Abstract
Although pain reduction is commonly the primary outcome in chronic pain clinical trials, physical functioning is also important. A challenge in designing chronic pain trials to determine efficacy and effectiveness of therapies is obtaining appropriate information about the impact of an intervention on physical function. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) and Outcome Measures in Rheumatology (OMERACT) convened a meeting to consider assessment of physical functioning and participation in research on chronic pain. The primary purpose of this article is to synthesize evidence on the scope of physical functioning to inform work on refining physical function outcome measurement. We address issues in assessing this broad construct and provide examples of frequently used measures of relevant concepts. Investigators can assess physical functioning using patient-reported outcome (PRO), performance-based, and objective measures of activity. This article aims to provide support for the use of these measures, covering broad aspects of functioning, including work participation, social participation, and caregiver burden, which researchers should consider when designing chronic pain clinical trials. Investigators should consider the inclusion of both PROs and performance-based measures as they provide different but also important complementary information. The development and use of reliable and valid PROs and performance-based measures of physical functioning may expedite development of treatments, and standardization of these measures has the potential to facilitate comparison across studies. We provide recommendations regarding important domains to stimulate research to develop tools that are more robust, address consistency and standardization, and engage patients early in tool development.
Keywords: OMERACT, IMMPACT, pain, physical performance measures, patient-reported outcomes, physical functioning, physical activity, accelerometer, work, employment, HRQOL, social participation, caregiver burden, clinical trials
1. Introduction
There is little question that the presence of chronic pain has impact on all areas of functioning, including emotional, social, as well as physical.53,61,170,186 With the persistence of pain, the extent and depth of these consequences expands and evolves. Focus groups and surveys have shown that physical functioning is altered in people living with chronic pain, with the majority of participants expressing functional problems in activities or increased symptoms during or following the activity.191 Physical functioning outcome measures are important because they provide data on the impact of pain and treatment effects beyond symptom reduction alone, moving into the impact on individuals’ lives beyond symptoms, a primary concern for patients.191 The objective of this article is to synthesize current evidence and expert deliberations and discussions (held at a joint Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)/Outcome Measures in Rheumatology (OMERACT) meeting) to present considerations and recommendations for the comprehensive assessment of physical functioning and related outcomes in chronic pain clinical trials. Although many additional factors may affect functioning, we included participation and caregiver burden as contextual outcomes because they have received scant attention in other reviews.
Central to the deliberations at the meeting was the concept of the relationship between physical functioning and “participation” and restrictions in activities of daily living (ADLs). The aim was to better define and contextualize these relationships and to discuss whether chronic pain clinical trials should consider participation as an additional patient-centered domain in outcome measurement. Hammel et al.79 found that individuals in their study conceptualized participation as a cluster of values (e.g., active and meaningful engagement/being a part of, choice and control, access and opportunity/enfranchisement, personal and societal responsibilities, having an impact and supporting others, and social connection, inclusion, and membership). People living with chronic pain may view the outcomes of a pain intervention to be more clinically meaningful if the outcomes assessed include evaluation of their ability to participate in various activities, in addition to pain and performance in clinic or laboratory assessments.
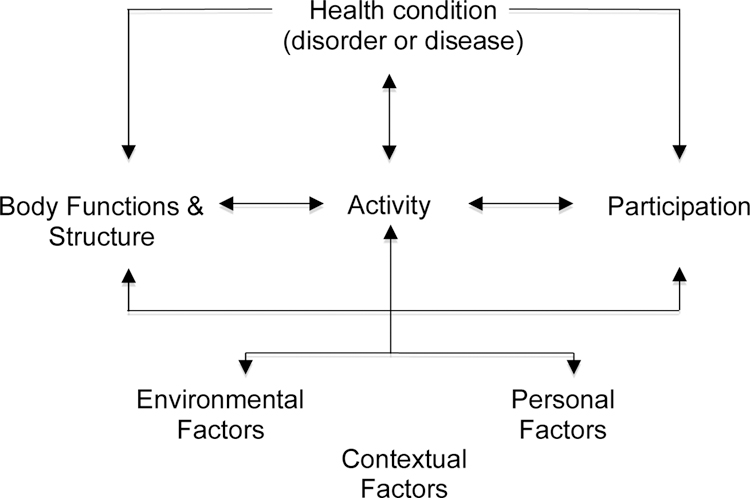
In a previous article, IMMPACT recommended that in order to assess HRQOL, physical, emotional, and social functioning should be included as core outcome domains in all chronic pain clinical trials.190 Investigators should include measures of physical functioning that provide evaluations of meaningful aspects of an individual’s life, including the ability to carry out ADLs such as household chores, walking, work, and self-care, as well as strength, endurance, and flexibility.190 These aspects have been categorized in an interrelated arrangement of domains in the International Classification of Functioning, Disability, and Health (ICF)210 (see Figure 1 for the model and Table 1 for ICF Chapters), in which physical functioning is found at the level of very specific task demands (taking a step), broader acts (walking a block), or participation restrictions (role functioning, meeting physical demands of work). Physical functioning is an important independent outcome domain. However, to date, the literature has failed to reveal a strong linear relationship between decreased pain and increase in activity.189 Outcome measures of physical functioning, activity, and social functioning can be useful in establishing the global impact of treatment and whether the adverse effects of a treatment affect the potential benefits of pain reduction. Although the discussions that provided the basis of our recommendations focused on analgesic clinical trials, the issues discussed in this article have implications and are relevant for research on the efficacy and effectiveness of interventions for patients with chronic pain more broadly.
Figure 1.
A model of disability that is the basis of ICF.210
ICF = International Classification of Functioning, Disability, and Health
Table 1.
The ICF chapters209
| Body | |
|---|---|
| Function: Mental functions Sensory functions and pain Voice and speech functions Functions of the cardiovascular, haematological, immunological and respirator systems Functions of the digestive, metabolic, endocrine systems Genitourinary and reproductive functions Neuromusculoskeletal and movement related functions Functions of the skin and related structures |
Structure: Structure of the nervous system The eye, ear and related structures Structures involved in the voice and speech Structure of the cardiovascular, immunological and respiratory systems Structures related to the digestive, metabolic and endocrine systems Structures related to genitourinary and reproductive systems Structure related to movement Skin and related structures |
| Activities and participation Learning and applying knowledge General tasks and demands Communication Mobility Self-Care Domestic life Interpersonal interactions and relationships Major life areas Community, social and civic life | |
| Environment Products and technology Natural environment and human-made changes to the environment Support and relationships Attitudes Services, systems and policies | |
ICF = International Classification of Functioning, Disability, and Health
2. Methods
In order to inform the design of future clinical trials, IMMPACT convened a meeting in collaboration with the members of the OMERACT Pain Working Group to review the evidence and discuss issues in measuring the broad construct of physical functioning as an outcome domain in chronic pain clinical trials. Prior to the meeting, two systematic literature searches were undertaken reviewing the evidence for physical functioning measures, one aimed at PRO measures and the other at observer, laboratory, and other performance-based outcome measures of pain-related physical functioning (Table 2); the results are presented in Tables 3 and 4. We have included references in these tables so the reader can examine the studies and ascertain the metrics, methods, and patient groups. The authors do not recommend any of the specific measures that are included, as it would be impossible to assess each of them in requisite detail and choose from among a variety of available measures within the context of a brief consensus conference. These are included only to illustrate commonly used measures of relevant concepts associated with the board construct of physical functioning.
Table 2.
Considerations for outcome measures: search strategies
|
Outcome measures were identified searching electronic databases: Medline, Embase, PsychINFO, CINAHL Hand searching and backward chaining was undertaken Key words for patient-reported outcomes and performance measures: physical activity, functioning, function, motor activity, activities of daily living, activity, exercise questionnaire*, scale, tool, assessment, self-report, measure* chronic disease, chronic condition, pain, chronic pain, musculoskeletal, rheumat*, long term conditions, older adults Key words for observer, laboratory and other outcome measures: physical activity, physical function, physical functioning, exercise, motor activity, pain, chronic pain, musculoskeletal pain, performance measures, observation, six minute walk test, timed up and go test Inclusion criteria: research from 1980 to present day, in English, published in peer review journals Exclusion criteria: pre 1980 unless a seminal or key paper, studies looking at athletic performance, sports, children and adolescents, studies looking at the aging process devoid of long term conditions and in post-acute care rehabilitation. |
In the place of a character in the search term indicates that any number of characters can be substituted in place of the asterisk
Table 3:
Patient-reported outcome measures of physical functioning
| Pain related physical functioning/activity measures | The Pain Disability Questionnaire5 Chronic Pain Self Efficacy Scale6 Pain Self-Efficacy Questionnaire136 Questionnaire for Physical Activity Decline in Pain (PAD)197 Daily Activity Diary for Chronic Pain Patients60 The Pain Disability Index151 The Multidimensional Pain Inventory104 The Brief Pain Inventory43 |
| General physical functioning/activity outcome measures | London Handicap Scale80 MOS 36-Item Short Form Health Survey (SF-36)200 The Impact on Participation and Autonomy (IPA)39 The Impact on Participation and Autonomy Questionnaire (IPAQ)38 The Physical Activity and Disability Survey (PADS)155 The Physical Activity Questionnaire117 The Quality of Well-Being Scale, Version 1.04 (QWB)96 The Sickness Impact Profile (SIP)20 Work Limitations Questionnaire (WLQ)112 Human Activity Profile49 Motor Fitness Scale106 Short Questionnaire to Assess Health-Enhancing Physical Activity (SQUASH)199 PROMIS3,4,21,30,34 |
| Activities of daily living measures | Population Surveys of Chronic Disease and Disability (Section 1)66 The Centres for Disease Control and Prevention’s Healthy Days Measures (the CDC HRQOL-14)132 The Duke-UNC Health Profile144 Katz Index of Independence in Activities of Daily Living99 Rosow Breslau Index of Mobility160 Nagi’s Upper or Lower Extremity Functional Index135 |
| Disease specific physical activity/functioning measures | Bath Ankylosing Spondylitis Functional Index37 Roland Morris Disability Questionnaire157 Fibromyalgia Impact Questionnaire36 Health Assessment Questionnaire64 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)18 Functional Status Assessment Instrument94 The FAST Functional Performance Inventory154 The Leisure Time Physical Activity Instrument (LPAI)124 The McMaster Toronto Arthritis (MACTAR) Patient Preference Disability Questionnaire188 The Physical Activity at Home or at Work Instrument (PAHWI)124 Short Musculoskeletal Function Assessment Questionnaire180 Modified Health Assessment Questionnaire (MHAQ)149 Musculoskeletal Functional Limitation Index98 Patient-specific activity scoring scheme177 |
| Site specific physical functioning/activity measures | Disabilities of the Arm, Shoulder and Head (DASH)92 QuickDASH76 Boston Carpal Tunnel Questionnaire114 Bournemouth Questionnaire25,26 Hip Injury and Osteoarthritis Outcome Score (HOOS)137 Knee Injury and Osteoarthritis Outcome Score (KOOS)159 Lequesne Hip and Knee Scores111 Neck Disability Index198 The Western Ontario Rotator Cuff Index107,108 The Oswestry Disability Index56 Patient-rated Tennis Elbow Evaluation121 Foot and Ankle Ability Measure (FAAM)156 |
Table 4:
Clinical, observer, and laboratory tools grouped by sub-domain (online summary of some measures at http://www.rehabmeasures.org/default.aspx)
| Mobility or Activity measures | Timed Up and Go (TUG)149 6 minute walk test (6MWT)16 Walk, Fast paced71 Self-paced42 Multi paced27 Actigraphy200 |
| General physical functioning measures | Stair climb test42 Chair stand test 5 times in a row (timed)24 Number of times in 30 seconds95 Balance One legged hop118 Standing stork147 |
| Multi-activity tests of physical functioning | Continuous Scale – Physical Function Performance Test (CS-PFP)165 Short Physical Performance Battery (SPPB)77 Physical Performance Test (PPT)162 Physical Activity Restrictions (PAR)153 Aggregated Locomotor Function (ALF)126 Functional Assessment System (FAS)139 Lin battery115 Steultjens battery173 Stratford battery177 |
| Site-specific physical functioning | Loaded forward-reach test for chronic low back pain7 Shoulder Range of motion202 Knee range of motion14 Hand grip strength test57 Single leg hop test139 |
The meeting brought together an international group of participants from academia, government agencies, industry, and public advocacy organizations selected based on research, personal experience living with pain, and clinical expertise relevant to evaluating physical activity and functioning. There were 28 academic and related participants, including 22 from the United States of America (USA), 3 from Canada, and 3 from the United Kingdom (UK). There were 9 government participants, 2 patient representatives from the USA, and 7 industry participants, all of whom were from the USA.
At the start of the meeting, background lectures provided overviews of the following topics: (1) United States Food and Drug Administration (FDA) guidance for the development and evaluation of PRO measures (A.S.); (2) an FDA perspective on review of outcome measures for drug approval and labeling (E.P.); (3) a conceptual overview defining physical function (D.B.); (4) PRO measures of physical functioning (A.M.T.); (5) clinician, observer, laboratory, and other outcome measures of physical functioning (K.P.); (6) actigraphy (K.V.P.); (7) social participation outcome measures (D.W.); (8) work participation outcome measures (M.G.); (9) outcome measures involving caregiver burden (J.M.); and (10) interpretation of the clinical importance of improvements in patient reported and “objective” assessments of physical functioning (D.J.C.). Slides supporting these presentations are available on the IMMPACT website: http://www.immpact.org/meetings/Immpact17/participants17.html. We recommend that readers view the slides, as appraisal of the evidence is more detailed than we are able to cover in depth in the present article.
Using the information presented in the background lectures, the subsequent discussions during extensive question and answer sessions, and informed by the background readings, participants deliberated on physical functioning outcome measures, including participation and caregiver burden as contextual factors. The rationale for the meeting was to examine the scope of physical functioning and the issues affecting it in order to provide recommendations and a research agenda for a detailed and thorough plan of activity following the meeting. The meeting was recorded and transcribed to retain ideas about important content and structure. A.M.T., K.P. and K.V.P., the lead authors, prepared a draft manuscript that reflected the deliberations. They circulated the initial draft to all participants for consideration and comment on content, structure, and proposed recommendations. The lead authors revised the manuscript iteratively until all authors approved its content. In this paper, we have incorporated the most salient points of these discussions.
3. Physical functioning: primary outcomes
3.1. Patient-reported outcome (PRO) measures
Authors often use different terms interchangeably in describing physical functioning without defining them precisely. Therefore, it is often unclear whether a particular outcome measure in a clinical trial is assessing organ-level impairments, task completion, or interference with specific activities. A number of studies indicate that different interventions used to treat chronic pain may have differential effects on physical impairment, activity limitation, as well as restrictions in participation.13 Ideal PRO physical function outcome measures differentiate among these to illustrate the true value of an intervention.13
A conceptual model for pain and physical functioning needed, for this initiative, to integrate the key concepts of the ICF with additional features that have particular clinical relevance, for example, the disease defining component(s), the mechanism of action of the treatment that an investigator is studying, and the primary outcome warranting study. Understanding of physical functioning requires consideration of the individual’s environment and context (see Tomey & Sowers185). Physical functioning is considered as the person’s capacity in set situations, however it also can acknowledge that persons can manage barriers to functioning such as stairs, temperature, and neighborhood accessibility by aids and adaptations or avoidance of them. Investigators must consider the physical functioning of an individual within the appropriate context to permit comparisons across respondents. For example, individuals with chronic conditions (pain or other sources of limitations in physical functioning) often learn to accommodate their condition through adaptations of techniques or learning to work around any limitations. Whether or not adaptations are considered is, for example, an important conceptual question and depends on the research question.
Investigators can achieve the measurement of physical functioning using a number of methods each providing a certain ‘window’ on the concept. These can range from direct observation and monitoring devices, to PROs. The FDA defines PRO measures as, “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else”.193 As illustrated in Tables 3–4, there is a plethora of PRO measures of physical functioning, some generic and others disease specific, each with its own strengths and weaknesses. In evaluating such measures, several points are particularly noteworthy (see also Williams et al.206). A primary consideration is the content validity of the instrument. None of the currently available measures is sufficiently comprehensive, covering all key domains of physical functioning. Some questionnaires focus solely on physical functioning, whereas others include multiple domains in which physical functioning is only one component or subscale of the assessment tool (Table 3). However, there needs to be a balance between comprehensiveness and respondent burden. It may not be feasible to ask patients to complete a very lengthy PRO measure or sets of measures.
Advanced psychometric methods, such as Item Response Theory (IRT) and the use of Computer Adaptive Testing (CAT), have the potential to reduce respondent burden by increasing the efficiency of the assessments.40,81,122 The National Institutes of Health Patient Reported Outcome Measurement Information System (PROMIS) is an important development that may make a significant contribution to clinical trial data capture as it evolves.3,4,21,30,34 It is a domain-oriented, mixed method approach to health outcomes intended to facilitate within- and between-disease comparisons, that included patient input from the earliest stages (see http://www.immpact.org/static/meetings/Immpact17/Williams.pptx). The PROMIS investigators developed items using advanced psychometric qualitative and quantitative methods. PROMIS uses IRT, which enables CAT, in order to improve precision latent trait estimation while also minimizing patient respondent burden. PROMIS physical functioning (PF) items may act as a standard for comparative effectiveness research (Patient-Centered Outcomes Research Institute has funded such research). PROMIS PF measures are available for both adults (full bank = 121 items) and pediatric populations (PF-mobility = 23 items, PF-upper extremity = 29 items); they are also available as part of a PROMIS Profile (29, 43 or 57-item versions).
It is also important to consider the target population of a PRO. The experience might be different across populations (chronic episodic versus chronic constant pain), and attribution within the PRO might need attention to match with a target population Researchers should ask whether appropriate normative information is available regarding the sample included in a particular target study. Various investigators have developed questionnaires for a range of populations; however, limitations experienced by some groups may not be applicable to others:
Instruments developed for use with adults over 18 and under a certain age may not be appropriate for use with older adults and are likely to be virtually completely irrelevant for children and adolescents;
measures developed on native language speakers may not be transferrable to second language samples;
and measures of physical functioning developed specifically for use with patients with low back pain might not be the best instruments to use with a sample of patients with neck and shoulder pain.
Further, as role and job responsibilities have evolved over time, the initially applied measures may become less applicable as respondents’ current roles and employment evolve.
Finally, it is important to consider that a range of contextual factors, in addition to pain, will likely influence PRO measures of physical function and participation. Various models of health and disability emphasize that a range of personal, health, social, political, and environmental factors often relate to PRO measures of function and performance. Although this makes them more complex to include in studies, the IMMPACT/OMERACT recommendation is that researchers should consider them. A previous IMMPACT recommendation, that we endorse, is that investigators consider using both a disease-specific physical functioning measure and a generic measure to be able to compare results across populations with different disorders.52 Investigators should also consider inclusion and exclusion criteria that capture specific contextual factors. This will increase the generalizability of the outcomes to certain contexts; however, generalizations to groups without certain contextual factors will be limited. It also may mean that investigators need to complete multiple studies to address a range of contextual factors and this may not be feasible. Qualitative studies may help in understanding the contextual factors better.
A range of self-reported measures of physical functioning has appeared in the literature; we divided these measures and approaches into broad categories (Table 3), with the clinical, observer, and laboratory tools grouped by sub-domain (Table 4). We did not intend for the lists included in Tables 3 and 4 to be exhaustive as there are many disease and location specific instruments, but rather to illustrate measures that researchers have used in clinical trials in persons with chronic pain.
3.2. Performance-based measures
Researchers generally recognize that self-report and performance-based measures appear to provide different but complementary information. Performance-based measures assess discrete physical actions in a standardized manner across study participants and sites, so that investigators can compare physical functioning across different clinical populations and countries. Investigators are increasingly using performance-based measures in observational and intervention studies, particularly in older adults146, as they can assess physical functioning in a standardized manner. For these measures, investigators ask participants to perform a specific task and they then evaluate these in an objective manner using predefined criteria, such as recording the number of repetitions completed or the time to complete a task. The development and use of performance-based measures aimed to address limitations in self-report measures of physical functioning that can be prone to contextual and psychosocial bias. Some performance-based measures have amassed a considerable amount of normative data and have established minimally clinical important differences (Table 4).16
Performance-based tasks, however, may not be relevant to the particular needs of individuals or capture their real-world limitations and environment. The perceptions and motivation of the respondents are also important to consider as they affect their behavior. Performance-based measures (e.g., 6MWT16, Timed Up and Go test – TUG150, Short Physical Performance Battery – SPPB77) are not devoid of subjectivity and behavioral responses as they depend on effort. Many factors, such as fear of pain or injury, lack of motivation due to emotional distress, and learned behavior may be independent of actual physical ability and could influence performance on such voluntary tasks; but investigators can, nevertheless, use these measures to provide a more objective assessment of the total impact of pain. Training and retraining of study staff in test administration are critical for maintaining standardized, reliable assessments of physical performance.
A number of systematic reviews have been published describing performance-based measures of physical functioning specifically pertaining to pain. For example, in collaboration with members of OMERACT, the Osteoarthritis Research Society International (OARSI) has assessed measurement properties of many performance measures specifically developed for use with patients who have hip or knee osteoarthritis pain.110 Currently, there are few performance-based tests that are widely used for any specific pain condition and their use in chronic pain clinical trials has been very limited. This is an important future direction for research in order to establish normative data in the field of pain research.
Researchers have developed a growing number of methods to assess task-related physical performance (e.g., functional capacity examinations using devices and instruments such as dynamometers and force plates) directly. Each of these approaches targets a particular physical function or set of physical functions (e.g., range of motion, muscle strength, gait speed and pattern). It is beyond the scope of this article to review each of these measures and their strengths and weaknesses. The references we used in this paper have been included to support further consideration of physical function outcomes. Once again, we encourage the interested reader to examine the appropriate references to inform their decisions regarding the appropriateness of any measure for their purposes. However, we will discuss a more general measure of activity, accelerometry (actigraphy) in greater depth in the next section to illustrate some of the complexities, strengths, and weaknesses inherent in technologically advanced measurement devices.
3.2.1. Actigraphy/Accelerometry
Actigraphy, also called accelerometry, is becoming more widely used both in research and for clinical applications to monitor physical activity objectively. Accelerometers are small, lightweight devices that can measure acceleration along 1, 2, or 3 axial planes and provide time-stamped data on intensity of physical activity and sedentary behavior. These data provide valuable complementary information to PROs and performance-based measures of physical function.
Self-reported physical activity is typically subject to recall bias and biased estimation and reporting, and is poorly correlated with estimates from accelerometers. For example, self-reported data from the 2005–2006 National Health and Nutrition Examination Survey showed that 60% of adults in the USA met physical activity guidelines of either 150 minutes per week of moderate activity or 75 minutes per week of vigorous physical activity, accumulated in 10-minute bouts.187 In contrast, accelerometer data showed that fewer than 10% met these guidelines.187 Weak correlations between self-reported and objectively measured physical activity have also been observed elsewhere.128 It is likely that the self-reported and accelerometer data are measuring different dimensions of physical activity (e.g., perceived versus actual intensity of activity), but both methods can provide important information. We need additional research to understand the meaning of this discordance between self-reported measures of physical activity and actigraphy data.
The reliability of actigraphy is well-established.28,201 Validity studies show that accelerometer counts are moderately to strongly associated with oxygen consumption during activity using direct and indirect calorimetry.54,62,85 Further, actigraphy is strongly associated with measures of physical performance, such as the 6MWT, and predicts physical disability82,109 and mortality.15 Accordingly, actigraphy provides valuable objective data on the intensity and extent of physical activity and is increasingly used in studies of chronic pain. Currently, however, the interpretation of actigraphy data beyond general levels of activity and sleep171 is problematic, although this will likely change with the application of advances in machine learning to the data. Indeed, emerging methodological studies are now capitalizing on the richness of raw accelerometer data to classify time spent in different activities (e.g., walking, sitting, lying down). Newer devices are available that also include inclinometers to measure posture and more reliably quantify time spent in different activities.73,120
Despite increasing use of accelerometers in observational studies of pain48, 133, 134,145,207, relatively few studies have used these devices in clinical trials. One exception is a randomized, placebo-controlled, crossover trial of celecoxib for knee osteoarthritis, which showed that accelerometer data can be combined with pain intensity ratings as a composite outcome measure and that peak activity alone responded to treatment.51 Another example is an open-label study of a transdermal fentanyl patch that showed greater improvement with actigraphy measures than with a numerical rating scale.1 Actigraphy can be useful for evaluating sleep, and can serve as an efficient assessment of both daily physical function and sleep using a single device in the same individual. However, lax standards in activity metrics (e.g., counts) as well as differences in hardware and software across devices may hinder progress in using accelerometers. Researchers need to conduct further methodological work to establish good practices and standards for using accelerometers and analyzing the resulting data in chronic pain clinical trials.
4. Physical functioning: contextual outcomes
Poor physical functioning can lead to the inability to participate in or impede interpersonal relations. Thus, impaired social functioning may reflect poor physical functioning, and it can contribute to decreased function. In addition, increased caregiver burden associated with chronic pain may likewise contribute to or reflect poor physical functioning. These themes are important to explore in relation to physical functioning given that they are contextual factors within the conceptual framework and have had scant coverage in reviews.
4.1. Participation
Assessment of social participation largely evolved out of the ICF framework but the construct of social participation is not universally defined, and often confused with general participation, activities of daily living, support, and other social factors. For this general construct, there are many instruments with varying degrees of psychometric adequacy,83,84,86,90,175,205 and in most cases, the construct is embedded within a more general scale or domain that is being assessed. Although the International Classification of Impairments, Disabilities, and Handicaps (ICIDH) framework is outdated, investigators are still making use of questionnaires based on it.
Studies have examined the impact of social support and its benefits and even deleterious influences59,158 on individuals living with pain and on functional disability.102,192,196 Emotional support tends to have a more beneficial impact in people living with pain than does instrumental support (doing things for people) as the latter can lead to learned helplessness.12 Studies have shown that both network size and quality of social support at diagnosis of rheumatoid arthritis patients, for example, predicts pain and functional status 3–5 years later.55 Given the emerging relevance of social participation, we recommend that assessment of social participation be considered in chronic pain clinical trials when the investigator believes that this construct may be perceived as a moderator or mediator in shaping the outcomes for a particular population under study101,196 (See http://www.immpact.org/static/meetings/Immpact17/Williams.pptx). We identified 37 potential measures in the literature of which 17 were particularly relevant to the discussion. Table 5 lists some of the established measures covering this domain.
Table 5:
Focused participation outcome measures
| Groups | Measures |
|---|---|
| Social participation as a construct | Sickness Impact Profile72 The Nottingham Health Profile93 Reintegration to Normal Living Index207 Social Role Participation Questionnaire68 PROMIS Social Functioning78 |
| Instruments that operationalize the ICIDH model | Craig Handicap Scale & Reporting Techniques (CHART)203 Perceived Handicap Questionnaire (PHQ)182 London Handicap Scale (LHS)80 Community Integration Questionnaire (CIQ)96 |
| Instruments aligned to the ICF and included the concept of social participation | Assessment of Life Habits (LIFE-H)137 Participation Objective, Participation Subjective (POPS)33 Participation Survey/Mobility (PARTS/M)74 Participation Measure for Post-Acute Care (PM-PAC)65 Vestibular Activities and Participation (VAP)2 WHO Disability Assessment Schedule 2193 |
| Measures that address specific aspects of social participation | Life Satisfaction Questionnaire-9 (LSQ)163 Impact on Participation and Autonomy Questionnaire (IPA)39 Measurement of Quality of the Environment (MQE)141 Participation Enfranchisement83 Work Limitations Questionnaire17,161,180 Workplace Activity Limitations Scale17,67,180 |
4.1.1. Participation in work and social activities within and outside the home
Participation in work, within and outside the home, and family, social, and leisure activities are important aspects of physical functioning. Measurement of work outcomes, including worker productivity, is complex; often these outcomes are characterized in terms of a variety of different theoretical frameworks (ICF31,165). The factors contributing to participation go beyond pain, and health-related factors (see Table 1, ICF Chapters) and work participation outcomes (e.g., measures of work loss, absenteeism, and productivity) are interconnected. Therefore, it may be necessary for investigators to measure different types of work outcomes to evaluate these relationships adequately. It is not sufficient to consider compensated work outside the home alone because, for example, as few as 20–50% of people with rheumatoid arthritis may be working outside the home41,130,169 and retired people typically are not engaged in full-time paid work. However, they may volunteer or perform comparable activities (e.g., yard work, hobbies) without receiving financial compensation. For the purpose of this section, we will use the generic term “work” to describe activities within and outside the home, paid and unpaid. For school-aged children and adolescents, school attendance and participation in school-related activities are comparable to “work”. We will not discuss these in this article.
Investigators have used employment status as an outcome in many clinical trials, typically using a simple binary (yes/no) response. Sick leave or short-term disability is another potential outcome. Absenteeism (absent from work) and presenteeism (in work but unable to function due to illness) are also used as well as work scheduling (e.g., full to part time) and work disruptions (e.g., arriving late, leaving early). Large survey or population health data can separate some of these diverse factors, but it is challenging to determine what should be included as outcome measures in a clinical trial. Several validated instruments are available that assess changes in the activities that are important to patients, for example, the Work Productivity Survey (WPS, the only questionnaire to query family, social, and leisure activities as well as work within the home)141, the Work Limitations Questionnaire (WLQ)9,112,113, and the Workplace Activity Limitations Scale (WALS).17,67,69 Such outcome measures should be considered for inclusion in clinical trials where there is an expectation that work will be affected by the treatment.
The fact that outcomes are interrelated raises the possibility of developing a global measure of participation or productivity for application to clinical trials (at least long-term trials where there would be sufficient time to expect that work would be affected). Pain can interfere with physical functioning and therefore work, and work can have an impact on pain; outcome assessments need to address this bi-directionality of work being both a cause and effect of pain and pain being a cause and effect of work loss. The work context (e.g., its physical demands and the accommodations and adaptations used to reduce pain and improve physical functioning) and the nature of the pain will make a difference in how participants will respond to work-related outcome measures. Given the complexities surrounding work participation measures, and the lack of clear definitions, the OMERACT Worker-Productivity Working Group was established to examine and understand the issues related to employment and select or develop relevant measures for clinical trials (please see Tang et al.181). As previously described, a number of measures can be used to assess the impact of pain on work functioning, within and outside of the home, as well as overall productivity.17,67,162,176,181,182 However, work participation does need consideration in terms of other family, social, and leisure roles. The ongoing efforts of the OMERACT working group will make it possible for researchers to develop specific recommendations regarding the assessment of participation in chronic pain clinical trials. In the meantime, investigators can use the WPS, WLQ, and WALS to inform the results of chronic pain clinical trials. However, non-paid work is under studied; it needs its own research agenda to understand better the impact of pain on work that does not have monetary benefits.
4.2. Decreasing physical functioning and caregiver burden
Caregiver burden may be a result of decreasing physical functioning in the person living with pain or may lead to decreased physical function as the caregiver feels compelled to take on more responsibility. Therefore, caregiver burden may act as a proxy indicator of physical functioning in the patient living with chronic pain. Researchers should consider the amount of time a caregiver saves and the reduced burden in caring for a patient as physical functioning improves as potential outcome measures for a clinical trial in patients with chronic pain. These outcomes may be important to examine in those clinical trials where investigators anticipate such effects.
Caregiver burden may be different if the individual living with pain is a child rather than an adult (see Palermo et al.143). Cultural and ethnic factors can play a major role, and investigators need to see outcome measures of caretaker burden in the context of the culture and ethnicity of the group that investigators are studying.
There are a number of tools to measure caregiver burden.50,58,75,89,100,125,167 However, to date this concept has not received sufficient attention, especially in the field of pain. Caregiver burden has been included as an outcome measure in phase 3 clinical trials in Alzheimer’s disease, the index condition for studying caregiver burden29, and such measures may have a role in later-phase pain clinical trials.
However, there are problems with caregiver burden assessment in clinical trials (see Lingler et al.116). There are certainly challenges in developing approaches that can isolate caregiver burden that is specifically attributable to pain from the burden associated with the condition causing the pain and impairment (e.g., neurological impairment due to motor or sensory loss or musculoskeletal impairment associated with a limb fracture or amputation). There are also challenges in discriminating burden associated with pain versus co-morbid mental health conditions, particularly emotional distress and depression.
There is the potential to develop reliable measures of caregiver burden associated with pain that are also responsive to change. To date, it is unclear whether caregiver burden associated with chronic pain is a contributing factor or a separate outcome of poor physical functioning. This may depend on the conceptual framework for physical functioning and on the specific intervention, an investigator plans to examine. However, we need further development of the theoretical basis for examining caregiver burden in chronic pain and definitions of caregiver outcomes. Therefore, caregiver burden may be an important outcome measure in future chronic pain clinical trial and hence its inclusion in the framework we propose.
5. Considerations regarding the use of physical functioning measures in chronic pain clinical trials.
Investigators need to give greater attention to the important role of conceptual models in framing physical functioning measures in chronic pain clinical trials. The models discussed delineate the various concepts that are relevant to physical functioning; the hypothesized relationships among the concepts, and a framework to support potential treatment targets.160 Researchers should develop these early when considering physical functioning measures for use in clinical trials.
The ICF is a classification system of health and health-related domains, and used here as an example of a conceptual model. As the functioning and disability of an individual occur in a context, ICF also includes a list of environmental factors. We recommend that behaviors and tasks associated with a particular condition be mapped to the physical functioning outcome measure(s) that would be used. Ideally, investigators should match a large proportion of behaviors and tasks relevant to a specific chronic pain condition to the outcome measures that they will use in the trial. If not, further consideration of which measures or methods to use is required. A number of mapping exercises have been published describing the process.44,45,88,172,176 These mapping exercises provide strong empirical support to recommend mapping to a conceptual model.
5.1. Outcome measures should include consideration of psychosocial factors that impact functioning
We have already noted that many if not most “objective” measures of physical functioning are dependent on individuals’ co-operation. These “voluntary” behaviors are potentially influenced by the range of HRQOL domains, including psychosocial factors (e.g., mood, attention, pain-related attitudes and beliefs)8,10,32,15,119,23,35,47,87,103,123,152, and thus cannot be considered to be “pure” measures of physical ability. Depression, pain-related fear, and catastrophizing are associated with increased interference, awareness of pain, and impaired disengagement from pain, and can moderate the effects of attentional coping leading to poor clinical outcomes.11,19, 23,35,47,87,103,123,152,179,195 Similarly, fear of injury or harm has an influence on physical effort and activity.199 Given such data, it is recommended that psychosocial outcome measures also be administered when assessing physical functioning, as changes, or the lack of changes, in physical functioning following an intervention may be accounted for by existing fear, catastrophizing, and depression. Therefore, investigators should interpret lack of improvement in physical functioning in chronic pain clinical trials taking into account whether participants’ scores are also elevated in the psychological domains. Psychosocial constructs and processes may be particularly relevant when considering measures of caregiver burden. Given the amount of research examining the impact of psychosocial factors on physical functioning, there is strong evidence to suggest that investigators should evaluate psychosocial factors in tandem with assessments of physical functioning.
5.2. Ensure outcome measures assess the core disease-defining concepts
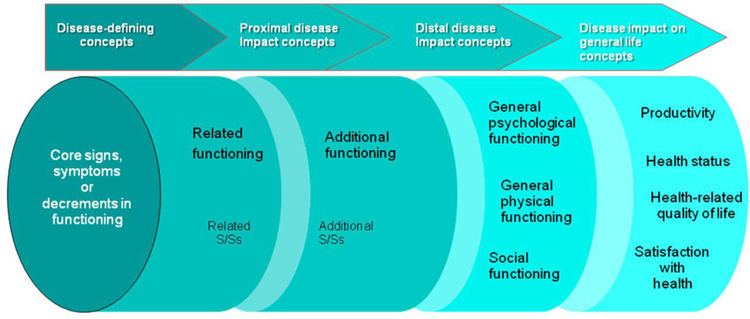
When evaluating treatment benefits on physical functioning, outcome evaluations need to consider a range of measures, all of which are important; however, the interpretation of trial results depends on knowing how treatment affects the core disease-defining concepts. Figure 2 illustrates this. On the left, measures are specific and represent the core disease. However, moving towards the right, the measures address concepts that can be modified by many factors other than just the core disease. These measures may also follow a different time course than the core disease-defining concept, in that they may take longer to recover following an intervention. We recommend that investigators identify the core disease-defining concepts before more downstream effects, and that they match the core disease-defining concepts with measures that will be the primary outcomes in a clinical trial. Although this recommendation appears to be reasonable, further research is required to address the complexities involved in identifying the core disease-defining concepts in those living with chronic pain.164
Figure 2.
Treatment benefit: what to measure? Schematic diagram depicting the impact of inter-related domains and sub-domains of assessment for chronic diseases.168
S/Ss = signs and symptoms
5.3. Assess performance properties for each physical functioning measure
Investigators should give careful attention to content validity and instrument design to insure that they present these precisely in their articles. A number of resources are available to support the identification of robust outcome measures for use in clinical trials including: OMERACT Filter 2.022, COSMIN46,131, and the PROMIS physical function domain.63,105 The International Society for Pharmaco-economic and Outcomes Research (ISPOR)127,147 also publishes consensus recommendations for the development and evaluation of measures. Investigators should match valid and reliable measures to the specific chronic pain condition being studied, and should include a generic measure of physical functioning to enhance interpretation of study outcomes by providing a context of data from other disorders. Considered together, the results of previous chronic pain clinical trials and on-going work provide sufficient evidence to support this recommendation.
5.4. The context of use of function measures and the environment of the respondent need to be identified
The presence of contextual factors defines the situation in which the abilities of individuals are being tested or quantified.129 It is important to also consider the person as a contextual factor68,70; for example, individuals adapting to their pain condition to conserve energy or accepting help from others. Differentiating whether a specific act (walking), identifiable task (walking outside), or participation (getting to work on time) is the outcome of interest is ultimately up to the investigators for a given study. Researchers will need to develop a general model of function to address capacity to function, the environmental pressures on that capacity, and the ability of coping and adaptation to modify the relationship between capacity and what study participants actually perform. In addition, it is essential to consider meaning; measures may miss what is truly important to those living with pain beyond what they are obligated to do on a day-to-day basis; for example, discretionary, recreational, and social activities. Investigators should not use physical functioning measures without appropriate consideration of contextual factors; they should collect relevant contextual information and present this information as part of the demographic and clinical description of the sample investigators plan to study. To ascertain how pain affects physical functioning from an individual perspective and weighing the contextual factors that are relevant will require a large body of evidence obtained initially from qualitative studies. This is, therefore, something that researchers will need to consider carefully as the evidence may not necessarily exist to guide the context of use of functional measures in specific patient groups.
6. Conclusions and specific recommendations
Physical functioning and activity are important components of health status119 and functional limitations in daily activities are common among adults with chronic pain.91 Researchers should develop physical functioning assessments for individuals with chronic pain within a conceptual model. Measures should be directed at the specific definition of physical functioning (i.e., activity, participation) that is of interest, and contextual factors and the environment should be considered. Investigators need to assess the appropriateness of a given measure for the specific research objectives of the research. There is a great deal of variability in the content and format of PRO measures of physical functioning, some of which undoubtedly reflects the diversity of painful conditions studied. Previous reviews have found variation in the number of recall periods used, inconsistencies in the development and validation methods of questionnaires, and limited, if any, discussion of the conceptual framework underlying the assessments. Therefore, future research needs to address these previous limitations to develop robust outcome measures for physical and social functioning.
Investigators did not develop most currently used measures with patient involvement, input, or perspective. We recommend the inclusion of patient input in the earliest stages of the development process. This can take the form of focus groups and qualitative studies to establish what are important outcomes for those living with persistent pain. Patient involvement is an important consideration in the FDA PRO guidance.193 Future research will need to base physical functioning measures on a conceptual framework that includes patient input. Researchers can conduct Delphi studies combining the views of patients living with persistent pain, health professionals, and other stakeholders. OMERACT has a long history of working collaboratively with patient research partners using a variety of research methods and workshops to ensure that researchers accurately capture patient views and incorporate these into meaningful outcome measures.
We recommend that all chronic pain clinical trials include measures of physical functioning that are appropriate for the specific population investigators are studying and are valid and reliable; caution is needed, however, when extending the use of any measures, even well-established ones, to populations that differ substantially. There are many influences on physical functioning, including moderators and mediating processes. Given this, chronic pain clinical trials need to be able to demonstrate the robustness of their outcome measures not only for reasons of data accuracy, but for the interpretation of the data and generalizability to clinical practice. Measures for pediatric populations are beyond the scope of this article and warrant dedicated consideration; however, many of the general assessment considerations discussed in this article also apply to children and adolescents.
As described in previous IMMPACT recommendations, investigators should give consideration to use of disease-specific measures combined with generic measures of physical functioning when designing a chronic pain clinical trial.190 Investigators may not capture specific impairments inherent in a particular pain disorder with generic outcome measures. Therefore, disease-specific measures may more readily detect clinically important improvement or deterioration in functioning that is a consequence of the treatment under study. We recommend consideration of a combination of both types of physical functioning measures (generic and disease specific) such as those listed in Tables 2 and 3. There are 3 key domains in the realm of physical function assessment: self-reported physical functioning, measured physical function, and measured activity levels. As discussed in this article, each has strengths and weaknesses and until more outcome methods research has been undertaken, these 3 domains may all need to be assessed when physical function is a key outcome domain in a chronic pain clinical trial. Actigraphy is a more objective measure and with improvements in technology, this may become the definitive physical functioning measure in future trials. At present, however, it is important for research groups to ascertain what it is that they want to measure as an outcome and to identify valid and reliable tools that minimize bias.
Objective measures of physical functioning can complement PROs and provide some unique information. We recommend actigraphy as an objective measure of physical activity if additional research demonstrates that the measure captures the physical activity of interest. For performance measures of physical functioning, we suggest that investigators should consider the TUG150, the 6MWT16, and the SPPB77 if appropriate for the context of use. Other performance measures may be more relevant for a specific clinical trial, or may have better measurement properties for a specific population (e.g., pediatric or geriatric populations) and investigators should also consider these. We listed a summary of the recommendations made throughout this article in Table 6.
Table 6:
Summary of Recommendations
| • Physical functioning assessments should be developed within a conceptual model |
| • Patient input should be included in the earliest stages of the development process for any outcome measure. |
| • Investigators should assess the appropriateness of any measure of physical functioning that they are considering for the specific population they are studying. |
| • Investigators should assess the appropriateness of a given measure for the specific research objectives of the research. |
| • Investigators should give consideration to use of disease-specific measures combined with generic measures of physical functioning when designing a chronic pain clinical trial. |
| • Consideration should be given to use of a combination of both types of physical functioning outcomes, that is, patient-reported measures and more objective assessments of activity or performance |
| • Investigators should consider actigraphy as an objective measure of physical activity if they can demonstrate that the measure captures the physical activity of interest. |
Future research directions include: (1) examination of the discrepancies between PRO and objective measures of physical functioning; (2) assessment of caregiver burden and workplace participation; and (3) examination of sleep, emotional functioning, and sexual functioning and their relationships to the physical functioning outcomes discussed in this article. Although our emphasis was clinical trials, consideration of the issues raised in this article should inform research and facilitate comparison across studies of chronic pain more broadly by improving understanding of the impact of pain on functioning, as well as potentially advancing the development of effective treatments for patients with diverse chronic pain conditions.
Acknowledgements
The reader should infer no official endorsement by the US Department of Veterans Affairs, US Food and Drug Administration, or the US National Institutes of Health. Support for the meeting was provided by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the FDA, which has received grants, contracts and other revenue for its activities from the FDA, multiple pharmaceutical and device companies, and other sources. The authors thank Valorie Thompson and Andrea Speckin for their invaluable assistance in the organization of the IMMPACT meeting, and Drs. Sharon Hertz, Allison Lin, Elektra Papadopoulos, Ashley Slagle, and Sara Yim for their contributions to the meeting.
Footnotes
The views expressed in this article are those of the authors, none of whom have financial conflicts of interest relevant to the issues discussed in this manuscript.
References
- [1].Agarwal S, Polydefkis M, Block B, Haythornthwaite J, Raja SN. Transdermal fentanyl reduces pain and improves functional activity in neuropathic pain states. Pain Med 2007;8:554–62. [DOI] [PubMed] [Google Scholar]
- [2].Alghwiri AA, Whitney SL, Baker CE, Sparto PJ, Marchetti GF, Rogers JC, Furman JM. The development and validation of the vestibular activities and participation measure. Arch Phys Med Rehabil 2012;93:1822–31. [DOI] [PubMed] [Google Scholar]
- [3].Alonso J, Bartlett SJ, Rose M, Aaronson NK, Chaplin JE, Efficace F, Leplege A, Lu A, Tulsky DS, Raat H, Ravens-Sieberer U, Revicki D, Terwee CB, Valderas JM, Cella D, Forrest CB, Group PI. The case for an international patient-reported outcomes measurement information system (PROMIS(R)) initiative. Health and quality of life outcomes 2013;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Amtmann D, Cook KF, Johnson KL, Cella D. The PROMIS initiative: involvement of rehabilitation stakeholders in development and examples of applications in rehabilitation research. Arch Phys Med Rehabil 2011;92(10 Suppl):S12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anagnostis C, Gatchel RJ, Mayer TG. The pain disability questionnaire: a new psychometrically sound measure for chronic musculoskeletal disorders. Spine (Phila Pa 1976) 2004;29:2290–302; discussion 2303. [DOI] [PubMed] [Google Scholar]
- [6].Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. PAIN 1995;63:77–84. [DOI] [PubMed] [Google Scholar]
- [7].Andersson EI, Lin CC, Smeets RJ. Performance tests in people with chronic low back pain: responsiveness and minimal clinically important change. Spine 2010;35:E1559–63. [DOI] [PubMed] [Google Scholar]
- [8].Ang DC, Bair MJ, Damush TM, Wu J, Tu W, Kroenke K. Predictors of pain outcomes in patients with chronic musculoskeletal pain co-morbid with depression: results from a randomized controlled trial. Pain Med 2010;11:482–91. [DOI] [PubMed] [Google Scholar]
- [9].Arumugam V, MacDermid JC. The Work Limitations Questionnaire (WLQ-25). J Physiother 2013;59(4):276. [DOI] [PubMed] [Google Scholar]
- [10].Asenlof P, Soderlund A. A further investigation of the importance of pain cognition and behaviour in pain rehabilitation: longitudinal data suggest disability and fear of movement are most important. Clin Rehabil 2010;24:422–30. [DOI] [PubMed] [Google Scholar]
- [11].Asmundson GJ, Kuperos JL, Norton GR. Do patients with chronic pain selectively attend to pain-related information?: preliminary evidence for the mediating role of fear. PAIN 1997;72:27–32. [DOI] [PubMed] [Google Scholar]
- [12].Avorn J, Langer E. Induced disability in nursing home patients: a controlled trial. J Am Geriatr Soc 1982;30:397–400. [DOI] [PubMed] [Google Scholar]
- [13].Ayis S, Arden N, Doherty M, Pollard B, Johnston M, Dieppe P. Applying the impairment, activity limitation, and participation restriction constructs of the ICF model to osteoarthritis and low back pain trials: a reanalysis. J Rheumatol 2010;37:1923–31. [DOI] [PubMed] [Google Scholar]
- [14].Bade MJ, Kittelson JM, Kohrt WM, Stevens-Lapsley JE. Predicting functional performance and range of motion outcomes after total knee arthroplasty. Am J Physical Med Rehabil 2014;93:579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bai J, Goldsmith J, Caffo B, Glass TA, Crainiceanu CM. Movelets: a dictionary of movement. Electronic J Statistics 2012;6:559–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Balke B A Simple Field Test for the Assessment of Physical Fitness. Rep 63–6. [Report] Civil Aeromedical Research Institute 1963:1–8. [PubMed]
- [17].Beaton DE, Tang K, Gignac MA, Lacaille D, Badley EM, Anis AH, Bombardier C. Reliability, validity, and responsiveness of five at-work productivity measures in patients with rheumatoid arthritis or osteoarthritis. Arthritis Care Res (Hoboken) 2010;62:28–37. [DOI] [PubMed] [Google Scholar]
- [18].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- [19].Bergbom S, Boersma K, Overmeer T, Linton SJ. Relationship among pain catastrophizing, depressed mood, and outcomes across physical therapy treatments. Phys Ther 2011;91:754–64. [DOI] [PubMed] [Google Scholar]
- [20].Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981;19:787–805. [DOI] [PubMed] [Google Scholar]
- [21].Bjorner JB, Rose M, Gandek B, Stone AA, Junghaenel DU, Ware JE Jr. Method of administration of PROMIS scales did not significantly impact score level, reliability, or validity. J Clin Epidemiol 2014;67:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d’Agostino MA, Conaghan PG, Bingham CO, Brooks P, Landewé R, March L, Simon LS, Singh JA, Strand V, Tugwell P. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol 2014;67:745–53. [DOI] [PubMed] [Google Scholar]
- [23].Boersma K, Linton SJ. How does persistent pain develop? An analysis of the relationship between psychological variables, pain and function across stages of chronicity. Behav Res Ther 2005;43:1495–1507. [DOI] [PubMed] [Google Scholar]
- [24].Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills 2006;103:215–22. [DOI] [PubMed] [Google Scholar]
- [25].Bolton JE, Breen AC. The Bournemouth Questionnaire: a short-form comprehensive outcome measure. I. Psychometric properties in back pain patients. J Manipulative Physiol Ther 1999;22:503–10. [DOI] [PubMed] [Google Scholar]
- [26].Bolton JE, Humphreys BK. The Bournemouth Questionnaire: a short-form comprehensive outcome measure. II. Psychometric properties in neck pain patients. J Manipulative Physiol Ther 2002;25:141–8. [DOI] [PubMed] [Google Scholar]
- [27].Borjesson M, Weidenhielm L, Elfving B, Olsson E. Tests of walking ability at different speeds in patients with knee osteoarthritis. Physiotherapy Res Int 2007;12:115–21. [DOI] [PubMed] [Google Scholar]
- [28].Brage S, Wedderkopp N, Franks PW, Andersen LB, Froberg K. Reexamination of validity and reliability of the CSA monitor in walking and running. Med Sci Sports Exercise 2003;35:1447–54. [DOI] [PubMed] [Google Scholar]
- [29].Brenner R, Madhusoodanan S, Brodsky E, Soberano W, Shack M, Nelson-Sasson A, Czobor P. Psychological symptoms in paid caregivers of dementia patients: a pilot study. Psychiatry 2006;3:46–9. [PMC free article] [PubMed] [Google Scholar]
- [30].Broderick JE, DeWitt EM, Rothrock N, Crane PK, Forrest CB. Advances in Patient-Reported Outcomes: the NIH PROMISR Measures. Egems 2013;1(1):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brouwer WB, Meerding WJ, Lamers LM, Severens JL. The relationship between productivity and health-related QOL: an exploration. Pharmacoeconomics 2005;23:209–18. [DOI] [PubMed] [Google Scholar]
- [32].Brown CA, Seymour B, Boyle Y, El-Deredy W, Jones AK. Modulation of pain ratings by expectation and uncertainty: Behavioral characteristics and anticipatory neural correlates. Pain 2008;135:240–50. [DOI] [PubMed] [Google Scholar]
- [33].Brown M, Dijkers M, Gordon W, Ashman T, Charatz H, Cheng Z. Participation Objective, Participation Subjective: A measure of participation combining outsider and insider perspectives. J Head Trauma Rehabil 2004;19:459–81. [DOI] [PubMed] [Google Scholar]
- [34].Bruce B, Fries J, Lingala B, Hussain YN, Krishnan E. Development and assessment of floor and ceiling items for the PROMIS physical function item bank. Arthritis Res Ther 2013;15(5):R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Buck R, Morley S. A daily process design study of attentional pain control strategies in the self-management of cancer pain. Eur J Pain 2006;10:385–98. [DOI] [PubMed] [Google Scholar]
- [36].Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol 1991;18:728–33. [PubMed] [Google Scholar]
- [37].Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, Jenkinson T. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- [38].Cardol M, de Haan RJ, de Jong BA, van den Bos GA, de Groot IJ. Psychometric properties of the Impact on Participation and Autonomy Questionnaire. Arch Phys Med Rehabil 2001;82:210–16. [DOI] [PubMed] [Google Scholar]
- [39].Cardol M, de Haan RJ, van den Bos GA, de Jong BA, de Groot IJ. The development of a handicap assessment questionnaire: the Impact on Participation and Autonomy (IPA). Clin Rehabil 1999;13:411–19. [DOI] [PubMed] [Google Scholar]
- [40].Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chung CP, Sokka T, Arbogast PG, Pincus T. Work disability in early rheumatoid arthritis: higher rates but better clinical status in Finland compared with the US. Ann Rheum Dis 2006;65:1653–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cibulka MT, White DM, Woehrle J, Harris-Hayes M, Enseki K, Fagerson TL, Slover J, Godges JJ. Hip pain and mobility deficits--hip osteoarthritis: clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopaedic section of the American Physical Therapy Association. J Orthopaed Sports Phys Ther 2009;39:A1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann the Acad Med (Singapore) 1994;23:129–38. [PubMed] [Google Scholar]
- [44].Coenen M, Cieza A, Stamm TA, Amann E, Kollerits B, Stucki G. Validation of the International Classification of Functioning, Disability and Health (ICF) Core Set for rheumatoid arthritis from the patient perspective using focus groups. Arthritis Res Ther 2006;8(4):R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Coenen M, Kus S, Rudolf KD, Müller G, Berno S, Dereskewitz C, MacDermid J. Do patient-reported outcome measures capture functioning aspects and environmental factors important to individuals with injuries or disorders of the hand? J Hand Ther 2013;26:332–42; quiz 342. [DOI] [PubMed] [Google Scholar]
- [46].Conijn AP, Jens S, Terwee CB, Breek JC, Koelemay MJ. Assessing the quality of available patient reported outcome measures for intermittent claudication: a systematic review using the COSMIN checklist. Eur J Vascular Endovascular Surg 2015;49:316–34. [DOI] [PubMed] [Google Scholar]
- [47].Crombez G, Eccleston C, Baeyens F, van Houdenhove B, van den Broeck A. Attention to chronic pain is dependent upon pain-related fear. J Psychosom Res 1999;47:403–10. [DOI] [PubMed] [Google Scholar]
- [48].Dansie EJ, Turk DC, Martin KR, Van Domelen DR, Patel KV. Association of chronic widespread pain with objectively measured physical activity in adults: findings from the National Health and Nutrition Examination survey. J Pain 2014;15:507–15. [DOI] [PubMed] [Google Scholar]
- [49].Daughton DM, Fix AJ, Kass I, Bell CW, Patil KD. Maximum oxygen consumption and the ADAPT quality-of-life scale. Arch Phys Med Rehabil 1982;63:620–2. [PubMed] [Google Scholar]
- [50].Davis KL, Marin DB, Kane R, Patrick D, Peskind ER, Raskind MA, Puder KL. The Caregiver Activity Survey (CAS): development and validation of a new measure for caregivers of persons with Alzheimer’s disease. Int J Geriatric Psychiatry 1997;12:978–88. [DOI] [PubMed] [Google Scholar]
- [51].Dunlop DD, Song J, Semanik PA, Sharma L, Bathon JM, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Mysiw WJ, Nevitt MC, Chang RW. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: prospective cohort study. BMJ 2014;348:g2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [53].Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 1999;125:356–66. [DOI] [PubMed] [Google Scholar]
- [54].Esliger DW, Rowlands AV, Hurst TL, Catt M, Murray P, Eston RG. Validation of the GENEA Accelerometer. Medicine and science in sports and exercise 2011;43:1085–93. [DOI] [PubMed] [Google Scholar]
- [55].Evers AW, Kraaimaat FW, Geenen R, Jacobs JW, Bijlsma JW. Pain coping and social support as predictors of long-term functional disability and pain in early rheumatoid arthritis. Behav Res Ther 2003;41:1295–310. [DOI] [PubMed] [Google Scholar]
- [56].Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940–52; discussion 2952. [DOI] [PubMed] [Google Scholar]
- [57].Falconer J, Hughes SL, Naughton BJ, Singer R, Chang RW, Sinacore JM. Self report and performance-based hand function tests as correlates of dependency in the elderly. J Am Geriatr Soc 1991;39:695–9. [DOI] [PubMed] [Google Scholar]
- [58].Feldman H, Gauthier S, Hecker J, Vellas B, Emir B, Mastey V, Subbiah P, Donepezil MSIG. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer’s disease and the effect on caregiver burden. J Am Geriatr Soc 2003;51:737–44. [DOI] [PubMed] [Google Scholar]
- [59].Flor H, Turk DC, Rudy TE. Relationship of pain impact and significant other reinforcement of pain behaviors: the mediating role of gender, marital status and marital satisfaction. PAIN 1989;38:45–50. [DOI] [PubMed] [Google Scholar]
- [60].Follick MJ, Ahern DK, Laser-Wolston N. Evaluation of a daily activity diary for chronic pain patients. PAIN 1984;19:373–82. [DOI] [PubMed] [Google Scholar]
- [61].Foster NE, Pincus T, Underwood MR, Vogel S, Breen A, Harding G. Understanding the process of care for musculoskeletal conditions--why a biomedical approach is inadequate. Rheumatology (Oxford) 2003;42:401–4. [PubMed] [Google Scholar]
- [62].Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exer 1998;30:777–81. [DOI] [PubMed] [Google Scholar]
- [63].Fries JF, Cella D, Rose M, Krishnan E, Bruce B. Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. J Rheumatol 2009;36:2061–6. [DOI] [PubMed] [Google Scholar]
- [64].Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 1982;9:789–93. [PubMed] [Google Scholar]
- [65].Gandek B, Sinclair SJ, Jette AM, Ware JE. Development and initial psychometric evaluation of the participation measure for post-acute care (PM-PAC). Am J Phys Med Rehabil 2007;86:57–71. [DOI] [PubMed] [Google Scholar]
- [66].Garrad J, Bennett AE. A validated interview schedule for use in population surveys of chronic disease and disability. Br J Prev Soc Med 1971;25:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gignac MA. Arthritis and employment: an examination of behavioral coping efforts to manage workplace activity limitations. Arthritis Rheum 2005;53:328–36. [DOI] [PubMed] [Google Scholar]
- [68].Gignac MA, Backman CL, Davis AM, Lacaille D, Cao X, Badley EM. Social role participation and the life course in healthy adults and individuals with osteoarthritis: are we overlooking the impact on the middle-aged? Soc Sci Med 2013;81:87–93. [DOI] [PubMed] [Google Scholar]
- [69].Gignac MA, Badley EM, Lacaille D, Cott CC, Adam P, Anis AH. Managing arthritis and employment: making arthritis-related work changes as a means of adaptation. Arthritis Rheum 2004;51:909–16. [DOI] [PubMed] [Google Scholar]
- [70].Gignac MA, Lacaille D, Beaton DE, Backman CL, Cao X, Badley EM. Striking a balance: work-health-personal life conflict in women and men with arthritis and its association with work outcomes. J Occup Rehabil 2014. 24:573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gill S, McBurney H. Reliability of performance-based measures in people awaiting joint replacement surgery of the hip or knee. Physiotherapy Res Int 2008;13:141–52. [DOI] [PubMed] [Google Scholar]
- [72].Gilson BS, Gilson JS, Bergner M, Bobbit RA, Kressel S, Pollard WE, Vesselago M. The sickness impact profile. Development of an outcome measure of health care. Am J Public Health 1975;65:1304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Grant PM, Ryan CG, Tigbe WW, Granat MH. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br J Sports Med 2006;40:992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gray DB, Hollingsworth HH, Stark SL, Morgan KA. Participation survey/mobility: psychometric properties of a measure of participation for people with mobility impairments and limitations. Arch Phys Med Rehabil 2006;87:189–97. [DOI] [PubMed] [Google Scholar]
- [75].Guerra-Silla MG, Gutierrez-Robledo LM, Villalpando-Berumen JM, Perez-Zepeda MU, Montana-Alvarez M, Reyes-Guerrero J, Rosas-Carrasco O. Psychometric evaluation of a Spanish language version of the Screen for Caregiver Burden (SCB) in caregivers of patients with mixed, vascular and Alzheimer’s dementia. J Clin Nursing 2011;20:3443–51. [DOI] [PubMed] [Google Scholar]
- [76].Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord 2006;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- [78].Hahn EA, DeVellis RF, Bode RK, Garcia SF, Castel LD, Eisen SV, Bosworth HB, Heinemann AW, Rothrock N, Cella D, on behalf of the PROMIS Cooperative Group. (2010). Measuring social health in the Patient-Reported Outcomes Measurement Information System (PROMIS): item bank development and testing. Qual Life Res 2010;19:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hammel J, Magasi S, Heinemann A, Whiteneck G, Bogner J, Rodriguez E. What does participation mean? An insider perspective from people with disabilities. Disabil Rehabil 2008;30:1445–60. [DOI] [PubMed] [Google Scholar]
- [80].Harwood RH, Rogers A, Dickinson E, Ebrahim S. Measuring handicap: the London Handicap Scale, a new outcome measure for chronic disease. Qual Health Care 1994;3:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hays RD, Liu H, Spritzer K, Cella D. Item response theory analyses of physical functioning items in the medical outcomes study. Med Care 2007;45(5 Suppl 1):S32–8. [DOI] [PubMed] [Google Scholar]
- [82].He B, Bai J, Zipunnikov VV, Koster A, Caserotti P, Lange-Maia B, Glynn NW, Harris TB, Crainiceanu CM. Predicting human movement with multiple accelerometers using movelets. Med Sci Sports Exercise 2014;46:1859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Heinemann AW, Lai JS, Magasi S, Hammel J, Corrigan JD, Bogner JA, Whiteneck GG. Measuring participation enfranchisement. Arch Phys Med Rehabil 2011;92:564–71. [DOI] [PubMed] [Google Scholar]
- [84].Heinemann AW, Magasi S, Bode RK, Hammel J, Whiteneck GG, Bogner J, Corrigan JD. Measuring enfranchisement: importance of and control over participation by people with disabilities. Arch Phys Med Rehabil 2013;94:2157–65. [DOI] [PubMed] [Google Scholar]
- [85].Hendelman D, Miller K, Baggett C, Debold E, Freedson P. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Med Sci Sports Exercise 2000;32(9 Suppl):S442–9. [DOI] [PubMed] [Google Scholar]
- [86].Hermsen LA, Terwee CB, Leone SS, van der Zwaard B, Smalbrugge M, Dekker J, van der Horst HE, Wilkie R. Social participation in older adults with joint pain and comorbidity; testing the measurement properties of the Dutch Keele Assessment of Participation. BMJ open 2013;3(8):e003181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Heyneman N, Fremouw wJ, Gano D, Kirkland F, Heiden D. Individual differences and the effectiveness of coping strategies for pain. Cogn Ther Res 1990;14:63–77. [Google Scholar]
- [88].Hieblinger R, Coenen M, Stucki G, Winkelmann A, Cieza A. Identification of essential elements of functioning in chronic widespread pain based on a statistical approach. Am J Phys Med Rehabil 2011;90:979–91. [DOI] [PubMed] [Google Scholar]
- [89].Hirschman KB, Shea JA, Xie SX, Karlawish JH. The development of a rapid screen for caregiver burden. J Am Geriatr Soc 2004;52:1724–9. [DOI] [PubMed] [Google Scholar]
- [90].Hirsh AT, Braden AL, Craggs JG, Jensen MP. Psychometric properties of the community integration questionnaire in a heterogeneous sample of adults with physical disability. Arch Phys Med Rehabil 2011;92:1602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum 2006;54:226–9. [DOI] [PubMed] [Google Scholar]
- [92].Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med 1996;29:602–8. [DOI] [PubMed] [Google Scholar]
- [93].Hunt SM, McKenna SP, McEwen J, Backett EM, Williams J, Papp E. A quantitative approach to perceived health status: a validation study. J Epidemiol Community Health 1980;34:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jette AM, Deniston OL. Inter-observer reliability of a functional status assessment instrument. J Chronic Dis 1978;31:573–80. [DOI] [PubMed] [Google Scholar]
- [95].Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exer Sport 1999;70:113–19. [DOI] [PubMed] [Google Scholar]
- [96].Kaplan CP. The community integration questionnaire with new scoring guidelines: concurrent validity and need for appropriate norms. Brain Injury 2001;15:725–31. [DOI] [PubMed] [Google Scholar]
- [97].Kaplan RM, Patterson TL, Kerner DN, Atkinson JH, Heaton RK, Grant I. The Quality of Well-Being scale in asymptomatic HIV-infected patients. HNRC Group. HIV Neural Behavioral Research Center. Qual Life Res 1997;6:507–14. [DOI] [PubMed] [Google Scholar]
- [98].Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord 2009;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30. [DOI] [PubMed] [Google Scholar]
- [100].Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, MacMillan A, Ketchel P, DeKosky ST. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 1998;46:210–15. [DOI] [PubMed] [Google Scholar]
- [101].Kavanaugh A, Gladman D, van der Heijde D, Purcaru O, Mease P. Improvements in productivity at paid work and within the household, and increased participation in daily activities after 24 weeks of certolizumab pegol treatment of patients with psoriatic arthritis: results of a phase 3 double-blind randomised placebo-controlled study. Ann Rheum Dis 2015;74:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Keefe FJ, Smith SJ, Buffington AL, Gibson J, Studts JL, Caldwell DS. Recent advances and future directions in the biopsychosocial assessment and treatment of arthritis. J Consult Clin Psychol 2002;70:640–55. [DOI] [PubMed] [Google Scholar]
- [103].Keogh E, Dillon C, Georgiou G, Hunt C. Selective attentional biases for physical threat in physical anxiety sensitivity. J Anxiety Disord 2001;15:299–315. [DOI] [PubMed] [Google Scholar]
- [104].Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;23:345–56. [DOI] [PubMed] [Google Scholar]
- [105].Khanna D, Krishnan E, Dewitt EM, Khanna PP, Spiegel B, Hays RD. The future of measuring patient-reported outcomes in rheumatology: Patient-Reported Outcomes Measurement Information System (PROMIS). Arth Care Res 2011;63 Suppl 11:S486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kinugasa T, Nagasaki H. Reliability and validity of the Motor Fitness Scale for older adults in the community. Aging (Milano) 1998;10:295–302. [DOI] [PubMed] [Google Scholar]
- [107].Kirkley A, Alvarez C, Griffin S. The development and evaluation of a disease-specific quality-of-life questionnaire for disorders of the rotator cuff: The Western Ontario Rotator Cuff Index. Clin J Sport Med 2003;13:84–92. [DOI] [PubMed] [Google Scholar]
- [108].Kirkley A, Griffin S, Dainty K. Scoring systems for the functional assessment of the shoulder. Arthroscopy 2003;19:1109–20. [DOI] [PubMed] [Google Scholar]
- [109].Koster A, Caserotti P, Patel KV, Matthews CE, Berrigan D, Van Domelen DR, Brychta RJ, Chen KY, Harris TB. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PloS one 2012;7(6):e37696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kroman SL, Roos EM, Bennell KL, Hinman RS, Dobson F. Measurement properties of performance-based outcome measures to assess physical function in young and middle-aged people known to be at high risk of hip and/or knee osteoarthritis: a systematic review. Osteoarthritis Cartilage 2014;22:26–39. [DOI] [PubMed] [Google Scholar]
- [111].Lequesne MG. The algofunctional indices for hip and knee osteoarthritis. J Rheumatol 1997;24:779–81. [PubMed] [Google Scholar]
- [112].Lerner D, Amick BC 3rd, Rogers WH, Malspeis S, Bungay K, Cynn D. The Work Limitations Questionnaire. Med Care 2001;39:72–85. [DOI] [PubMed] [Google Scholar]
- [113].Lerner D, Reed JI, Massarotti E, Wester LM, Burke TA. The Work Limitations Questionnaire’s validity and reliability among patients with osteoarthritis. J Clin Epidemiol 2002;55:197–208. [DOI] [PubMed] [Google Scholar]
- [114].Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am 1993;75:1585–92. [DOI] [PubMed] [Google Scholar]
- [115].Lin YC, Davey RC, Cochrane T. Tests for physical function of the elderly with knee and hip osteoarthritis. Scand J Med Science Sports 2001;11:280–6. [DOI] [PubMed] [Google Scholar]
- [116].Lingler JH, Martire LM, Schulz R. Caregiver-specific outcomes in antidementia clinical drug trials: a systematic review and meta-analysis. J Am Geriatr Soc 2005;53:983–90. [DOI] [PubMed] [Google Scholar]
- [117].Liu B, Woo J, Tang N, Ng K, Ip R, Yu A. Assessment of total energy expenditure in a Chinese population by a physical activity questionnaire: examination of validity. Int J Food Sci Nutr 2001;52:269–82. [DOI] [PubMed] [Google Scholar]
- [118].Logerstedt D, Grindem H, Lynch A, Eitzen I, Engebretsen L, Risberg MA, Axe MJ, Snyder-Mackler L. Single-legged hop tests as predictors of self-reported knee function after anterior cruciate ligament reconstruction: the Delaware-Oslo ACL cohort study. Am J Sports Med 2012;40:2348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Louie GH, Reveille JD, Ward MM. Challenges comparing functional limitations in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol 2009;27(4 Suppl 55):S83–91. [PMC free article] [PubMed] [Google Scholar]
- [120].Lyden K, Kozey Keadle SL, Staudenmayer JW, Freedson PS. Validity of two wearable monitors to estimate breaks from sedentary time. Med Sci Sports Exercise 2012;44:2243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Macdermid J Update: The Patient-rated Forearm Evaluation Questionnaire is now the Patient-rated Tennis Elbow Evaluation. J Hand Ther 2005;18:407–10. [DOI] [PubMed] [Google Scholar]
- [122].Magland JF, Tjoa CW, Childress AR. Spatio-temporal activity in real time (STAR): optimization of regional fMRI feedback. Neuroimage 2011;55:1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Main CJ, Sullivan MJL, Watson PJ. Pain Management: Practical Applications of the Biopsychosocial Perspective in Clinical and Occupational Settings. Edinburgh: Churchill Livingstone, 2007.
- [124].Mannerkorpi K, Hernelid C. Leisure Time Physical Activity Instrument and Physical Activity at Home and Work Instrument. Development, face validity, construct validity and test-retest reliability for subjects with fibromyalgia. Disabil Rehabil 2005;27:695–701. [DOI] [PubMed] [Google Scholar]
- [125].Martinez-Rodriguez S, Ortiz-Marques N, Iraurgi I, Carrasco M, Miguel JJ. Adaptation and analysis of psychometric features of the Caregiver Risk Screen: a tool for detecting the risk of burden in family caregivers. Int Psychogeriatrics 2013;25:755–64. [DOI] [PubMed] [Google Scholar]
- [126].McCarthy CJ, Oldham JA. The reliability, validity and responsiveness of an aggregated locomotor function (ALF) score in patients with osteoarthritis of the knee. Rheumatology 2004;43:514–17. [DOI] [PubMed] [Google Scholar]
- [127].McGhan WF, Al M, Doshi JA, Kamae I, Marx SE, Rindress D. The ISPOR Good Practices for Quality Improvement of Cost-Effectiveness Research Task Force Report. Value in Health 2009;12:1086–99. [DOI] [PubMed] [Google Scholar]
- [128].McLoughlin MJ, Colbert LH, Stegner AJ, Cook DB. Are women with fibromyalgia less physically active than healthy women? Medicine and science in sports and exercise 2011;43:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med 2006;63:1011–22. [DOI] [PubMed] [Google Scholar]
- [130].McWilliams DF, Varughese S, Young A, Kiely PD, Walsh DA. Work disability and state benefit claims in early rheumatoid arthritis: the ERAN cohort. Rheumatology (Oxford) 2014;53:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mokkink LB, Terwee CB, Gibbons E, Stratford PW, Alonso J, Patrick DL, Knol DL, Bouter LM, de Vet HC. Inter-rater agreement and reliability of the COSMIN (COnsensus-based Standards for the selection of health status Measurement Instruments) checklist. BMC Med Res Methodology 2010;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Moriarty DG, Zack MM, Kobau R. The Centers for Disease Control and Prevention’s Healthy Days Measures - population tracking of perceived physical and mental health over time. Health Qual Life Outcomes 2003;1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Murphy SL, Kratz AL, Williams DA, Geisser ME. The association between symptoms, pain coping strategies, and physical activity among people with symptomatic knee and hip osteoarthritis. Frontiers in Psychology 2012;3:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Rheum 2008;59:849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc 1976;54:439–67. [PubMed] [Google Scholar]
- [136].Nicholas MK. The Pain Self-Efficacy Questionnaire: taking pain into account. Eur J Pain 2007;11:153–63. [DOI] [PubMed] [Google Scholar]
- [137].Nilsdotter AK, Lohmander LS, Klässbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 2003;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Noreau L, Fougeyrollas P, Vincent C. The LIFE-H: assessment of the quality of social participation. Technology and Disability 2002;14:113–18. [Google Scholar]
- [139].Öberg U, Öberg B, Öberg T. Validity and reliability of a new assessment of lower-extremity dysfunction. Phys Ther 1994;74:861–71. [DOI] [PubMed] [Google Scholar]
- [140].Orishimo KF, Kremenic IJ. Effect of fatigue on single-leg hop landing biomechanics. J Appl Biomechanics 2006;22:245–54. [DOI] [PubMed] [Google Scholar]
- [141].Osterhaus JT, Purcaru O, Richard L. Discriminant validity, responsiveness and reliability of the rheumatoid arthritis-specific Work Productivity Survey (WPS-RA). Arthritis Res Ther 2009;11(3):R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Pafougeyrollas F, Noreau L, Dion S, Lepage C, Sévigny M, St-Michel G. La Mesure des habitudes de vie (MHAVIE 3.0), Instrument abrégé. Lac Saint-Charles, Québec: CQCIDIH, 1999. [Google Scholar]
- [143].Palermo TM, Valrie CR, Karlson CW. Family and parent influences on pediatric chronic pain: a developmental perspective. Am Psychol 2014;69:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Parkerson GR, Gehlbach SH, Wagner EH, James SA, Clapp NE, Muhlbaier LH. The Duke-UNC Health Profile: an adult health status instrument for primary care. Med Care 1981;19:806–28. [DOI] [PubMed] [Google Scholar]
- [145].Patel KV, Dansie EJ, Turk DC. Impact of chronic musculoskeletal pain on objectively measured daily physical activity: a review of current findings. Pain Manage 2013;3:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain amoung older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain 2013;154:2649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L. Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1--eliciting concepts for a new PRO instrument. Value in Health 2011;14(8):967–77. [DOI] [PubMed] [Google Scholar]
- [148].Peltenburg AL, Erich WB, Bernink MJ, Huisveld IA. Selection of talented female gymnasts, aged 8 to 11, on the basis of motor abilities with special reference to balance: a retrospective study. Int J Sports Med 1982;3:37–42. [DOI] [PubMed] [Google Scholar]
- [149].Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a MDHAQ [corrected] for standard care of patients with rheumatic diseases. J Rheumatol 2005;32:1432–9. [PubMed] [Google Scholar]
- [150].Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- [151].Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills 1984;59:974. [DOI] [PubMed] [Google Scholar]
- [152].Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009;9:745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Rainville P, Bao QV, Chretien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain 2005;118:306–18. [DOI] [PubMed] [Google Scholar]
- [154].Rejeski WJ, Ettinger WH Jr., Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage 1995;3:157–67. [DOI] [PubMed] [Google Scholar]
- [155].Rimmer JH, Riley BB, Rubin SS. A new measure for assessing the physical activity behaviors of persons with disabilities and chronic health conditions: the Physical Activity and Disability Survey. Am J Health Promot 2001;16:34–42. [DOI] [PubMed] [Google Scholar]
- [156].RobRoy LM, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity of the ankle and foot ability measure. Foot Ankle Int 2005;26:968–83. [DOI] [PubMed] [Google Scholar]
- [157].Roland M, Morris R. A study of the natural history of low-back pain. Part II: development of guidelines for trials of treatment in primary care. Spine (Phila Pa 1976) 1983;8:145–50. [DOI] [PubMed] [Google Scholar]
- [158].Romano JM, Jensen MP, Turner JA, Good Hyman Hops AB. Chronic pain patient-partner interactions: further support for a chronic behavioral model of pain. Behav Ther 2000;31:415–40. [Google Scholar]
- [159].Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS)--validation of a Swedish version. Scand J Med Sci Sports 1998;8:439–48. [DOI] [PubMed] [Google Scholar]
- [160].Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol 1966;21:556–9. [DOI] [PubMed] [Google Scholar]
- [161].Rothman ML, Beltran P, Cappelleri JC, Lipscomb J, Teschendorf B, Group MFP-ROCM. Patient-reported outcomes: conceptual issues. Value Health 2007;10 Suppl 2:S66–75. [DOI] [PubMed] [Google Scholar]
- [162].Roy JS, MacDermid JC, Amick BC, Shannon HS, McMurtry R, Roth JH, Grewal R, Tang K, Beaton D. Validity and responsiveness of presenteeism scales in chronic work-related upper-extremity disorders. Phys Ther 2011;91:254–66. [DOI] [PubMed] [Google Scholar]
- [163].Rozzini R, Frisoni GB, Bianchetti A, Zanetti O, Trabucchi M. Physical Performance Test and Activities of Daily Living scales in the assessment of health status in elderly people. Journal of the American Geriatrics Society 1993;41:1109–13. [DOI] [PubMed] [Google Scholar]
- [164].Salokangas RK, Joukamaa M, Mattila V. Measurement of life satisfaction. Developing a life satisfaction scale. Comprehensive gerontology Section B, Behavioural, social, and applied sciences 1988;2:69–74. [PubMed] [Google Scholar]
- [165].Sandqvist JL, Henriksson CM. Work functioning: a conceptual framework. Work 2004;23:147–57. [PubMed] [Google Scholar]
- [166].Schenkman M, Cutson TM, Zhu CW, Whetten-Goldstein K. A longitudinal evaluation of patients’ perceptions of Parkinson’s disease. Gerontologist 2002;42:790–8. [DOI] [PubMed] [Google Scholar]
- [167].Schreiner AS, Morimoto T, Arai Y, Zarit S. Assessing family caregiver’s mental health using a statistically derived cut-off score for the Zarit Burden Interview. Aging Mental Health 2006;10:107–11. [DOI] [PubMed] [Google Scholar]
- [168].Slagle AF. Update on the Clinical Assessment Qualification Program. PRO Consortium Workshop Washington: IMMPACT, 2014. [Google Scholar]
- [169].Sokka T Long-term outcomes of rheumatoid arthritis. Curr Opin Rheumatol 2009;21:284–90. [DOI] [PubMed] [Google Scholar]
- [170].Sprangers MA, de Regt EB, Andries F, van Agt HM, Bijl RV, de Boer JB, Foets M, Hoeymans N, Jacobs AE, Kempen GI, Miedema HS, Tijhuis MA, de Haes HC. Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol 2000;53:895–907. [DOI] [PubMed] [Google Scholar]
- [171].Spritzer KL, Hays RD. MOS Sleep Scale: A manual for use and scoring. Version 1. Los Angeles, CA, 2003. [Google Scholar]
- [172].Stamm T, Hieblinger R, Boström C, Mihai C, Birrell F, Thorstensson C, Fialka-Moser V, Meriaux-Kratochvila S, Smolen J, Coenen M. Similar problem in the activities of daily living but different experience: a qualitative analysis in six rheumatic conditions and eight European countries. Musculoskeletal Care 2014;12:22–33. [DOI] [PubMed] [Google Scholar]
- [173].Stamm TA, Cieza A, Coenen M, Machold KP, Nell VP, Smolen JS, Stucki G. Validating the International Classification of Functioning, Disability and Health Comprehensive Core Set for Rheumatoid Arthritis from the patient perspective: a qualitative study. Arthritis Rheum 2005;53:431–9. [DOI] [PubMed] [Google Scholar]
- [174].Steultjens MP, Dekker J, van Baar ME, Oostendorp RA, Bijlsma JW. Internal consistency and validity of an observational method for assessing disability in mobility in patients with osteoarthritis. Arthritis Care Res 1999;12(1):19–25. [DOI] [PubMed] [Google Scholar]
- [175].Stewart G, Sara G, Harris M, Waghorn G, Hall A, Sivarajasingam S, Gladman B, Mowry B. A brief measure of vocational activity and community participation: development and reliability of the Activity and Participation Questionnaire. Australian and New Zealand J Psychiatry 2010;44:258–66. [DOI] [PubMed] [Google Scholar]
- [176].Stolwijk C, Castillo-Ortiz JD, Gignac M, Luime J, Boonen A, Group OWP. Importance of contextual factors when measuring work outcome in ankylosing spondylitis: a systematic review by the OMERACT worker productivity group. Arthritis Care Research 2015. [DOI] [PubMed]
- [177].Stratford P, Gill C, Westaway M, Binkley J. Assessing disability and change on individual patients: a report of a patient specific measure. Physiotherapy Canada 1995;47:258–63. [Google Scholar]
- [178].Stratford PW, Kennedy DM. Performance measures were necessary to obtain a complete picture of osteoarthritic patients. J Clin Epidemiol 2006;59:160–7. [DOI] [PubMed] [Google Scholar]
- [179].Sullivan M, Bishop S, Pivik P. The Pain Catastrophizing Scale: development and validation. Psychol Assessment 1995;7:524–32. [Google Scholar]
- [180].Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short musculoskeletal function assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am 1999;81:1245–60. [DOI] [PubMed] [Google Scholar]
- [181].Tang K, Boonen A, Verstappen SM, Escorpizo R, Luime JJ, Lacaille D, Fautrel B, Bosworth A, Cifaldi M, Gignac MA, Hofstetter C, Leong A, Montie P, Petersson IF, Purcaru O, Bombardier C, Tugwell PS, Beaton DE. Worker productivity outcome measures: OMERACT filter evidence and agenda for future research. J Rheumatol 2014;41:165–76. [DOI] [PubMed] [Google Scholar]
- [182].Tang K, Escorpizo R, Beaton DE, Bombardier C, Lacaille D, Zhang W, Anis AH, Boonen A, Verstappen SM, Buchbinder R, Osborne RH, Fautrel B, Gignac MA, Tugwell PS. Measuring the impact of arthritis on worker productivity: perspectives, methodologic issues, and contextual factors. J Rheumatol 2011;38:1776–90. [DOI] [PubMed] [Google Scholar]
- [183].Tate D, Forchheimer M, Maynard F, Dijkers M. Predicting depression and psychological distress in persons with spinal cord injury based on indicators of handicap. Am J Phys Med Rehabil 1994;73:175–83. [DOI] [PubMed] [Google Scholar]
- [184].Taylor AM, Philips K, Taylor JO, Singh JA, Conaghan PG, Choy EH, Tugwell PS, Kaiser U, Strand V, Simon LS, Mease PJ. Is chronic pain a disease in its own right? Discussions from a pre-OMERACT 2014 workshop on chronic pain. J Rheumatol 2015;2:1947–1953. [DOI] [PubMed] [Google Scholar]
- [185].Tomey KM, Sowers MR. Assessment of physical functioning: a conceptual model encompassing environmental factors and individual compensation strategies. Phys Ther 2009;89:705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur J Pain 2010;14:380–6. [DOI] [PubMed] [Google Scholar]
- [187].Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prevent Med 2011;40:454–61. [DOI] [PubMed] [Google Scholar]
- [188].Tugwell P, Bombardier C, Buchanan WW, Goldsmith CH, Grace E, Hanna B. The MACTAR Patient Preference Disability Questionnaire--an individualized functional priority approach for assessing improvement in physical disability in clinical trials in rheumatoid arthritis. J Rheumatol 1987;14:446–51. [PubMed] [Google Scholar]
- [189].Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain 2002;18:355–65. [DOI] [PubMed] [Google Scholar]
- [190].Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003;106:337–45. [DOI] [PubMed] [Google Scholar]
- [191].Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain 2008;137:276–85. [DOI] [PubMed] [Google Scholar]
- [192].Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull 1996;119:488–531. [DOI] [PubMed] [Google Scholar]
- [193].US Department of Health and Human Services Food and Drug Administration. Guidance for industry on patient-reported outcome measures: Use in medical product development to support labeling claims. Federal Register, Vol. 74, 2009. pp. 65132–3. [Google Scholar]
- [194].Ustin TB, Kostanjsek N (2000) Measuring Health and Disability. Manual for WHO Disability Assessment Schedule. WHODAS 2.0. World Health Organisation: Geneva. [Google Scholar]
- [195].Van Damme S, Crombez G, Van Nieuwenborgh-De Wever K, Goubert L. Is distraction less effective when pain is threatening? An experimental investigation with the cold pressor task. Eur J Pain 2008;12:60–7. [DOI] [PubMed] [Google Scholar]
- [196].Veenhuizen Y, Cup EH, Groothuis JT, Hendriks JC, Adang EM, van Engelen BG, Geurts A. Effectiveness and cost-effectiveness of a self-management group program to improve social participation in patients with neuromuscular disease and chronic fatigue: protocol of the Energetic study. BMC Neurology 2015;15(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Verbunt JA. Reliability and validity of the PAD questionnaire: a measure to assess pain-related decline in physical activity. J Rehabil Med 2008;40:9–14. [DOI] [PubMed] [Google Scholar]
- [198].Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14:409–15. [PubMed] [Google Scholar]
- [199].Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. PAIN 1995;62:363–72. [DOI] [PubMed] [Google Scholar]
- [200].Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- [201].Welk GJ, Schaben JA, Morrow JR Jr. Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exercise 2004;36:1637–45. [PubMed] [Google Scholar]
- [202].Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003;56:1163–9. [DOI] [PubMed] [Google Scholar]
- [203].Westerberg CE, Solem-Bertoft E, Lundh I. The reliability of three active motor tests used in painful shoulder disorders. Presentation of a method of general applicability for the analysis of reliability in the presence of pain. Scan J Rehabil Med 1996;28:63–70. [PubMed] [Google Scholar]
- [204].Whiteneck GG, Charlifue SW, Gerhart KA, Overholser JD, Richardson GN. Quantifying handicap: a new measure of long-term rehabilitation outcomes. Arch Phys Med Rehabil 1992;73:519–26. [PubMed] [Google Scholar]
- [205].Whiteneck GG, Dijkers MP, Heinemann AW, Bogner JA, Bushnik T, Cicerone KD, Corrigan JD, Hart T, Malec JF, Millis SR. Development of the participation assessment with recombined tools-objective for use after traumatic brain injury. Archives of physical medicine and rehabilitation 2011;92:542–51. [DOI] [PubMed] [Google Scholar]
- [206].Williams K, Frei A, Vetsch A, Dobbels F, Puhan MA, Rüdell K. Patient-reported physical activity questionnaires: a systematic review of content and format. Health Qual Life Outcomes 2012;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Wilson AC, Palermo TM. Physical activity and function in adolescents with chronic pain: a controlled study using actigraphy. J Pain 2012;13:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Wood-Dauphinee SL, Opzoomer MA, Williams JI, Marchand B, Spitzer WO. Assessment of global function: the Reintegration to Normal Living Index. Arch Phys Med Rehabil 1988;69:583–90. [PubMed] [Google Scholar]
- [209].Woolf AD, Akesson K. Understanding the burden of musculoskeletal conditions. The burden is huge and not reflected in national health priorities. BMJ 2001;322(7294):1079–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].World Health Organisation. (2001). International Classification of Functioning, Disability, and Health (ICF). World Health Organisation, Geneva. [Google Scholar]