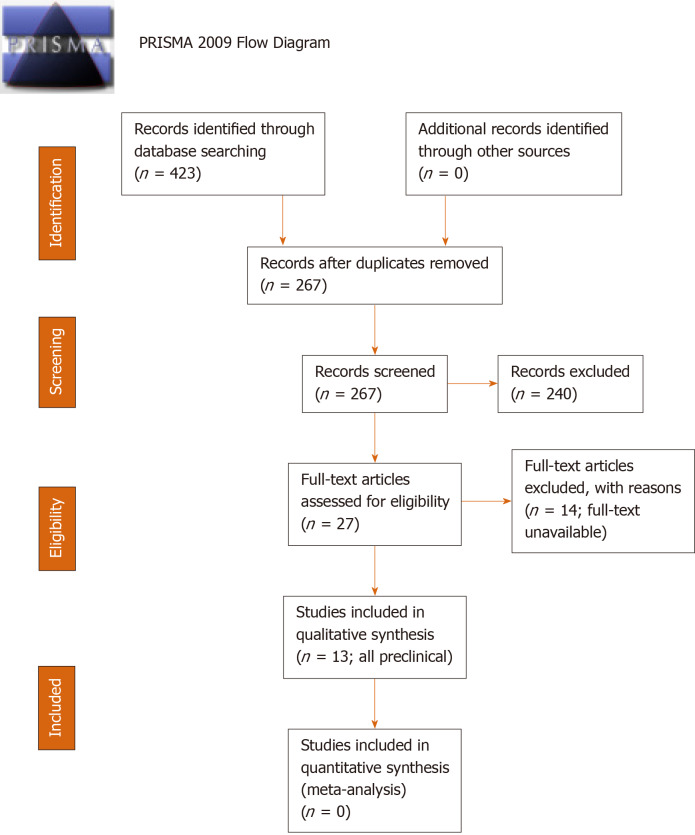
Our search identified 267 unique articles, of which 27 underwent full-text review and 13 studies met criteria for inclusion[17,19-21,30-38] (Figure 1). All fourteen excluded studies were eliminated for lack of full-text availability. Of the 13 included studies – all preclinical – five employed spinal fusion models[19-21,31,33] (Table 1), two employed critical-sized alveolar defect models[35,37] (Table 2), and six employed critical-sized calvarial defect models[17,30,32,34,36,38] (Table 3). Across all studies there were 7 unique oxysterols evaluated (Table 4).
Figure 1.
PRISMA 2009 flow diagram for article selection.
Table 1.
Descriptive summaries of the identified studies investigating the effect of oxysterols on bone formation in spinal fusion models (n = 5)
| Ref. (level of evidence) | Species (n) | Surgical model and scaffold(s) used | Experimental groups (n) | Assessment method and time point | Fusion rate (no. fused/total) |
| Johnson et al[21], 2011 (V) | Rat (n = 59) | L4–L5 PLF using a collagen sponge supplemented with Oxy341, Oxy49, or rhBMP-2 | A. DMSO only (control) (n = 9) | No motion bilaterally via manual palpation at 9 wk post-operatively | A. 0% (0/9) |
| B. 5-μg rhBMP-2 (n = 10) | B. 100% (10/10) | ||||
| C. 0.2-mg Oxy34 (n = 10) | C. 0% (0/10) | ||||
| D. 2-mg Oxy34 (n = 10) | D. 50% (5/10) | ||||
| E. 20-mg Oxy34 (n = 10) | E. 100% (10/10) | ||||
| F. 20-mg Oxy49 (n = 10) | F. 100% (10/10) | ||||
| Stappenbeck et al[20], 2012 (V) | Rat (n = 30) | L4-L5 PLF using a collagen sponge supplemented with Oxy41, Oxy18, or Oxy212 | A. 2-mg Oxy4 (n = 10) | Fusion rate was not reported; however, via μ-computed tomographic analysis, treatment with Oxy21 led to significantly larger de novo bone volumes compared to treatment with Oxy4, which was comparable to treatment with Oxy18 | |
| B. 2-mg Oxy18 (n = 10) | |||||
| C. 2-mg Oxy21 (n = 10) | |||||
| Montgomery et al[19], 2014 (V) | Rat (n = 38) | L4-L5 PLF using a collagen sponge supplemented with Oxy1332 or rhBMP-2 | A. DMSO only (control) (n = 7) | No motion bilaterally via manual palpation at 8 wk post-operatively | A. 0% (0/7) |
| B. 5-μg rhBMP-2 (n = 8) | B. 100% (8/8) | ||||
| C. 0.2-mg Oxy133 (n = 8) | C. 0% (0/8) | ||||
| D. 2-mg Oxy133 (n = 8) | D. 50% (4/8) | ||||
| E. 20-mg Oxy133 (n = 7) | E. 86% (6/7) | ||||
| Scott et al[31], 2015 (V) | Rabbit (n = 22) | L4-L5 PLF using iliac crest autograft supplemented with Oxy133 or rhBMP-2 | A. Saline only (control) (n = 5) | No motion bilaterally via manual palpation at 8 wk post-operatively | A. 0% (0/5) |
| B. 30-μg rhBMP-2 (n = 6) | B. 83.3% (5/6) | ||||
| C. 20-mg Oxy133 (n = 6) | C. 83.3% (5/6) | ||||
| D. 60-mg Oxy133 (n = 5) | D. 80% (4/5) | ||||
| Buser et al[33], 2017 (V) | Rat (n = 64) | L3-L5 posterolateral fusion (PLF) using a collagen sponge supplemented with Oxy133 and/or rhBMP-2 | A. DMSO only (control) (n = 32, 8 rats/4 segments each) | No motion at the operated segment via manual palpation at 8 wk post-operatively | A. 0% (0/32) |
| B. 0.5-µg rhBMP-2 (n = 32, 8 rats/4 segments each) | B. 69% (22/32) | ||||
| C. 5-µg rhBMP-2 (n = 32, 8 rats/4 segments each) | C. 100% (32/32) | ||||
| D. 5-mg Oxy133 (n = 32, 8 rats/4 segments each) | D. 100% (32/32) | ||||
| E. 20-mg Oxy133 (n = 32, 8 rats/4 segments each) | E. 100% (32/32) | ||||
| F. 0.5-µg rhBMP2 + 5-mg Oxy133 (n = 32, 8 rats/4 segments each) | F. 100% (32/32) | ||||
| G. 0.5-µg rhBMP2 + 20-mg Oxy133 (n = 32, 8 rats/4 segments each) | G. 100% (32/32) | ||||
| H. 5-µg rhBMP2 + 20-mg Oxy133 (n = 32, 8 rats/4 segments each) | H: 100% (32/32) | ||||
Oxy4 and Oxy34 are identical.
Oxy21 and Oxy133 are identical. PLF: Posterolateral inter-transverse process spinal fusion; rhBMP-2: Recombinant human bone morphogenetic protein-2; DMSO: Dimethyl sulfoxide.
Table 2.
Descriptive summaries of the identified studies investigating the effect of oxysterols on bone formation in critical-sized alveolar defect models (n = 2)
| Ref. (level of evidence) | Species (n) | Surgical model and scaffold(s) used | Experimental groups (n) | Assessment method and time point | Bone regeneration (%):1 | |
| Lee et al[35], 2017 (V) | Rat (n = 9) | Regeneration of critical-size alveolar defects (left and right maxillary first molars) using equimolar amounts of 20(S)-OHC and 22(S)-OHC (dissolved in 1% DMSO in PBS) or rhBMP-2 in solution | A. DMSO only (control) (n = 3) | Bone regeneration as assessed via µ-CT at 15 days of healing. | A. Approximately 45% | |
| B. 15-µg rhBMP-2 (n = 3) | B. Approximately 53% | |||||
| C. 0.40-µg of 20(S)-OHC and 22(S)-OHC (n = 3) | C. Approximately 65% | |||||
| Bakshi et al[37], 2019 (V) | Rat (n = not reported) | Regeneration of a critical-size alveolar defect (7 mm × 4 mm × 3 mm) using a collagen sponge supplemented with rhBMP-2 or a mixture of 20(S)-OHC and 22(R)-OHC oxysterols | A. No treatment | Bone regeneration as assessed via µ-CT at 8 weeks of healing. | A. Approximately 65% | |
| Collagen sponge | B. No additive | B. Approximately 72% | ||||
| C. 12.5-µg of rhBMP-2 | C. Approximately 92%a | |||||
| D. 20-mg of 20(S)-OHC and 22(R)-OHC | D. Approximately 90%a | |||||
Percent bone regeneration of critical-sized defect.
P < 0.05, compared to collagen sponge only and to no treatment. rhBMP-2: Recombinant human bone morphogenetic protein-2; OHC: Hydroxycholesterol; DMSO: Dimethyl sulfoxide; PBS: Phosphate buffer solution; µ-CT: Micro-computed tomography.
Table 3.
Descriptive summaries of the identified studies investigating the effect of oxysterols on bone formation in critical-sized calvarial defect models (n = 6)
| Ref. (level of evidence) | Species (n) | Surgical model and scaffold(s) used | Experimental groups (n) | Assessment method and time point | Bone regeneration (%):1 |
| Aghaloo et al[17], 2007 (V) | Rat (n = 15) | Regeneration of critical-sized, bilateral parietal defects (5-mm) using PLGA (porosity: 92%) scaffolds supplemented with 20(S)-OHC and 22(S)-OHC. | A. PBS only (control) (n = 12 defects) | Bone regeneration as assessed via µ-CT at 6 weeks of healing. | A. approximately 12% |
| B. 70-ng 20(S)-OHC + 70-ng 22(S)-OHC (n = 9 defects) | B. approximately 21%a | ||||
| C. 700-ng 20(S)-OHC + 700-ng 22(S)-OHC (n = 9 defects) | C. approximately 22%a | ||||
| Hokugo et al[30], 2014 (V) | Rat (n = not reported) | Regeneration of critical-sized, right parietal defects (5-mm) using a gelatin hydrogel soaked with 20(S)-OHC or incorporating 20(S)-OHC-containing micelles. | A. No implantation (empty defect) (n = 4 defects) | Percent bone regeneration was not reported; however, via μ-CT observation at 6 wk, “no” bone regeneration was observed in untreated defects, “some” bone formation was noted in the periphery of defects implanted with gelatin hydrogel and gelatin hydrogel + 20(S)-OHC, and “robust” bone formation was observed in defects treated with gelatin hydrogel + 20(S)-OHC-micelle. | |
| B. Gelatin hydrogel alone (n = 4 defects) (mass not reported) | |||||
| C. 20(S)-OHC-gelatin hydrogel (without micelle) (n = 4 defects) (mass not reported) | |||||
| D. 20(S)-OHC-micelle-gelatin hydrogel (n = 4 defects) (mass not reported) | |||||
| Hokugo et al[32], 2016 (V) | Rabbit (n = 25) | Regeneration of critical-sized cranial defect (6-mm; 4 defects/cranium) using a collagen sponge supplemented with rhBMP-2 and/or Oxy49. | A. No treatment (empty defect) (n = 5) | Bone regeneration as assessed via µ-CT at 6 weeks of healing. | A. approximately 10%a |
| B. Collagen sponge only (n = 5) | B. approximately 35% | ||||
| C. 75-μg rhBMP-2 (n = 5) | C. approximately 65%a | ||||
| D. 1-mg Oxy49 (n = 5) | D. approximately 55%a | ||||
| E. 10-mg Oxy49 (n = 5) | E. approximately 65%a | ||||
| Li et al[36], 2017 (V) | Rabbit (n = not reported) | Regeneration of critical-sized cranial defect (8-mm; 4 defects/cranium) using a collagen sponge supplemented with Oxy1332 and/or rhBMP-2. | A. No treatment (n ≥ 6 defects) | Percent bone regeneration was not reported; however, via micro-CT analysis at 6 wk, significantly greater bone formation was observed following treatment with either 10-mg Oxy133 or 7-μg rhBMP-2 relative to treatment with collagen alone. | |
| B. Collagen sponge only (n ≥ 6 defects) | |||||
| C. 7-μg rhBMP-2 (n ≥ 6 defects) | |||||
| D. 1-mg Oxy133 (n ≥ 6 defects) | |||||
| E. 10-mg Oxy133 (n ≥ 6 defects) | |||||
| Cui et al[34], 2017 (V) | Mouse (n = 12) | Regeneration of critical-sized, right side cranial defects (3-mm) using MeGC hydrogels supplemented with BMSCs and either SA/Cholesterol or SA/20(S)-OHC sterosomes. | A. No treatment (empty defect) (n = 4) | Bone regeneration as assessed via µ-CT at 6 wk of healing. | A. 7% |
| B. SA/Cholesterol sterosome (n = 4) (mass not reported) | B. 31% | ||||
| C. SA/20(S)-OHC sterosome (n = 4) (mass not reported) | C. 61%a | ||||
| Huang et al[38], 2019 (V) | Rabbit (n = 6) | Regeneration of critical-sized cranial defect (6-mm; 4 defects/cranium) using an inorganic bovine bone graft (Bio-Oss) supplemented with SVA and/or 20(S)-OHC. | A. No supplementation (Bio-Oss only; control) (n = 6 defects) | Percent bone regeneration was not reported; however, via histological analysis at 4 wk, bone regeneration was increased following treatment with SVA, 20(S)-OHC, and, especially, SVA + 20(S)-OHC, relative to no supplementation (Bio-Oss only control). A synergistic effect was observed with combinatorial treatment of SVA and 20(S)-OHC. | |
| B. 0.5-mg of SVA (n = 6 defects) | |||||
| C. 1.0-μg of 20(S)-OHC (n = 6 defects) | |||||
| D. 0.5-mg of SVA + 1.0-μg of 20(S)-OHC (n = 6 defects) | |||||
Percent bone regeneration of critical-sized defect.
Oxy133 and Oxy21 are identical.
P < 0.05 compared to PBS only (Aghaloo et al[17]), collagen sponge only (Hokugo et al[32]), or no treatment (empty defect) (Cui et al[34]). rhBMP-2: Recombinant human bone morphogenetic protein-2; PLGA: Poly(lactic-co-glycolic acid); SVA: Simvastatin; OHC: Hydroxycholesterol; µ-CT: Micro-computed tomography; MeGC: Methacrylated chitosan; SA: Stearylamine; BMSCs: Bone marrow-derived mesenchymal stem/stromal cells.
Table 4.
Summary of unique oxysterols investigated for in vivo bone tissue engineering in animal models
| Oxysterol name | Change from cholesterol1 | Effect on in vivo bone formation (defect model) | Ref. |
| 20(S)-OHC | OH group at C20(S) | Increase2 (alveolar and calvarial) | Aghaloo et al[17], 2007; Hokugo et al[30], 2014; Lee et al[35], 2017; Cui et al[34], 2017; Bakshi et al[37], 2019; Huang et al[38], 2019 |
| 22(R)-OHC | OH group at C22(R) | Increase2 ,3 (alveolar) | Bakshi et al[37], 2019 |
| 22(S)-OHC | OH group at C22(S) | Increase2 ,3 (calvarial) | Aghaloo et al[17], 2007 |
| Oxy4/Oxy34 | OH group at C20(S), single bond between C5 and C6, OH group at C6(S) | Increase2 (spinal fusion) | Johnson et al[21], 2011; Stappenbeck et al[20], 2012 |
| Oxy18 | OH group at C20(S), single bond between C5 and C6, OH group at C6(S), deuterated carbons at C22 and C23 | Increase4 (spinal fusion) | Stappenbeck et al[20], 2012 |
| Oxy21/Oxy133 | OH group at C20(S), single bond between C5 and C6, OH group at C6(S), n-hexane at C20(S) | Increase2 (spinal fusion, calvarial) | Stappenbeck et al[20], 2012; Montgomery et al[19], 2014; Scott et al[31], 2015; Li et al[36], 2015; Buser et al[33], 2017 |
| Oxy49 | OH group at C20(S), single bond between C5 and C6, OH group at C6(S), double bond between C25 and C27 | Increase2 (spinal fusion, calvarial) | Johnson et al[21], 2011; Hokugo et al[32], 2016 |
(S) and (R) denote stereoisomerism. Refer to Figure 3 for molecular structures.
Statistically significant relative to control.
Used in combination with 20(S)-OHC.
Significantly greater bone formation relative to Oxy4/Oxy34 (internal positive control). OHC: Hydroxycholesterol.
Quality assessment
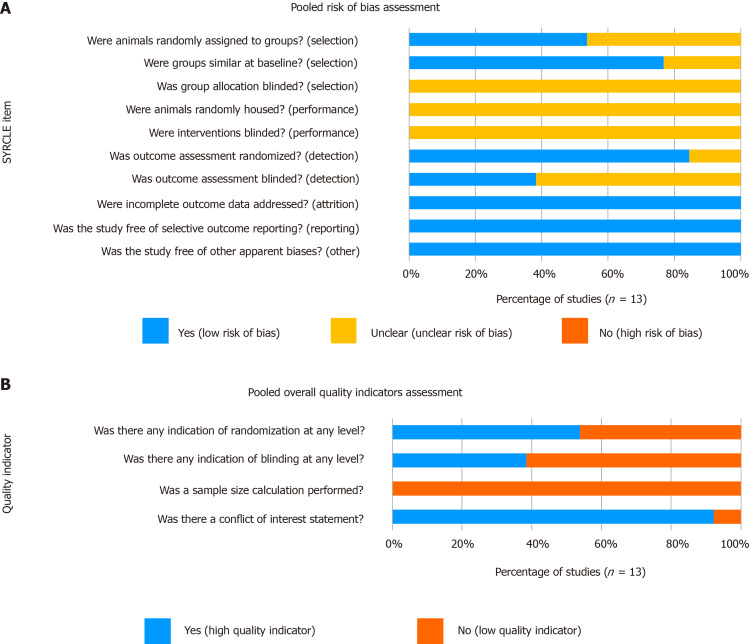
Methodological quality assessment of the included studies is summarized in Figure 2. Though all included studies were found to have a low risk of both attrition and reporting biases, they all presented an “unclear risk of bias” with regard to group allocation and intervention blinding. Additionally, 62% of studies had an “unclear risk” of assessment blinding bias, creating an overall unclear risk of bias among the included studies. Considering quality indicators, 54% of studies reported randomization at any level and 38% reported blinding at any level. Although no study provided a sample size calculation, 92% provided conflict of interest statements that at minimum identified sources of funding.
Figure 2.
Quality assessment summary of the included studies (n = 13). A: SYRCLE risk of bias items; B: Overall quality indicators.
Oxysterols in spinal fusion models
Descriptive summaries of the identified spinal fusion studies are provided in Table 1. Of these 5 studies (n = 213 animals), four employed rat models (n = 191) and one utilized a rabbit model (n = 22). All studies used posterolateral inter-transverse process lumbar fusion models with four employing a one-level model (L4-L5) and the fifth utilizing a two-level model (L3-L5). For grafting, all studies used a collagen sponge scaffold, with the exception of Scott et al[31], who employed iliac crest autograft. In total, four unique oxysterols were evaluated: Oxy4/Oxy34, Oxy18, Oxy21/Oxy133, and Oxy49 (Oxy4 and Oxy34 are identical, and Oxy21 and Oxy133 are identical).
Oxysterols significantly augmented arthrodesis rates in all four studies with a negative control group, with Oxy4/Oxy34 and Oxy21/Oxy133 showing a dose-dependent effect. Furthermore, in all four studies with a rhBMP-2 positive control group, similar osteoinductive effects were observed between rhBMP-2- and oxysterol-treated animals (Table 1). These findings are most strongly highlighted by Johnson et al[21], who utilized a collagen scaffold supplemented with 0.2, 2, or 20 mg of Oxy34 in a rat model[21]. These different doses led to reported fusion rates of 0%, 50%, and 100% (10 animals per group), respectively. The oxysterol group receiving the highest dose had an identical fusion rate to controls treated with 5 µg of rhBMP-2, suggesting non-inferiority. Both agents significantly improved fusion relative to that seen in negative controls treated with inert dimethyl sulfoxide (DMSO). Similar findings were reported by Montgomery et al[19], who reported fusion rates of 0%, 50%, and 86% using a collagen scaffold supplemented with 0.2, 2, and 20 mg of Oxy133, respectively, compared to rates of 100% using rhBMP-2 and 0% using a control solution. Across the five identified studies, fusion rates of 100% were achieved in some cases with the use of Oxy34, Oxy49, and Oxy133. Stappenbeck et al[20] reported significantly larger bone volumes using Oxy21 (Oxy133) relative to Oxy4 (Oxy34) in a rat spinal fusion model.
Oxysterols in critical-sized alveolar defect models
Table 2 contains descriptive summaries of the 2 studies examining oxysterols in critical-sized alveolar defect models, both of which employed rat models. Lee et al[35] employed a two-site defect model and injected solutions of DMSO, rhBMP-2, or a combination of 20(S)-hydroxycholesterol and 22(S)-hydroxycholesterol into the extracted sockets. These treatments produced mean bone regeneration of 45%, 53%, and 65%, respectively[35]. While suggesting oxysterols to have osteoinductive properties, the regeneration rates were not significantly different across groups.
In contrast, using a one-site defect model, Bakshi et al[37] found significant differences in bony regeneration rates among animals treated with rhBMP-2 (92%) or oxysterol-supplemented collagen scaffolds (90%) relative to negative controls that were untreated (65%) or treated with unadulterated collagen scaffolds (72%). Similar to the findings of Johnson et al[21] and Montgomery et al[19] in the spinal fusion models, bony regeneration rates were equivocal in animals treated with rhBMP-2 and those receiving a combination of 20(S)-hydroxycholesterol and 22(R)-hydroxycholesterol et al[37].
Oxysterols in critical-sized calvarial defect models
Descriptive summaries of the critical-sized calvarial defect studies are provided in Table 3. Of the six studies, one employed a mouse model, two employed rat models, and three employed rabbit models. Two studies employed single defect models (one defect per skull), 1 study employed a two-defect model, and 3 studies employed four-defect models. Scaffold materials varied widely across studies and included poly(lactic-co-glycolic acid) polymer, gelatin hydrogel, collagen, methacrylated chitosan (MeGC) hydrogel, and inorganic bovine bone graft. A single study co-implanted bone marrow-derived mesenchymal stromal/stem cells (BMSCs) with oxysterols[34]. In total, four unique oxysterols were evaluated: 20(S)-hydroxycholesterol, 22(S)-hydroxycholesterol, Oxy49, and Oxy133.
As seen in Table 3, the studies cumulatively suggested oxysterols significantly increase bone regeneration relative to control treatments. The largest study by Hokugo et al[32] compared bony regeneration rates in 25 rabbits receiving no treatment or treatment with a collagen sponge. Animals treated with collagen sponges supplemented by either oxysterols or rhBMP-2 had improved bony regeneration rates relative to controls, with the 1 mg Oxy49, 10 mg of Oxy49, and 75 µg rhBMP-2 groups having mean percent bone regeneration of approximately 55%, 65%, and 65%, respectively. Animals treated with collagen alone experienced only approximately 35% bone regeneration, which was significantly less than all treated groups[32].
Similarly, in a murine single-defect model, Cui et al[34] directly compared bony regeneration in untreated animals to those treated with MeGC hydrogel scaffolds supplemented with BMSCs and sterosomes containing either cholesterol or 20(S)-hydroxycholesterol. Bone regeneration was greater in animals treated with 20(S)-hydroxycholesterol (61%) versus cholesterol (31%), and both provided better bony regeneration than was seen in untreated controls (7%)[34]. The above results combined with the four remaining studies showed significant osteoinductive effects in animals treated with 20(S)-hydroxycholesterol, 22(S)-hydroxycholesterol, Oxy49, and Oxy133.
Osteoinductive effects by specific oxysterol
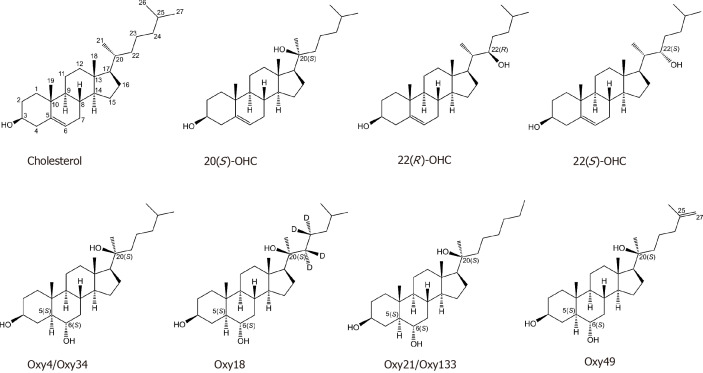
The osteoinductive effects seen across all studies are summarized for each specific oxysterol in Table 4. To date, seven oxysterols have been investigated, all of which have been shown to enhance bone formation in at least one in vivo model. Of these seven oxysterols, 20(S)-hydroxycholesterol, 22(R)-hydroxycholesterol, and 22(S)-hydroxycholesterol are naturally occurring species, where the remaining four are synthetic analogues designed for enhanced osteoinductive properties (Figure 3).
Figure 3.
Molecular structures of the identified oxysterols that have been investigated for in vivo bone tissue engineering in animal models. Cholesterol is shown for reference. OHC: Hydroxycholesterol.
DISCUSSION
Globally, millions of patients undergo bone grafting each year[1,2]. Though autologous bone remains the “gold standard” for grafting procedures, it is by its very nature in limited supply and does not ensure optimal bony outcomes. Additionally, the harvest of orthotopic autograft (e.g., iliac crest graft) is associated with increased surgical risk and donor-site complications, making it a less-than-ideal option in more medically complex patients[2-5]. Consequently, there exists an obvious need for implantable bone substitute materials, the creation of which is the focus of bone tissue engineering[9,10]. Despite prodigious efforts by the field, relatively few commercially viable substitutes have been generated that are capable of providing outcomes equivalent or superior to autograft. One such product is the rhBMP-2 treated collagen sponge (Infuse®, Medtronic Spinal & Biologics; Memphis, TN), which has been used in hundreds of thousands of patients and generated billions of dollars in revenue since its market introduction in 2002[12]. Along these same lines, though, it can significantly increase care costs, has limited FDA-approved indications, possesses a worrisome side effect profile, and does not ensure optimal bony outcomes[14,15]. Consequently, there remains an outstanding need for additional osteoinductive and osteoconductive agents, of which oxysterols are one promising candidate currently undergoing preclinical investigations.
Here, we present the first systematic review of the available evidence for oxysterols as osteoinductive agents. Our analysis included 13 preclinical in vivo studies investigating 7 unique oxysterols in three common animal models of bone healing – spinal fusion, calvarial critical-sized defect, and alveolar critical-sized defect. In aggregate these studies found that oxysterols produced significantly greater arthrodesis rates and bony regeneration relative to the rates seen in untreated animals. Additionally, in the subset of studies directly comparing oxysterol-treated animals to those treated with rhBMP-2 containing scaffolds, non-inferior outcomes were seen in the oxysterol group, suggesting that they may have clinically comparable osteoinductive properties to rhBMP-2. Importantly, though, these properties appear to be stereoisomer-specific[39].
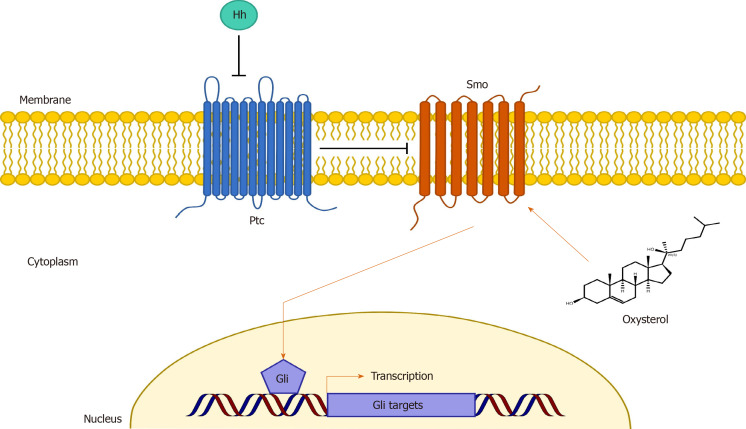
A visual representation of the proposed mechanism by which oxysterols exert their osteoinductive effects is illustrated in Figure 4. At present, it is believed that oxysterol-mediated osteoinduction occurs via activation of the Hedgehog (Hh) signaling pathway, a signaling pathway known to be essential for normal bone development[19,20,31,34-37,40,41]. Though multiple signaling cascades have been demonstrated for Hh signaling, canonical signaling involves the interaction of the transmembrane proteins Patched (Ptc) and Smoothened (Smo)[40]. Under normal circumstances, Ptc binds to and represses Smoothened, thereby inhibiting Smo signaling. But upon binding Hh ligand, Ptc is inactivated, allowing for Smo activation[40]. Activated Smo increases levels of the active forms of Gli transcription factors, which translocate to the nucleus and upregulate multiple genes, including those key for bone formation[40]. It is well-known that Smo activity can be modulated by small molecules[42,43], and more recent evidence has suggested oxysterols may directly activate Smo, thereby promoting Hh signaling[39,44]. Consistent with this, it has been shown that treatment of bone marrow-derived MSCs with both sonic Hh (a member of the Hh ligand family) and Oxy133 results in synergistic activation of Hh signaling and upregulation of osteogenic differentiation markers in vitro[19]. As a possible negative feedback mechanism, it is thought that activated Ptc transports oxysterols away from Smo to maintain Smo inactivation[44].
Figure 4.
Conceptual illustration of the proposed primary mechanism by which oxysterols exert their osteoinductive effects. It is believed that oxysterol-mediated osteoinduction occurs via activation of the Hedgehog (Hh) signaling pathway, which is known to be essential for normal bone development. In canonical Hh signaling, the transmembrane protein Patched (Ptc) inhibits Smoothened (Smo) activity. However, upon binding Hh ligand, Ptc is inactivated, allowing for Smo activation. Activated Smo increases levels of the active forms of Gli transcription factors, which translocate to the nucleus and upregulate multiple genes, including those keys for bone formation. In a stereoisomer-specific manner, it is believed that oxysterols may directly activate Smo, thereby promoting Hh signaling and osteoinduction. Ptc: Patched; Smo: Smoothened; Hh: Hedgehog.
Conventional growth factors, such as rhBMP-2, have several limitations that render them suboptimal for many clinical applications. In general, they are limited by high dose requirements, poor shelf-stability, low in vivo half-life, and high costs[45-47]. They are also potentially immunogenic and have low diffusion potential owing to their large size[45-47]. By contrast, as small molecules, oxysterols are attractive alternatives. Small molecules are low molecular weight (< 1000 Dalton) organic compounds that are generally highly diffusible, allowing for better tissue penetration than growth factors. These small molecules may additionally be designed to activate the same intracellular signaling cascades as growth factors[45-47]. Small molecules also frequently possess the advantages of high shelf-stability and lower manufacturing cost, making them financially advantageous relative to peptides. Unlike peptides, small molecules are not prone to denaturation and accordantly can be transported more easily with lower logistical costs, making them potentially more desirable from an efficacy and cost perspective[45]. Furthermore, small molecules are less immunogenic[46] and are highly amenable to chemical modification, allowing for better control of their bioactivity and tissue targeting[19,21,48]. Lastly, the pharmaceutical industry has greater experience with small molecules and there is therefore a concordantly larger knowledge base from which targeted delivery mechanisms can be developed. These mechanisms can reduce the likelihood of off-target effects and optimize pharmacokinetic properties for optimal therapeutic effect[45-47].
For all of the above reasons, interest in small molecules for bone tissue engineering has been growing rapidly, with a near-exponential rise in articles published on this topic since the early 2000s[46]. It is likely that this literature will continue to expand, as high-throughput screening allows for the concomitant testing of thousands of compounds, accelerating the identification of osteogenic compounds[45,49,50]. To exemplify this, Genthe et al[51] recently used high-throughput screening of a library of approximately 600000 small molecules to identify a new class of osteogenic small molecules, called “ventromorphins”. These previously undescribed agents were found to directly activate BMP signaling[51]. Other groups around the world have used similar techniques to identify promising small molecules for bone tissue engineering across a wide-range of drug classes[50,52-55]. Future studies are necessary to evaluate the osteoinductive effects and safety profiles of these and other small molecules. Yet it is likely that some of these osteogenic small molecules – potentially oxysterols – will see clinical translation for the purposes of augmenting in vivo bone formation. Whether this is as an adjunct or alternative to growth-factor based therapies remains unclear.
There are several limitations to our study. First, our analysis was limited to a primary outcome of macroscopic evidence of bony fusion or regeneration. We did not consider changes in osteoblast, osteocyte, or osteoclast gene expression or proliferation, nor did we consider histological findings. To definitively conclude that it is the oxysterols mediating improved bony healing through osteoinduction we would likely need to include these endpoints. However, said outcomes are not readily available in the in vivo literature and from a clinical perspective, evidence of bone formation via imaging or manual palpation is considered the “gold standard” for the models considered. Furthermore, we were unable to perform a meta-analysis secondary to the limited and heterogeneous nature of the available data. Along these lines, the data we were able to include in the review was found to have an overall “unclear risk of bias” as assessed by the SYRCLE Risk of Bias tool. Consequently, the generalizability of our findings may be limited. However, given the consistent findings across the included studies, it appears there is sufficient in vivo evidence to support the osteogenic nature of oxysterols. Despite this, the absence of available clinical data means that we are unable to determine the clinical effectiveness of these agents in mediating in vivo bone formation. Given these limitations, it is apparent that additional, high-quality research is needed to more fully evaluate the efficacy, safety, and cost-effectiveness of oxysterols on bone tissue engineering.
In conclusion, oxysterols appear to be promising osteoinductive small molecules with potential for bone tissue engineering in the fields of orthopaedic surgery, neurosurgery, and oral maxillofacial surgery. The present preclinical literature has an unclear risk of bias, but the presence of positive outcomes across multiple animal species and surgical models suggests there is sufficient evidence to explore their application in larger animal models and eventually early phase clinical trials. Based upon prior in vitro work and the observation of dose-dependency, it appears that the observed effects are secondary to oxysterol-mediated upregulation of osteogenic gene products. Future work is necessary however to identify the therapeutically optimal oxysterol and to optimize its pharmacokinetic properties for use in orthopaedic surgery.
ARTICLE HIGHLIGHTS
Research background
Bone grafting is performed on millions of patients each year, and autologous bone remains the “gold standard” bone graft material. However, autologous bone is limited in supply, is associated with increased surgical risk and donor-site complications and does not ensure optimal bony outcomes. Consequently, there exists an obvious need for implantable bone substitute materials, the creation of which is the focus of bone tissue engineering.
Research motivation
Recently, oxysterols – oxygenated derivatives of cholesterol – have been proposed as a novel class of osteoinductive small molecules for bone tissue engineering. However, there has yet to be a review examining the effect of oxysterols on in vivo bone formation. To address this, here we provide the first systematic review of the effect of oxysterols on in vivo bone formation as a means of evaluating the potential therapeutic utility of oxysterols for bone tissue engineering.
Research objectives
Following PRISMA guidelines, we aimed to systematically review the available literature examining the effect of oxysterols on in vivo bone formation.
Research methods
Using the PubMed/MEDLINE, Embase, and Web of Science databases, we queried all publications in the English-language literature investigating the effect of oxysterols on in vivo bone formation. Articles were screened for eligibility using PICOS criteria and assessed for potential bias using an expanded version of the SYRCLE Risk of Bias assessment tool. All full-text articles examining the effect of oxysterols on in vivo bone formation were included. Extracted data included: Animal species, surgical/defect model, description of therapeutic and control treatments, and method for assessing bone growth. Primary outcome was fusion rate for spinal fusion models and percent bone regeneration for critical-sized defect models. Data were tabulated and described by both surgical/defect model and oxysterol employed. Additionally, data from all included studies were aggregated to posit the mechanism by which oxysterols may mediate in vivo bone formation.
Research results
Thirteen studies (all preclinical) met our inclusion/exclusion criteria. Of the 13 included studies, 5 employed spinal fusion models, 2 employed critical-sized alveolar defect models, and 6 employed critical-sized calvarial defect models. Based upon SYRCLE criteria, the included studies were found to possess an overall “unclear risk of bias”; 54% of studies reported treatment randomization and 38% reported blinding at any level. Overall, seven unique oxysterols were evaluated: 20(S)-hydroxycholesterol, 22(R)-hydroxycholesterol, 22(S)-hydroxycholesterol, Oxy4/Oxy34, Oxy18, Oxy21/Oxy133, and Oxy49. All had statistically significant in vivo osteoinductive properties, with Oxy4/Oxy34, Oxy21/Oxy133, and Oxy49 showing a dose-dependent effect in some cases. In the eight studies that directly compared oxysterols to rhBMP-2-treated animals, similar rates of bone growth occurred in the two groups. Biochemical investigation of these effects suggests that they may be primarily mediated by direct activation of Smoothened in the Hedgehog signaling pathway.
Research conclusions
Present preclinical evidence suggests oxysterols significantly augment in vivo bone formation. Based upon prior in vitro work and the observation of dose-dependency, it appears that the observed effects are secondary to oxysterol-mediated upregulation of osteogenic gene products. The present preclinical literature has an unclear risk of bias, but the presence of positive outcomes across multiple animal species and surgical models suggests there is sufficient evidence to explore their application in larger animal models and eventually early phase clinical trials.
Research perspectives
Oxysterols appear to be promising osteoinductive small molecules with potential for bone tissue engineering in the fields of orthopaedic surgery, neurosurgery, and oral maxillofacial surgery. Future work is necessary to identify the therapeutically optimal oxysterol and to optimize its pharmacokinetic properties for use in orthopaedic surgery.
Footnotes
Conflict-of-interest statement: The authors declare no relevant conflicts of interest. Cottrill E receives non-study-related grant support from National Institute on Aging. Theodore N is a consultant for Globus and receives royalties from Globus and Depuy Synthes; Sciubba D is a consultant for Baxter, DePuy Synthes, Globus, K2M, Medtronic, NuVasive, and Stryker, and receives non-study-related grant support from Baxter, North American Spine Society, and Stryker. Witham T is a consultant for DePuy Synthes, is an advisory board member and shareholder of Augmedics, and receives non-study-related grant support from the Gordon and Marilyn Macklin Foundation.
PRISMA 2009 Checklist statement: PRISMA guidelines were followed for this systematic review.
Manuscript source: Unsolicited manuscript
Peer-review started: January 14, 2020
First decision: April 22, 2020
Article in press: July 1, 2020
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cartmell SH, Yukata K S-Editor: Dou Y L-Editor: A E-Editor: Wang LL
Contributor Information
Ethan Cottrill, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States. ecottri1@jhmi.edu.
Julianna Lazzari, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
Zach Pennington, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
Jeff Ehresman, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
Andrew Schilling, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
Naomi Dirckx, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
Nicholas Theodore, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
Daniel Sciubba, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
Timothy Witham, Department of Neurosurgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
References
- 1.Brown A, Stock G, Patel AA, Okafor C, Vaccaro A. Osteogenic protein-1: a review of its utility in spinal applications. BioDrugs. 2006;20:243–251. doi: 10.2165/00063030-200620040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36 Suppl 3:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin P, Li DJ, Auston DA, Mir HS, Yoon RS, Koval KJ. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J Orthop Trauma. 2019;33:203–213. doi: 10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez de Grado G, Keller L, Idoux-Gillet Y, Wagner Q, Musset AM, Benkirane-Jessel N, Bornert F, Offner D. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng. 2018;9:2041731418776819. doi: 10.1177/2041731418776819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honeybul S, Morrison DA, Ho KM, Lind CR, Geelhoed E. A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty. J Neurosurg. 2017;126:81–90. doi: 10.3171/2015.12.JNS152004. [DOI] [PubMed] [Google Scholar]
- 7.Hsu WK, Nickoli MS, Wang JC, Lieberman JR, An HS, Yoon ST, Youssef JA, Brodke DS, McCullough CM. Improving the clinical evidence of bone graft substitute technology in lumbar spine surgery. Global Spine J. 2012;2:239–248. doi: 10.1055/s-0032-1315454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumetti S, Galli C, Manfredi E, Consolo U, Marchetti C, Ghiacci G, Toffoli A, Bonanini M, Salgarelli A, Macaluso GM. Correlation between density and resorption of fresh-frozen and autogenous bone grafts. Biomed Res Int. 2014;2014:508328. doi: 10.1155/2014/508328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatara AM, Mikos AG. Tissue Engineering in Orthopaedics. J Bone Joint Surg Am. 2016;98:1132–1139. doi: 10.2106/JBJS.16.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottrill E, Ahmed AK, Lessing N, Pennington Z, Ishida W, Perdomo-Pantoja A, Lo SF, Howell E, Holmes C, Goodwin CR, Theodore N, Sciubba DM, Witham TF. Investigational growth factors utilized in animal models of spinal fusion: Systematic review. World J Orthop. 2019;10:176–191. doi: 10.5312/wjo.v10.i4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaidya R. Transforaminal interbody fusion and the "off label" use of recombinant human bone morphogenetic protein-2. Spine J. 2009;9:667–669. doi: 10.1016/j.spinee.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Hustedt JW, Blizzard DJ. The controversy surrounding bone morphogenetic proteins in the spine: a review of current research. Yale J Biol Med. 2014;87:549–561. [PMC free article] [PubMed] [Google Scholar]
- 15.James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev. 2016;22:284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo KW, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev. 2012;64:1277–1291. doi: 10.1016/j.addr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aghaloo TL, Amantea CM, Cowan CM, Richardson JA, Wu BM, Parhami F, Tetradis S. Oxysterols enhance osteoblast differentiation in vitro and bone healing in vivo. J Orthop Res. 2007;25:1488–1497. doi: 10.1002/jor.20437. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths WJ, Wang Y. Oxysterol research: a brief review. Biochem Soc Trans. 2019;47:517–526. doi: 10.1042/BST20180135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery SR, Nargizyan T, Meliton V, Nachtergaele S, Rohatgi R, Stappenbeck F, Jung ME, Johnson JS, Aghdasi B, Tian H, Weintraub G, Inoue H, Atti E, Tetradis S, Pereira RC, Hokugo A, Alobaidaan R, Tan Y, Hahn TJ, Wang JC, Parhami F. A novel osteogenic oxysterol compound for therapeutic development to promote bone growth: activation of hedgehog signaling and osteogenesis through smoothened binding. J Bone Miner Res. 2014;29:1872–1885. doi: 10.1002/jbmr.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stappenbeck F, Xiao W, Epperson M, Riley M, Priest A, Huang D, Nguyen K, Jung ME, Thies RS, Farouz F. Novel oxysterols activate the Hedgehog pathway and induce osteogenesis. Bioorg Med Chem Lett. 2012;22:5893–5897. doi: 10.1016/j.bmcl.2012.07.073. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JS, Meliton V, Kim WK, Lee KB, Wang JC, Nguyen K, Yoo D, Jung ME, Atti E, Tetradis S, Pereira RC, Magyar C, Nargizyan T, Hahn TJ, Farouz F, Thies S, Parhami F. Novel oxysterols have pro-osteogenic and anti-adipogenic effects in vitro and induce spinal fusion in vivo. J Cell Biochem. 2011;112:1673–1684. doi: 10.1002/jcb.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WR, Ishikawa T, Umetani M. The interaction between metabolism, cancer and cardiovascular disease, connected by 27-hydroxycholesterol. Clin Lipidol. 2014;9:617–624. doi: 10.2217/clp.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luu W, Sharpe LJ, Capell-Hattam I, Gelissen IC, Brown AJ. Oxysterols: Old Tale, New Twists. Annu Rev Pharmacol Toxicol. 2016;56:447–467. doi: 10.1146/annurev-pharmtox-010715-103233. [DOI] [PubMed] [Google Scholar]
- 24.Kha HT, Basseri B, Shouhed D, Richardson J, Tetradis S, Hahn TJ, Parhami F. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J Bone Miner Res. 2004;19:830–840. doi: 10.1359/JBMR.040115. [DOI] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooijmans CR, Hlavica M, Schuler FAF, Good N, Good A, Baumgartner L, Galeno G, Schneider MP, Jung T, de Vries R, Ineichen BV. Remyelination promoting therapies in multiple sclerosis animal models: a systematic review and meta-analysis. Sci Rep. 2019;9:822. doi: 10.1038/s41598-018-35734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PM, Buchan A, van der Worp HB, Traystman R, Minematsu K, Donnan GA, Howells DW. Good laboratory practice: preventing introduction of bias at the bench. Stroke. 2009;40:e50–e52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- 30.Hokugo A, Saito T, Li A, Sato K, Tabata Y, Jarrahy R. Stimulation of bone regeneration following the controlled release of water-insoluble oxysterol from biodegradable hydrogel. Biomaterials. 2014;35:5565–5571. doi: 10.1016/j.biomaterials.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Scott TP, Phan KH, Tian H, Suzuki A, Montgomery SR, Johnson JS, Atti E, Tetratis S, Pereira RC, Wang JC, Daubs MD, Stappenbeck F, Parhami F. Comparison of a novel oxysterol molecule and rhBMP2 fusion rates in a rabbit posterolateral lumbar spine model. Spine J. 2015;15:733–742. doi: 10.1016/j.spinee.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hokugo A, Sorice S, Parhami F, Yalom A, Li A, Zuk P, Jarrahy R. A novel oxysterol promotes bone regeneration in rabbit cranial bone defects. J Tissue Eng Regen Med. 2016;10:591–599. doi: 10.1002/term.1799. [DOI] [PubMed] [Google Scholar]
- 33.Buser Z, Drapeau S, Stappenbeck F, Pereira RC, Parhami F, Wang JC. Effect of Oxy133, an osteogenic oxysterol, on new bone formation in rat two-level posterolateral fusion model. Eur Spine J. 2017;26:2763–2772. doi: 10.1007/s00586-017-5149-9. [DOI] [PubMed] [Google Scholar]
- 34.Cui ZK, Kim S, Baljon JJ, Doroudgar M, Lafleur M, Wu BM, Aghaloo T, Lee M. Design and Characterization of a Therapeutic Non-phospholipid Liposomal Nanocarrier with Osteoinductive Characteristics To Promote Bone Formation. ACS Nano. 2017;11:8055–8063. doi: 10.1021/acsnano.7b02702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JS, Kim E, Han S, Kang KL, Heo JS. Evaluating the oxysterol combination of 22(S)-hydroxycholesterol and 20(S)-hydroxycholesterol in periodontal regeneration using periodontal ligament stem cells and alveolar bone healing models. Stem Cell Res Ther. 2017;8:276. doi: 10.1186/s13287-017-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li A, Hokugo A, Segovia LA, Yalom A, Rezzadeh K, Zhou S, Zhang Z, Parhami F, Stappenbeck F, Jarrahy R. Oxy133, a novel osteogenic agent, promotes bone regeneration in an intramembranous bone-healing model. J Tissue Eng Regen Med. 2017;11:1490–1499. doi: 10.1002/term.2047. [DOI] [PubMed] [Google Scholar]
- 37.Bakshi R, Hokugo A, Zhou S, Zhang Z, Wang L, Rezzadeh K, Segovia LA, Jarrahy R. Application of Hydroxycholesterols for Alveolar Cleft Osteoplasty in a Rodent Model. Plast Reconstr Surg. 2019;143:1385–1395. doi: 10.1097/PRS.0000000000005528. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Lin Y, Rong M, Liu W, He J, Zhou L. 20(S)-hydroxycholesterol and simvastatin synergistically enhance osteogenic differentiation of marrow stromal cells and bone regeneration by initiation of Raf/MEK/ERK signaling. J Mater Sci Mater Med. 2019;30:87. doi: 10.1007/s10856-019-6284-0. [DOI] [PubMed] [Google Scholar]
- 39.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Andre P, Ye L, Yang YZ. The Hedgehog signalling pathway in bone formation. Int J Oral Sci. 2015;7:73–79. doi: 10.1038/ijos.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 42.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCabe JM, Leahy DJ. Smoothened goes molecular: new pieces in the hedgehog signaling puzzle. J Biol Chem. 2015;290:3500–3507. doi: 10.1074/jbc.R114.617936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharpe HJ, de Sauvage FJ. Signaling: An oxysterol ligand for Smoothened. Nat Chem Biol. 2012;8:139–140. doi: 10.1038/nchembio.774. [DOI] [PubMed] [Google Scholar]
- 45.Aravamudhan A, Ramos DM, Nip J, Subramanian A, James R, Harmon MD, Yu X, Kumbar SG. Osteoinductive small molecules: growth factor alternatives for bone tissue engineering. Curr Pharm Des. 2013;19:3420–3428. doi: 10.2174/1381612811319190008. [DOI] [PubMed] [Google Scholar]
- 46.Laurencin CT, Ashe KM, Henry N, Kan HM, Lo KW. Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug Discov Today. 2014;19:794–800. doi: 10.1016/j.drudis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo KW, Ashe KM, Kan HM, Laurencin CT. The role of small molecules in musculoskeletal regeneration. Regen Med. 2012;7:535–549. doi: 10.2217/rme.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merk D, Friedrich L, Grisoni F, Schneider G. De Novo Design of Bioactive Small Molecules by Artificial Intelligence. Mol Inform. 2018:37. doi: 10.1002/minf.201700153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han CY, Wang Y, Yu L, Powers D, Xiong X, Yu V, Nguyen Y, Jean DJ, Jr, Babij P. Small molecules with potent osteogenic-inducing activity in osteoblast cells. Bioorg Med Chem Lett. 2009;19:1442–1445. doi: 10.1016/j.bmcl.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 50.Brey DM, Motlekar NA, Diamond SL, Mauck RL, Garino JP, Burdick JA. High-throughput screening of a small molecule library for promoters and inhibitors of mesenchymal stem cell osteogenic differentiation. Biotechnol Bioeng. 2011;108:163–174. doi: 10.1002/bit.22925. [DOI] [PubMed] [Google Scholar]
- 51.Genthe JR, Min J, Farmer DM, Shelat AA, Grenet JA, Lin W, Finkelstein D, Vrijens K, Chen T, Guy RK, Clements WK, Roussel MF. Ventromorphins: A New Class of Small Molecule Activators of the Canonical BMP Signaling Pathway. ACS Chem Biol. 2017;12:2436–2447. doi: 10.1021/acschembio.7b00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook B, Rafiq R, Lee H, Banks KM, El-Debs M, Chiaravalli J, Glickman JF, Das BC, Chen S, Evans T. Discovery of a Small Molecule Promoting Mouse and Human Osteoblast Differentiation via Activation of p38 MAPK-β. Cell Chem Biol. 2019;26:926–935.e6. doi: 10.1016/j.chembiol.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradford STJ, Ranghini EJ, Grimley E, Lee PH, Dressler GR. High-throughput screens for agonists of bone morphogenetic protein (BMP) signaling identify potent benzoxazole compounds. J Biol Chem. 2019;294:3125–3136. doi: 10.1074/jbc.RA118.006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vrijens K, Lin W, Cui J, Farmer D, Low J, Pronier E, Zeng FY, Shelat AA, Guy K, Taylor MR, Chen T, Roussel MF. Identification of small molecule activators of BMP signaling. PLoS One. 2013;8:e59045. doi: 10.1371/journal.pone.0059045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alves H, Dechering K, Van Blitterswijk C, De Boer J. High-throughput assay for the identification of compounds regulating osteogenic differentiation of human mesenchymal stromal cells. PLoS One. 2011;6:e26678. doi: 10.1371/journal.pone.0026678. [DOI] [PMC free article] [PubMed] [Google Scholar]