Abstract
Background
This study was designed to explore the combined effects of repetitive transcranial magnetic stimulation (rTMS) and human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) transplantation on neural stem cell proliferation in rats with spinal cord injury (SCI).
Material/Methods
SCI was induced in 90 rats by laminectomy at T10. Fifteen rats each were treated with 0.5 Hz rTMS or 10 Hz rTMS or underwent hUCB-MSC transplantation; 15 each were treated with 0.5 Hz rTMS+hUCB-MSCs or 10 Hz rTMS+hUCB-MSCs; and 15 were untreated (control group). The Basso, Beattie, and Bresnahan (BBB) scores and motor evoked potentials (MEPs) were measured, and all rats underwent biotin dextran-amine (BDA) tracing of the corticospinal tract (CST). The levels of expression of neural stem cell proliferation related proteins, including BrdU, nestin, Tuj1, Ng2+ and GFAP, were measured, and the levels of bFGF and EGF determined by Western blotting.
Results
BBB scores and MEPs were increased after rTMS and hUCB-MSC transplantation, while histologically determined SCI–induced neuron apoptosis was attenuated. The numbers of BDA-positive fibers and Brdu-, nestin- and Tuj1-positive cells were markedly increased and the numbers of Ng2+- and GFAP-positive cells were markedly decreased following treatment with rTMS alone or rTMS plus hUCB-MSC transplantation. The levels of expression of bFGF and EGF were significantly upregulated following rTMS treatment and hUCB-MSC transplantation. Higher performance was observed after combined treatment with rTMS and hUCB-MSC transplantation than after either alone.
Conclusions
The combination of rTMS treatment and hUCB-MSC transplantation could attenuate SCI-induced neural stem cell apoptosis and motor dysfunction in rats.
MeSH Keywords: Mesenchymal Stromal Cells, Spinal Cord Injuries, Transcranial Magnetic Stimulation
Background
Spinal cord injury (SCI) triggers a variety of acute and chronic disabilities, damaging neurological and pathological plasticity [1,2]. SCI induces a cascade of degenerative events, including motor-sensory dysfunction and defects in autonomic systems [2]. These dysfunctions are mainly caused by limited neuronal regeneration after SCI or acute traumatic injury [1].
Repetitive transcranial magnetic stimulation (rTMS) is a new tool in the treatment of traumatic brain injury, especially for neurological rehabilitation [3]. rTMS has been shown to promote neural stem cell proliferation and may be successful in the treatment of ischemia and traumatic brain injury in rat models [4,5]. The ability of rTMS to protect against brain and neural injuries was found to be related to genetic factors, including microRNA-25 [4] and the expression of brain derived neurotrophic factor (BDNF)-TrkB signaling [6].
Human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) can differentiate into cells of several lineages, with full proliferative potential [7,8]. These cells have therefore been used to treat traumatic brain injuries and SCI [9]. Transplantation of hUCB-MSCs partly improves the outcomes of rats with traumatic brain injury by stimulating neurogenesis and angiogenesis [10].
Patients with SCI who underwent rTMS were found to experience better pain relief than patients who underwent sham rTMS [11]. rTMS was found to promote sensorimotor recovery in incomplete SCI [12], and transplantation of hUCB-MSCs promoted recovery of the damaged function of spinal cord nerves in SCI rats [13]. However, few studies have assessed the effects of rTMS on the transplantation efficacy of hUCB-MSCs and none, to our knowledge, has evaluated whether the combination of rTMS treatment and hUCB-MSCs transplantation could promote neural stem cell proliferation in SCI rats.
This study was designed to assess the individual and combined effects of rTMS and hUCB-MSC transplantation on the proliferation of neural stem cells in rats with SCI. The effects of these treatments on cell apoptosis and proliferation and the recovery of rat motor behavior were evaluated. The results of this study may suggest creative methods for the treatment of SCI.
Material and Methods
Animals and SCI models
All experiments were carried out on adult male Sprague-Dawley rats (body weight 230–250 g) and followed the guidelines of the Institutional Animal Care and use Committee of the Department of Neurosurgery of the First Affiliated Hospital of Zhengzhou University. Rats were pre-anesthetized and SCI was induced by laminectomy at T10 using a modification of the Allen method [14,15]. Rats were injected intramuscularly with prophylactic kanamycin (1 mg/kg) once daily and underwent bladder evacuation every other day [16]. All animal experiments were performed in accordance with institutional guidelines, following a protocol approved by the Ethics Committees of the First Affiliated Hospital of Zhengzhou University, Henan, China.
rTMS and hUCB-MSC transplantation
The 90 rats with SCI were randomly assigned to six groups of 15 rats each. One group was not further treated (Model); one was treated with 0.5 Hz rTMS (pulse frequency, at an intensity of 90% active motor threshold, for 500 ms); one was treated with 10 Hz rTMS, one underwent hUCB-MSC transplantation alone, one was treated with 0.5 Hz rTMS+hUCB-MSCs and one was treated with 10 Hz rTMS+hUCB-MSCs. For rTMS treatment, the rats were placed on a fixator to immobilize the head and body. The center of an elliptical magnetic coil, 80 mm wide, 50 mm high, and 6 mm thick, was placed above the bregma. Each stimulation, at a frequency of 0.5 Hz or 10 Hz, lasted for 3 seconds, followed by a rest for 6 seconds, with each rat receiving a total of 500 stimulations per day.
For hUCB-MSC transplantation, fresh blood samples were collected from newborns after obtaining the mother’s informed consent. The blood samples were anticoagulated with heparin, followed by separation of mononuclear cells by Ficoll-Hypaque (Sigma, USA). The isolated mononuclear cells were diluted to a concentration of 1.0×106/mL and incubated for 5 days at 37°C under 5% CO2 atmosphere in specialized MSC medium (Gibco, USA) [17]. hUCB-MSCs were transplanted on the second day after SCI surgery. Briefly, rats were anesthetized and skin incisions were made [17], and each rate was administered 10 μL of 6.67×104/μL hUCB-MSCs, which was repeated three times.
Behavioral tests
Basso, Beattie, and Bresnahan (BBB) scores and motor evoked potentials (MEPs) were measured as described [18,19]. BBB scores were measured 1 day and 1, 2, 4 and 8 weeks after rTMS and hUCB-MSC administration. Ten rats in each group were sacrificed under anesthesia, and injured spinal cords (T10–T11) were removed for hematoxylin-eosin (HE) staining, western blotting, and immunohistochemistry, with all of these assays performed by two observers unaware of the experimental procedures.
Anterograde biotin dextran-amine (BDA) tract tracing
Five rats in each group were subjected to biotin dextran-amine (BDA) tracing of the corticospinal tracts (CST) [20]. The rats were anesthetized, followed by stereotactic injection of 1 μL 5% BDA (Thermo Fisher, Shanghai, China) into each of eight bregma sites using a Hamilton microliter syringe (Hamilton Instruments, Las Vegas, NV, USA). Three weeks later, the rats were sacrificed, and their brains were removed and sectioned, followed by immunofluorescence staining and analysis of the nerve fibers in the CST using a fluorescence microscope.
Western blotting
Injured spinal cords were cut into pieces and treated with trypsin. The proteins were extracted using RIPA lysis and extraction buffer (Sangon Biotech, China) and their concentrations were measured using a Thermo Scientific microBCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). The proteins were separated on 10% SDS-PAGE and transferred onto Millipore PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked with silk and incubated at 4ºC for 12 h with primary antibodies against bFGF (1: 500 dilution, Millipore), EGF (1: 500 dilution, Millipore), and GAPDH (1: 1,000 dilution, Biogenesis, Bournemouth, UK). After washing, the membranes were incubated with HRP-conjugated secondary antibodies, and immunoreactive bands were analyzed using an ECL kit (Amersham Biosciences, Little Chalfont, UK).
Immunohistochemical analysis and HE staining
For immunohistochemistry assay, spinal cords were fixed with formalin and embedded in paraffin. The specimens were hydrated with ethanol and serially sectioned (5-μm thick) on a rotary paraffin section machine (RM2255, Leica, Wetzlar, Germany). Slides were dewaxed, blocked and incubated overnight at 4°C with primary antibodies against BrdU (1: 500 dilution, Abcam, Cambridge, MA, USA), Nestin (1: 200 dilution, Millipore), Tuj1 (1: 500 dilution, Millipore), Ng2+ (1: 200 dilution, Millipore), and GFAP (1: 500 dilution, Millipore). After three washes with PBS, the slides were incubated with biotin-conjugated secondary antibody, and binding was visualized by 3, 3′-diaminobenzidine (DAB) staining (Boster Biological Engineering Company, Wuhan, China). Samples were histologically evaluated using a double-blind scoring method. Cells with brown granules were considered positive, and the percentages of positive cells relative to total cells were calculated. For HE staining, the sections were stained with HE staining solution (Nanjing Jiancheng Biotechnology Institute).
Statistical analysis
All experiments were performed in triplicate, and data were expressed as mean±standard deviation (SD) of three sets of triplicates. Behavioral scores were compared by repeated measures analysis of variance (RMANOVA), and differences among groups were analyzed by one-way analysis of variance (ANVOA). All statistical analyses were performed using SPSS19.0 software (SPSS Inc., Chicago, IL, USA) and all graphs were constructed using Graph Prism 6.0 software (GraphPad Prism, San Diego, CA). P<0.05 was considered statistically significant and P<0.01 was considered highly statistically significant.
Results
RTMS and hUCB-MSCs improve rat motor performance
All the rats had lower BBB scores at 1 day after than before SCI (Figure 1A), with scores of all rats being higher 8 weeks after injury. Significant differences were observed between untreated rats and those treated with rTMS and hUCB-MSCs. BBB scores of rats administered rTMS alone or together with hUCB-MSCs transplantation increased as rTMS pulse frequency increased. Moreover, higher BBB scores were observed in rats treated with hUCB-MSCs or hUCB-MSCs plus rTMS than in rats treated with rTMS alone (p<0.05).
Figure 1.
(A, B) rTMS and hUCB-MSCs transplantation improve rat behavior. Rats subjected to spinal cord injury (SCI) were treated with rTMS and hUCB-MSCs. Basso, Beattie, and Bresnahan (BBB) scores were measured after 1 day and 1, 2, 4 and 8 weeks, and motor evoked potentials (MEPs) were measured after 8 weeks. * p<0.05 and ** p<0.01 compared with rats subjected to SCI alone; # p<0.05 compared with rTMS.
MEPs were lower in all rats 1 d after than before SCI (p<0.01, Figure 1B). As expected, MEPs of all rats were subsequently improved by treatments with rTMS and hUCB-MSC transplantation. MEP was higher in rats that underwent hUCB-MSC transplantation, alone or together with rTMS, than in those treated with rTMS alone. Moreover, MEP was higher at higher rTMS pulse frequency. These results demonstrated that rTMS and hUCB-MSCs transplantation resulted in improved rat motor performance.
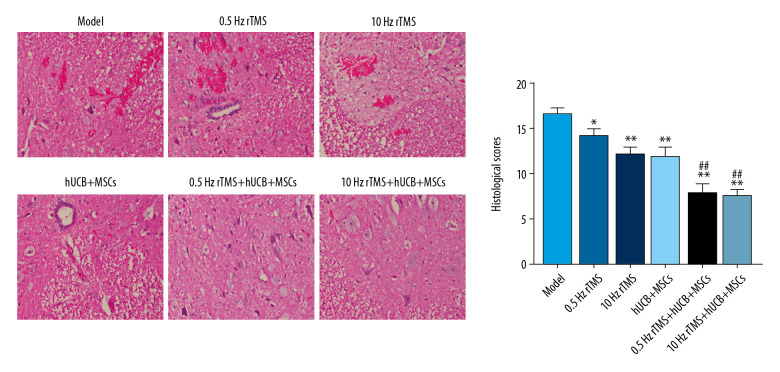
RTMS and hUCB-MSCs restore SCI induced spinal cord tissue damage
Rats that underwent SCI showed obvious damage to neurons and neuron swelling, accompanied by nuclear pyknosis (Figure 2). These acute injuries, however, were ameliorated by combined rTMS treatment and hUCB-MSCs transplantation. No apparent necrosis, swelling or nuclear pyknosis was observed in spinal cord neurons, and there were significant differences in neuron structures of rats treated with 0.5 Hz rTMS+hUCB-MSCs and 10 Hz rTMS+hUCB-MSCs. HE staining also showed that rTMS and hUCB-MSCs could reverse SCI-induced damage to neuron structures.
Figure 2.
HE staining analysis. Rats with spinal cord injury (SCI) were treated with rTMS and hUCB-MSCs transplantation for 8 weeks. * p<0.05 and ** p<0.01 compared with rats subjected to SCI alone; # p<0.05 and ## p<0.01 compared with rTMS.
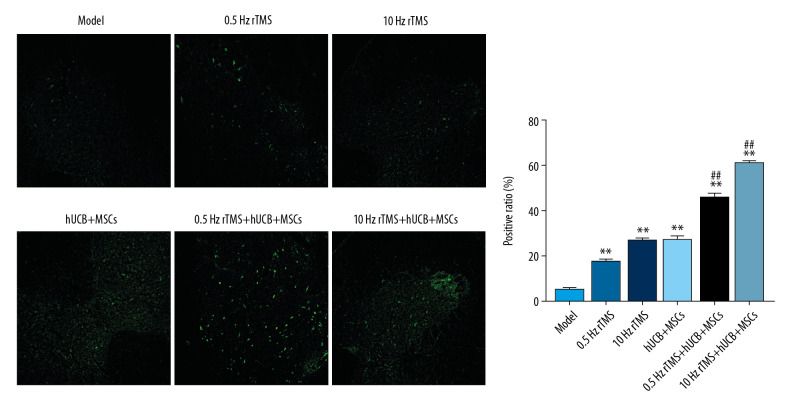
RTMS and hUCB-MSCs improve BDA-positive fibers in CST of SCI rats
BDA-labeled corticospinal fibers in CST were traced using a fluorescence microscope (Figure 3). The numbers of BDA-positive fibers in the intact CST were lower in rats that underwent SCI than in normal control rats. rTMS treatment or hUCB-MSCs transplantation increased the numbers of BDA-positive fibers in the intact CST, suggesting that rTMS or hUCB-MSCs transplantation inhibited neuron apoptosis and necrosis.
Figure 3.
Biotin dextran-amine (BDA) tracing of rat corticospinal tract (CST). Fluorescent anterograde tract tracing with BDA and immunofluorescence staining to analyze nerve fibers in the CST using fluorescence microscopy. Bar=50 μm. * p<0.05 and ** p<0.01 compared with rats subjected to SCI alone; # p<0.05 and ## p<0.01 compared with rTMS.
Immunohistochemical analysis
To further determine the effects of rTMS and hUCB-MSCs on the proliferation of neurons in rats following SCI, the expression of proteins involved in neural stem cell proliferation was analyzed by immunohistochemistry. The expression of BrdU and nestin in injured spinal cords was found to gradually increase over time following treatment with rTMS and hUCB-MSCs (Figure 4). Moreover, the effect of rTMS and hUCB-MSCs transplantation on the proliferation of neurons, oligodendrocytes and astrocytes was assessed by immunohistochemical analysis of the expression of Tuj1, Ng2+, and GFAP, respectively. Treatment with rTMS and hUCB-MSCs increased the numbers of Tuj1-positive cells, while decreasing the numbers of Ng2+- and GFAP-positive cells in injured spinal cords, suggesting that rTMS and hUCB-MSCs transplantation increased neural stem cell proliferation and the number of newly generated neurons.
Figure 4.
(A, B) Immunohistochemical analysis of neuron proliferation related proteins. Samples were incubated with antibodies to the neural stem cell proliferation related proteins BrdU and nestin and to Tuj1, Ng2+, and GFAP, which are markers for neurons, oligodendrocytes and astrocytes, respectively. rTMS and hUCB-MSCs transplantation promoted the proliferation of neuron stem cells, oligodendrocytes and astrocytes. * p<0.05 and ** p<0.01 compared with rats subjected to SCI alone; # p<0.05 compared with rTMS.
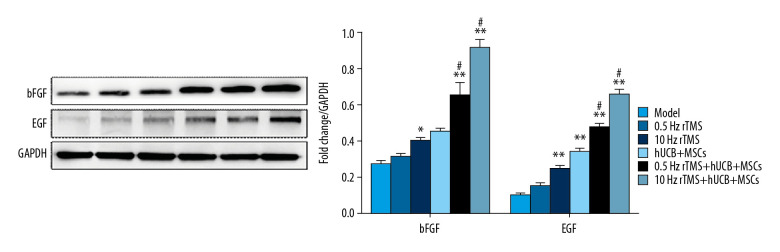
RTMS and hUCB-MSCs upregulate expression of bFGF and EGF in SCI rats
bFGF and EGF were shown to promote the proliferation of neural stem cells [21–24], suggesting that rTMS and hUCB-MSCs transplantation may reduce neuron apoptosis by enhancing the expression of bFGF and EGF proteins. Western blotting showed that the levels of bFGF and EGF proteins in injured spinal cords of rats were gradually increased by rTMS treatment and hUCB-MSCs transplantation (Figure 5) and that the level of expression of these proteins was positively associated with rTMS pulse frequency, suggesting that the effects of rTMS treatment and hUCB-MSCs transplantation were related to bFGF and EGF.
Figure 5.
Effects of rTMS and hUCB-MSCs on the expression of bFGF and EGF proteins. Western blotting analysis, showing the expression of the neural stem cells proliferation related proteins bFGF and EGF. * p<0.05 and ** p<0.01 compared with rats subjected to SCI alone; # p<0.05 compared with rTMS. Bar in B=50 μm.
Discussion
SCI can trigger various of acute and chronic disabilities and a cascade of degenerative events as a result of the limited neuronal regeneration after SCI [1]. Both rTMS treatment and hUCB-MSCs transplantation have been reported to improve sensorimotor recovery, which could reduce the intractable neuropathic pain and alleviate the mechanical allodynia experienced by patients with SCI [11–13]. For example, rTMS treatment improved MEPs in 13 men and three women with SCI [21]. Moreover, implantation of bionic-designed acellular spinal cord scaffolds seeded with hUCB-MSCs was found to increase BBB scores and histological status in SCI rats [22]. Similarly, the present study confirmed that the administration of rTMS alone or together with hUCB-MSCs transplantation could improve rat behavior, while alleviating obvious neuron damage and swelling, accompanied by nuclear pyknosis.
BDA has been widely used an effective long-range tracer of primary afferent projections in the spinal cord [23]. Injury to the spinal cord was found to reduce the level of BDA-positive fibers [24]. Similarly, the present study showed that BDA-positive fibers in the intact CST were increased after SCI rats were treated with rTMS or underwent hUCB-MSCs transplantation. BrdU and nestin are proteins related to neural stem cell proliferation. The proportion of BrdU-positive cells was observed to be significantly upregulated after rTMS treatment [25], and transplantation of hUCB-MSCs was found to upregulate the expression of nestin and Tuj1 [26]. In the present study, we found the administration of rTMS alone or together with hUCB-MSCs transplantation markedly increased the numbers of BrdU-, nestin- and Tuj1-positive cells while reducing the proportions of Ng2+- and GFAP-positives cells.
SCI-induced apoptosis has been associated with genetic factors, including the PI3K/Akt pathway [27], IKK/NF-κB signaling [28], and bFGF/EGF [29]. Although rTMS could promote neural stem cell proliferation and has been suggested as a new tool in the treatment of SCI [4], few studies have focused on the molecular mechanism [6]. The present study found that rTMS, whether alone or together with transplantation of hUCB-MSCs, inhibited SCI-induced neural stem cell apoptosis and enhanced neural cell proliferation via the expression of bFGF and EGF. Moreover, treatment with 10 Hz rTMS was more effective and induced higher expression of bFGF and EGF than 0.5 Hz rTMS in these rats, suggesting that the protective effects of rTMS against SCI-induced neurons apoptosis may be associated with bFGF and EGF.
Both rTMS treatment and hUCB-MSCs transplantation have been shown to have anti-apoptotic and neuro-protective effects in acute optic nerve injury [4,9,10]. These protective effects were reported associated with activation of the PI3K/Akt pathway, ERK1/2 signaling, and BDNF-TrkB signaling [30], showing the crucial role of hUCB-MSCs transplantation in neuro-protection. Comparative analysis in this study showed that the neuro-protective effects of hUCB-MSCs transplantation were higher than those of rTMS treatment in SCI rats. Transplantation of hUCB-MSCs resulted in better motor performance, higher proliferation rates and enhanced expression of bFGF and EGF proteins than treatment with rTMS alone, with combined treatment being more effective than either alone.
Taken together, these results indicate that hUCB-MSCs transplantation was more effective than rTMS, and that their combination was more effective than either alone. Further investigations of other potential signaling pathways are needed to comprehensively understand the molecular mechanisms by which rTMS and hUCB-MSCs transplantation counteract the effects of SCI.
Conclusions
This study confirmed that rTMS, alone or together with hUCB-MSCs transplantation, enhanced the proliferation of neurons, the expression of the proliferation related proteins bFGF and EGF, and improved motor performance in SCI rats. Moreover, the combination of rTMS and hUCB-MSCs transplantation was more effective than either alone. These findings indicate that the combination of rTMS treatment and hUCB-MSCs transplantation may be effective in the treatment of SCI and traumatic brain injury.
Footnotes
Source of support: Departmental sources
References
- 1.Kabu S, Gao Y, Kwon BK, Labhasetwar V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J Control Release. 2015;219:141–54. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang BT, Cregg JM, DePaul MA, et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015;518:404–8. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neville IS, Hayashi CY, El Hajj SA, et al. Repetitive transcranial magnetic stimulation (rTMS) for the cognitive rehabilitation of traumatic brain injury (TBI) victims: Study protocol for a randomized controlled trial. Trials. 2015;16:440. doi: 10.1186/s13063-015-0944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo F, Han X, Zhang J, et al. Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation via the regulation of MiR-25 in a rat model of focal cerebral ischemia. PLoS One. 2014;9:e109267. doi: 10.1371/journal.pone.0109267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Wang L, Zhang R, et al. Anti-depressive mechanism of repetitive transcranial magnetic stimulation in rat: The role of the endocannabinoid system. J Psychiatr Res. 2014;51:79–87. doi: 10.1016/j.jpsychires.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Zhang Z, Su Y, et al. Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF-TrkB signal pathway in cultured hippocampal neurons. Neurochem Int. 2013;62:84–91. doi: 10.1016/j.neuint.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–92. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 8.Hua J, Gong J, Meng H, et al. Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: Proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biol Int. 2014;38:198–210. doi: 10.1002/cbin.10188. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Cheng H, Dai G, et al. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;1532:76–84. doi: 10.1016/j.brainres.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen SH, Wang JJ, Chen CH, et al. Umbilical cord blood-derived CD34+ cells improve outcomes of traumatic brain injury in rats by stimulating angiogenesis and neurogenesis. Cell Transplant. 2014;23:959–79. doi: 10.3727/096368913X667006. [DOI] [PubMed] [Google Scholar]
- 11.Gao F, Chu H, Li J, et al. Repetitive transcranial magnetic stimulation for pain after spinal cord injury: A systematic review and meta-analysis. J Neurosurg Sci. 2017;61:514–22. doi: 10.23736/S0390-5616.16.03809-1. [DOI] [PubMed] [Google Scholar]
- 12.Ellaway PH, Vásquez N, Craggs M. Induction of central nervous system plasticity by repetitive transcranial magnetic stimulation to promote sensorimotor recovery in incomplete spinal cord injury. Front Integr Neurosci. 2014;8:42. doi: 10.3389/fnint.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui B, Li E, Yang B, Wang B. Human umbilical cord blood-derived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp Ther Med. 2014;7:1233–36. doi: 10.3892/etm.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faden AI, Simon RP. A potential role for excitotoxins in the pathophysiology of spinal cord injury. Ann Neurol. 1988;23:623–26. doi: 10.1002/ana.410230618. [DOI] [PubMed] [Google Scholar]
- 15.Allen AR. Surgery of experimental lesion of spinal equivalent to crush injury of fracture dislocation of spinal column: A preliminary report. JAMA. 1911;57:878–80. [Google Scholar]
- 16.Kim JY, Choi GS, Cho YW, et al. Attenuation of spinal cord injury-induced astroglial and microglial activation by repetitive transcranial magnetic stimulation in rats. J Korean Med Sci. 2013;28:295–99. doi: 10.3346/jkms.2013.28.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung HJ, Chung WH, Lee JH, et al. Expression of neurotrophic factors in injured spinal cord after transplantation of human-umbilical cord blood stem cells in rats. J Vet Sci. 2016;17:97–102. doi: 10.4142/jvs.2016.17.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwatsuki K, Yoshimine T, Sankai Y, et al. Involuntary muscle spasm expressed as motor evoked potential after olfactory mucosa autograft in patients with chronic spinal cord injury and complete paraplegia. J Biomed Sci Eng. 2013;6:908–16. [Google Scholar]
- 19.Basso DM, Beattie MS, Bresnahan J. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Zhao M, Xiao T, et al. Constraint-induced movement therapy overcomes the intrinsic axonal growth-inhibitory signals in stroke rats. Stroke. 2013;44:1698–705. doi: 10.1161/STROKEAHA.111.000361. [DOI] [PubMed] [Google Scholar]
- 21.Nojima K, Iramina K. Relationship between rTMS effects and MEP features before rTMS. Neurosci Lett. 2018;664:110–15. doi: 10.1016/j.neulet.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Chen J, Liu B, et al. Acellular spinal cord scaffold seeded with mesenchymal stem cells promotes long-distance axon regeneration and functional recovery in spinal cord injured rats. J Neurol Sci. 2013;325:127–36. doi: 10.1016/j.jns.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Novikov LN. Labeling of central projections of primary afferents in adult rats: A comparison between biotinylated dextran amine, neurobiotin and Phaseolus vulgaris-leucoagglutinin. J Neurosci Methods. 2001;112:145–54. doi: 10.1016/s0165-0270(01)00461-7. [DOI] [PubMed] [Google Scholar]
- 24.Zuo YC, Xiong NX, Zhao HY. Stereotactic injection of shRNA GSK-3β-AAV promotes axonal regeneration after spinal cord injury. J Huazhong Univ Sci Technolog Med Sci. 2016;36:548–53. doi: 10.1007/s11596-016-1623-6. [DOI] [PubMed] [Google Scholar]
- 25.Bueno C, Ramirez C, Rodríguez-Lozano FJ, et al. Human adult periodontal ligament-derived cells integrate and differentiate after implantation into the adult mammalian brain. Cell Transplant. 2013;22:2017–28. doi: 10.3727/096368912X657305. [DOI] [PubMed] [Google Scholar]
- 26.Jurga M, Lipkowski AW, Lukomska B, et al. Generation of functional neural artificial tissue from human umbilical cord blood stem cells. Tissue Eng Part C Methods. 2009;15:365–72. doi: 10.1089/ten.tec.2008.0485. [DOI] [PubMed] [Google Scholar]
- 27.Jung SY, Kim DY, Yune TY, et al. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med. 2014;7:587–93. doi: 10.3892/etm.2013.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu M, Wang S, Han X, Lv D. Butein inhibits NF-κB activation and reduces infiltration of inflammatory cells and apoptosis after spinal cord injury in rats. Neurosci Lett. 2013;542:87–91. doi: 10.1016/j.neulet.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Rabchevsky AG, Fugaccia I, Turner AF, et al. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. 2000;164:280–91. doi: 10.1006/exnr.2000.7399. [DOI] [PubMed] [Google Scholar]
- 30.Lim JY, Park SI, Oh JH, et al. Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J Neurosci Res. 2008;86:2168–78. doi: 10.1002/jnr.21669. [DOI] [PubMed] [Google Scholar]