Abstract
Addictions to licit and illicit drugs are chronic relapsing brain disorders that affect circuits that regulate reward, motivation, memory, and decision-making. Drug-induced pathological changes in these brain regions are associated with characteristic enduring behaviors that continue despite adverse biopsychosocial consequences. Repeated exposure to these substances leads to egocentric behaviors that focus on obtaining the drug by any means and on taking the drug under adverse psychosocial and medical conditions. Addiction also includes craving for the substances and, in some cases, involvement in risky behaviors that can cause death. These patterns of behaviors are associated with specific cognitive disturbances and neuroimaging evidence for brain dysfunctions in a diverse population of drug addicts. Postmortem studies have also revealed significant biochemical and/or structural abnormalities in some addicted individuals. The present review provides a summary of the evidence that has accumulated over the past few years to implicate brain dysfunctions in the varied manifestations of drug addiction. We thus review data on cerebrovascular alterations, brain structural abnormalities, and postmortem studies of patients who abuse cannabis, cocaine, amphetamines, heroin, and “bath salts”. We also discuss potential molecular, biochemical, and cellular bases for the varied clinical presentations of these patients. Elucidation of the biological bases of addiction will help to develop better therapeutic approaches to these patient populations.
Keywords: Cannabis, Cocaine, Methamphetamine, MDMA, Heroin, Cathinones, Bath salts, Dopamine, Toxicity, Frontal cortex, Hippocampus, Nucleus accumbens, Striatum, Reward mechanisms
Introduction
Addictions are brain disorders that affect neural pathways that subsume reward, motivation, and memory. Because drugs such as amphetamines, cannabis, cocaine, and certain opiates are illegal, it is difficult to know the exact prevalence of drug use. An estimated 149–271 million people have been reported to use an illicit drug worldwide in 2009. These numbers represented 125–203 million cannabis users and 15–39 million users of opioids, amphetamines, or cocaine [47]. The levels and patterns of drug addiction vary with the pharmacological agents being used. For example, cocaine, marijuana, and methamphetamine can create various degrees of psychological dependence that is related, in part, to drug-induced euphoria and an obsessive desire to repeat the initial experience of “getting high”. The repeated use of opiates induces physical dependence in addition to psychological dependence. Nevertheless, addiction to any of these substances is associated with adverse consequences that impact biological, psychological, and social spheres. Addiction also causes severe financial burdens on health-care systems throughout the world. The enduring clinical manifestations of drug addiction are dependent on plastic changes that occur in various brain regions involved in learning and reward processes (Fig. 1). The psychological consequences are manifested by an inability to abstain from drug seeking and taking, unbearable craving, and other characteristic behavioral impairments [198]. The social costs of addiction also include interactions with the legal system because of behaviors focusing on procuring illicit drugs, substantial loss of productivity at work, and multiple incarcerations secondary to criminal behaviors. The National Institute on Drug Abuse has estimated the cost of drug and alcohol abuse/addiction at around $600 billion annually [141]. The psychosocial problems associated with addictions might be related to the various biological effects of these drugs on the central nervous system (CNS). Indeed, these agents can impact both brain structure and function [198]. Drug-induced CNS functions have been documented in clinical settings using cognitive tests and neuroimaging studies [39]. Moreover, a number of investigators have reported significant biochemical and structural changes in brains of humans addicted to illicit substances. The present review documents some of the evidence to support the notion that the commonly used illicit substances do cause pathological changes in the brain. We also propose some potential biological mechanisms for these abnormalities.
Fig. 1.
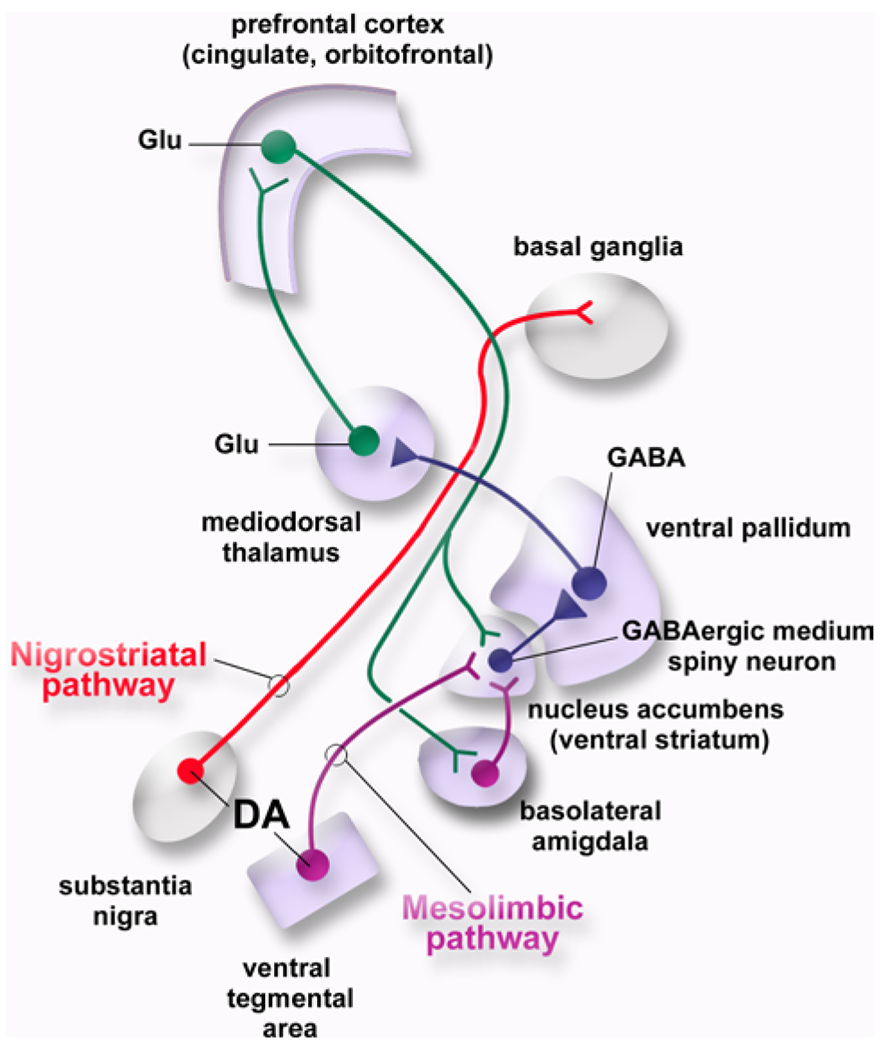
A model of the brain reward system. The nucleus accumbens (ventral striatum) is the major target of the mesolimbic dopaminergic system. It contains GABAergic medium spiny neurons (MSNs) that project to the ventral pallidum (VP). Natural rewards and addictive drugs increase the release of dopamine from the terminals of dopaminergic projections emanating from the ventral tegmental area (VTA). Nigrostriatal dopaminergic pathways (substantia nigra pars compacta to dorsal striatum) also participate in reward-related processes. DA dopamine, GABA g-aminobutyric acid, glu glutamate
Marijuana use disorder
Cannabis remains the most widely produced and consumed illicit substance globally, ahead of amphetamines, opioids, and cocaine [143]. In 2009, between 2.8 and 4.5 % of the world population aged 15–64 years had used cannabis at least once. Cannabis use has increased globally, particularly in Asia, since 2009 [143]. Cannabis sativa is a plant that grows wild in many temperate and tropical climates throughout the world. The cannabis plant contains 489 known compounds representing almost all classes of chemicals, including 70 cannabinoids, of which delta-9-tetrahydrocannabinol (THC) is the most psychoactive [51]. Smoking is the preferred route of delivery, but the drug can also be taken orally. The acute psychopharmacological effects associated with cannabis use include euphoria, increased self-confidence, relaxation, and a general sense of well-being [75]. Acute psychiatric and behavioral abnormalities, such as anxiety, panic, and attentional abnormalities, have also been reported [75]. Negative side effects including psychological and physical dependence have also been described in chronic cannabis users [75]. Risks of psychotic disorders or symptoms are higher in regular cannabis users [137].
The pharmacological effects of cannabis occur through stimulation of cannabinoid receptors by Δ9-tetrahydrocannabinol (THC) [51]. These are the CB1 and CB2 receptors that are abundant in brain’s reward circuitries implicated in addiction [112]. CB1 is found on presynaptic terminals and modulates neurotransmission through inhibition of adenylyl cyclase activation and cAMP production, decrease of Ca2+ influx, and increase of K+ conductance [120]. CB1 receptors are distributed heterogeneously throughout the human brain and are widespread in the neocortex, with particularly high concentrations in frontal regions and areas of associational function [66]. Thalamic nuclei known to be involved in behavioral and cognitive function (anterior, mediodorsal, midline, and intralaminar) have higher receptor density, as do other limbic regions, including the hippocampus, amygdalar complex, and entorhinal cortex [66]. Within the basal ganglia receptor, distribution is highest in the globus pallidus and ventral pallidum [66]. Hindbrain localization is found primarily in the cerebellar cortex, whereas the brainstem and spinal cord are almost deprived of any CB1 receptors [66]. CB1 receptors are also found in human peripheral tissue, but in much lower concentrations. On the other hand, the second type of cannabinoid receptor, CB2 [139], is present mainly in immune tissues and cells [61, 139], with more recent evidence showing that it is also present in the CNS [207].
The accumulated evidence supports a role of the endocannabinoid system in various aspects of drug addiction including influencing rewarding properties of drugs, drug-seeking behavior, and craving/relapse [112]. Cannabis users suffer from deficits in broad cognitive domains that include memory, attention, decision-making, and psychomotor speed [8]. Additionally, poorer cognitive performance in areas of risk taking, decision-making, and episodic memory may influence the degree to which cannabis users engage in risky behaviors with negative health consequences [173].
Cerebrovascular effects
Marijuana use also impacts the cardiovascular system [179]. Marijuana use increases blood pressure through sympathetic stimulation and reduced parasympathetic tone [63]. Marijuana use is associated with cardiac ischemia in susceptible individuals, presumably by causing catecholamine release that, in turn, increases resting heart rate, ischemia, and arrhythmias [179]. Coronary vasospasm precipitated by marijuana smoking has also been proposed as a mechanism for precipitation of acute myocardial infarction in individuals with either normal or minimally diseased coronary arteries [28]. Myocardial infarction in adults [135] and children [134] has also been reported with marijuana use. A recent study reported higher mortality rates associated with marijuana use among patients with high cardiovascular risks [59]. Interestingly, smoking marijuana decreases vascular resistance, causes orthostatic hypotension, and limits oxygen uptake by increasing levels of carboxyhemoglobin [179]. Some of the vascular effects of the drug might be related to the significant increases in serum apoC-III levels found in heavy marijuana users [83] since there is a positive relationship between apoC-III levels and risk of vascular disturbances.
In addition, marijuana smoking may also be related to the precipitation of acute coronary syndrome (ACS) [181]. Although the mechanism by which marijuana use might precipitate ACS is not yet clear, marijuana smoking may lead to the disruption of plaques due to hemodynamic stresses and the formation of occlusive thrombi [135]. Further evidence for increased vascular risk factors in marijuana users was provided in a paper that documented high pulsatility index, a measure of cerebrovascular resistance, and increased systolic velocity in marijuana users [78]. These vascular changes might be secondary to marijuana-induced changes in receptor expression in the vasculature, since the drug causes increased receptor expression after its chronic use [79]. In addition to high consumption of cannabis [125, 211], potential triggering factors of stroke in these patients include concomitant alcohol consumption [125, 204]. These observations support the notion that cerebrovascular consequences of marijuana abuse may be underestimated [203].
Biochemical and structural abnormalities
Several groups of scientists have investigated the possibility that the drug might cause alterations in tissue volume, morphology, and composition in the brain [36, 118, 128, 182, 210]. These investigators have found marijuana-induced changes in the hippocampus [210] and cerebellum [36]. These structural changes might be related, in part, to THC-induced decreases in the number of neurons and synapses in animal models [98]. Marijuana abusers also show lower gray matter density in the right parahippocampal gyrus and greater density bilaterally near the precentral gyrus and the right thalamus as well as lower gray matter volume in the right anterior hippocampus [17]. This patient population also suffers from lower white matter density in the left parietal lobe and higher density around the parahippocampal and fusiform gyri on the left side compared to non-users [118]. Within the group of heavy cannabis users, reduced gray matter volume strongly correlated with individual differences in cannabis use and dependence. Specifically, current weekly cannabis use was associated with reduced hippocampal volume, and the level of cannabis dependence was associated with reduced amygdala volume [36]. Other investigators have reported that cannabis users showed gender-dependent changes in the volume of the prefrontal cortex [128].
The discovery of cannabinoid receptors in oligodendroglial cells [136] motivated a new line of investigation on white matter status in marijuana users. For example, cannabinoid receptors have been detected in white matter structures of the fetal and post-natal rat brain, including the corpus callosum, anterior commissure, fornix, stria terminalis, and stria medullaris [166]. In adults, the density of cannabinoid receptors is reduced in these structures [66, 166]. These observations suggest the existence of a developmental period during which white matter structures might be particularly sensitive to the enduring effects of exogenous cannabis exposure. Down-regulation of the endogenous cannabinoid system due to long-term cannabis exposure during this developmental period [40] may result in apoptosis of oligodendrocyte progenitors [136] and, thereby, alter white matter development [6, 96, 183]. The age during which regular cannabis use begins might be a key factor determining the severity of any white matter alteration in marijuana users [212]. In their study, radial and axial diffusivity correlated positively with the age at which regular use commenced [212]. Axonal connectivity was found to be impaired in the right fimbria of the hippocampus (fornix), splenium of the corpus callosum, and commissural fibers extending to the precuneus. These observations are consistent with the high levels of cannabinoid receptors in the fornix and corpus callosum of the developing rat brain [136, 166]. All users in the Zalesky et al. study [212] had started regular use of the drug during adolescence or early adulthood, times of continuing white matter development [6].
In summary, the wealth of information gathered from neuroimaging studies has indicated that marijuana abuse disorder is accompanied with both functional and structural changes in various regions of the brain. There is still an almost complete lack of information generated from postmortem studies. The one study that we located reported an increase in the density of cannabinoid-1 receptors in the caudate and putamen of subjects who had recently ingested cannabis [46], indicating that the drug can indeed impact the neurochemistry of the cannabinoid system. To better understand the potential clinical impact of this drug, more neuropathological studies that use modern neurobiological techniques are necessary to elucidate the long-term impact of this substance on the brain.
Cocaine use disorder
Cocaine is a highly addictive drug due to its short biologic half-life and strong reinforcing properties that can lead to compulsive usage. The United Nations Office on Drugs and Crime (UNODC) estimates the annual prevalence of cocaine use to be between 0.3 and 0.5 % of the world population aged 15–64, or about 14.2–20.5 million people in that age range [142].
The acute effects of the drug include intense euphoria, increased energy, and alertness that are linked to cocaine’s inhibition of dopamine (DA) transporter function and consequent increased DA levels in the synaptic cleft [163] (Fig. 2). In contrast, cocaine overdose is associated with seizures, status epilepticus, headaches, and strokes [145]. The long-term effects of the drug that include anxiety, depression, drug craving, and hypersomnolence [199] might involve disturbances in various neurotransmitter systems including neuropeptidergic stress systems [91]. Cocaine use is also accompanied by adverse consequences on the cardiovascular, thermoregulatory, and respiratory systems in addition to its neuropsychiatric sequelae [145]. These neuropsychiatric manifestations include decrements in several cognitive domains including executive function, decision-making, impulsivity, visuoperception, psychomotor speed, manual dexterity, verbal learning, and memory [145]. These cognitive deficits are probably related to functional dysfunctions in the prefrontal lobe [16, 68], since neurological patients who suffer damage in this brain region manifest similar cognitive problem [12].
Fig. 2.
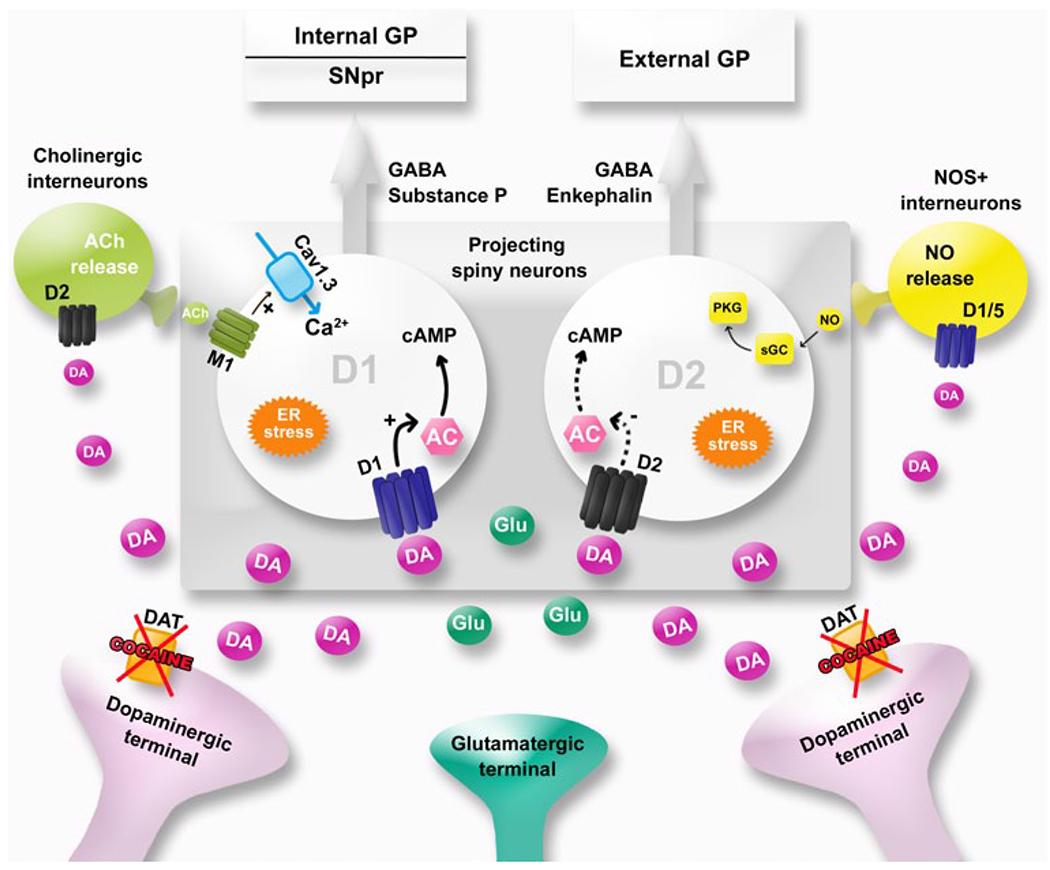
Cocaine potentiates the action of dopamine on ventral and dorsal striatal neurons. Cocaine blocks dopamine transporters (DAT) and increases DA content in the synaptic cleft. Cocaine also disrupts calcium homeostasis and increases endoplasmic reticulum (ER) stress in GABAergic cells within the striatum. Medium-size GABA-containing spiny neurons that represent the main (95 %) striatal neuronal population participate in the modulation of output signals from the basal ganglia. These neurons interact with parvalbumin-containing GABA-releasing interneurons, NADPH diaphorase-, and somatostatin-positive interneurons. They also interact with large cholinergic aspiny interneurons. D1 receptors are found predominantly in striatonigral neurons of the ‘direct pathway’, whereas D2 receptors are mainly expressed by the striatopallidal neurons of the ‘indirect pathway’. Corticostriatal neurons project onto GABA-releasing striatal output neurons where they exert glutamate-mediated excitation. AC adenylyl cyclase, Ach acetylcholine, GP globus pallidus, NO nitric oxide, PKG protein kinase G, sGC soluble guanylyl cyclase
Other neurological manifestations of cocaine abuse include movement disorders that are probably due to disturbances in nigrostriatal dopaminergic transmission [31]. Tourette-like symptoms, dystonic reactions, prolonged dyskinetic movements, and choreic movements have all been reported with heavy use of the drug [15]. Some patients also suffer from cocaine-induced excited delirium [194], a syndrome similar to neuroleptic-induced neuroleptic malignant syndrome which is characterized by hyperthermia, extrapyramidal signs, altered consciousness, and autonomic dysfunction [92, 194].
Cerebrovascular effects
Chronic cocaine abuse is known to be associated with destructive nasal, and midfacial bones and soft tissue destruction [110]. These necrotic changes are secondary to the combined effects of cocaine-induced vasoconstriction and damage to endothelial cells. The hard palate, lateral nasal wall, and nasal septum are the most commonly affected sites [110]. Myocardial infarction, life-threatening ventricular arrhythmias, and cerebral infarction are associated with cocaine [145, 195]. Cocaine-induced seizures are usually single, tonic-clonic, and may resolve without intervention [154, 199]. Neurological patients with known seizure disorders are twice as likely to suffer from cocaine-induced seizures [54]. However, these events can also be pre-terminal and the risk of patients dying increases with multiple seizures, hyperthermia, acidosis, cardiac dysrhythmia, and cardiac arrest [199]. The abuse of large doses of cocaine by young patients [99] can also cause hemorrhagic strokes when the drug is taken either intravenously or smoked [156]. The cause of these strokes might be related to drug-induced hypertension [67]. Ischemic strokes have also been reported [90], with vasospasm, cerebral vacuities, and arterial thrombosis having been suggested as causative agents [16].
In addition, cocaine use might cause stroke by dislodging thromboemboli [171]. Importantly, Nolte et al. [146] have reported that 59 % of cases of fatal intracranial hemorrhages followed over a period of 1 year were associated with cocaine abuse [146]. This prospective study found no specific pathologic lesions to support either inflammatory or vasculotoxic etiologies. The authors suggested that cocaine-mediated pharmacodynamic changes such as vasospasm and hypertension as possible mechanisms that would not have left any pathological traces [146]. Other important mechanisms for cocaine-induced strokes include impaired cerebral auto-regulation of blood flow with cerebral artery dilatation or reperfusion injury [15]. Others have reported the presence of underlying aneurysms or arteriovascular malformations as causative factors for cocaine-associated intracerebral and subarachnoid hemorrhages [24]. For example, there are reported cases of cocaine-associated aneurysm rupture [105], with these occurring at an earlier age than expected in comparison to non-drug-using patients [151]. Interestingly, Fessler et al. [56] reported that the average size of the berry aneurysm observed in 12 cocaine users was 4.9 mm, compared to 11 mm in non-drug-using patients. Also, this study suggested that chronic cocaine use appears to predispose patients who harbor incidental neurovascular anomalies to present with ruptures of aneurysm at an earlier point in the natural history of the disorder [56]. Nevertheless, much remains to be done to better understand the pathobiological bases of the cardiovascular and cerebrovascular effects of this drug.
Biochemical and structural abnormalities
A number of abnormalities in gene and protein expression patterns have been found in postmortem brains of cocaine addicts. Specifically, DAT levels and DA uptake are increased in the ventral striatum of cocaine abusers [116]. Cocaine addicts who died because of cocaine-induced delirium did not show similar increases in DAT expression, suggesting that this patient population might represent an important subgroup of patients with differential responses to the abuse of the drug [115]. Although cocaine exerts its biochemical effects in the brain by increasing DA levels in dopaminergic synaptic areas, there is no evidence that the expression of either D1 or D2 receptor gene is affected in the nucleus accumbens, caudate, putamen, or substantia nigra of cocaine addicts [127]. There is, however, increased D3 receptor mRNA expression in the nucleus accumbens in victims of cocaine overdoses [176]. Serotonin transporter (SERT) densities are also increased in the nucleus accumbens and striatum in victims of cocaine overdose [117]. Moreover, decreased levels of enkephalin mRNA, mu opioid receptor binding, and dopamine uptake sites have been reported [88, 194]. In contrast, the levels of dynorphin mRNA and kappa opioid receptor binding are increased [82, 185]. Other neuropeptides of interest include cocaine- and amphetamine-regulated transcript (CART) that is increased in the nucleus accumbens of cocaine abusers [1]. Of interest to the discussion of white matter abnormalities described above are the observations that a number of myelin-related genes, including myelin basic protein (MBP), proteolipid protein (PLP), and myelin-associated oligodendrocyte basic protein (MOBP) are decreased in the brains of cocaine abusers [95]. These decreases occur in the ventral and dorsal regions of the caudate and putamen, suggesting that widespread changes to the myelin structure could be associated with neuroadaptive changes in the brain of chronic cocaine abusers.
Additionally, a study of brain biomarkers showed that fatal cases classified as excited delirium exhibited elevated levels of heat shock protein 70 (HSP70). These changes in HSP70 in conjunction with dopamine transporter levels were stated to be a reliable marker of excited delirium [115]. A recent study also reported that HSPA1A and HSPA1B gene expression, the HSP70-encoding genes, was increased in cocaine-related deaths in comparison with drug-free controls [86]. Because elevated HSP70 expression was related to a prolonged survival period, HSP70 might not be a reliable brain biomarker of excited delirium [86].
Cocaine addicts suffer from cognitive dysfunctions that are probably secondary to abnormal functions in the prefrontal cortex (PFC) [74]. Structural abnormalities in the PFC have indeed been found in cocaine addicts [55, 119, 180]. Specifically, Fein et al. [55] reported that dependence on crack cocaine (with or without concomitant alcohol dependence) was associated with reduced prefrontal cortical volume (dependence on both substances was not associated with greater prefrontal volume reductions). Reduced prefrontal cortical volume was associated with cognitive impairments in frontal cortex-mediated abilities (executive function). These observations suggest that reduced cerebral volume might have functional consequences. Matochick et al. [119] also found lower gray matter tissue density in the frontal cortex of cocaine addicts. Reduced frontal gray volume was also observed in cocaine addicts. There was also reduced gray volume in the temporal cortex and cerebellum of these patients [180]. Cocaine abusers showed reduced total cortical volume and reduced thickness, including paralimbic cortices such as the insula and dorsal prefrontal cortex [111]. Recently, another study documented lower gray matter volumes in the orbitofrontal cortex, right Inferior frontal gyrus, right insula, left amygdala and parahippocampal gyrus, temporal gyrus, and bilateral caudate in cocaine-dependent individuals [138]. Also, volume reduction in the striatum and the right supramarginal gyrus has been found in this patient population [7].
Cocaine dependence is also associated with impaired integrity of the white matter in the frontal lobe and corpus callosum [100, 101]. Xu et al. [208] described reduced integrity of the white matter, measured by diffusion tensor imaging, in the frontal, parietal, temporal, and occipital lobes of cocaine abusers. Diffusion tensor imaging (DTI) is a quantitative MRI technique that involves measuring the fractional anisotropy of the random motion of water molecules in the brain. Since anisotropy is modulated by the presence of myelin, diffusion indices have been used to measure changes in white matter integrity in many diseases that involve myelin degeneration [58]. Although multiple indexes of tissue abnormalities have been described using DTI, these changes will need to be linked to histological alterations to validate the relationship of DTI to tissue changes on a microscopic level.
Recently, Bell et al. [13] provided more evidence for significant structural changes in the white matter of abstinent cocaine addicts. These abnormalities were observed in the left anterior callosal fibers, left genu of the corpus callosum, right superior longitudinal fasciculus, right callosal fibers, and the superior corona radiata bilaterally. The reason for these abnormalities includes cocaine-induced repeated vasoconstriction and hypoperfusion of the brain as suggested by Lim et al. [101]. This idea is supported by findings that chronic cerebral hypoperfusion causes white matter abnormalities in an animal model [97]. Other potential mechanisms include toxic effects of prolonged use of the drug through mechanisms that include increased cellular calcium [98] and/or endoplasmic reticulum stress [27]. This argument is consistent with the report of decreased dopamine cell numbers (−16 %) in the anterior midbrain of human cocaine users [103]. The presence of reactive gliosis in this brain region area suggests that the decreased number of dopamine cells found in cocaine users might be a relatively recent event [103]. More studies are necessary to document the potential neurodegenerative effects of this drug on the brain.
Amphetamine analog use disorders
Use of amphetamines remains widespread globally and appears to be increasing [143]. Amphetamines are psychoactive substances with stimulant, euphoric, anorectic, and, in some cases, empathogenic, entactogenic, and hallucinogenic properties [32]. These compounds enter the brain very easily and cause significant changes in monoaminergic neurotransmission [32]. Amphetamines act as competitive substrates at the membrane transporters of noradrenaline (NAT), dopamine (DAT), and serotonin (5-HTT; SERT), reducing the reuptake of endogenous neurotransmitters while inducing the reverse transport of endogenous neurotransmitters [50]. Amphetamines also promote the release of DA, 5-HT, and NE from storage vesicles and cause increases in their cytoplasmic concentrations, rendering them readily available for reverse transport [93], (Fig. 3). This phenomenon occurs because the amphetamines can disrupt the pH gradient required for vesicular DA sequestration, with a secondary release of DA into the cytoplasmic compartment [186, 187]. Of these amphetamines, methamphetamine is taken by oral, intravenous, nasal, or smoking routes [93], whereas 3,4-methylenedioxymethamphetamine (MDMA) is usually ingested orally [32].
Fig. 3.
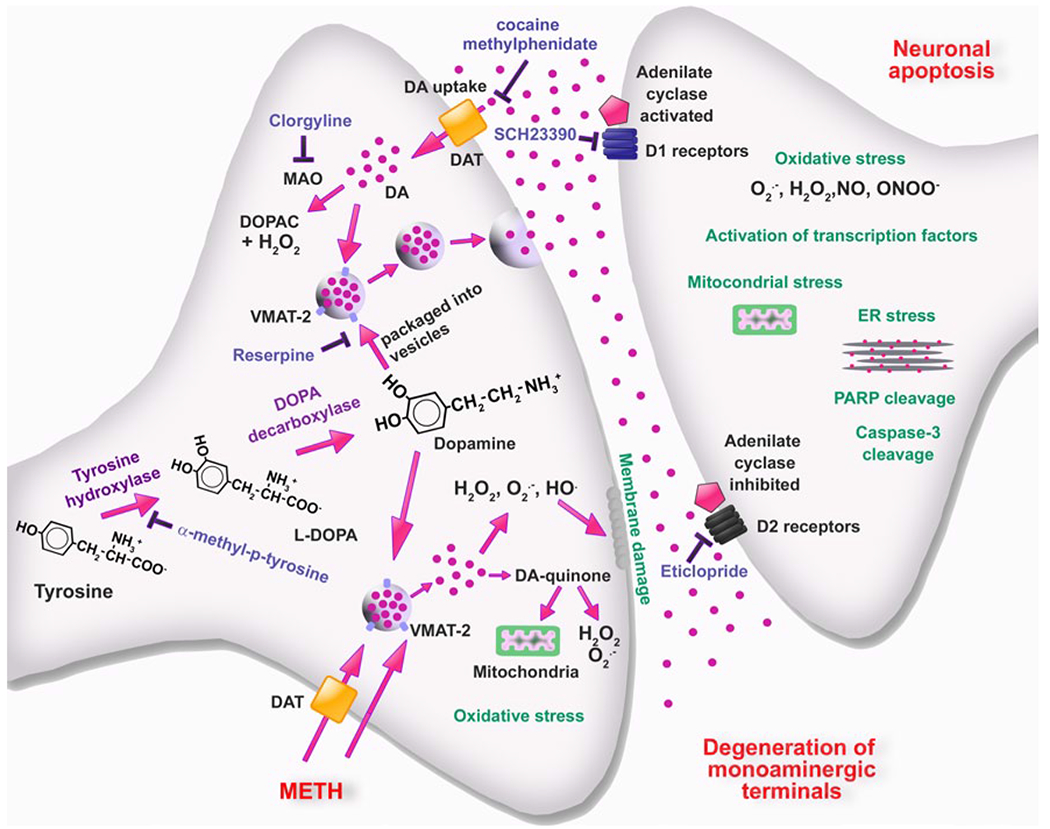
Schematic representation of cellular and molecular events involved in METH-induced DA neurotoxicity within the striatum. METH enters dopaminergic neurons via DAT and passive diffusion. Within these neurons, METH enters synaptic vesicles through VMAT-2 and causes DA release into the cytoplasm by disturbing pH balance. In the cytoplasm, DA auto-oxidizes to form toxic DA quinones followed by generation of superoxide radicals and hydrogen peroxides. Subsequent formation of hydroxyl radicals through interactions of superoxides and hydrogen peroxide with transition metals leads to oxidative stress, mitochondrial dysfunctions, and peroxidative damage to pre-synaptic membranes. The involvement of endogenous DA in METH toxicity is supported by findings that the TH inhibitor, α-methyl-p-tyrosine, which blocks DA synthesis, affords protection against METH toxicity. Also, the role of DA is supported by observations that use of the MAO inhibitor, clorgyline, and of the irreversible inhibitor of vesicular transport, reserpine, which results in increases in cytoplasmic DA levels can exacerbate METH-induced toxicity. These events are thought to be partly responsible for the loss of DA terminals. Toxic effects of released DA appear to depend on activation of DA receptors, because DA receptor antagonists block degeneration of DA terminals. Interactions of DA with D1 receptors on post-synaptic membrane cause activation of various transcription factors and subsequent up-regulation of death cascades in post-synaptic neurons. Modified from Krasnova and Cadet [93]
Methamphetamine use disorder
Abuse of methamphetamine has become an international public health problem [143]. Annual prevalence estimates for amphetamine-like substances ranged between 0.3 and 1.3 % in 2009, or about 14–57 million people aged 15–64 years [143]. Amphetamine abuse is highly prevalent in Africa, the Americas, and Asia. The inexpensive production of methamphetamine, its low cost, and long duration of action are factors that contribute to its abuse profile [93]. Methamphetamine users experience a sense of euphoria, increased productivity, hypersexuality, decreased anxiety, and increased energy after acute intake [81]. These effects can last for several hours, because the elimination half-life of methamphetamine ranges from 10 to 12 h [171]. Long-term negative consequences of methamphetamine abuse range from anxiety and insomnia to convulsions, paranoia, and brain damage [93]. Consistent with abnormalities in brain structure and function, cognitive impairments have also been observed in methamphetamine abusers [45]. A meta-analysis study by Scott et al. [175] identified significant deficits of a medium magnitude in several different cognitive processes that are dependent on the functions of frontostriatal and limbic circuits. These include episodic memory, executive functions, complex information processing speed, and psychomotor functions [175]. Methamphetamine dependence is associated with complaints of cognitive dysfunctions including memory problems and self-reported deficits in everyday functioning [160].
Cerebrovascular effects
Methamphetamine is a potent sympathomimetic that causes DA and NE release in the vascular system, with resulting increases in pulse rate and blood pressure [93]. In a retrospective study, Ho et al. [80] studied cases from patients who used methamphetamine, documented by history or urine toxicology screening. They found an association of methamphetamine usage with ischemic strokes, intracerebral hemorrhages, and subarachnoid hemorrhages. Other investigators have reported similar findings [76, 150, 155, 201]. There was a predominance of vascular pathology in the anterior circulation and these included vascular stenoses, atherosclerosis, and aneurysms. Intracerebral hemorrhage is also associated with the use of amphetamines and other related sympathomimetic drugs [76]. Methamphetamine-induced transient elevated blood pressure might be a causative factor for the presentation of intracerebral hemorrhage [20], a mechanism similar to the known consequences of idiopathic hypertension [102]. This idea is consistent with the report that methamphetamine-induced strokes occur in regional distributions typical for hypertensive hemorrhages (e.g., basal ganglia, thalamus, pons, and subcortical white matter). In several reports, patients who used illicit drugs (including methamphetamine) were reported to have irregular beading of vessels and small artery occlusive changes [126, 168]. More work is needed before firm conclusions can be drawn on this issue because other studies [209] have failed to confirm these findings.
Biochemical and structural brain findings
As mentioned above, methamphetamine causes massive DA release in the synaptic cleft in brain regions that receive dopaminergic projections from the midbrain [93, 186]. Postmortem studies have documented reduced levels of DA, TH, and DAT in the caudate of chronic methamphetamine users [202]. Consistent with these observations, neuroimaging studies also found significantly lower DAT binding in the brains of methamphetamine users [124, 196]. Lower VMAT-2 binding was also reported in some methamphetamine addicts [85]. DAT binding levels improved significantly in the caudate nucleus after a period or protracted abstinence from methamphetamine use [85, 196]. Methamphetamine abusers also show significant alterations in brain metabolism in several brain regions [174, 189]. These abnormalities include hypometabolism in the frontal cortex [88] and the anterior cingulate cortex (ACC) [104]. Higher parietal metabolic activity has been reported in methamphetamine abusers [14, 197]. Methamphetamine addicts also show structural abnormalities that include volume loss of cortical gray in the temporal and the insular cortices that are age dependent [140]. Methamphetamine users also suffer from smaller intracranial [174] and hippocampal volume [189]. Changes in white matter tissue integrity and/or organization (prefrontal white matter, corona radiata, genu of the corpus callosum, and perforant path were also reported in these patients [190].
The idea that methamphetamine abuse might increase the risk of developing Parkinson’s disease (PD) was stimulated by studies in animals that had documented methamphetamine-induced neurotoxic effects in nigrostriatal dopaminergic terminal areas [93], (Fig. 3). Interestingly, a recent epidemiological study found that the risk of developing PD was 75 % higher among methamphetamine abusers than in a group of control subjects [29]. The risk of PD was also higher in the methamphetamine group compared with a group of cocaine abusers [31]. One of the consequences of methamphetamine toxicity is reactive astrocytosis [19] and microglial activation in various brain regions [158, 177]. The presence of increased microgliosis in the brains of methamphetamine addicts [177] and in PD patients [49] together with the significant loss of dopaminergic markers in both groups [69] might provide a partial explanation for the epidemiological data cited [29]. It is also possible that methamphetamine-induced astrocytosis might account for larger volume of the basal ganglia reported in some methamphetamine addicts [33, 84].
MDMA use disorder
MDMA is a popular illicit drug in North America, Europe, and Oceania [143]. However, MDMA use has been declining in some parts of the world [143]. MDMA is associated with experience of euphoria, increased self-confidence, increased sensory perceptions, tachycardia, and hyperthermia [64]. These effects wear off after 24–48 h, with patients experiencing muscle aches, depression, fatigue, and decreased concentration [64]. Long-term use of the drug is reported to be associated with serotonergic dysfunctions that impact cognitive domains [70, 153]. Cognitive deficits include verbal fluency, verbal memory, working memory, executive function, and short and long-term prospective memory [153]. Heavy MDMA users scored higher than controls in a number of psychiatric symptoms that include paranoid ideation, somatization, obsessionality, phobic anxiety, altered appetite, and restless sleep [152].
Cerebrovascular effects
In human studies, acute MDMA administration can cause arrhythmias, hypertension, increased myocardial oxygen consumption, cardiac ischemia, and heart failure [70, 114]. Intracranial hemorrhages have been reported as a result of pre-existing congenital abnormalities [93]. Damage to vessel walls is also a possible cause of strokes concurrent with MDMA use [87]. Additionally, several deaths in MDMA users have been reported related to hyponatremia that can cause severe cerebral edema [131]. Patients usually present with confusion, convulsions, delirium or both that can rapidly progress to coma and death [74]. Some of these clinical observations are probably secondary to MDMA-induced sustained high levels of circulating biogenic amines [192]. Interestingly, MDMA can also stimulate catecholamine release from cardiovascular cells in vitro [57]. Alterations of calcium homeostasis might also contribute to the acute and chronic cardiovascular changes associated with MDMA use [192].
Biochemical and structural abnormalities
MDMA is a substrate of the serotonin transporter (SERT) which enters the neurons and releases serotonin (5-hydroxytryptophan) from storage vesicles [32, 76]. MDMA also inhibits the activity of tryptophan hydroxylase (TPH), the rate-limiting enzyme for serotonin synthesis, and inhibits serotonin degradation by monoamine oxidase B [30]. MDMA is also a serotonin2A (5-HT2A) receptor agonist [9]. Some of the biochemical actions of MDMA might be relevant to the clinical observations that MDMA users show reduced SERT ligand binding across multiple brain regions in PET studies [121–123]. Another study also reported that MDMA-preferring users showed significant decreases in SERT binding in the neocortex, striatum, and amygdala [52]. SERT binding was negatively correlated with the number of lifetime MDMA exposures [52]. The clinical data are consistent with postmortem evidence that MDMA users do exhibit decreased SERT protein levels in the striatum, and occipital, frontal, and temporal cortices [89]. Active MDMA users also showed decreased 5-HT2A receptor levels in their brains [161]. MDMA-treated rats have consistently been shown to have abnormalities in their serotonergic system [107]. These include decreased levels of 5-HT and of its major metabolite, 5-HIAA, decreased number of 5-HT transporters, and decreased activity of the rate-limiting enzyme of 5-HT synthesis, TPH [34]. These abnormalities are reported to last for months or even years after drug administration [107]. Consistent with these findings, lower 5-HIAA levels were measured in the cerebral spinal fluid (CSF) of active recreational MDMA users [121].
MDMA abuse is also associated with long-lasting effects on glucose metabolism in the human brain [22]. Reduced glucose metabolic uptake has been reported in the amygdala, hippocampus, and Brodmann area II [147]. There was reduced glucose uptake within the striatum and cingulate cortex in MDMA users, effects that were more severe with a history of very early abuse [147]. MDMA users also suffer from gray matter reduction in several brain regions and these abnormalities might be relevant to reported cognitive impairments in these patients [37]. Cowan et al. [38] compared MDMA users to non-MDMA users and found that there was decreased gray matter in the Brodmann area, the cerebellum, and the brainstem. Daumann et al. [43] also reported that experienced MDMA users showed several regions of lower gray matter volume in the medial orbitofrontal cortex and the medial dorsal anterior cingulate cortex in comparison to individuals with low levels of MDMA exposure. The thalamus was also affected in MDMA users [44]. The clinical findings are consistent with the animal literature. DA-induced oxidative stress in 5-HT terminals, and 5-HT2A and dopamine D1 receptor-mediated hyperthermia are potential causative factors for MDMA neurotoxicity [44, 186]. There was no specific toxic or fatal concentration of MDMA, though higher concentrations were observed in cases of MDMA toxicity, when compared with polydrug use and trauma cases [130]. In determining the cause of death, the postmortem concentration of MDMA cannot be taken to provide the cause of death without consideration of the history and the other autopsy findings [129]. In a MDMA-related autopsy, histological necrosis of the globus pallidus was reported, with intimal proliferation in large blood vessels [184].
New drugs and new challenges: the emergence of “bath salts”
Drug culture varies between different geographical locations and changes with time as different drugs emerge and with their availability on the drug scene. This may be related to availability or synthesis of new drugs of abuse. One evolving phenomenon is the use of synthetic cathinones, commonly referred to as “bath salts” [10, 133]. They are often snorted and this may result in foreign material in the alveoli of the lungs and can be seen on microscopy at autopsy. These β-ketoamphetamines are structurally related to methamphetamine and MDMA. They are psychostimulants and the effects of these drugs in humans have been increasingly reported in the last few years [42, 206]. They have been shown to have powerful cocaine-like actions in experimental models [11]. Commonly encountered drugs include mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV). Use of these drugs in humans is associated, among other things, with dizziness, euphoria, anxiety, and depression [42].These drugs can cause hot flushes and feelings of hyperthermia, known as mephedrone sweats. Deaths have been associated with hyperthermia, but so far reported organ pathology in humans has been non-specific [130].
Animal models have shown central nervous system effects with locomotor effects [113], affecting long-term cognitive function [48]. A study of mice given a binge-like regimen of mephedrone caused hyperthermia and locomotor stimulation, but did not show decreased levels of dopamine, tyrosine hydroxylase, or dopamine transporter [3]. There was no microglial activation in the striatum and no increase was seen in glial fibrillary acidic protein levels [3]. The same group found that, while mephedrone did not induce toxicity to dopamine nerve endings in the striatum, it did potentiate the effects of other neurotoxic drugs of abuse [4]. This could be very significant in view of the known polydrug use of users of stimulant drugs and that tablets may contain both mephedrone and MDMA [65].
Heroin use disorder
Use of opiates (heroin and opium) has been stable around the world (16.5 million people, or 0.4 % of the population aged 15–64 years), although a higher prevalence for opiate is found in southwest and central Asia, eastern and southeastern Europe, and North America [143]. Heroin is the most commonly abused drug within opiates [25]. Heroin is a semi-synthetic drug derived from opium. The drug is taken orally, via inhalation, and/or by intravenous injections [23]. Opiates have their biochemical and physiological effects via stimulation of three types of opiate receptors that are the μ, κ, and δ opioid receptors [191]. When administered via the oral route under medical supervision, opioids do not appear to produce major adverse events; the risk of developing dependence was reported to be rather small [25]. Dependence develops most rapidly after intravenous use [144]. Acute and chronic effects of heroin include neurovascular disorders, leukoencephalopathy, and atrophy [23]. Heroin users show several cognitive deficits in neuropsychological tests. These deficits include visual memory, working memory, processing speed, and executive function [71].
Cerebrovascular effects
In up to 90 % of all cases, the brain of patients dying of heroin overdose shows cerebral edema with increased brain weight at autopsy [148, 162]. Bilateral, symmetric ischemic lesions/necrosis of the globus pallidus was found in 5–10 % of heroin addicts after intravenous or intranasal abuse [162, 193]. These pathological findings are thought to be due to recurrent heroin-induced episodes of hypoxia [2, 162]. Single-photon emission computed tomography (SPECT) studies in opiate-dependent subjects have also consistently reported decreased regional cerebral blood flow (rCBF), especially in the frontal and temporal cortex [41, 167].
Heroin use is associated with reports of ischemic strokes [73]. These strokes are probably due to cardio-embolic phenomena in the setting of infective endocarditis [106]. Another source for embolic phenomena in the heroin abuser is dislodgment of foreign bodies added to heroin mixtures. These foreign bodies include starch, sugar, quinine, caffeine, and talcum powder that may enter the circulation and become lodged in the lungs [106]. Granulomatous reactions from these substances can lead to pulmonary hypertension [106]. Arteritis and vasculitis have also been implicated as causes of heroin-related strokes [21]. Other potential causes of stroke include hypotension and hypoxemia induced by opiate overdose that can result in global hypoxic-ischemic injury [2]. “Beading” on angiography has been reported, but strong pathological evidence is lacking [21]. In addition to acute ischemia, white matter changes from microvascular disease have been reported in chronic heroin abusers [25]. Diffuse, symmetric, and bilateral T2 hyperintensities have been observed in the subcortical or periventricular white matter, in a manner consistent with the diagnosis of vascular leukoencephalopathy [159].
Biochemical and structural abnormalities
Postmortem studies have reported that heroin addicts also show decrease of alpha adrenergic-2 in the frontal cortex, hypothalamus, and caudate nucleus [60]. Astrocytes of the frontal cortex of heroin addicts showed decreased density of I2-imidazoline receptors and decreased immunoreactivity of the related imidazoline receptor protein [170]. Heroin addicts also showed alterations in the cortical density of G-protein subunits [53, 77]. Specifically, there was a significant increase in the Gβ subunit immunoreactivity in the temporal cortex [77] and increased density of Gα subunits in the frontal cortex of heroin addicts [53, 77]. These results appear to be consistent with the known coupling of opiate receptors to guanosine triphosphate (GTP) binding (G) proteins [53]. Other findings of interest include decreased NF-L protein levels in the frontal cortex of heroin addicts [62] that suggest that opiate drugs might cause axonal damage after their chronic abuse.
Heroin addiction is also associated with profound alterations in brain structure and composition [25, 54]. Heroin addicts show alterations in gray and white matter morphometry [149, 169] and functions [109] in the brain. Preclinical studies of opioid-induced effects on neural systems support the idea that clinical changes in brain function and structure (e.g., altered dendritic spine density and neuronal apoptosis) are consequences of chronic heroin exposure [165]. There are enlarged ventricular spaces [41] and loss of frontal volume loss in heroin addicts [157]. Opiate-dependent subjects on methadone maintenance therapy showed decreased gray matter density in the prefrontal, insular, temporal, and fusiform cortex [108]. Thalamic volume is also reduced in heroin-dependent patients [159].
A distinct entity, spongiform leukoencephalopathy, has been described after inhalation of pre-heated heroin [94]. Other modes of heroin intake do not seem to be associated with the disease. Although it has been suggested that the disorder was related to a contaminant (poisoned heroin vapors, paralysis)-induced lipophilic toxin-mediated process that was enhanced by cerebral hypoxia, the exact etiology for these abnormalities has remained an enigma [172, 205]. The neurological deficits associated with this entity are irreversible with progression to death in some patients, and there is no effective therapy [200]. Scans of these patients usually show symmetrical non-enhancing hypodense areas in the cerebral and cerebellar white matter [178, 188, 200]. Selective involvement of the corticospinal tract, the solitary tract, and the lemniscus medialis have also been reported in heroin abusers [188]. MRI scans revealed areas of decreased signal intensity (hypointense) on T1-weighted images and increased signal intensity (hyperintense) on T2-weighted images [178, 188, 200]. The neuroradiological findings are usually not correlated with the extent of the neurological deficits [205]. On neuropathological examination, spongiform degeneration of the white matter is characterized by vacuoles surrounded by a network of thin myelinated fibers, reduced number of oligodendrocytes, and reduction of axons [164, 178]. The absence of typical hypoxic lesions and the presence of spongiosis with massive astrogliosis distinguish these cases from those with delayed leukoencephalopathy following severe hypoxia [164]. Increased prevalence of white matter hyperintensity lesions was reported in the frontal lobes of opiate-dependent patients compared with control subjects [108]. Recently, Bora et al. [18] also reported that long-term opiate abusers had significant decreases in axial diffusivity in superior longitudinal fascicule and right frontal white matter, suggesting that prolonged use is associated with axonal injury in these regions. Changes in the expression of myelin-related genes and their products, drug-induced oxidative stress/mitochondrial dysfunction, and/or apoptosis are potential mechanisms for the appearance of white matter lesions in heroin addicts [18]. Additional neuropathological evidence indicates that heroin addiction might cause deposition of hyperphosphorylated tau and neuroinflammation in the brain [5, 26].
Approach to autopsy in drug-related deaths
A pathologist conducting an autopsy on a potentially drug-related death or a neuropathologist requested to examine a brain from a drug-related case needs to be aware of the value and limitations of any examination. Also in assessing drug-related findings, consideration of the effects of polydrug use needs to be determined. Polydrug use is now common and the findings of organ damage may be related to more than one drug. One of the questions that have to be dealt with is whether organ retention is justified or legally allowed [72]. Drug-related deaths are typically performed as medico-legal autopsies, which may limit the ability to retain whole organs or tissue for future research [35] that is sorely needed in this area.
The approach to the investigation of a drug-related death requires knowledge of the history, scene, and toxicological findings. The neuropathologist needs to be cognizant of potential pathological findings in such cases [132]. In many deaths where cannabis, stimulant, or opiate drugs have been used, there will be no specific findings when routine neuropathological techniques are used. However, pathology related to drug use will be encountered in cases as detailed above, and anticipation of the needs for neuropathology and specific tissue retention and examination may yield better outcomes. This issue is important for both clinical and research purposes in view of a lack of enough strong supporting evidence that certain drugs do cause incontrovertible neuropathological changes that form the substrates for the clinical presentations associated with drug addiction.
Conclusions
Substance dependence is characterized by abnormal goal-directed behaviors that are the manifestations of pathological changes in the cortico-striatal-limbic circuitries that subsume reward-related behaviors. However, acute and chronic effects of illegal drugs appear to reach beyond the boundaries of these circuits. The data reviewed here indicate that the abuse of illicit drug is accompanied by moderate to severe neuropsychological impairments that appear to be secondary to functional and structural changes in various brain regions, including both cortical and subcortical regions of the human brain. These abnormalities are secondary, in part, to drug-induced vascular abnormalities, oxidative stress, and the induction of other biochemical cascades that have been identified as causative factors in other classical neurologic disorders such as PD or strokes. Because drug dependence develops over many months, it is highly likely that drug-related changes of behaviors are mediated in part by these pathological phenomena in such a way that these alterations might significantly impact the clinical course of addiction. Thus, impaired learning of verbal instruction due to specific brain dysfunctions might negatively impact a patient’s ability to follow instruction, with subsequent increase in recidivism. This argument suggests that the addictions might need to be classified as neurological disorders, with emphasis on an approach that includes thorough neuropsychological and neuroimaging assessments patients who present for treatment of these severe disorders. Additionally, more efforts are needed to encourage postmortem evaluations of the brains of these patients. This approach has recently been successfully applied in the cases of affective and schizophrenic disorders and has stimulated important potential new leads toward the elucidation of the molecular pathology of these disorders.
Acknowledgments
This paper is supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), NIH, and DHHS. V. Bisagno is a Fulbright Fellow, United States Department of State and is also supported by grants PIP11420100100072 and PICT 2012-0924, Argentina.
Contributor Information
Jean Lud Cadet, NIDA Intramural Research Program, Molecular Neuropsychiatry Research Branch, NIDA/NIH/DHHS, 251 Bayview Boulevard, Baltimore, MD 21224, USA.
Veronica Bisagno, Instituto de Investigaciones Farmacológicas (ININFA-UBA-CONICET), Junín 956, piso 5, C1113 Buenos Aires, Argentina.
Christopher Mark Milroy, Eastern Ontario Forensic Pathology, Unit of the Ontario Forensic Pathology Service, Division of Anatomical Pathology, The Ottawa Hospital, 501 Smyth Road, Ottawa, ON K2A 2L4, Canada.
References
- 1.Albertson DN, Pruetz B, Schmidt CJ et al. (2004) Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem 88:1211–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen SN, Skullerud K (1999) Hypoxic/ischaemic brain damage, especially pallidal lesions, in heroin addicts. Forensic Sci Int 102:51–59 [DOI] [PubMed] [Google Scholar]
- 3.Angoa-Pérez M, Kane MJ, Briggs DI et al. (2013) Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J Neurochem 125:102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angoa-Pérez M, Kane MJ, Francescutti DM et al. (2012) Mephedrone, an abused psychoactive component of “bath salts” and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J Neurochem 120:1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony IC, Norrby KE, Dingwall T et al. (2010) Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain 133:3685–3698 [DOI] [PubMed] [Google Scholar]
- 6.Barnea-Goraly N, Menon V, Eckert M et al. (2005) White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral cortex (New York, NY: 1991) 15:1848–1854 [DOI] [PubMed] [Google Scholar]
- 7.Barrós-Loscertales A, Garavan H, Bustamante JC et al. (2011) Reduced striatal volume in cocaine-dependent patients. NeuroImage 56:1021–1026 [DOI] [PubMed] [Google Scholar]
- 8.Batalla A, Bhattacharyya S, Yücel M et al. (2013) Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS ONE 8:e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB (1988) Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol 149:159–163 [DOI] [PubMed] [Google Scholar]
- 10.Baumann MH, Partilla JS, Lehner KR (2013) Psychoactive “bath salts”: not so soothing. Eur J Pharmacol 698:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumann MH, Partilla JS, Lehner KR et al. (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology 38:552–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50:7–15 [DOI] [PubMed] [Google Scholar]
- 13.Bell RP, Foxe JJ, Nierenberg J et al. (2011) Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend 114:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman SM, Voytek B, Mandelkern MA et al. (2008) Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry 13:897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boghdadi MS, Henning RJ (1997) Cocaine: pathophysiology and clinical toxicology. Heart Lung 26:466–483 [DOI] [PubMed] [Google Scholar]
- 16.Bolla KI, Cadet JL, London ED (1998) The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci 10:280–289 [DOI] [PubMed] [Google Scholar]
- 17.Bolla KI, Eldreth DA, Matochik JA, Cadet JL (2005) Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage 26:480–492 [DOI] [PubMed] [Google Scholar]
- 18.Bora E, Yücel M, Fornito A et al. (2012) White matter micro-structure in opiate addiction. Addict Biol 17:141–148 [DOI] [PubMed] [Google Scholar]
- 19.Bowyer JF, Davies DL, Schmued L et al. (1994) Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther 268:1571–1580 [PubMed] [Google Scholar]
- 20.Brisman JL, Song JK, Newell DW (2006) Cerebral aneurysms. N Engl J Med 355:928–939 [DOI] [PubMed] [Google Scholar]
- 21.Brust JC (1997) Vasculitis owing to substance abuse. Neurol Clin 15:945–957 [DOI] [PubMed] [Google Scholar]
- 22.Buchert R, Obrocki J, Thomasius R et al. (2001) Long-term effects of “ecstasy” abuse on the human brain studied by FDG PET. Nucl Med Commun 22:889–897 [DOI] [PubMed] [Google Scholar]
- 23.Büttner A (2011) Review: the neuropathology of drug abuse. Neuropathol Appl Neurobiol 37:118–134 [DOI] [PubMed] [Google Scholar]
- 24.Büttner A, Mall G, Penning R et al. (2003) The neuropathology of cocaine abuse. Leg Med 5(Suppl 1):S240–S242 [DOI] [PubMed] [Google Scholar]
- 25.Büttner A, Mall G, Penning R, Weis S (2000) The neuropathology of heroin abuse. Forensic Sci Int 113:435–442 [DOI] [PubMed] [Google Scholar]
- 26.Büttner A, Rohrmoser K, Mall G et al. (2006) Widespread axonal damage in the brain of drug abusers as evidenced by accumulation of beta-amyloid precursor protein (beta-APP): an immunohistochemical investigation. Addiction 101:1339–1346 [DOI] [PubMed] [Google Scholar]
- 27.Cadet JL, Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32:117–131 [DOI] [PubMed] [Google Scholar]
- 28.Caldicott DGE, Holmes J, Roberts-Thomson KC, Mahar L (2005) Keep off the grass: marijuana use and acute cardiovascular events. Eur J Emerg Med 12:236–244 [DOI] [PubMed] [Google Scholar]
- 29.Callaghan RC, Cunningham JK, Sykes J, Kish SJ (2012) Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend 120:35–40 [DOI] [PubMed] [Google Scholar]
- 30.Capela JP, Carmo H, Remião F et al. (2009) Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol 39:210–271 [DOI] [PubMed] [Google Scholar]
- 31.Cardoso FE, Jankovic J (1993) Cocaine-related movement disorders. Mov Disord 8:175–178 [DOI] [PubMed] [Google Scholar]
- 32.Carvalho M, Carmo H, Costa VM et al. (2012) Toxicity of amphetamines: an update. Arch Toxicol 86:1167–1231 [DOI] [PubMed] [Google Scholar]
- 33.Chang L, Cloak C, Patterson K et al. (2005) Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry 57:967–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Commins DL, Vosmer G, Virus RM et al. (1987) Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther 241:338–345 [PubMed] [Google Scholar]
- 35.Cordner S, Leahy F (2013) Ethical research on bodies in the jurisdiction of coroners or medical examiner. Acad Forensic Pathol 3:301–313 [Google Scholar]
- 36.Cousijn J, Wiers RW, Ridderinkhof KR et al. (2012) Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. NeuroImage 59:3845–3851 [DOI] [PubMed] [Google Scholar]
- 37.Cowan RL, Lyoo IK, Sung SM et al. (2003) Reduced cortical gray matter density in human MDMA (Ecstasy) users: a voxel-based morphometry study. Drug Alcohol Depend 72:225–235 [DOI] [PubMed] [Google Scholar]
- 38.Cowan RL, Roberts DM, Joers JM (2008) Neuroimaging in human MDMA (Ecstasy) users. Ann N Y Acad Sci 1139:291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daglish MRC, Nutt DJ (2003) Brain imaging studies in human addicts. Eu Neuropsychopharmacol 13:453–458 [DOI] [PubMed] [Google Scholar]
- 40.Dalton VS, Zavitsanou K (2010) Cannabinoid effects on CB1 receptor density in the adolescent brain: an autoradiographic study using the synthetic cannabinoid HU210. Synapse 64:845–854 [DOI] [PubMed] [Google Scholar]
- 41.Danos P, Kasper S, Grünwald F et al. (1998) Pathological regional cerebral blood flow in opiate-dependent patients during withdrawal: a HMPAO-SPECT study. Neuropsychobiology 37:194–199 [DOI] [PubMed] [Google Scholar]
- 42.Dargan PI, Albert S, Wood DM (2010) Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. QJM 103:875–879 [DOI] [PubMed] [Google Scholar]
- 43.Daumann J, Koester P, Becker B et al. (2011) Medial prefrontal gray matter volume reductions in users of amphetamine-type stimulants revealed by combined tract-based spatial statistics and voxel-based morphometry. Neuroimage 54:794–801 [DOI] [PubMed] [Google Scholar]
- 44.De Win MML, Jager G, Booij J et al. (2008) Neurotoxic effects of ecstasy on the thalamus. Br J Psychiatry 193:289–296 [DOI] [PubMed] [Google Scholar]
- 45.Dean AC, Groman SM, Morales AM, London ED (2013) An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38:259–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dean B, Sundram S, Bradbury R et al. (2001) Studies on [3H] CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 103:9–15 [DOI] [PubMed] [Google Scholar]
- 47.Degenhardt L, Hall W (2012) Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 379:55–70 [DOI] [PubMed] [Google Scholar]
- 48.den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanperä I, Korpi ER (2013) Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol Biochem Behav 103:501–509. doi: 10.1016/j.pbb.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 49.Doorn KJ, Lucassen PJ, Boddeke HW et al. (2012) Emerging roles of microglial activation and non-motor symptoms in Parkinson’s disease. Progress Neurobiol 98:222–238 [DOI] [PubMed] [Google Scholar]
- 50.Elliott JM, Beveridge TJR (2005) Psychostimulants and monoamine transporters: upsetting the balance. Curr Opin Pharmacol 5:94–100 [DOI] [PubMed] [Google Scholar]
- 51.Elsohly MA, Slade D (2005) Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci 78:539–548 [DOI] [PubMed] [Google Scholar]
- 52.Erritzoe D, Frokjaer VG, Holst KK et al. (2011) In vivo imaging of cerebral serotonin transporter and serotonin(2A) receptor binding in 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) and hallucinogen users. Arch Gen Psychiatry 68:562–576 [DOI] [PubMed] [Google Scholar]
- 53.Escriba PV, Sastre M, Garcia-Sevilla JA (1994) Increased density of guanine nucleotide-binding proteins in the postmortem brains of heroin addicts. Arch Gen Psychiatry 51:494–501 [DOI] [PubMed] [Google Scholar]
- 54.Esse K, Fossati-Bellani M, Traylor A, Martin-Schild S (2011) Epidemic of illicit drug use, mechanisms of action/addiction and stroke as a health hazard. Brain Behav 1:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fein G, Di Sclafani V, Meyerhoff DJ (2002) Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend 68:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fessler RD, Esshaki CM, Stankewitz RC et al. (1997) The neurovascular complications of cocaine. Surg Neurol 47:339–345 [DOI] [PubMed] [Google Scholar]
- 57.Fitzgerald JL, Reid JJ (1994) Sympathomimetic actions of methylenedioxymethamphetamine in rat and rabbit isolated cardiovascular tissues. J Pharm Pharmacol 46:826–832 [DOI] [PubMed] [Google Scholar]
- 58.Fox RJ, Cronin T, Lin J, Wang X, Sakaie K, Ontaneda D, Mahmoud SY, Lowe MJ, Phillips MD (2011) Measuring myelin repair and axonal loss with diffusion tensor imaging. Am J Neuroradiol 32(1):85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frost L, Mostofsky E, Rosenbloom JI et al. (2013) Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am Heart J 165:170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabilondo AM, Meana JJ, Barturen F et al. (1994) mu-Opioid receptor and alpha 2-adrenoceptor agonist binding sites in the postmortem brain of heroin addicts. Psychopharmacology 115:135–140 [DOI] [PubMed] [Google Scholar]
- 61.Galiègue S, Mary S, Marchand J et al. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232: 54–61 [DOI] [PubMed] [Google Scholar]
- 62.Garcéa-Sevilla JA, Ventayol P, Busquets X et al. (1997) Marked decrease of immunolabelled 68 kDa neurofilament (NF-L) proteins in brains of opiate addicts. NeuroReport 8:1561–1565 [DOI] [PubMed] [Google Scholar]
- 63.Gash A, Karliner JS, Janowsky D, Lake CR (1978) Effects of smoking marihuana on left ventricular performance and plasma norepinephrine: studies in normal men. Ann Intern Med 89:448–452 [DOI] [PubMed] [Google Scholar]
- 64.Geibprasert S, Gallucci M, Krings T (2010) Addictive illegal drugs: structural neuroimaging. Am J Neuroradiol 31:803–808. doi: 10.3174/ajnr.A1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.German CL, Fleckenstein AE, Hanson GR (2013) Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. doi: 10.1016/j.lfs.2013.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glass M, Dragunow M, Faull RL (1997) Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77:299–318 [DOI] [PubMed] [Google Scholar]
- 67.Glauser J, Queen JR (2007) An overview of non-cardiac cocaine toxicity. J Emerg Med 32:181–186 [DOI] [PubMed] [Google Scholar]
- 68.Goldstein RZ, Leskovjan AC, Hoff AL et al. (2004) Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia 42:1447–1458 [DOI] [PubMed] [Google Scholar]
- 69.Granado N, Ares-Santos S, Moratalla R (2013) Methamphetamine and Parkinson’s disease. Parkinson’s disease 308052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green AR, Mechan AO, Elliott JM et al. (2003) The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 55:463–508 [DOI] [PubMed] [Google Scholar]
- 71.Gruber SA, Silveri MM, Yurgelun-Todd DA (2007) Neuropsychological consequences of opiate use. Neuropsychol Rev 17:299–315 [DOI] [PubMed] [Google Scholar]
- 72.Haden-Pinneri K, Weedn VW (2013) Organ and tissue retention. Acad Forensic Pathol 3:294–300 [Google Scholar]
- 73.Hagan IG, Burney K (2007) Radiology of recreational drug abuse. Radiographics 27:919–940 [DOI] [PubMed] [Google Scholar]
- 74.Hall AP, Henry JA (2006) Acute toxic effects of “Ecstasy” (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth 96:678–685 [DOI] [PubMed] [Google Scholar]
- 75.Hall W, Degenhardt L (2009) Adverse health effects of non-medical cannabis use. Lancet 374:1383–1391 [DOI] [PubMed] [Google Scholar]
- 76.Harrington H, Heller HA, Dawson D et al. (1983) Intracerebral hemorrhage and oral amphetamine. Arch Neurol 40:503–507 [DOI] [PubMed] [Google Scholar]
- 77.Hashimoto E, Frölich L, Ozawa H et al. (1996) Alteration of glutamyltranspeptidase binding proteins in postmortem brains of heroin addicts. Alcohol Clin Exp Res 20:301A–304A [PubMed] [Google Scholar]
- 78.Herning RI, Better WE, Tate K, Cadet JL (2005) Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology 64:488–493 [DOI] [PubMed] [Google Scholar]
- 79.Hillard CJ (2000) Endocannabinoids and vascular function. J Pharmacol Exp Ther 294:27–32 [PubMed] [Google Scholar]
- 80.Ho EL, Josephson SA, Lee HS, Smith WS (2009) Cerebrovascular complications of methamphetamine abuse. Neurocrit Care 10:295–305 [DOI] [PubMed] [Google Scholar]
- 81.Homer BD, Solomon TM, Moeller RW et al. (2008) Methamphetamine abuse and impairment of social functioning: a review of the underlying neurophysiological causes and behavioral implications. Psychol Bull 134:301–310 [DOI] [PubMed] [Google Scholar]
- 82.Hurd YL, Herkenham M (1993) Molecular alterations in the neostriatum of human cocaine addicts. Synapse 13:357–369 [DOI] [PubMed] [Google Scholar]
- 83.Jayanthi S, Buie S, Moore S et al. (2010) Heavy marijuana users show increased serum apolipoprotein C-III levels: evidence from proteomic analyses. Mol Psychiatry 15:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jernigan TL, Gamst AC, Archibald SL et al. (2005) Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry 162:1461–1472 [DOI] [PubMed] [Google Scholar]
- 85.Johanson C-E, Frey KA, Lundahl LH et al. (2006) Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 185:327–338 [DOI] [PubMed] [Google Scholar]
- 86.Johnson MM, David JA, Michelhaugh SK et al. (2012) Increased heat shock protein 70 gene expression in the brains of cocaine-related fatalities may be reflective of postdrug survival and intervention rather than excited delirium. J Forensic Sci 57:1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalant H (2001) The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ 165:917–928 [PMC free article] [PubMed] [Google Scholar]
- 88.Kim Y-T, Lee S-W, Kwon D-H et al. (2009) Dose-dependent frontal hypometabolism on FDG-PET in methamphetamine abusers. J Psychiatr Res 43:1166–1170 [DOI] [PubMed] [Google Scholar]
- 89.Kish SJ, Fitzmaurice PS, Chang LJ et al. (2010) Low striatal serotonin transporter protein in a human polydrug MDMA (ecstasy) user: a case study. J Psychopharmacol 24:281–284 [DOI] [PubMed] [Google Scholar]
- 90.Konzen JP, Levine SR, Garcia JH (1995) Vasospasm and thrombus formation as possible mechanisms of stroke related to alkaloidal cocaine. Stroke 26:1114–1118 [DOI] [PubMed] [Google Scholar]
- 91.Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kosten TR, Kleber HD (1988) Rapid death during cocaine abuse: a variant of the neuroleptic malignant syndrome? Am J Drug Alcohol Abuse 14:335–346 [DOI] [PubMed] [Google Scholar]
- 93.Krasnova IN, Cadet JL (2009) Methamphetamine toxicity and messengers of death. Brain Res Rev 60:379–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krinsky CS, Reichard R (2012) Chasing the dragon: a review of toxic leukoencephalopathy. Acad Forensic Pathol 2:67–73 [Google Scholar]
- 95.Kristiansen LV, Bannon MJ, Meador-Woodruff JH (2009) Expression of transcripts for myelin related genes in postmortem brain from cocaine abusers. Neurochem Res 34:46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumra S (2007) Schizophrenia and cannabis use. Minn Med 90:36–38 [PubMed] [Google Scholar]
- 97.Kurumatani T, Kudo T, Ikura Y, Takeda M (1998) White matter changes in the gerbil brain under chronic cerebral hypoperfusion. Stroke 29:1058–1062 [DOI] [PubMed] [Google Scholar]
- 98.Landfield PW, Cadwallader LB, Vinsant S (1988) Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Res 443:47–62 [DOI] [PubMed] [Google Scholar]
- 99.Levine SR, Brust JC, Futrell N et al. (1991) A comparative study of the cerebrovascular complications of cocaine: alkaloidal versus hydrochloride—a review. Neurology 41:1173–1177 [DOI] [PubMed] [Google Scholar]
- 100.Lim KO, Choi SJ, Pomara N et al. (2002) Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry 51:890–895 [DOI] [PubMed] [Google Scholar]
- 101.Lim KO, Wozniak JR, Mueller BA et al. (2008) Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend 92:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lineberry TW, Bostwick JM (2006) Methamphetamine abuse: a perfect storm of complications. Mayo Clin Proc 81:77–84. doi: 10.4065/81.1.77 [DOI] [PubMed] [Google Scholar]
- 103.Little KY, Ramssen E, Welchko R et al. (2009) Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res 168:173–180 [DOI] [PubMed] [Google Scholar]
- 104.London ED, Simon SL, Berman SM et al. (2004) Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 61:73–84 [DOI] [PubMed] [Google Scholar]
- 105.Lowenstein DH, Massa SM, Rowbotham MC et al. (1987) Acute neurologic and psychiatric complications associated with cocaine abuse. Am J Med 83:841–846 [DOI] [PubMed] [Google Scholar]
- 106.Lucas CE (2005) The impact of street drugs on trauma care. J Trauma 59:S57–S60 (discussion S67-S75) [DOI] [PubMed] [Google Scholar]
- 107.Lyles J, Cadet JL (2003) Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Rev 42:155–168 [DOI] [PubMed] [Google Scholar]
- 108.Lyoo IK, Pollack MH, Silveri MM et al. (2006) Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology 184:139–144 [DOI] [PubMed] [Google Scholar]
- 109.Ma N, Liu Y, Li N et al. (2010) Addiction related alteration in resting-state brain connectivity. Neuroimage 49:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Magro CM, Dyrsen M (2008) Angiocentric lesions of the head and neck. Head Neck Pathol 2:116–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Makris N, Gasic GP, Kennedy DN et al. (2008) Cortical thickness abnormalities in cocaine addiction—a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron 60:174–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maldonado R, Berrendero F, Ozaita A, Robledo P (2011) Neurochemical basis of cannabis addiction. Neuroscience 181:1–17 [DOI] [PubMed] [Google Scholar]
- 113.Marusich JA, Grant KR, Blough BE, Wiley JL (2012) Effects of synthetic cathinones contained in “bath salts” on motor behaveior and a functional observational battery in mice. Neurotoxicology 33:1305–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mas M, Farré M, De la Torre R et al. (1999) Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 290:136–145 [PubMed] [Google Scholar]
- 115.Mash DC, Duque L, Pablo J et al. (2009) Brain biomarkers for identifying excited delirium as a cause of sudden death. Forensic Sci Int 190:e13–e19 [DOI] [PubMed] [Google Scholar]
- 116.Mash DC, Pablo J, Ouyang Q et al. (2002) Dopamine transport function is elevated in cocaine users. J Neurochem 81:292–300 [DOI] [PubMed] [Google Scholar]
- 117.Mash DC, Staley JK, Izenwasser S et al. (2000) Serotonin transporters upregulate with chronic cocaine use. J Chem Neuroanat 20:271–280 [DOI] [PubMed] [Google Scholar]
- 118.Matochik JA, Eldreth DA, Cadet J-L, Bolla KI (2005) Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend 77:23–30 [DOI] [PubMed] [Google Scholar]
- 119.Matochik JA, London ED, Eldreth DA et al. (2003) Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage 19:1095–1102 [DOI] [PubMed] [Google Scholar]
- 120.McAllister SD, Glass M (2002) CB(1) and CB(2) receptor-mediated signalling: a focus on endocannabinoids. Prostaglandins Leukot Essent Fatty Acids 66:161–171 [DOI] [PubMed] [Google Scholar]
- 121.McCann UD, Ridenour A, Shaham Y, Ricaurte GA (1994) Serotonin neurotoxicity after (±)3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”): a controlled study in humans. Neuropsychopharmacology 10:129–138 [DOI] [PubMed] [Google Scholar]
- 122.McCann UD, Szabo Z, Seckin E et al. (2005) Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology 30:1741–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCann UD, Szabo Z, Vranesic M et al. (2008) Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (±)3,4-methylenedioxymethamphetamine (“ecstasy”) users: relationship to cognitive performance. Psychopharmacology 200:439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McCann UD, Wong DF, Yokoi F et al. (1998) Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 18:8417–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCarron MO, Thomas AM (1997) Cannabis and alcohol in stroke. Postgrad Med J 73:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McIntosh A, Hungs M, Kostanian V, Yu W (2006) Carotid artery dissection and middle cerebral artery stroke following methamphetamine use. Neurology 67:2259–2260 [DOI] [PubMed] [Google Scholar]
- 127.Meador-Woodruff JH, Little KY, Damask SP et al. (1993) Effects of cocaine on dopamine receptor gene expression: a study in the postmortem human brain. Biol Psychiatry 34:348–355 [DOI] [PubMed] [Google Scholar]
- 128.Medina KL, McQueeny T, Nagel BJ et al. (2009) Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol 14:457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Milroy CM (2011) “Ecstasy” associated deaths: what is a fatal concentration? Analysis of a case series. Forensic Sci Med Pathol 7:248–252 [DOI] [PubMed] [Google Scholar]
- 130.Milroy CM (2012) The “Bath Salt” Mephedrone—a new(ish) kid on the block. Acad Forensic Pathol 250–254 [Google Scholar]
- 131.Milroy CM, Clark JC, Forrest AR (1996) Pathology of deaths associated with “ecstasy” and “eve” misuse. J Clin Pathol 49:149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milroy CM, Parai JL (2011) The histopathology of drugs of abuse. Histopathology 59:579–593 [DOI] [PubMed] [Google Scholar]
- 133.Miotto K, Striebel J, Cho AK, Wang C (2013) Clinical and pharmacological aspects of bath salt use: a review of the literature and case reports. Drug Alcohol Depend 132:1–12 [DOI] [PubMed] [Google Scholar]
- 134.Mir A, Obafemi A, Young A, Kane C (2011) Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics 128:e1622–e1627. doi: 10.1542/peds.2010-3823 [DOI] [PubMed] [Google Scholar]
- 135.Mittleman MA, Lewis RA, Maclure M et al. (2001) Triggering myocardial infarction by marijuana. Circulation 103:2805–2809 [DOI] [PubMed] [Google Scholar]
- 136.Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell NJ (2002) Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J Neurosci Res 67:829–836 [DOI] [PubMed] [Google Scholar]
- 137.Moore THM, Zammit S, Lingford-Hughes A et al. (2007) Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370:319–328 [DOI] [PubMed] [Google Scholar]
- 138.Moreno-López L, Catena A, Fernández-Serrano MJ et al. (2012) Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend 125:208–214 [DOI] [PubMed] [Google Scholar]
- 139.Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65 [DOI] [PubMed] [Google Scholar]
- 140.Nakama H, Chang L, Fein G et al. (2011) Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction 106:1474–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.National Institute on Drug Abuse N Costs of Substance Abuse. http://www.drugabuse.gov/related-topics/trends-statistics#costs)
- 142.United Nations (2011) World drug report 2011. http://www.unodc.org/documents/data-and-analysis/WDR2011/World_Drug_Report_2011_ebook.pdf
- 143.United Nations (2013) World drug report 2013. http://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf
- 144.Neiman J, Haapaniemi HM, Hillbom M (2000) Neurological complications of drug abuse: pathophysiological mechanisms. Eu J Neurol 7:595–606 [DOI] [PubMed] [Google Scholar]
- 145.Nnadi CU, Mimiko OA, McCurtis HL, Cadet JL (2005) Neuropsychiatric effects of cocaine use disorders. J Natl Med Assoc 97:1504–1515 [PMC free article] [PubMed] [Google Scholar]
- 146.Nolte KB, Brass LM, Fletterick CF (1996) Intracranial hemorrhage associated with cocaine abuse: a prospective autopsy study. Neurology 46:1291–1296 [DOI] [PubMed] [Google Scholar]
- 147.Obrocki J, Buchert R, Vaterlein O et al. (1999) Ecstasy—long-term effects on the human central nervous system revealed by positron emission tomography. Br J Psychiatry 175:186–188 [DOI] [PubMed] [Google Scholar]
- 148.Oehmichen M, Meissner C, Reiter A, Birkholz M (1996) Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol 91:642–646 [DOI] [PubMed] [Google Scholar]
- 149.Offiah C, Hall E (2008) Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol 63:146–152 [DOI] [PubMed] [Google Scholar]
- 150.Olsen ER (1977) Intracranial hemorrhage and amphetamine usage. Review of the effects of amphetamines on the central nervous system. Angiology 28:464–471 [DOI] [PubMed] [Google Scholar]
- 151.Oyesiku NM, Colohan AR, Barrow DL, Reisner A (1993) Cocaine-induced aneurysmal rupture: an emergent negative factor in the natural history of intracranial aneurysms? Neurosurgery 32:518–525 [DOI] [PubMed] [Google Scholar]
- 152.Parrott AC, Sisk E, Turner JJ (2000) Psychobiological problems in heavy “ecstasy” (MDMA) polydrug users. Drug Alcohol Depend 60:105–110 [DOI] [PubMed] [Google Scholar]
- 153.Parrott AC (2006) MDMA in humans: factors which affect the neuropsychobiological profiles of recreational ecstasy users, the integrative role of bioenergetic stress. J Psychopharmacology (Oxford, England) 20:147–63 [DOI] [PubMed] [Google Scholar]
- 154.Pascual-Leone A, Dhuna A, Altafullah I, Anderson DC (1990) Cocaine-induced seizures. Neurology 40:404–407 [DOI] [PubMed] [Google Scholar]
- 155.Perez JA, Arsura EL, Strategos S (1999) Methamphetamine-related stroke: four cases. J Emerg Med 17:469–471 [DOI] [PubMed] [Google Scholar]
- 156.Peterson PL, Roszler M, Jacobs I, Wilner HI (1991) Neurovascular complications of cocaine abuse. J Neuropsychiatry Clin Neurosci 3:143–149 [DOI] [PubMed] [Google Scholar]
- 157.Pezawas LM, Fischer G, Diamant K et al. (1998) Cerebral CT findings in male opioid-dependent patients: stereological, planimetric and linear measurements. Psychiatry Res 83:139–147 [DOI] [PubMed] [Google Scholar]
- 158.Raineri M, Gonzalez B, Goitia B et al. (2012) Modafinil abrogates methamphetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS ONE 7:e46599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reid AG, Daglish MRC, Kempton MJ et al. (2008) Reduced thalamic grey matter volume in opioid dependence is influenced by degree of alcohol use: a voxel-based morphometry study. J Psychopharmacol 7–10 [DOI] [PubMed] [Google Scholar]
- 160.Rendell PG, Mazur M, Henry JD (2009) Prospective memory impairment in former users of methamphetamine. Psychopharmacology 203:609–616 [DOI] [PubMed] [Google Scholar]
- 161.Reneman L, Endert E, De Bruin K et al. (2002) The acute and chronic effects of MDMA (“ecstasy”) on cortical 5-HT2A receptors in rat and human brain. Neuropsychopharmacology 26:387–396 [DOI] [PubMed] [Google Scholar]
- 162.Richter RW, Pearson J, Bruun B et al. (1973) Neurological complications of addiction to heroin. Bull N Y Acad Med 49:3–21 [PMC free article] [PubMed] [Google Scholar]
- 163.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223 [DOI] [PubMed] [Google Scholar]
- 164.Rizzuto N, Morbin M, Ferrari S et al. (1997) Delayed spongiform leukoencephalopathy after heroin abuse. Acta Neuropathol 94:87–90 [DOI] [PubMed] [Google Scholar]
- 165.Robinson TE, Kolb B (1999) Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 33:160–162 [DOI] [PubMed] [Google Scholar]
- 166.Romero J, Garcia-Palomero E, Berrendero F et al. (1997) Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse 26:317–323 [DOI] [PubMed] [Google Scholar]
- 167.Rose JS, Branchey M, Buydens-Branchey L et al. (1996) Cerebral perfusion in early and late opiate withdrawal: a technetium-99m-HMPAO SPECT study. Psychiatry Res 67:39–47 [DOI] [PubMed] [Google Scholar]
- 168.Rothrock JF, Rubenstein R, Lyden PD (1988) Ischemic stroke associated with methamphetamine inhalation. Neurology 38:589–592 [DOI] [PubMed] [Google Scholar]
- 169.Ryan A, Molloy FM, Farrell MA, Hutchinson M (2005) Fatal toxic leukoencephalopathy: clinical, radiological, and necropsy findings in two patients. J Neurol Neurosurg Psychiatry 76:1014–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sastre M, Ventayol P, García-Sevilla JA (1996) Decreased density of I2-imidazoline receptors in the postmortem brain of heroin addicts. NeuroReport 7:509–512 [DOI] [PubMed] [Google Scholar]
- 171.Schepers RJF, Oyler JM, Joseph RE et al. (2003) Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem 49:121–132 [DOI] [PubMed] [Google Scholar]
- 172.Schiffer D, Brignolio F, Giordana MT et al. (1985) Spongiform encephalopathy in addicts inhaling pre-heated heroin. Clin Neuropathol 4:174–180 [PubMed] [Google Scholar]
- 173.Schuster RM, Crane NA, Mermelstein R, Gonzalez R (2012) The influence of inhibitory control and episodic memory on the risky sexual behavior of young adult cannabis users. JINS 18:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SHHW (2010) Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage 50:1392–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scott JC, Woods SP, Matt GE et al. (2007) Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17:275–297 [DOI] [PubMed] [Google Scholar]
- 176.Segal DM, Moraes CT, Mash DC (1997) Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res 45:335–339 [DOI] [PubMed] [Google Scholar]
- 177.Sekine Y, Ouchi Y, Sugihara G et al. (2008) Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28:5756–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sempere AP, Posada I, Ramo C, Cabello A (1991) Spongiform leucoencephalopathy after inhaling heroin. Lancet 338:320. [DOI] [PubMed] [Google Scholar]
- 179.Sidney S (2002) Cardiovascular consequences of marijuana use. J Clin Pharmacol 42:64S–70S [DOI] [PubMed] [Google Scholar]
- 180.Sim ME, Lyoo IK, Streeter CC et al. (2007) Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology 32: 2229–2237 [DOI] [PubMed] [Google Scholar]
- 181.Singla S, Sachdeva R, Mehta JL (2012) Cannabinoids and atherosclerotic coronary heart disease. Clin Cardiol 35:329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Solowij N, Walterfang M, Lubman DI et al. (2013) Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophr Res 143:179–184 [DOI] [PubMed] [Google Scholar]
- 183.Solowij N, Yücel M, Respondek C et al. (2011) Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychol Med 41:2349–2359 [DOI] [PubMed] [Google Scholar]
- 184.Squier MV, Jalloh S, Hilton-Jones D, Series H (1995) Death after ecstasy ingestion: neuropathological findings. J Neurol Neurosurg Psychiatry 58:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Staley JK, Rothman RB, Rice KC et al. (1997) Kappa2 opioid receptors in limbic areas of the human brain are upregulated by cocaine in fatal overdose victims. J Neurosci 17:8225–8233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sulzer D, Rayport S (1990) Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron 5:797–808 [DOI] [PubMed] [Google Scholar]
- 187.Sulzer D, Sonders MS, Poulsen NW, Galli A (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Progr Neurobiol 75:406–433 [DOI] [PubMed] [Google Scholar]
- 188.Tan TP, Algra PR, Valk J, Wolters EC (1994) Toxic leukoencephalopathy after inhalation of poisoned heroin: MR findings. AJNR 15:175–178 [PMC free article] [PubMed] [Google Scholar]
- 189.Thompson PM, Hayashi KM, Simon SL et al. (2004) Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci 24:6028–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tobias MC, O’Neill J, Hudkins M et al. (2010) White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology 209:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Trescot AM, Datta S, Lee M, Hansen H (2008) Opioid pharmacology. Pain Physician 11:S133–S153 [PubMed] [Google Scholar]
- 192.Turillazzi E, Riezzo I, Neri M et al. (2010) MDMA toxicity and pathological consequences: a review about experimental data and autopsy findings. Curr Pharm Biotechnol 11:500–509 [DOI] [PubMed] [Google Scholar]
- 193.Vila N, Chamorro A (1997) Ballistic movements due to ischemic infarcts after intravenous heroin overdose: report of two cases. Clin Neurol Neurosurg 99:259–262 [DOI] [PubMed] [Google Scholar]
- 194.Vilke GM, DeBard ML, Chan TC et al. (2012) Excited Delirium Syndrome (ExDS): defining based on a review of the literature. J Emerg Med 43:897–905 [DOI] [PubMed] [Google Scholar]
- 195.Virmani R, Robinowitz M, Smialek JE, Smyth DF (1988) Cardiovascular effects of cocaine: an autopsy study of 40 patients. Am Heart J 115:1068–1076 [DOI] [PubMed] [Google Scholar]
- 196.Volkow ND, Chang L, Wang GJ et al. (2001) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382 [DOI] [PubMed] [Google Scholar]
- 197.Volkow ND, Chang L, Wang GJ et al. (2001) Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry 158:383–389 [DOI] [PubMed] [Google Scholar]
- 198.Volkow ND, Wang G-J, Fowler JS, Tomasi D (2012) Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 52:321–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Warner EA (1993) Cocaine abuse. Ann Int Med 119:226–235 [DOI] [PubMed] [Google Scholar]
- 200.Weber W, Henkes H, Möller P et al. (1998) Toxic spongiform leucoencephalopathy after inhaling heroin vapour. Eur Radiol 8:749–755 [DOI] [PubMed] [Google Scholar]
- 201.Weiss SR, Raskind R, Morganstern NL et al. (1970) Intracerebral and subarachnoid hemorrhage following use of methamphetamine (“speed”). Int Surg 53:123–127 [PubMed] [Google Scholar]
- 202.Wilson JM, Kalasinsky KS, Levey AI et al. (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703 [DOI] [PubMed] [Google Scholar]
- 203.Wolff V, Armspach J-P, Lauer V et al. (2013) Cannabis-related Stroke: myth or reality? Stroke 44:558–563 [DOI] [PubMed] [Google Scholar]
- 204.Wolff V, Lauer V, Rouyer O et al. (2011) Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke 42:1778–1780 [DOI] [PubMed] [Google Scholar]
- 205.Wolters EC, Van Wijngaarden GK, Stam FC et al. (1982) Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet 2:1233–1237 [DOI] [PubMed] [Google Scholar]
- 206.Wood DM, Greene SL, Dargan PI (2011) Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. EMJ 28:280–282 [DOI] [PubMed] [Google Scholar]
- 207.Xi Z-X, Peng X-Q, Li X et al. (2011) Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat Neurosci 14:1160–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu J, DeVito EE, Worhunsky PD et al. (2010) White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology 35:1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yen DJ, Wang SJ, Ju TH, Chen CC, Liao KK, Fuh JL, Hu HH (1994) Stroke associated with methamphetamine inhalation. Eur Neurol 34(1):16–22 [DOI] [PubMed] [Google Scholar]
- 210.Yücel M, Solowij N, Respondek C et al. (2008) Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry 65:694–701 [DOI] [PubMed] [Google Scholar]
- 211.Zachariah SB (1991) Stroke after heavy marijuana smoking. Stroke 22:406–409 [DOI] [PubMed] [Google Scholar]
- 212.Zalesky A, Solowij N, Yucel M et al. (2012) Effect of long-term cannabis use on axonal fibre connectivity. Brain 135:2245–2255 [DOI] [PubMed] [Google Scholar]


