Abstract
Background
The aim of this study was to analyze the clinical application of cortex phellodendri compound fluid (CPCF) in the treatment of diabetic foot ulcers.
Material/Methods
From January 2012 to December 2015, a total of 720 cases of diabetic foot ulcers (DFU) were randomly assigned into an experimental group (n=540) that was treated by CPCF and a control group (n=180) that was treated by a Kangfuxin solution (KFS). After 4 weeks of treatment, their ulcer area, serum growth factor, clinical total effective rate, and incidence of adverse events were assessed.
Result
There were 720 patients who completed the trial. The experimental group was superior to the control group in reducing ulcer area, increasing growth factor content, and total effective rate (P<0.05). There was no significant difference in the adverse events rates between the 2 groups.
Conclusion
CPCF external treatment of diabetic foot ulcer can promote ulcer healing and increase the concentration of growth factors, and it is safe and reliable.
MeSH Keywords: Diabetic Foot; Lipid Metabolism Disorders; Medicine, Chinese Traditional
Background
Diabetic foot (DF) is one of the crucial complications of diabetic patients. The World Health Organization (WHO) defines diabetes combined with lower-extremity sensory neuropathy, microcirculation ischemia resulting in infection, ulcers, and deep tissue damage as DF [1]. There are about 150 million diabetic patients worldwide, of which 15% have diabetic foot ulcers (DFU) [2]. About 80% of diabetic foot amputations are caused by improper treatment of foot ulcers [3]. Statistical analysis revealed that DFU patients had a higher amputation risk than non-diabetic patients [4]. With the advancement of medicine, especially the good effect of traditional Chinese medicine treatment of DFU, domestic and foreign scholars are paying increasing attention to research on DFU, which is of great value in preventing foot ulcers and avoiding amputation [5]. Clinical studies have shown that topical administration of Cortex phellodendri compound fluid (CPCF) can reduce the purulent secretions of wounds, alleviate pain, promote inflammatory resolution of wounds, and effectively promote wound healing in DFU treatment [5–10].
CPCF consists of Huangbai (Phellodendron Chinese Schneid), Lianqiao (Forsythia suspensa), Jinyinhua (Lonicera japonica Thunb), Pugongying (Taraxacum mongolicum Hand.-Mazz), and Wugong (Scolopendra). TCM uses Huangbai to treat conditions such as cirrhosis, chronic hepatitis, and tuberculosis. In addition to treating these diseases, it also is used for diarrhea and detoxification. In vitro experiments have shown that Huangbai is effective against staphylococcus aureus, pneumococcal, diphtheria, streptococcus viridans, and shigella dysenteriae. The effects of Lianqiao are detoxifying, reducing swelling, and dispersing. Jinyinhua can treat skin itching such as eczema, and alleviates swollen ulcer, erysipelas, cellulitis, and other symptoms. Pugongying can be used for a variety of infections and suppurative diseases. Wugong has detoxification, anti-cancer, and anti-inflammatory effects. Those allowed CPCF to have the effects of promoting angiogenesis, wound healing, anti-inflammatory and improving immunity [11].
The pathogenesis of DF is multi-faceted and complicated, and there are many theories about the cause. In recent years, the toxicity of DF has become the focus of research. The accumulation of advanced glycosylation end products (AGEs) in skin tissue has led to the change of histological cytology behavior has become an important research topic [12]. After many years of clinical practice and basic experiments, our department has attained reliable results in the treatment of DFU with traditional Chinese medicine. Traditional Chinese medicine believes that damp heat and toxicity are the main pathogenic factors of DFU. CPCF can clear away dampness, heat, and toxins, promoting wound healing, and reduces the amputation rate [13]. Kangfuxin solution (KSF) can significantly promote the growth of granulation tissue, promote angiogenesis, accelerate the shedding of necrotic tissue, and repair ulcers when used externally. It has been shown to heal diabetic foot ulcers [14,15]. In this study, we conducted a multicenter clinical trial to evaluate the clinical efficacy and safety of CPCF compared with KSF in DFU treatment.
Material and Methods
General information
According to the Guiding Principles for Variety Protection of Traditional Chinese Medicines (TCM), 720 DFU patients who met the inclusion criteria from January 2012 to December 2015 were studied, including 136 cases from the Peripheral Vascular Department of Dongzhimen Hospital (Beijing University of Chinese Medicine), 120 from the Ulcerous Vascular Surgical Department of Beijing Hospital of TCM, 120 from the Surgical Department of the First Teaching Hospital of Tianjin University of TCM, 120 from the Surgical Department of Luoyang No. 1 Hospital of TCM, 80 from the Surgical Department of Shaanxi University of Chinese Medicine Hospital, 80 from the Surgical Department of Hubei Hospital of TCM, and 64 cases from the Surgical Department of Handan Hospital of TCM. The patients were randomly assigned for the 4-week treatment to an experimental group (CPCF, n=540) or a control group (KFS group, n=180) using a random-number generator. The effective sample size was calculated based on Lehr’s formula, and the α and 1-β values are set at 0.05% and 80%, respectively.
Diagnostic criteria
Diagnostic criteria of DFU were based on the “Second Diabetic Foot Conference, Diabetes Association of Chinese Medical Association in 2000: the appearance of acral skin blisters, ulcers, erosions, gangrene and or necrosis in diabetic patients”.
The standard of TCM differentiation included the following primary factors: ulcer area, ulcer depth, redness and swelling around the ulcer, granulation conditions, and pus condition; and the following secondary symptoms: pain, burning around the ulcer, thirst, dry stool, dark urine, red tongue or tongue with yellow coating, and rapid pulse [16].
Inclusion and exclusion criteria
Inclusion criteria were: (1) DFU patient age 30–75 years; (2) wound area ≤10 cm2; (3) signed informed consent, volunteered to participate in this investigation as required, and attended follow-up appointments. Exclusion criteria were: (1) Serious local necrosis and amputation was required; (2) skin ulcers caused by radiation or other reason; (3) patients with severe infection; (4) other serious diseases such as cancer or with short expected life; (5) liver and kidney dysfunction, ALT >60; (6) severe malnutrition; (7) mental disorders; (8) pregnant or intending to become pregnant; (9) alcohol or drug addiction; (10) allergic constitution or patients who were allergic to CPCF; (11) participating in other trials or had participated within the past 4 weeks.
Intervention methods
The basic treatment for each patient included control of blood sugar, low-salt and low-fat diet, reasonable regular exercise, anti-infection measures, nourishing the nerves, and improving microcirculation.
Topical treatment with iodine was applied to disinfect the wound, eliminate metamorphic and necrotic tissue as soon as possible, and rinse the wound with saline. Then, external treatment was applied, in which the ulcers in the experimental group were covered with gauze soaked with CPCF (Shandong Hanfang Pharmaceutical Co. (100 ml/bottle; code number approved by SFDA: Z10950097; batch number: 1208301), and the dose of CPCF was in accordance with the instructions. For deeper DFU, 20 ml of CPCF was used to rinse the wound with a syringe. External treatment was applied once daily. For the control group, the ulcer was treated as above but with KFS (Sichuan Good Doctor Panxi Pharmaceutical Co. (100 ml/bottle; code number approved by SFDA: Z51021834, batch number: 1206060), and the dose of KFS was in accordance with the instructions. During the 4 weeks of the study, patients did not receive DFU treatment with any other drugs, and all patients enrolled in the study completed 4 weeks of treatment.
Observation index
Wound area
The ulcers of both groups were photographed before treatment (day 0) and on days 7, 14, 21, and 28 after treatment, and the corresponding ulcer area was measured by Image Pro Plus image processing software by the same clinician, who did not know the patient grouping.
Growth factor indexes
The serum quantities of VEGF (vascular endothelial growth factor), EGF (epidermis growth factor), and bFGF (basic fibroblast growth factor) were tested by enzyme-linked immunosorbent assay (ELISA) kits (VEGF, DRE64578–201306, EGF, DRE67548–201306 and bFGF, DRE75893–201306) acquired from Biolianshuo Co. (www.biolianshuo.com). Clinical efficacy assessment was based on primary symptoms and secondary symptoms [17].
The primary symptoms were scored as P0: sore area healed, sore depth healed, granulation wound healed, and no pus and no swelling round sore; P2: sore area ≤3 cm2, deep epidermis ulcer, red granulation, pus-like serum secretion and swelling round the edge of the wound <0.5 cm; P4: sore area 4–6 cm2, dermis ulcer, pink granulation, thick pus and swelling around the edge of the wound 0.5–1 cm and P6: sore area >6 cm2, sores deep in the skin, granulation edema, pus volume, contamination and swelling round the edge of the wound >1 cm [16].
The secondary symptoms were scored as S0: no pain, no thirst, no dry stool, and no dark urine; S1: mild pain without disturbing work and daily life, thirst, dry stool, and dark urine; S2: moderate pain disturbing work and daily life and S3: obvious pain, disturbing work and daily life, bed rest required.
The treatment efficacy was calculated by syndrome score (%), which is syndrome scores before treatment minus after treatment/scores before treatment. Recovery designated as reduction of syndrome score ≥90% with disappearance or basic disappearance of clinical symptoms and signs (CSS); efficiency designated as 70% reduction of syndrome score ≥70% with obvious improvement of CSS (<90%); valid designated as reduction of syndrome score ≥30% and <70% of improvement of CSS; invalid was the reduction of syndrome score <30% with no improvement of CSS, or even aggravated.
Adverse events
Adverse reactions during the study were mainly occasional hypoglycemia, ulcerative itching, or wound bleeding.
Statistical methods
Statistical analysis using SPSS 22.0 was performed for each index. The results are shown as mean±standard deviation (Mean±SD). Normal distribution and homogeneity of variance were assessed by the Kruskal-Wallis test. Statistical differences between the groups were calculated by t test. One-way ANOVA followed by Newman-Keus test (for F value <0.05 among the analyzed data) was used to estimate the statistical significance among the different time points in the 2 groups. P<0.05 was set as statistically significant.
Results
Baseline information
Trial group consisted of 540 cases, including 292 males and 248 females, ages 53–74 years, with an average of 63.7±9.56 years. The duration of disease was 1–26 years with an average of 13.21±9.83 years. The control group included 180 cases (105 males and 75 females) ages 51–75 years, with an average of 63.12±0.53 years. The duration of disease was 3–24 years, with an average of 13.54±8.55 years. No significant differences were found in male/female ratio, age, or duration between the 2 groups
Comparison of total clinical efficacy
The total efficacy rate for the experimental group was of 96.48%, and 66.67% for the control group, showing that clinical efficacy was significantly higher in the experimental group (P=0.0004, Table 1).
Table 1.
Comparison of total clinical efficacy between 2 groups before and after treatment.
| Groups | Recovery (%) | Efficiency (%) | Valid (%) | Invalid (%) | Total clinical efficacy |
|---|---|---|---|---|---|
| Control (KFS) | 21 (11.67) | 67 (37.22) | 32 (17.78) | 60 (33.33) | 66.67% |
| Experimental (CPCF) | 189 (35.00) | 242 (44.81) | 90 (16.67) | 19 (3.52) | 96.48%* |
P=0.00004 compared to KFS group.
Comparison of primary and secondary syndrome scores before and after treatment
There was no significant difference in the total score or the score of the original syndrome and the score of the secondary syndrome in the 2 groups before intervention. After 4 weeks of treatment, the total scores (15.83±1.78 vs. 9.62±2.21), the primary syndrome score (13.32±1.32 vs. 8.48±1.21), and the secondary syndrome score (2.51±0.11 vs. 1.42±0.43) of the experimental group were better than those of the control group (P<0.05, Figure 1).
Figure 1.
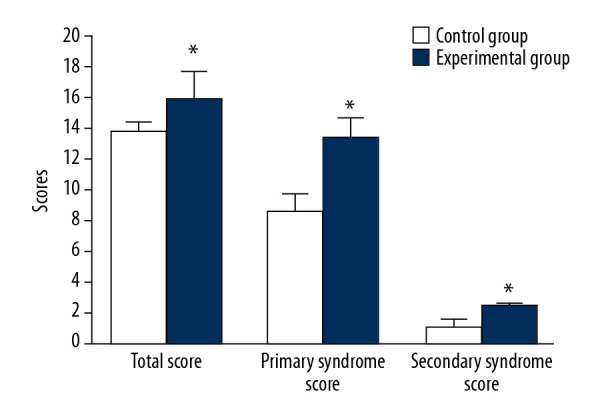
Comparison of main and secondary syndrome scores before and after treatment. * P<0.05 compared to the KFS group.
Comparison of changes in average area of wounds
The average wound area in both groups decreased with prolonged treatment time (Figure 2). The mean wound area in the control group were 7.73±2.11 cm2, 6.83±2.95 cm2 and 5.66±2.58 cm2 on days 0, 7, and 14 of treatment, respectively, and there was no significant difference in mean wound area observed between days of treatment. The average wound area of the control group on day 21 (4.42±2.87 cm2) and day 28 (2.78±3.32 cm2) began to be significantly smaller than that on days 0, 7, and 14 (Figure 2, * P<0.05). In the experimental group, the average wound area was 7.58 ±2.13 cm2 and 6.48±3.02 cm2 on days 0 and 7, respectively, and there was no significant change between time points. However, the average area of wounds on day 14 (3.83±3.13 cm2), 21 (2.39±2.53 cm2), and day 28 (1.18±2.49 cm2) began to be significantly smaller than that on days 0 and 7 (** P<0.05). There were significant differences in the average wound area between days 14, 21, and 28 (# P<0.05). There were significant differences in the average area of wounds between the 2 groups on days 14, 21, and 28 (# P<0.05). Figure 3 shows representative cases before, during, and after CPCF and KFS treatments.
Figure 2.
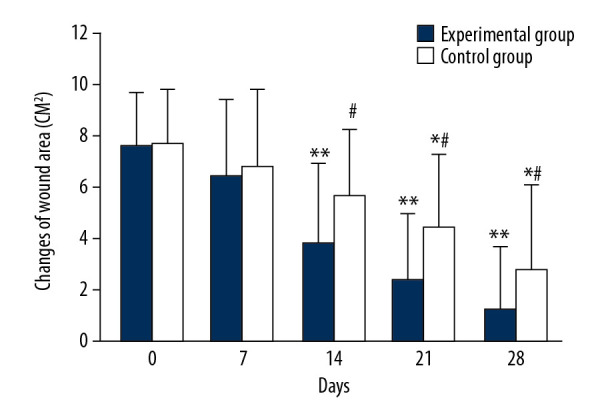
Comparison of changes in average area of wounds. * P<0.05 compared to day 0, 7 and 14 of KFS group; ** P<0.05 compared to day 0 and 7 of CPCF group; # P<0.05 compared to KFS group on the same day.
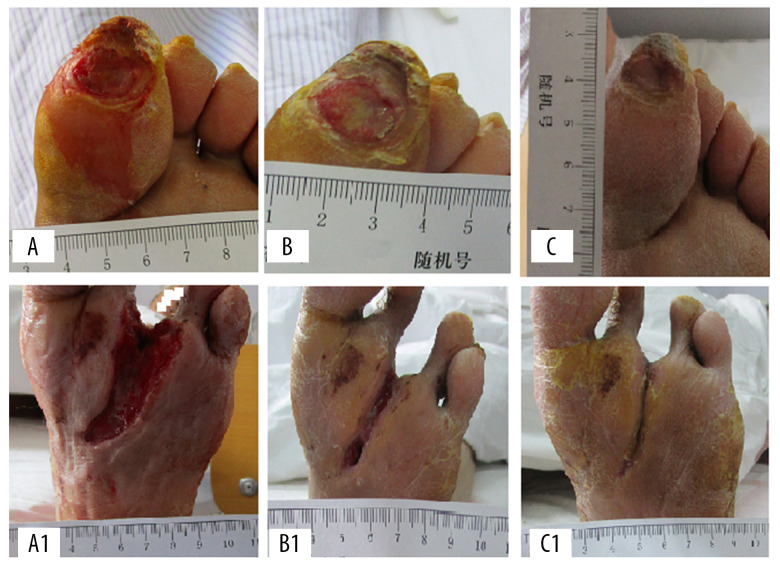
Figure 3.
The representative cases before, during, and after CPCF and KFS treatments. (A) DFU before treatment. (B) DFU treated by KFS for 14 days. (C) DFU treated by KFS for 28 days. (A1) DFU before treatment. (B1) DFU treated by CPCF for 14 days. (C1) DFU treated by CPCF for 28 days.
Comparison of VEGF changes during treatment
The mean serum VEGF concentration of the control group on days 0, 7, 14, 21, and 28 were 87.13±7.11 ng/ml, 102.05±4.36 ng/ml, 116.0±7.24 ng/ml, 124.1±8.09 ng/ml, and 144.9±6.94 ng/ml, respectively, and there were significant differences between each tested day (Figure 4, * P<0.05). In the experimental group, the average serum VEGF levels on days 0, 7, 14, 21, and 28 were 85.3±7.27 ng/ml, 110.32±7.78 ng/ml, 135.18±7.21 ng/ml, 152.37±8.64 ng/ml, and 162.5±7.18 ng/ml, respectively, and there were significant differences between each test day (Figure 4, ** P<0.05). There were significant differences in mean VEGF values between the 2 groups on days 7, 14, 21, and 28 (Figure 4, # P<0.05).
Figure 4.
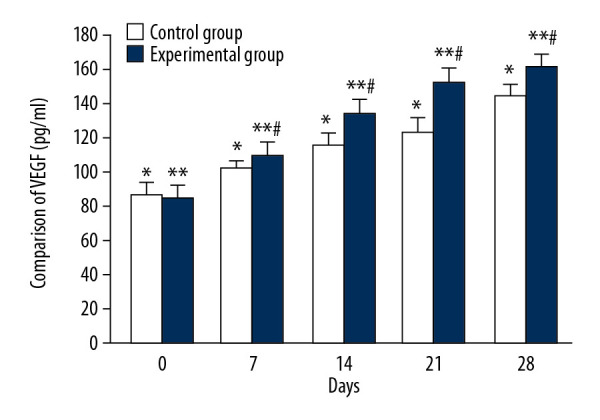
Comparison of VEGF changes between CPCF and KFS groups. * P<0.05 compared to each tested day in KFS group; ** P<0.05 compared to each tested day of CPCF group; # P<0.05 compared to KFS on the same day.
Comparison of EGF changes during treatment
The mean serum EGF levels of the control group on days 0, 7, 14, 21, and 28 were 419.51±10.32 ng/ml, 439.75±19.21 ng/ml, 556.27±24.76 ng/ml, 623.24±20.43 ng/ml, and 732.25±23.78 ng/ml, respectively, and there were significant differences between each tested day (Figure 5, * P<0.05). The mean serum EGF concentration of the experimental group on days 0, 7, 14, 21, and 28 were 424.18±11.41 ng/ml, 551.66±10.17 ng/ml, 721.82±21.27 ng/ml, 810.17±20.65 ng/ml, and 948.53±27.74 ng/ml, respectively, and there were significant differences between each tested day (Figure 5, ** P<0.05). There were significant differences in mean EGF values between the 2 groups on days 7, 14, 21, and 28 (Figure 5, # P<0.05).
Figure 5.
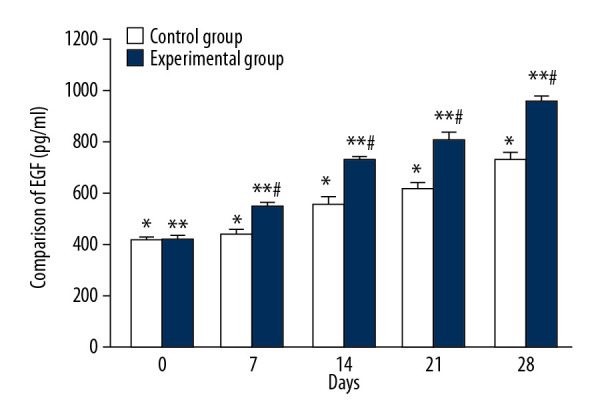
Comparison of EGF changes between CPCF and KFS during treatment. * P<0.05 compared to each tested day of KFS group; ** P<0.05 compared to each tested day of CPCF group; # P<0.05 compared to KFS group on the same day.
Comparison of bFGF changes during treatment
The average serum bFGF levels of the control group on days 0, 7, 14, 21, and 28 were 4.29±1.12 ng/ml, 5.23±1.71 ng/ml, 9.53±1.23 ng/ml, 10.74±1.14 ng/ml, and 11.52±1.41 ng/ml, respectively, and there were significant differences between each tested day (Figure 6, * P<0.05). The mean serum bFGF levels of the experimental group on days 0, 7, 14, 21, and 28 were 5.14±1.09 ng/ml, 9.65±1.78 ng/ml, 14.3±1.43 ng/ml, 19.25±1.67 ng/ml, and 21.35±1.19 ng/ml, respectively, and there were significant differences between each tested day (Figure 6, ** P<0.05). There were significant differences in the mean bFGF values between the 2 groups on days 7, 14, 21, and 28 (Figure 6, # P<0.05).
Figure 6.
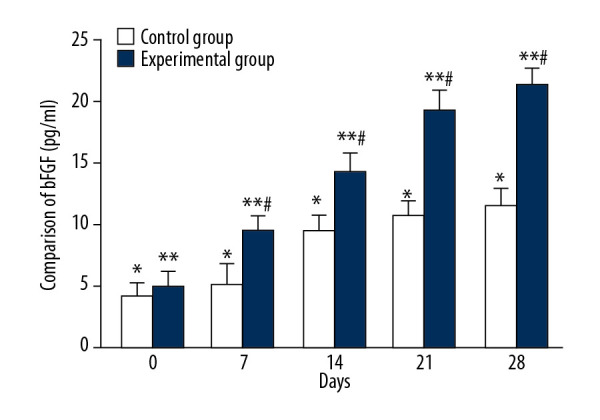
Comparison of bFGF changes between CPCF and KFS groups during treatment. * P<0.05 compared to each tested day of KFS group; ** P<0.05 compared to each tested day of CPCF group; # P<0.05 compared to KFS group on the same day.
Comparison of adverse events
There was no significant difference observed in adverse events rates between the experimental and control groups. In line with clinical judgment, no adverse events were related to treatment (Table 2).
Table 2.
Comparison of adverse events between the 2 groups during treatment.
| Groups | Total events (%) | Hypoglycemia (%) | Pruritus on the ulcer surface (%) | Bleeding on the ulcer surface (%) |
|---|---|---|---|---|
| Control (KFS) | 17 (9.44) | 6 (3.33) | 9 (5.00) | 2 (1.11) |
| Experimental (CPCF) | 51 (9.44) | 16 (2.96) | 29 (5.37) | 6 (1.11) |
Discussion
The incidence, disability rate, and treatment cost of DFU are all high, and the incidence rate is increasing yearly [17]. TCM believes that DFU is the dysfunction of spleen qi and kidney yang deficiency, as well as the invasion of cold and dampness, and the main disease mechanism is qi blood stagnation and channel obstruction. As the course of the disease becomes longer, ulcers of the extremities appear due to blood yin deficiency and loss of nutrition of the limbs. In recent years, TCM has continuously demonstrated its advantages in internal and external treatment of diseases, in which TCM has been widely applied to systemic or topical treatment of diabetes and its long-term complications, with good results. CPCF treatment is becoming more widely used in DFU. The present study discovered that the total effective rate of CPCF for topical administration of DFU was 96.48%, which was considerably better than KFS (66.67%), indicating that the drug has an obvious clinical effect on DFU. After 4 weeks of intervention, the total scores, the primary syndrome score, and the secondary syndrome score in the CPCF group were considerably lower than in the KFS group. From the perspective of wound healing, the ulcer area of both groups was narrower than before treatment. For the reduction of DFU area, CPCF was superior to KFS. In comparison of changes in mean wound area, we found that the experimental group showed significant differences starting on the second week, while the control group showed significant differences on the third week. Compared to KFS, CPCF treatment showed a faster effect in DUF treatment and the combination of basic therapy and topical CPCF therapy is an effective method for treating DFU.
The serum growth factor concentrations of both groups after treatment were significantly higher than before treatment. VEGF plays an important role in promoting vascular growth. EGF can stimulate the repair and regeneration of damaged epidermis. bFGF has a vital role in angiogenesis, promoting wound healing, tissue repair, tissue regeneration, and neural tissue growth and development. Our data showed that the levels of VEGF, EGF, and bFGF in the experimental and control groups were significantly higher than before treatment on different days after CPCF treatment. The serum level of growth factor in the experimental group was significantly increased at 2 weeks, and it was significantly higher than in the control group at the same time point. The control serum growth factor concentration showed a significant increase at 3 weeks, indicating that CPCF has a faster effect than KFS.
Recent pharmacological findings have revealed that the berberine in Huangbai has antibacterial and anti-ulcer effects. Jinyinhua has a strong inhibitory effect on pathogenic microorganisms, and it has anti-inflammatory and antipyretic effects and enhances immunity. Forsythia in Lianqiao has antibacterial activity. Pugongying has strong antibacterial, anti-tumor, and immune functions. Wugong has obvious analgesic and anti-inflammatory effects.
The consequences of a diabetic foot infection, especially a deep infection, are more severe than infections in other parts of the body. Because of the anatomical features of the foot, infection can spread from one area to another, and the lack allows the patient to continue to walk and further expand infection. About 15% of diabetic patients have DFU; 15–20% of these need amputation and almost 85% have amniotic foot ulcers before amputation [18–20]. Patients with DFU tend to have diabetes-related complications and comorbidities.
It has been pointed out that the main underlying cause is ischemia caused by peripheral neuropathy and peripheral vascular disease. In DFU, free-tissue grafting is usually the last treatment applied before amputation, so optimizing the preoperative plan to prevent flap rupture is critical. Preoperative lower-extremity arteriography helps to diagnose peripheral vascular diseases and helps select a suitable intervention with a lower incidence of complications [21]. CPCF treatment has broad-spectrum antibacterial activity, which can quickly eliminate swelling, reduce secretions, and accelerate wound healing [22,23]. CPCF also can stimulate various growth factors in the process of wound repair to assist in many biological effects such as chemotaxis, synthetic secretion, proliferation and differentiation, induction of apoptosis, and stimulation of angiogenesis, thereby promoting wound healing [24]. The results revealed no significant difference in the incidence of adverse events between CPCF and KFS. CPCF is effective and safe in DFU treatment. The weaknesses of this study are its short observation time (4 weeks), short follow-up time, and small number of cases. Future research will increase the number of cases and observe long-term effects of CPCF.
Conclusions
In summary, in the external treatment of DFU, CPCF can promote ulcer healing and increase the serum concentration of growth factors, and it is safe and reliable in clinical external use.
Footnotes
Ethics approval and consent to participate:
This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of our hospital on Oct. 20, 2012. (No. ECSL-BDY-2012-43). This was an interventional study registered with the Chinese Clinical Trial Registry (chiCTR-IPR-15007182) on Oct. 11, 2015.
Availability of data and material
The data are available from the corresponding author upon request.
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Al-Busaidi IS, Mason R, Lunt H. Diabetic charcot neuroarthropathy: The diagnosis must be considered in all diabetic neuropathic patients presenting with a hot, swollen foot. N Z Med J. 2015;128(1424):77–80. [PubMed] [Google Scholar]
- 2.Pabón-Carrasco M, Juárez-Jiménez JM, Reina-Bueno M, et al. Behavior of provisional pressure-reducing materials in diabetic foot. J Tissue Viability. 2016;12(16):20–26. doi: 10.1016/j.jtv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Walsh JW, Hoffstad OJ, Sullivan MO, Margolis J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2015;33(11):1493–98. doi: 10.1111/dme.13054. [DOI] [PubMed] [Google Scholar]
- 4.Williams ML, Holewinski JE. Use of a human acellular dermal wound matrix in patients with complex wounds and comorbidities. J Wound Care. 2015;24(6):261–67. doi: 10.12968/jowc.2015.24.6.261. [DOI] [PubMed] [Google Scholar]
- 5.Zhou LJ, Li BH. [Comparison of the clinical efficacy between compound fluid of cortex phellodendri and povidone in the treatment of infected wounds in diabetic foot]. Chi J Bioche Pharmac. 2016;36(5):171–73. [in Chinese] [Google Scholar]
- 6.Zhang W, Yu ZJ, Jin L. [Efficacy analysis of heat-dispelling, toxin-removal, circulation-boosting formulation combined with compound fluid of cortex phellodendri in diabetic foot patients]. Chi J Experi Trad Med Formulae. 2016;22(12):177–81. [in Chinese] [Google Scholar]
- 7.Hou XL, Xu J, Wang PH, et al. [Clinical efficacy analysis of compound fluid of cortex phellodendri as supplemental therapy for diabetic foot ulcers]. Chi J Experi Trad Med Formulae. 2016;22(12):159–63. [in Chinese] [Google Scholar]
- 8.Li YS, Yang FH. [Effects of compound fluid of cortex phellodendri on inflammatory and growth factors during external therapy of diabetic foot ulcers]. Chi J New Drugs. 2014;23(10):1163–66. [in Chinese] [Google Scholar]
- 9.Yao J, Zhao X. [Advance in clinical application of compound Phellodendron solution]. Chi J New Drugs. 2014;3:308–12. [in Chinese] [Google Scholar]
- 10.Wang N, Ju S, Yang FH, et al. [Efficacy and safety of compound Cortex Phellodendri Liquid for diabetic foot ulcer: A meta-analysis]. Chi J New Drugs. 2018;27(15):1771–75. [in Chinese] [Google Scholar]
- 11.Zhang SF, Jia ZH, Wu JH, et al. [Effects on wound healing and microbicidal activity of compound fluid of cortex phellodendri on skin wounds in rabbits]. Chi J New Drugs. 2016;11:1330–32. [in Chinese] [Google Scholar]
- 12.Lu SL, Qing C, Xie T, et al. [Study on the mechanism of “hidden damage” in diabetic skin]. Chi J Trauma. 2004;20:468–71. [in Chinese] [Google Scholar]
- 13.Liao XB, Zhu T, Lin JX. [A general survey of the mechanism of topical Chinese medicine to promote wound healing]. J Trad Chi Med. 2010;25:861. [in Chinese] [Google Scholar]
- 14.Zhao JY, Yang ZH. [Observation of the effect of MEBO Wound Ulcer Patch and Kangfuxin solution chronic skin ulcer]. Chinese and Foreign Med Res. 2020;18(08):147–14. [in Chinese] [Google Scholar]
- 15.Lan Y. Observation on the effect of insulin plus Kangfuxin solution on diabetic foot. The Med Forum. 2016;20(22):3118. [in Chinese] [Google Scholar]
- 16.State administration of traditional Chinese medicine. Chinese medicine industry standard (ZY/T001.1.1-001.9-94) diagnostic efficacy standard for TCM syndrome. Nanjing: Nanjing Univ Press; 1994. pp. 243–44. [Google Scholar]
- 17.Xu ZR, Ran XW, Xian Y, et al. Ertapenem versus piperacillin/tazobactam for diabetic foot infections in China: A phase 3 multicentre, randomized, double-blind, active-controlled, non-inferiority trial. J Antimicrob Chemother. 2016;71(6):1688–96. doi: 10.1093/jac/dkw004. [DOI] [PubMed] [Google Scholar]
- 18.Palumbo PJ, Melton LJ. Peripheral vascular disease and diabetes. In: Harris MI, Hamman RF, editors. Diabetes in America. Washington: US Government Printing Office. NIH Pub; 1985. pp. 16–21. [Google Scholar]
- 19.Pendsey S. Diabetic foot: A clinical atlas. Jaypee Brothers Medical Publishers; 2003. [Google Scholar]
- 20.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: Basis for prevention. Diabetes Care. 1990;13:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 21.Janhofer DE, Lakhiani C, Kim PJ, et al. The utility of preoperative arteriography for free flap planning in patients with chronic lower extremity wounds. Plast Reconstr Surg. 2019;143(2):604–13. doi: 10.1097/PRS.0000000000005265. [DOI] [PubMed] [Google Scholar]
- 22.Zhao FJ, Zhang XY. [Efficacy observation of 30 patients with chronic lower limb ulcers treated with compound fluid of cortex phellodendri]. Yunnan J Trad Chi Med. 2014;35(7):56. [in Chinese] [Google Scholar]
- 23.Li SS, Zhang ZY, Li RH, et al. [Clinical efficacy observations of compound fluid of cortex phellodendri as wet compress in the treatment of Stage II and III pressure ulcers]. Chi J Clin Rational Drug Use. 2014;7(8):43–44. [in Chinese] [Google Scholar]
- 24.Tang QL, Han SS, Fu J, et al. [Research on the effects of MEBT/MEBO on VEGF, bFGF, and mRNA expression during the wound healing process in skin]. J Youjiang Med Univ Natio. 2012;34(5):597–601. [in Chinese] [Google Scholar]