Abstract
The ongoing worldwide increase in life expectancy portends a rising prevalence of age-related cardiovascular (CV) diseases in the coming decades that demands a deeper understanding of their molecular mechanisms. Inflammation has recently emerged as an important contributor for CV disease development. Indeed, a state of chronic sterile low-grade inflammation characterizes older organisms (also known as inflamm-ageing) and participates pivotally in the development of frailty, disability, and most chronic degenerative diseases including age-related CV and cerebrovascular afflictions. Due to chronic activation of inflammasomes and to reduced endogenous anti-inflammatory mechanisms, inflamm-ageing contributes to the activation of leucocytes, endothelial, and vascular smooth muscle cells, thus accelerating vascular ageing and atherosclerosis. Furthermore, inflamm-ageing promotes the development of catastrophic athero-thrombotic complications by enhancing platelet reactivity and predisposing to plaque rupture and erosion. Thus, inflamm-ageing and its contributors or molecular mediators might furnish targets for novel therapeutic strategies that could promote healthy ageing and conserve resources for health care systems worldwide. Here, we discuss recent findings in the pathophysiology of inflamm-ageing, the impact of these processes on the development of age-related CV diseases, results from clinical trials targeting its components and the potential implementation of these advances into daily clinical practice.
Keywords: Inflamm-ageing, Vascular ageing, Inflammation, Endothelial dysfunction, Cardiovascular disease
General introduction
A progressive decline in physiological processes characterizes ageing. Thus, the elderly comprise a highly vulnerable population burdened with morbidity and disability. In particular, ageing affects the cardiovascular (CV) system leading to increased incidence of CV disease including hypertension, heart failure, atherosclerosis, and its acute complications, i.e. myocardial infarction (MI) and stroke.1–5
An increased incidence of age-related CV disease accompanies the steady worldwide increase in life expectancy. This pandemic presents an urgent need to understand how age regulates CV pathophysiology to confront and control its impact.6 Inflammation has emerged as an independent CV risk factor and pathogenic contributor to CV disease.7–9 Accordingly, this article reviews the recent concept of inflamm-ageing and summarizes experimental and clinical evidence linking inflammation to age-dependent CV disease. Moreover, it discusses the potential role of inflamm-ageing as a target for future therapeutic interventions.
Inflamm-ageing
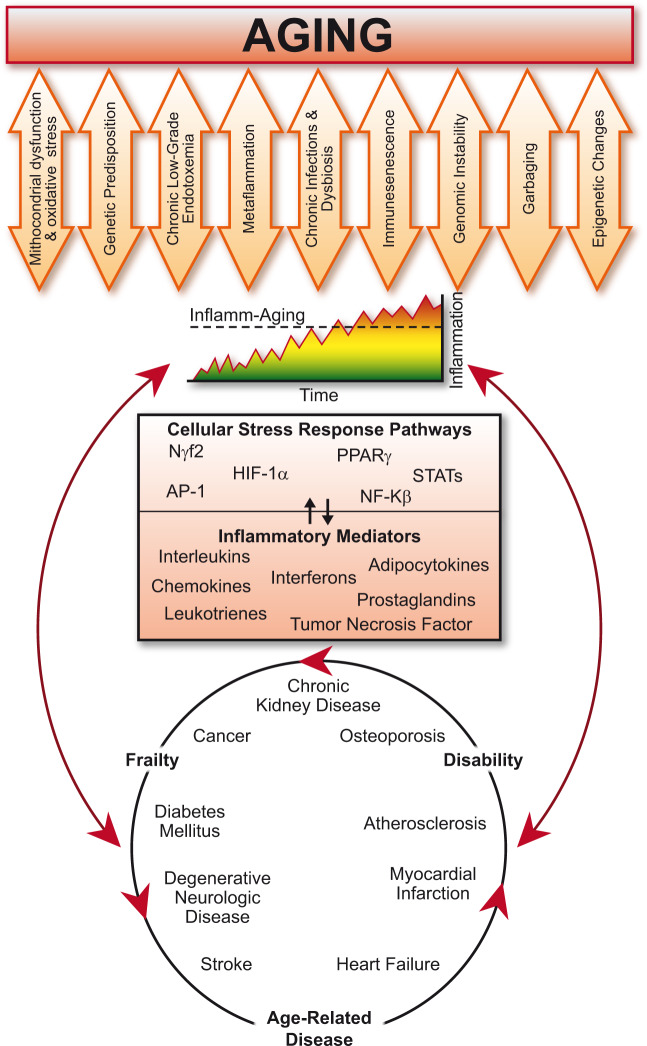
The term ‘inflamm-ageing’ emerged in the early 2000s to describe the state of chronic sterile low-grade inflammation observed in older organisms.10 Since then, this concept has evolved to indicate a broader immune dysregulation in elderly people during which persistent increased levels of pro-inflammatory mediators accompany a blunted inflammatory response to immunogenic triggers (reference 11 reviews these aspects in detail). Accordingly, hallmarks of inflamm-ageing include chronic activation of the innate immune system and increased circulating levels of pro-inflammatory mediators, such as interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF)-α, and of the biomarker C-reactive protein (CRP).12–15 Curiously, in contrast, adaptive immunity wanes with age, as demonstrated by reduced responsiveness to influenza vaccines in the elderly.16–18 Inflamm-ageing associates with frailty, motor, and cognitive disability and overall mortality as well as being a recognized risk factor for most chronic degenerative diseases including cancer, dementia, renal, and CV disease (Figure 1).19–26 Yet, establishing the causative role of inflamm-ageing for many others age-related conditions will require further longitudinal studies. Various lines of evidence suggest that high-sensitivity C-reactive protein (hsCRP) assesses CV risk factor in both young and old individuals.27,28 Indeed, hsCRP retains an independent association with CV events after adjusting for age as shown by stratification or multivariable statistical adjustment in the setting of CV risk prediction.29 Also, results from the Cardiovascular Health Study and the Rural Health Promotion Project demonstrate the ability of hsCRP to predict coronary events in the elderly after extensive adjustment for known risk factors and measures of subclinical atherosclerosis.30 A precise set of criteria for inflamm-ageing remains unsettled, nor do we advocate the use of a panel of biomarkers to identify inflamm-ageing. Rather, we wish to convey to practitioners a forward-looking view of the burgeoning basic science progress that provides novel mechanistic links between ageing, inflammation, and metabolic changes with ageing. We offer clinicians who confront an increasingly ageing population a perspective that the processes are not inevitable and unrelated degenerative processes. Rather, the emerging biological insights reviewed here promise to spur the development of novel strategies to combat the inflamm-ageing complex.
Figure 1.
The deep interactions between ageing, inflamm-ageing, and age-related conditions/disease. With ageing, several cellular and molecular mechanisms contribute to the chronic inappropriate activation of the inflammatory system (orange arrows). The resulting complex interaction between genetic predisposition and chronic exposure to a broad spectrum of exogenous and endogenous danger stimuli causes the continuous activation of a limited range of promiscuous sensors triggering different cellular stress-response pathways (upper half of the box). The resulting synthesis and release of different inflammatory mediators (lower half of the box) can drive a progressive increase of the inflammatory burden and lead to inflamm-ageing. Inflamm-ageing is the common pathophysiological mechanisms of frailty and several age-related diseases (included in the lowest circle), which in turn increase the rate of ageing and thus feed a vicious circle (red arrows).
Although no universally accepted theory thus far explains the chronic inappropriate activation of inflammation during ageing, several cellular and molecular mechanisms may contribute to this process (Figure 1). These mechanisms include cellular senescence and particularly immune-senescence, telomere shortening, genomic instability, defects in protein catabolism, dysregulation of autophagy and mitophagy, alteration of the host microbiota (i.e. dysbiosis) and chronic infections (e.g. cytomegalovirus and periodontitis), mitochondrial dysfunction, and chronic exposure to toxins at low level (e.g. chronic low-grade endotoxaemia, perhaps derived from the intestinal microbiome and accentuated by impaired epithelial barrier function with age or illness) (Figure 1).31 Production and accumulation of such danger signals as self damage-associated molecular patterns (DAMPs) or non-self pathogen-associated molecular patterns, may promote inflamm-ageing and mediate inflammation by acting on promiscuous sensors, such as Toll-like and NOD-like receptors (TLRs and NLRs, respectively).32 Recent work has implicated other receptors involved in cellular signalling, such as the Notch and Klotho/FGF23 pathways, in inflamm-ageing and in the development of age-related CV disease although evidence remains rudimentary.33,34 Although these pathways promote survival of younger organisms, with ageing, their pro-inflammatory activities—which includes NF-kB activation, production of ILs (mainly IL-1 and IL-18), Type 1 interferons, and other cytokines—increase chronically and can thus fuel inflamm-ageing (Figure 1).32 Genetic variants that affect systemic levels of cytokines and other inflammatory mediators may shape individual responses to these age-related challenges.35–38 Cellular changes that contribute to inflamm-ageing can also act at the epigenetic levels by regulating RNA transcription and translation through DNA methylation, histone modification, and by modulating non-coding RNAs.39Figure 1 presents an overview of the different pathways involved in the development of inflamm-ageing.
Recently, Prof. Franceschi et al.40—who coined the term inflamm-ageing— proposed that age-related metabolic diseases result from an age-related increased inflammatory tone (inflamm-ageing) and nutrient excess (metaflammation). As immune and metabolic response co-evolved over millennia,41 inflamm-ageing and metaflammation show similar molecular mechanisms with sterile inflammatory response as a critical determinant and macrophages as master cells.40,42,43 Ageing heightens metaflammation which in turn supports inflamm-ageing that may reflect accelerated ageing in response to caloric excess.44 At a crossroad between metabolism and inflammation, the gut microbiota may modulate both metaflammation and inflamm-ageing. Indeed, the myriad of bacteria that colonize our digestive tract transform dietary molecules into metabolites and regulate the functionality of different organs and tissues.45,46 The gut microbiota undergoes profound shaping with ageing and these changes may promote physiological nutrition-related inflammation.47,48 For example, transfer of gut microbiota from aged to young germ-free animals associates with increased intestinal permeability and accelerated inflamm-ageing (macrophage dysfunction, increased systemic TNF-α, and alteration of adaptive immunity).49,50 In humans, an increase in gut Proteobacteria with age correlates with the systemic increase of pro-inflammatory cytokines, such as IL-6 and IL-8.51 Also, with ageing the gut microbiota becomes more abundant but less diverse,52 features considered harmful for the host.52,53 In contrast, an increase in microbial diversity characterizes the composition of ultra-centenarian gut flora and accompanies healthy ageing in several populations.53
The impact of inflamm-ageing on age-related cardiovascular disease
Both low-grade systemic inflammation and ageing augment risk of CV morbidity and mortality.2,54 Not only does inflamm-ageing promote atherosclerosis per se but it also interacts with traditional CV risk factors (e.g. overweight/obesity, hypertension, and Type 2 diabetes mellitus) to exacerbate their deleterious CV effects. This relationship is reciprocal as CV conditions can promote inflammation and fuel a vicious cycle linking inflamm-ageing to the development of CV disease.55 Whether inflamm-ageing occurs in absence of metabolic diseases, such as obesity and diabetes remain uncertain and will require further study.
In overweight and obese patients, high-caloric intake associates with a chronic pro-inflammatory status (the above-mentioned metaflammation) as a result of sustained post-prandial inflammatory stimulation and activation of adipose tissue.56,57 During the post-prandial period, circulating triglyceride-rich lipoprotein particles directly induce the expression of adhesion molecules, cytokines, and pro-oxidants in endothelial cells and leucocytes and thus favour vascular inflammation.58–61 Moreover, adipocyte hypertrophy (i.e. increased cellular size) can lead to local hypoxia and endoplasmic reticulum stress. Those conditions can impair the protective AMPK and SIRT pathways and increase production of pro-inflammatory and chemotactic adipocytokines which promote inflammation and cooperate with inflamm-ageing to boost CV damage.62,63 Ageing augments monocyte and lymphocyte accumulation in adipose tissue where these cells contribute to increased production of inflammatory mediators.64 In contrast, caloric restriction and weight loss associate with the activation of longevity-promoting pathways and reduction of inflammation.1,57,62,65–69
The pro-inflammatory milieu characterizing high-caloric intake also promotes insulin resistance, a well-recognized CV risk factor associated with ageing.70,71 Indeed, the CV risk associated with diabetes increases strongly in elderly as compared with young individuals.72–74 Here, inflamm-ageing broadens the vicious circle that connects obesity, insulin resistance, ageing, and age-related CV disease. Elevated levels of pro-inflammatory cytokines as seen in inflamm-ageing and metaflammation promote the infiltration of immune cells within insulin-responsive tissue (i.e. fat and muscles), thereby increasing local oxidative stress and inflammation and reducing the expression of the insulin receptor.75–77 As a result, high circulating levels of glucose, lipids, free fatty acids, and reactive oxygen species (ROS) promote the transition from metabolically compensated obesity to acquisition of features of the metabolic syndrome cluster.78 Also, pro-inflammatory cytokines, glucose, and modified lipids favour endothelial dysfunction thus accelerating CV ageing. Indeed, vascular cells from diabetic patients show shorter telomeres and increased senescence biomarkers (e.g. β-galactosidase) related to the accentuated atherosclerotic risk observed in the elderly with diabetes.79,80
Inflammatory mediators also exacerbate vascular endothelial and smooth muscle cell dysfunction that accompanies hypertension in the elderly.7,81 Accumulation of inflammatory cells (i.e. dendritic cells, NK cells, macrophages) in arteries characterizes experimental hypertension.82 Monocytes express receptors for angiotensin II (AngII) and for mineralocorticoids, pathways that drive hypertension, and promote inflammatory polarization and increase ROS production.83 With inflamm-ageing, macrophages within the vessel wall produce higher amounts of ROS that reduce NO availability, boost adhesion molecule expression, stimulate VSMC hypertrophy and activate matrix metalloproteinases, processes implicated in vascular remodelling and dysfunction.84 Accordingly, mice with functionally deficient macrophages show blunted vascular oxidative stress, endothelial dysfunction, and resist hypertension.85,86 Mediators of inflamm-ageing, such as TNF-α, IL-1β, caspase 1, and other components of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome can contribute to age-related progression of hypertension and thus represent potential therapeutic targets in this setting.87 Indeed, the multimeric intracellular sensor, NLRP3 can detect different microbial peptides, endogenous danger signals, and non-self-irritants resulting in the assembly and activation of the NLRP3 inflammasome. Inflammasome activation requires two steps that tightly regulate its functions. A priming step augments the expression of individual inflammasome components and their assembly into the macromolecular multimer. A wide variety of potentially injurious stimuli then provide a second signal that confers activity on a proteolytic enzyme component of the inflammasome, caspase 1. This proteinase cleaves the inactive precursors of the pro-inflammatory cytokines IL-1β and IL-18, unleashing their biological actions.88 In inflamm-ageing, the sustained increase in inflammatory mediators together with the reduction of circulating anti-inflammatory cytokines (e.g. IL-10 and adiponectin) might exacerbate vascular extracellular matrix remodelling and arterial stiffening, thereby widening pulse pressure, promoting systolic hypertension, and accelerating plaque formation.89
Atherosclerosis and its thrombotic complications account for most CV morbidity and mortality. Inflamm-ageing drives atherosclerosis, a recognized chronic inflammatory condition. Cells within atherosclerotic plaques often show features of senescence.8,90 Cytokines and inflammatory cells participate in atherosclerosis at all stages, able to exert either pro- or anti-atherogenic effects.91–94 Pro-inflammatory cytokines can impair endothelial cell barrier function, vasodilator properties, and induce adhesion molecules and chemokines that recruit leucocytes to lesions. Cholesterol crystals and other DAMPs within the atherosclerotic lesion co-activate the NLRP3 inflammasome in macrophages and augment the production of active forms of the pro-inflammatory cytokines IL-1β and IL-18.83 The resulting inflammatory environment mediates the ongoing cellular recruitment with generation of foam cells and fatty streaks eventually leading to the development of complex plaques. NLRP3 activity also rises during endothelial cell senescence as a result of increased oxidative stress, and by defective autophagy in senescent cells.2 Accordingly, the NLRP3 inflammasome has emerged as a therapeutic target for inflamm-ageing that might counteract age-related CV disease. In atherosclerotic arteries, some studies show decreases in anti-inflammatory factors, such as glucocorticoids, IL-10, IL-1 receptor antagonist, and NO limiting a return to homoeostasis and promoting chronic unresolved inflammation.95
Recently, a class of molecules referred to as specialized pro-resolving mediators have garnered increasing interest. SPMs derive from polyunsaturated fatty acids via lipoxygenase, cyclooxygenase, cytochrome P450, or their combination.96 Levels of SPMs (such as resolvin D1) decrease significantly in vulnerable regions of atherosclerotic plaques where macrophages abundantly express nuclear 5-lipooxygenase. This enzyme converts arachidonic acid to pro-inflammatory leukotrienes potentially promoting plaque rupture.96
Senescent cells accumulate in advanced atherosclerotic plaques, as identified through specific markers, such as senescence-associated β galactosidase (SAβG), p16, and tumour suppressor alternative reading frame (ARF). Increased rate of SAβG-positive cells localizes in the intimal and medial layers from atherosclerotic arteries compared with age-matched healthy vessels.97,98 Cellular senescence refers to the irreversible loss of cell’s ability to divide2; furthermore, these cells acquire a defined senescence-associated secretory phenotype characterized by enhanced production of inflammatory cytokines, growth factors, and proteases.99 The increased production of matrix metalloproteinases by senescent cells can enhance extracellular matrix breakdown and augment remodelling of the advanced atherosclerotic plaque.100,101 Degradation of the extracellular matrix facilitates VSMC migration from the media, mediates arterial compensatory enlargement and can weaken the plaque’s protective fibrous cap.102 Indeed, under these conditions VSMC can sustain DNA damage and telomere shortening. These alterations promote stress-induced premature senescence (SIPS) with loss of proliferative abilities, defective autophagy, and induction of apoptosis.98,103 Shortening of telomeres (i.e. repetitive nucleotide sequences at the ends of chromosomes) normally occurs at each cell division but inflammation and oxidative stress can enhance this process. Telomere shortening regulates cellular senescence and associates with atherosclerosis and major CV events.104,105
Oxidative stress as a result of ROS overproduction or defective scavenging critically contributes to SIPS and participates in ageing of the CV system.1 Indeed, several genes that modulate ageing and influence lifespan (i.e. ageing and longevity genes) contribute critically to atherosclerosis by augmenting cellular oxidative stress and levels of inflammatory mediators.1,2 Also, the altered expression of those genes (e.g. p66Shc and JunD) can impair age-related angiogenesis, essential for repairing damaged vessels and maintaining vascular homoeostasis.106–108 Ageing, inflammation, and oxidative stress can alter the epigenetic regulation of such genes leading to defective response to hypoxia, blunted endothelial cell migration/proliferation and reduced number and functionality of stem and progenitor cells and thus favour premature CV ageing.39,109
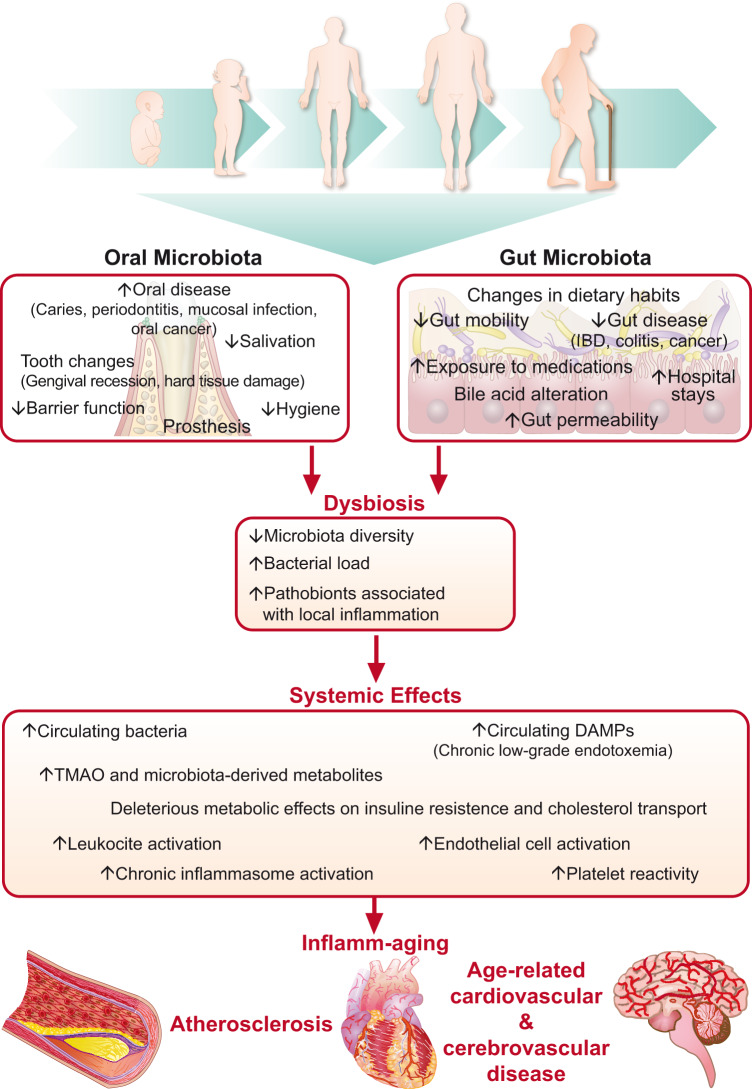
The human microbiome may also influence age-related CV inflammation, and several studies have reported the presence of bacteria in atherosclerotic plaques.110,111 Furthermore, bacterial DNA can circulate in patients with CV diseases and the personal microbiota fingerprint links to CV risk.112–115 As previously mentioned, during ageing microbiota composition undergoes modifications associated with rise of local and systemic inflammatory markers, altered intestinal permeability and increased circulating bacterial DNA which may facilitate the development of atherosclerosis (Figure 2).49,116 Although mechanistic studies implicated the ability of different microbial pathogens to directly invade vascular cells and leucocytes, thereby promoting inflammation, the intestinal microbiome also functions as an endocrine organ releasing different metabolites in response to dietary intake; in turn, such metabolites can enter the systemic circulation and act as hormones, signalling at a distance.116 Although some metabolites may promote health, dysbiotic conditions may generate toxic metabolites that can accumulate with age. One such microbial product, trimethylamine N-oxide (TMAO), associates in some, but not all, studies with coronary artery disease (CAD) and with major adverse cardiac events in some cohorts.117–119 Trimethylamine N-oxide links mechanistically to experimental CV disease. Indeed, several reports showed increased inflammation, foam cell formation, plaque burden, and thrombotic potential in animals fed with choline- or carnitine-rich diet (both substrates for TMAO synthesis) or in germ-free animals transplanted with synthetic microbial communities capable of producing TMAO.119–121 Mechanistically, TMAO may modulate vascular dysfunction by acting the NLRP3 inflammasome pathway, increasing oxidative stress, promoting forward cholesterol transport, and directly enhancing platelet thrombogenicity via increased Ca2+-signalling.122 Accordingly, recent experimental data suggest targeting TMAO synthesis by TMA lyase inhibitor as a potential anti-atherosclerosis therapy.123
Figure 2.
Age-related dysbiosis enhances inflamm-ageing eventually accelerating atherogenesis. Age-related dysbiosis enhances inflamm-ageing eventually accelerating atherogenesis. With ageing several endogenous and exogenous changes (included in the upper boxes), foster a less healthy oral and gut microbiota (left and right boxes, respectively). These alterations impair the homoeostatic symbiosis between the intestinal flora and the host. Total bacterial load increases, while microbiota diversity falls allowing pathobionts to establish a condition of chronic local inflammation known as dysbiosis (central box). Together with local consequences, dysbiosis also causes systemic effects (lower box) including increased circulating bacteria, danger-associated molecular pathways as well as trimethylamine N-oxide and other microbiota-derived metabolites eventually causing the systemic activation of inflammatory pathways, promoting age-related cardiovascular and cerebrovascular disease (bottom of the figure).
Inflammation as a target to blunt age-related cardiovascular disease: current evidence from clinical studies
Despite major remaining knowledge gaps, current evidence points towards inflamm-ageing as an important contributor of age-related CV disease; indeed, some of its molecular pathways have already proven effective as targets in the clinical setting. In keeping with the inflammatory nature of atherosclerosis and its acute thrombotic manifestations, patients with chronic extra-cardiac inflammatory diseases, such as rheumatoid arthritis or psoriatic arthritis have a higher risk of CV disease (adjusted relative risk of 2.0 for MI) and related mortality compared with the general population. Systemic inflammation may contribute independently to this increased CV risk and lead to MI, cerebrovascular disease, and heart failure.124–129 Furthermore, prospective cohort studies have consistently shown that elevated plasma levels of hsCRP and several other biomarkers of inflammation associate independently with increasing risk of future CV events in different populations.130–132 In a comprehensive meta-analysis of >50 prospective studies, the magnitude of risk associated with 1 SD elevation in hsCRP resembled that observed for a 1 SD elevation in total cholesterol or blood pressure.133 Results from the JUPITER study of rosuvastatin in primary prevention patients with above median hsCRP, LDL <130 mg/dL, and without extra-cardiac inflammatory disease indicated that the statin-induced reduction in CV events derived from both the observed reductions in vascular inflammation, as measured by hsCRP, and in LDL levels.134 Data from PROVE-IT also support the concept of targeting both LDL and inflammation for CV disease prevention.135 As they impact both inflammation and lipids, statin trials could not test whether selectively reducing inflammation (without lowering LDL) would improve CV outcomes. Taken together, these data along with those of multiple animal experiments bolstered the hypothesis that targeting mediators of inflammation may improve clinical outcomes in patients with atherosclerosis and encourage further clinical trials testing anti-inflammatory treatments in this setting (Table 1).
Table 1.
Main recent clinical trials of anti-inflammatory therapy in cardiovascular disease
| Trial, year | Drug | Target | Dosage | Size | Main result |
|---|---|---|---|---|---|
| ARISE136 | Succinobucol | OxLDL | 300 mg once daily | 6144 | Failure to reduce fatal and non-fatal cardiovascular events in patients with recent acute coronary syndromes |
| CANTOS137 | Canakinumab | IL-1β | 150 mg every 3 months | 10 061 | Reduction of recurrent fatal and non-fatal cardiovascular events |
| CIRT138 | Methotrexate | Purinergic signalling | 15–20 mg weekly | 4786 | Premature stop due to inefficacy in reduction of inflammatory mediators and cardiovascular events in patients with stable atherosclerosis |
| COLCOT139 | Colchicine | Microtubule assembly | 0.5 mg once daily | 4745 | Reduction of recurrent ischaemic cardiovascular events |
| LoDoCo140 | Colchicine | Microtubule assembly | 0.5 mg once daily | 532 | Reduction of cardiovascular events in patients with stable coronary artery disease |
| LoDoCo2 (EU Clinical Trial Regeister n 2015-005568-40) | Colchicine | Microtubule assembly | 0.5 mg once daily | 5000 | Ongoing |
| MRC-ILA Heart141 | Anakinra | IL-1R | 100 mg once daily | 182 | Reduction of inflammatory markers 14 days after non-ST elevation acute coronary syndrome. Excess of MACE events 1 year after the treatment |
| SELECT-ACS142 | Inclacumab | P-selectin | 20 mg/kg single dose | 544 | Reduction of peak Troponin I and myocardial creatine kinase after non-ST-elevation acute coronary |
| SELECT-CABG143 | Inclacumab | P-selectin | 20 mg/kg every 4 weeks | 384 | Failure to reduce saphenous vein graft disease following coronary artery bypass graft |
| SOLID-TIMI-52144 | Darapladib | Lp-PLA2 | 160 mg once daily | 13 026 | Failure to reduce major coronary events in patients with recent acute coronary syndrome |
| STABILITY145 | Darapladib | Lp-PLA2 | 160 mg once daily | 15 828 | Failure to reduce fatal and non-fatal cardiovascular events in patients with stable coronary artery disease |
| VCU-ART 3146 | Anakinra | IL-1 | 100 mg twice daily | 99 | Ongoing |
| VISTA-16147 | Varespladib | sPLA2 | 500 mg once daily | 5145 | Terminated for failure to reduce fatal and non-fatal cardiovascular events in patients with recent acute coronary syndrome. Warning for possible harm |
IL, interleukin; IL-1R, interleukin 1 receptor; Lp-PLA2, Lipoprotein-associated phospholipase A2; MACE, major adverse cardiovascular events; OxLDL, oxidized low-density lipoprotein cholesterol; sPLA2, soluble phospholipase A2; TNFα, tumour necrosis factor α.
Clinical trials have shown that the P-selectin antagonist inclacumab and an anti-inflammatory serpin both reduced myocardial damage after percutaneous coronary intervention performed for an acute MI.142,148 A 5-lipoxygenase inhibitor has also appeared to reduce levels of leukotrienes and hsCRP and slow atherosclerosis progression in another relatively small clinical study.149 Despite these results of intermediate endpoint studies, large-scale CV outcome studies have not shown benefit for these interventions.
Completed clinical trials of anti-inflammatory agents targeting cardiovascular clinical outcomes
Canakinumab anti-inflammatory thrombosis outcomes study (CANTOS) tested canakinumab, a monoclonal antibody that neutralizes the pro-inflammatory cytokine IL-1β, in a study of 10 061 patients with previous MI and an hsCRP concentration of 2 mg/L or above on full standard of care medications including effective statin treatment.137 Canakinumab administered subcutaneously every 3 months dose-dependently reduced hsCRP in the treated groups compared with placebo, without changes in atherogenic lipoprotein levels. At a median follow-up of 3.7 years, the middle dose met the stringent criteria for statistical significance, both for the primary endpoint of non-fatal MI, non-fatal stroke, or CV death and the secondary endpoint that included hospitalization for unstable angina that led to urgent revascularization. All-cause mortality did not differ significantly in the canakinumab groups compared with placebo. Canakinumab did associate with a small but significant increase in the incidence of fatal infections compared with placebo.
The Cardiovascular Inflammation Reduction trial (CIRT) tested the anti-inflammatory agent methotrexate in a randomized placebo-controlled study of 4786 patients with a previous MI or multivessel coronary disease, who also had Type 2 diabetes or the metabolic syndrome.138 CIRT halted prematurely after a median follow-up of 2.3 years for futility. As opposed to the effect of canakinumab in CANTOS, low-dose methotrexate did not affect blood levels of IL-1β, IL-6, or hsCRP.
Clinical studies of the anti-inflammatory agent colchicine
Colchicine, a widely available, inexpensive, and orally administered anti-inflammatory drug, has approval for the management of patients with gout (microcrystalline inflammatory arthritis). Colchicine may exert its effects through the inhibition of tubulin polymerization,150 interfere with the assembly of the multimeric NLRP3 inflammasome, and through effects on cellular adhesion molecules and inflammatory chemokines.151–154 Thus, colchicine can interfere with many functions of white blood cells including migration and degranulation. Through the disruption of the cytoskeleton, colchicine can suppress secretion of cytokines and chemokines as well as platelet aggregation in vitro.155,156 Considerable work has highlighted the potential of colchicine in the treatment of inflammatory CV diseases. Indeed, several clinical trials157–161 have demonstrated the efficacy of colchicine for the treatment and prevention of recurrent pericarditis, a therapy that has become standard of care. In a retrospective cross-sectional study of 1288 low-risk patients with gout, those treated prophylactically with colchicine for 1 year had a significantly lower incidence of MI compared with patients who did not receive colchicine, with a trend towards reduced all-cause mortality.162 Considerable confounding plagues such observational studies, emphasizing the necessity of rigorous randomized trials in this and in other domains.
Colchicine has also undergone study in patients with CAD. In the LoDoCo Trial, 532 patients with clinically stable coronary disease randomly received treatment with colchicine or no colchicine in addition to usual care for a minimum of 2 years in an unblinded PROBE design.140 Following a mean follow-up of 36 months, colchicine-treated patients experienced significantly fewer CV events (composite incidence of acute coronary syndromes, out-of-hospital cardiac arrest, or non-cardioembolic ischaemic stroke) as compared with no colchicine. A reduction in acute coronary events unrelated to stent disease largely drove this effect. The results of this relatively small trial indicated that colchicine may be a safe and effective agent for the prevention of major CV events in this population and that its mechanism of action may be through inhibiting the inflammatory pathway identified in unstable atherosclerotic plaques. Although LoDoCo was small, included a control group without placebo, and focused on patients with stable CAD, it has stimulated larger and more rigorous clinical studies.
Indeed, a 2016 Cochrane systematic review concluded that ‘colchicine may have substantial benefits in reducing MI in selected high-risk populations’ but ‘more evidence from large-scale randomized trials is needed’.163 Accordingly, the recently published COLchicine Cardiovascular Outcomes Trial (COLCOT) evaluated the effects of colchicine on CV events as well as its safety and tolerability in ∼4745 post-MI patients recruited within 30 days after the index event.139 Daily administration of colchicine (0.5 mg daily) for a median follow-up time of 22.6 months significantly reduced the risk of primary composite endpoint (death from CV causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina leading to coronary revascularization) as compared with placebo.139 This result derived mainly from lower risks of angina and stroke, while the treatment did not show effects on the risk of death from CV causes or MI. The gastrointestinal side effects of colchicine were somewhat more frequent in the treated group (i.e. nausea and flatulence). Pneumonia also increased in the colchicine-treated group, but infectious deaths and septic shock did not differ in the two groups.139 Currently, the LoDoCo-2 study is assessing the value of colchicine on clinical outcomes in ∼5500 patients with stable CAD and will provide an additional evaluation of anti-inflammatory therapy with colchicine in patients with coronary syndromes.
In sum, novel therapies that target inflammatory pathways can reduce atherosclerotic risk. Although not all inflammation blockers do so, neutralization of IL-1β and treatment with colchicine demonstrated effectiveness in secondary CV prevention. As expected, anti-inflammatory therapies can increase the risk of infections and future studies should strive to test interventions that interfere less with host defenses.
The strong age-dependency of clonal haematopoiesis and the consequent CV risk discussed below illustrates an example of accentuated inflammation directly related to ageing. Links between clonal hematopoiesis and telomere length provide another mechanism that links ageing to inflammation.164 These observations should spur a quest to identify biomarkers that could distinguish inflamm-ageing from chronic inflammation of other causes. This distinction could inform the design of clinical trials that could enrol the frequently underrepresented elderly population, and test directed anti-inflammatory therapies in a precision or personalized manner.
Future therapeutic perspectives
Recent findings have identified a novel connection between ageing, inflammation, and CV disease completely unsuspected until a few years ago: clonal haematopoiesis. With age, humans acquire somatic mutations in bone marrow stem cells that lead to accumulation in peripheral blood of clones of mutant leucocytes. This situation is common, and relates strongly to age. Seventy-year-old individuals have at least an 1 in 10 chance of harbouring >2% of the cells in their peripheral blood that bear a mutation associated with clonal haematopoiesis.165,166 The genes mutated that give rise to these clones represent a small subset of the some 40 well-characterized driver genes for leukaemia. Leukaemic transformation generally requires the successive acquisition of three or more mutations in the same clone. Thus, only a few individuals with clones in peripheral blood bearing just one such mutation will develop acute leukaemia. Therefore, this condition has been dubbed clonal haematopoiesis of indeterminate potential (CHIP).167 This situation is analogous to the development of monoclonal gammopathy of unknown significance, another age-related situation.
Although individuals with CHIP have only a 0.5–1% annual rate of conversion to acute leukaemia, they exhibit a 40% increase in CV risk, independent of traditional risk factors.166,168 Experiments in mice genetically altered to have loss of function of Tet2 a commonly mutated gene that generates CHIP exhibit accelerated atherosclerosis.169 Thus, CHIP does not merely accompany ageing, a strong risk factor for atherosclerosis, but appears causally related to aggravation of vascular disease. Analyses of leucocytes from these mice engineered to manifest CHIP indicate overexpression of pro-inflammatory genes, including the products of the NLRP3 inflammasome IL-1β and its downstream companion IL-6.169 The presence of CHIP mutations, drives not only excess risk of MI and stroke but also of heart failure and death due to heart failure.170 Thus, CHIP provides a newly recognized link between ageing, inflammation, major CV diseases, and cancer, another common affliction of ageing. The most common mutations associated with CHIP alter methylation of pyrimidines, strong evidence that epigenetic alterations contribute to the excess CV risk caused by CHIP.167
The advances in the understanding of the biology of ageing and inflammation provide a new perspective for potential therapies. One of the central hubs of inflammation signalling, the inflammasome, has received intense interest as a potential therapeutic target. This multimeric intracellular protein processes the precursors of the pro-inflammatory cytokines IL-1β and IL-18 to their active products.81 Small molecule inhibitors of the inflammasome are currently in clinical development. The major product of the NLRP3 inflammasome, the active forms of IL-1β and IL-18 could be targets of therapy with receptor antagonists or neutralizing antibodies. The success of the CANTOS illustrates the efficacy of neutralizing IL-1β in individuals with already established coronary heart disease.137 IL-1 and IL-18, in turn, trigger the expression of IL-6 in many cell types.81 This cascade amplifies inflammasome signalling considerably. IL-6 can promote thrombotic events by boosting fibrinogen and plasminogen activator inhibitor-1 production by the liver, hence rendering blood more coagulable and impairing fibrinolysis.171 Therapeutic antibodies can also target IL-6 and its receptor as well as IL-18. Thus, the inflammasome pathway provides a rich palette of potential therapeutic targets for combatting this aspect of inflamm-ageing.
In contrast to the promise of the inflammasome pathway, other hubs of inflammatory signalling appear less attractive as therapeutic targets. The very centrality of the NF-ĸB pathway indicates that its inhibition might impair host defenses in a more global fashion than inhibition of the NLRP3 inflammasome. Indeed, the boost in inflammatory gene expression due to IL-1 isoforms and TNF depends largely on NF-ĸB.172 Moreover, NF-ĸB activation can contribute to apoptosis, raising the possibility that its inhibition could promote tumour growth. Indeed, the increased lymphoma seen with anti-TNF therapies may reflect impaired tumour surveillance and/or decreased apoptosis.173 Targeting of IL-17/IL-23 requires caution due to some signals of increased CV risk.174
Other anti-inflammatory therapies ranging from a variety of anti-oxidant interventions and inhibition of p38 mitogen-associated kinase (MAP kinase) did not prevent coronary events in a well-conducted clinical trial.175 The recent demonstration that a high-dose pharmaceutical preparation of eicosapentaenoic acid can reduce CV events suggests another potential avenue for addressing inflamm-ageing therapeutically.176 Three recent randomized clinical trials evaluated low-dose aspirin in primary CV prevention and led the American Heart Association and the American College of Cardiology (AHA/ACC) to issue updated aspirin recommendations in March.177 Those trials compared low-dose aspirin (ca. 100 mg daily) with placebo over 5–7.5-year follow-up in diabetics (ASCEND), patients at moderate CV risk (ARRIVE) and elderly individuals (ASPREE).178–180 None of the above-mentioned trials demonstrated CV benefit (reduction in MI, stroke, or CV mortality). Indeed, ASPREE found an increased risk of all-cause mortality and gastrointestinal malignancies for the aspirin arm.178–180 As for secondary CV prevention, aspirin remains a cornerstone of therapy although some concerns pertain including (i) most studies were conducted decades ago and might not reflect current standard of care (e.g. statin treatment), (ii) most trials enrolled young and predominantly male patients, and (iii) the regimen used in those trials differs importantly from current recommendations.181 As with aspirin, other non-steroidal anti-inflammatory agents also appear not to provide CV benefit, and particularly in the case of cyclooxygenase-2 selective inhibitors may even augment CV events.182 Some of the benefit that accrued from the high-dose omega-3 fatty acid therapy could result from dampening of inflammation.183
Beyond pro-inflammatory interventions, the characterization of SPMs provides a way to enhance the resolution of inflammation without impairing host defenses, as mentioned above. The elegant chemistry and functional biology of SPMs provides an intriguing potential for further advances in the therapy of inflammation associated with ageing and other conditions.
The recognition of the nexus between ageing, inflammation, and CV disease has gained considerable new mechanistic understanding as summarized here. A number of novel potential therapeutic avenues have emerged from these advances in basic science. The use of biomarkers to assess over activity of specific pro-inflammatory pathways could help target therapies and enhance personalization of medical interventions in the growing population of elderly that will increasingly comprise a bulk of patients cared for cardiologists.
Conclusions
Older individuals commonly exhibit low-grade persistent inflammation, presenting a postulated mechanistic pillar of ageing biology. In the elderly, chronic inflammation predicts the risk of frailty, sarcopenia, disability, and age-related chronic disease, including CV conditions. Recent years have witnessed substantial progress in unveiling the mechanisms underlying inflamm-ageing. Clinical trials have validated the notion that the inhibition of selected inflammatory mediators can reduce CV events. However, studies focusing on anti-inflammatory agents and their effects on age-related CV conditions are scarce and controversial. This gap comprises an important area of unmet medical need that merits further focused clinical studies.
Novel pharmacological treatments that selectively target the pathways driving inflamm-ageing might prevent CV disease and retard the age-related decline in physiological processes. Translation of the basic findings into clinical tools able to challenge the burden of CV disease in an ageing population will require dedicated clinical trials designed to investigate the frequently underrepresented but growing segment of elderly individuals.
Funding
This work was supported by the Swiss National Science Foundation [310030_175546], the Swiss Heart Foundation, the Alfred and Annemarie von Sick Grants for Translational and Clinical Research Cardiology and Oncology and the Foundation for Cardiovascular Research–Zurich Heart House to G.G.C. G.G.C. is also the recipient of a Sheikh Khalifa's Foundation Ass. Professorship at the Faculty of Medicine, University of Zurich. P.L. received funding by National Heart, Lung, and Blood Institute [R01HL080472], American Heart Association [18CSA34080399], and RRM Charitable Fund.
Conflict of interest: P.L. is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron, and XBiotech, Inc. P.L. is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, IFM Therapeutics, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. P.L. serves on the Board of XBiotech. P.L.’s laboratory has received research funding in the last 2 years from Novartis.
References
- 1. Camici GG, Savarese G, Akhmedov A, Luscher TF.. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J 2015;36:3392–3403. [DOI] [PubMed] [Google Scholar]
- 2. Paneni F, Diaz Canestro C, Libby P, Luscher TF, Camici GG.. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol 2017;69:1952–1967. [DOI] [PubMed] [Google Scholar]
- 3. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 5. Lind L, Sundstrom J, Arnlov J, Lampa E.. Impact of aging on the strength of cardiovascular risk factors: a longitudinal study over 40 years. J Am Heart Assoc 2018;7:e007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camici GG, Liberale L.. Aging: the next cardiovascular disease? Eur Heart J 2017;38:1621–1623. [DOI] [PubMed] [Google Scholar]
- 7. Carbone F, Liberale L, Bonaventura A, Cea M, Montecucco F.. Targeting inflammation in primary cardiovascular prevention. Curr Pharm Des 2016;22:5662–5675. [DOI] [PubMed] [Google Scholar]
- 8. Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 9. Shi Y, Camici GG, Luscher TF.. Cardiovascular determinants of life span. Pflugers Arch 2010;459:315–324. [DOI] [PubMed] [Google Scholar]
- 10. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G.. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2006;908:244–254. [DOI] [PubMed] [Google Scholar]
- 11. Frasca D, Blomberg BB.. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016;17:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Puzianowska-Kuznicka M, Owczarz M, Wieczorowska-Tobis K, Nadrowski P, Chudek J, Slusarczyk P, Skalska A, Jonas M, Franek E, Mossakowska M.. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing 2016;13:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ershler WB, Keller ET.. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000;51:245–270. [DOI] [PubMed] [Google Scholar]
- 14. Stowe RP, Peek MK, Cutchin MP, Goodwin JS.. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci 2010;65:429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerli R, Monti D, Bistoni O, Mazzone AM, Peri G, Cossarizza A, Di Gioacchino M, Cesarotti ME, Doni A, Mantovani A, Franceschi C, Paganelli R.. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev 2001;121:37–46. [DOI] [PubMed] [Google Scholar]
- 16. Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D.. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol 2018;40:83–94. [DOI] [PubMed] [Google Scholar]
- 17. Osterholm MT, Kelley NS, Sommer A, Belongia EA.. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:36–44. [DOI] [PubMed] [Google Scholar]
- 18. Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD.. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine 2013;31:6030–6033. [DOI] [PubMed] [Google Scholar]
- 19. Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N.. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 20. Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB.. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation 2001;103:947–953. [DOI] [PubMed] [Google Scholar]
- 21. Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M, Health A.. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 2009;64:1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ.. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 1999;47:639–646. [DOI] [PubMed] [Google Scholar]
- 23. Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci 2010;1207:155–162. [DOI] [PubMed] [Google Scholar]
- 24. Kooman JP, Dekker MJ, Usvyat LA, Kotanko P, van der Sande FM, Schalkwijk CG, Shiels PG, Stenvinkel P.. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Renal Physiol 2017;313:F938–F950. [DOI] [PubMed] [Google Scholar]
- 25. Baylis D, Bartlett DB, Syddall HE, Ntani G, Gale CR, Cooper C, Lord JM, Sayer AA.. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordr) 2013;35:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trollor JN, Smith E, Agars E, Kuan SA, Baune BT, Campbell L, Samaras K, Crawford J, Lux O, Kochan NA, Brodaty H, Sachdev P.. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age (Dordr) 2012;34:1295–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zakai NA, Katz R, Jenny NS, Psaty BM, Reiner AP, Schwartz SM, Cushman M.. Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: the Cardiovascular Health Study. J Thromb Haemost 2007;5:1128–1135. [DOI] [PubMed] [Google Scholar]
- 28. Strandberg TE, Tilvis RS.. C-reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol 2000;20:1057–1060. [DOI] [PubMed] [Google Scholar]
- 29. Libby P, Ridker PM.. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med 2004;116 Suppl 6A:9S–16S. [DOI] [PubMed] [Google Scholar]
- 30. Tracy RP, Lemaitre RN, Psaty BM, Ives DG, Evans RW, Cushman M, Meilahn EN, Kuller LH.. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol 1997;17:1121–1127. [DOI] [PubMed] [Google Scholar]
- 31. Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, Morishita R.. Source of chronic inflammation in aging. Front Cardiovasc Med 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franceschi C, Salvioli S, Garagnani P, de Eguileor M, Monti D, Capri M.. Immunobiography and the heterogeneity of immune responses in the elderly: a focus on inflammaging and trained immunity. Front Immunol 2017;8:982.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balistreri CR, Madonna R, Melino G, Caruso C.. The emerging role of Notch pathway in ageing: focus on the related mechanisms in age-related diseases. Ageing Res Rev 2016;29:50–65. [DOI] [PubMed] [Google Scholar]
- 34. Liberale L, Camici GG.. The Role of Vascular Aging in Atherosclerotic Plaque Development and Vulnerability. Curr Pharm Des 2019;25:3098–3111. [DOI] [PubMed] [Google Scholar]
- 35. Rafiq S, Stevens K, Hurst AJ, Murray A, Henley W, Weedon MN, Bandinelli S, Corsi AM, Guralnik JM, Ferruci L, Melzer D, Frayling TM.. Common genetic variation in the gene encoding interleukin-1-receptor antagonist (IL-1RA) is associated with altered circulating IL-1RA levels. Genes Immun 2007;8:344–351. [DOI] [PubMed] [Google Scholar]
- 36.IL6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O'Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J.. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012;379:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, Pellikka N, Wallaschofski H, Kettunen J, Henneman P, Baumert J, Strachan DP, Fuchsberger C, Vitart V, Wilson JF, Pare G, Naitza S, Rudock ME, Surakka I, de Geus EJ, Alizadeh BZ, Guralnik J, Shuldiner A, Tanaka T, Zee RY, Schnabel RB, Nambi V, Kavousi M, Ripatti S, Nauck M, Smith NL, Smith AV, Sundvall J, Scheet P, Liu Y, Ruokonen A, Rose LM, Larson MG, Hoogeveen RC, Freimer NB, Teumer A, Tracy RP, Launer LJ, Buring JE, Yamamoto JF, Folsom AR, Sijbrands EJ, Pankow J, Elliott P, Keaney JF, Sun W, Sarin AP, Fontes JD, Badola S, Astor BC, Hofman A, Pouta A, Werdan K, Greiser KH, Kuss O, Meyer zu Schwabedissen HE, Thiery J, Jamshidi Y, Nolte IM, Soranzo N, Spector TD, Volzke H, Parker AN, Aspelund T, Bates D, Young L, Tsui K, Siscovick DS, Guo X, Rotter JI, Uda M, Schlessinger D, Rudan I, Hicks AA, Penninx BW, Thorand B, Gieger C, Coresh J, Willemsen G, Harris TB, Uitterlinden AG, Jarvelin MR, Rice K, Radke D, Salomaa V, Willems van Dijk K, Boerwinkle E, Vasan RS, Ferrucci L, Gibson QD, Bandinelli S, Snieder H, Boomsma DI, Xiao X, Campbell H, Hayward C, Pramstaller PP, van Duijn CM, Peltonen L, Psaty BM, Gudnason V, Ridker PM, Homuth G, Koenig W, Ballantyne CM, Witteman JC, Benjamin EJ, Perola M, Chasman DI.. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 2011;123:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium, Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, Guo Y, Chung C, Peasey A, Pfister R, Mooijaart SP, Ireland HA, Leusink M, Langenberg C, Li KW, Palmen J, Howard P, Cooper JA, Drenos F, Hardy J, Nalls MA, Li YR, Lowe G, Stewart M, Bielinski SJ, Peto J, Timpson NJ, Gallacher J, Dunlop M, Houlston R, Tomlinson I, Tzoulaki I, Luan J, Boer JM, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Ferrucci L, Bandenelli S, Tanaka T, Meschia JF, Singleton A, Navis G, Mateo Leach I, Bakker SJ, Gansevoort RT, Ford I, Epstein SE, Burnett MS, Devaney JM, Jukema JW, Westendorp RGJ, de Borst G, van der Graaf Y, de Jong PA, Mailand-van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Stephens JW, Eaton CB, Robinson JG, Manson JE, Fowkes FG, Frayling TM, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y, Redline S, Lange LA, Kumari M, Wareham NJ, Verschuren WM, Benjamin EJ, Whittaker JC, Hamsten A, Dudbridge F, Delaney JA, Wong A, Kuh D, Hardy R, Castillo BA, Connolly JJ, van der Harst P, Brunner EJ, Marmot MG, Wassel CL, Humphries SE, Talmud PJ, Kivimaki M, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Hakonarson H, Reiner AP, Keating BJ, Sattar N, Hingorani AD, Casas JP.. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet 2012;379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Costantino S, Libby P, Kishore R, Tardif JC, El-Osta A, Paneni F.. Epigenetics and precision medicine in cardiovascular patients: from basic concepts to the clinical arena. Eur Heart J 2018;39:4150–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A.. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018;14:576–590. [DOI] [PubMed] [Google Scholar]
- 41. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017;542:177–185. [DOI] [PubMed] [Google Scholar]
- 42. Prattichizzo F, De Nigris V, Spiga R, Mancuso E, La Sala L, Antonicelli R, Testa R, Procopio AD, Olivieri F, Ceriello A.. Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res Rev 2018;41:1–17. [DOI] [PubMed] [Google Scholar]
- 43. Nardini C, Moreau JF, Gensous N, Ravaioli F, Garagnani P, Bacalini MG.. The epigenetics of inflammaging: the contribution of age-related heterochromatin loss and locus-specific remodelling and the modulation by environmental stimuli. Semin Immunol 2018;40:49–60. [DOI] [PubMed] [Google Scholar]
- 44. Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, Monti D, Capri M, Salvioli S.. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne) 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee YK, Mazmanian SK.. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010;330:1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kundu P, Blacher E, Elinav E, Pettersson S.. Our gut microbiome: the evolving inner self. Cell 2017;171:1481–1493. [DOI] [PubMed] [Google Scholar]
- 47. Groussin M, Mazel F, Sanders JG, Smillie CS, Lavergne S, Thuiller W, Alm EJ.. Unraveling the processes shaping mammalian gut microbiomes over evolutionary time. Nat Commun 2017;8:14319.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saraswati S, Sitaraman R.. Aging and the human gut microbiota-from correlation to causality. Front Microbiol 2014;5:764.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge MI, Boekschoten MV, Smidt H, Faas MM, de Vos P.. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol 2017;8:1385.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larche MJ, Davidson DJ, Verdu EF, Surette MG, Bowdish D.. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017;21:455–466.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W.. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 2010;5:e10667.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M, Brigidi P, Candela M.. Gut microbiota and extreme longevity. Curr Biol 2016;26:1480–1485. [DOI] [PubMed] [Google Scholar]
- 53. Santoro A, Ostan R, Candela M, Biagi E, Brigidi P, Capri M, Franceschi C.. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci 2018;75:129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mason JC, Libby P.. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015;36:482–489c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Libby P, Ridker PM, Maseri A.. Inflammation and atherosclerosis. Circulation 2002;105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 56. Rocha VZ, Libby P.. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 2009;6:399–409. [DOI] [PubMed] [Google Scholar]
- 57. Carbone F, Adami G, Liberale L, Bonaventura A, Bertolotto M, Andraghetti G, Scopinaro N, Camerini GB, Papadia FS, Cordera R, Dallegri F, Montecucco F.. Serum levels of osteopontin predict diabetes remission after bariatric surgery. Diabetes Metab 2018;45:356–362. [DOI] [PubMed] [Google Scholar]
- 58. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 59. van Oostrom AJ, Rabelink TJ, Verseyden C, Sijmonsma TP, Plokker HW, De Jaegere PP, Cabezas MC.. Activation of leukocytes by postprandial lipemia in healthy volunteers. Atherosclerosis 2004;177:175–182. [DOI] [PubMed] [Google Scholar]
- 60. Higgins LJ, Rutledge JC.. Inflammation associated with the postprandial lipolysis of triglyceride-rich lipoproteins by lipoprotein lipase. Curr Atheroscler Rep 2009;11:199–205. [DOI] [PubMed] [Google Scholar]
- 61. Wang YI, Bettaieb A, Sun C, DeVerse JS, Radecke CE, Mathew S, Edwards CM, Haj FG, Passerini AG, Simon SI.. Triglyceride-rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)-1 expression via differential regulation of endoplasmic reticulum stress. PLoS One 2013;8:e78322.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brandhorst S, Longo VD.. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res 2019;124:952–965. [DOI] [PubMed] [Google Scholar]
- 63. Liberale L, Bonaventura A, Vecchie A, Casula M, Dallegri F, Montecucco F, Carbone F.. The role of adipocytokines in coronary atherosclerosis. Curr Atheroscler Rep 2017;19:10.. [DOI] [PubMed] [Google Scholar]
- 64. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr.. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M, Fuss PJ, Roberts SB, Holloszy JO, Fontana L.. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY) 2016;8:1416–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D.. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 2004;18:1657–1669. [DOI] [PubMed] [Google Scholar]
- 67. Mirzaei H, Suarez JA, Longo VD.. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab 2014;25:558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liberale L, Bonaventura A, Carbone F, Bertolotto M, Contini P, Scopinaro N, Camerini GB, Papadia FS, Cordera R, Camici GG, Dallegri F, Adami GF, Montecucco F.. Early reduction of matrix metalloproteinase-8 serum levels is associated with leptin drop and predicts diabetes remission after bariatric surgery. Int J Cardiol 2017;245:257–262. [DOI] [PubMed] [Google Scholar]
- 69. Bonaventura A, Liberale L, Carbone F, Scopinaro N, Camerini G, Papadia FS, Cordera R, Dallegri F, Adami GF, Montecucco F.. High baseline C-reactive protein levels predict partial type 2 diabetes mellitus remission after biliopancreatic diversion. Nutr Metab Cardiovasc Dis 2017;27:423–429. [DOI] [PubMed] [Google Scholar]
- 70. Hotamisligil GS, Shargill NS, Spiegelman BM.. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 71. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS.. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997;389:610–614. [DOI] [PubMed] [Google Scholar]
- 72. Cigolle CT, Blaum CS, Halter JB.. Diabetes and cardiovascular disease prevention in older adults. Clin Geriatr Med 2009;25:607–41, vii–viii. [DOI] [PubMed] [Google Scholar]
- 73. Stout MB, Justice JN, Nicklas BJ, Kirkland JL.. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda) 2017;32:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS.. Diabetes in older adults. Diabetes Care 2012;35:2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Halter JB, Musi N, McFarland Horne F, Crandall JP, Goldberg A, Harkless L, Hazzard WR, Huang ES, Kirkman MS, Plutzky J, Schmader KE, Zieman S, High KP.. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes 2014;63:2578–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wu H, Ballantyne CM.. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest 2017;127:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reilly SM, Saltiel AR.. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 2017;13:633–643. [DOI] [PubMed] [Google Scholar]
- 78. Vecchie A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, Fruhbeck G, Montecucco F.. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med 2018;48:6–17. [DOI] [PubMed] [Google Scholar]
- 79. Shakeri H, Lemmens K, Gevaert AB, De Meyer GRY, Segers V.. Cellular senescence links aging and diabetes in cardiovascular disease. Am J Physiol Heart Circ Physiol 2018;315:H448–H462. [DOI] [PubMed] [Google Scholar]
- 80. Aryan Z, Ghajar A, Faghihi-Kashani S, Afarideh M, Nakhjavani M, Esteghamati A.. Baseline high-sensitivity c-reactive protein predicts macrovascular and microvascular complications of type 2 diabetes: a population-based study. Ann Nutr Metab 2018;72:287–295. [DOI] [PubMed] [Google Scholar]
- 81. Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol 2017;70:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM.. Inflammation, immunity, and hypertension. Hypertension 2011;57:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liberale L, Dallegri F, Montecucco F, Carbone F.. Pathophysiological relevance of macrophage subsets in atherogenesis. Thromb Haemost 2017;117:7–18. [DOI] [PubMed] [Google Scholar]
- 84. Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL.. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens 2004;22:535–542. [DOI] [PubMed] [Google Scholar]
- 85. De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL.. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 2005;25:2106–2113. [DOI] [PubMed] [Google Scholar]
- 86. Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, Schiffrin EL.. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol 2007;292:H1789–H1795. [DOI] [PubMed] [Google Scholar]
- 87. De Miguel C, Rudemiller NP, Abais JM, Mattson DL.. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep 2015;17:507.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Swanson KV, Deng M, Ting JP.. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 2019;19:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tomiyama H, Shiina K, Matsumoto-Nakano C, Ninomiya T, Komatsu S, Kimura K, Chikamori T, Yamashina A.. The contribution of inflammation to the development of hypertension mediated by increased arterial stiffness. J Am Heart Assoc 2017;6:e005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tabas I, García-Cardeña G, Owens GK.. Recent insights into the cellular biology of atherosclerosis. J Cell Biol 2015;209:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bonaventura A, Liberale L, Carbone F, Vecchie A, Diaz-Canestro C, Camici GG, Montecucco F, Dallegri F.. The pathophysiological role of neutrophil extracellular traps in inflammatory diseases. Thromb Haemost 2018;118:6–27. [DOI] [PubMed] [Google Scholar]
- 92. Bonaventura A, Montecucco F, Dallegri F, Carbone F, Luscher TF, Camici GG, Liberale L.. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc Res 2019;115:1266–1285. [DOI] [PubMed] [Google Scholar]
- 93. Casula M, Montecucco F, Bonaventura A, Liberale L, Vecchie A, Dallegri F, Carbone F.. Update on the role of Pentraxin 3 in atherosclerosis and cardiovascular diseases. Vascul Pharmacol 2017;99:1–12. [DOI] [PubMed] [Google Scholar]
- 94. Liberale L, Diaz-Cañestro C, Bonetti NR, Paneni F, Akhmedov A, Beer JH, Montecucco F, Lüscher TF, Camici GG.. Post-ischaemic administration of the murine Canakinumab-surrogate antibody improves outcome in experimental stroke. Eur Heart J 2018;39:3511–3517. [DOI] [PubMed] [Google Scholar]
- 95. Tousoulis D, Oikonomou E, Economou EK, Crea F, Kaski JC.. Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur Heart J 2016;37:1723–1732. [DOI] [PubMed] [Google Scholar]
- 96. Thorp EB. Proresolving lipid mediators restore balance to the vulnerable plaque. Circ Res 2016;119:972–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I.. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002;105:1541–1544. [DOI] [PubMed] [Google Scholar]
- 98. Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M.. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 2006;99:156–164. [DOI] [PubMed] [Google Scholar]
- 99. Childs BG, Durik M, Baker DJ, van Deursen JM.. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 2015;21:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Soto-Gamez A, Demaria M.. Therapeutic interventions for aging: the case of cellular senescence. Drug Discov Today 2017;22:786–795. [DOI] [PubMed] [Google Scholar]
- 101. Wang M, Kim SH, Monticone RE, Lakatta EG.. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 2015;65:698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;368:2004–2013. [DOI] [PubMed] [Google Scholar]
- 103. Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer G.. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res 2018;114:622–634. [DOI] [PubMed] [Google Scholar]
- 104. Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH.. Telomere shortening in atherosclerosis. Lancet 2001;358:472–473. [DOI] [PubMed] [Google Scholar]
- 105. Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, Nordestgaard BG.. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol 2012;32:822–829. [DOI] [PubMed] [Google Scholar]
- 106. Paneni F, Costantino S, Krankel N, Cosentino F, Luscher TF.. Reprogramming ageing and longevity genes restores paracrine angiogenic properties of early outgrowth cells. Eur Heart J 2016;37:1733–1737. [DOI] [PubMed] [Google Scholar]
- 107. Paneni F, Osto E, Costantino S, Mateescu B, Briand S, Coppolino G, Perna E, Mocharla P, Akhmedov A, Kubant R, Rohrer L, Malinski T, Camici GG, Matter CM, Mechta-Grigoriou F, Volpe M, Luscher TF, Cosentino F.. Deletion of the activated protein-1 transcription factor JunD induces oxidative stress and accelerates age-related endothelial dysfunction. Circulation 2013;127:1229–1240.e1–e21. [DOI] [PubMed] [Google Scholar]
- 108. Spescha RD, Klohs J, Semerano A, Giacalone G, Derungs RS, Reiner MF, Rodriguez Gutierrez D, Mendez-Carmona N, Glanzmann M, Savarese G, Kränkel N, Akhmedov A, Keller S, Mocharla P, Kaufmann MR, Wenger RH, Vogel J, Kulic L, Nitsch RM, Beer JH, Peruzzotti-Jametti L, Sessa M, Lüscher TF, Camici GG.. Post-ischaemic silencing of p66Shc reduces ischaemia/reperfusion brain injury and its expression correlates to clinical outcome in stroke. Eur Heart J 2015;36:1590–1600. [DOI] [PubMed] [Google Scholar]
- 109. Costantino S, Camici GG, Mohammed SA, Volpe M, Luscher TF, Paneni F.. Epigenetics and cardiovascular regenerative medicine in the elderly. Int J Cardiol 2018;250:207–214. [DOI] [PubMed] [Google Scholar]
- 110. Lindskog Jonsson A, Hållenius FF, Akrami R, Johansson E, Wester P, Arnerlöv C, Bäckhed F, Bergström G.. Bacterial profile in human atherosclerotic plaques. Atherosclerosis 2017;263:177–183. [DOI] [PubMed] [Google Scholar]
- 111. Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, Hasan AA, Amar S.. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol 2018;72:2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dinakaran V, Rathinavel A, Pushpanathan M, Sivakumar R, Gunasekaran P, Rajendhran J.. Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS One 2014;9:e105221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Szeto CC, Kwan BC, Chow KM, Kwok JS, Lai KB, Cheng PM, Pang WF, Ng JK, Chan MH, Lit LC, Leung CB, Li PK.. Circulating bacterial-derived DNA fragment level is a strong predictor of cardiovascular disease in peritoneal dialysis patients. PLoS One 2015;10:e0125162.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Backhed F.. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA 2011;108 Suppl 1:4592–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Szeto CC, McIntyre CW, Li PK.. Circulating bacterial fragments as cardiovascular risk factors in CKD. J Am Soc Nephrol 2018;29:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tang WH, Kitai T, Hazen SL.. Gut microbiota in cardiovascular health and disease. Circ Res 2017;120:1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L.. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc 2017;6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C.. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–2956. [DOI] [PubMed] [Google Scholar]
- 119. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL.. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Skye SM, Zhu W, Romano KA, Guo CJ, Wang Z, Jia X, Kirsop J, Haag B, Lang JM, DiDonato JA, Tang WHW, Lusis AJ, Rey FE, Fischbach MA, Hazen SL.. Microbial transplantation with human gut commensals containing CutC is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circ Res 2018;123:1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Brown JM, Hazen SL.. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 2018;16:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL.. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC.. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003;107:1303–1307. [DOI] [PubMed] [Google Scholar]
- 125. Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, Gabriel SE.. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum 2005;52:412–420. [DOI] [PubMed] [Google Scholar]
- 126. Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE.. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 2005;52:722–732. [DOI] [PubMed] [Google Scholar]
- 127. Wolfe F, Freundlich B, Straus WL.. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol 2003;30:36–40. [PubMed] [Google Scholar]
- 128. del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A.. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737–2745. [DOI] [PubMed] [Google Scholar]
- 129. Bonetti NR, Diaz-Canestro C, Liberale L, Crucet M, Akhmedov A, Merlini M, Reiner MF, Gobbato S, Stivala S, Kollias G, Ruschitzka F, Luscher TF, Beer JH, Camici GG.. Tumour necrosis factor-alpha inhibition improves stroke outcome in a mouse model of rheumatoid arthritis. Sci Rep 2019;9:2173.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rader DJ. Inflammatory markers of coronary risk. N Engl J Med 2000;343:1179–1182. [DOI] [PubMed] [Google Scholar]
- 131. Ridker PM. Inflammation, C-reactive protein, and cardiovascular disease: moving past the marker versus mediator debate. Circ Res 2014;114:594–595. [DOI] [PubMed] [Google Scholar]
- 132. Ridker PM, Hennekens CH, Buring JE, Rifai N.. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 133.Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J.. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Trial Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 2009;373:1175–1182. [DOI] [PubMed] [Google Scholar]
- 135. Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E.. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol 2005;45:1644–1648. [DOI] [PubMed] [Google Scholar]
- 136. Tardif JC, McMurray JJ, Klug E, Small R, Schumi J, Choi J, Cooper J, Scott R, Lewis EF, L'Allier PL, Pfeffer MA; Aggressive Reduction of Inflammation Stops Events Trial Investigators. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:1761–1768. [DOI] [PubMed] [Google Scholar]
- 137. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 138. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F.. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 140. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL.. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–410. [DOI] [PubMed] [Google Scholar]
- 141. Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC.. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J 2015;36:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tardif JC, Tanguay JF, Wright SR, Duchatelle V, Petroni T, Gregoire JC, Ibrahim R, Heinonen TM, Robb S, Bertrand OF, Cournoyer D, Johnson D, Mann J, Guertin MC, L'Allier PL.. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J Am Coll Cardiol 2013;61:2048–2055. [DOI] [PubMed] [Google Scholar]
- 143. Stahli BE, Tardif JC, Carrier M, Gallo R, Emery RW, Robb S, Cournoyer D, Blondeau L, Johnson D, Mann J, Lesperance J, Guertin MC, L'Allier PL.. Effects of P-selectin antagonist inclacumab in patients undergoing coronary artery bypass graft surgery: SELECT-CABG trial. J Am Coll Cardiol 2016;67:344–346. [DOI] [PubMed] [Google Scholar]
- 144. O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CPSOLID-TIMI 52 InvestigatorsSteen DL.. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 2014;312:1006–1015. [DOI] [PubMed] [Google Scholar]
- 145.STABILITY Investigators, White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen M-F, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim H-S, Koenig W, Linhart A, Lonn E, López-Sendón J, Manolis AJ, Mohler ER, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos-Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse H-F, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L.. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 146. Van Tassell BW, Lipinski MJ, Appleton D, Roberts CS, Kontos MC, Abouzaki N, Melchior R, Mueller G, Garnett J, Canada J, Carbone S, Buckley LF, Wohlford G, Kadariya D, Trankle CR, Oddi Erdle C, Sculthorpe R, Puckett L, DeWilde C, Shah K, Angiolillo DJ, Vetrovec G, Biondi-Zoccai G, Arena R, Abbate A.. Rationale and design of the Virginia Commonwealth University-Anakinra Remodeling Trial-3 (VCU-ART3): a randomized, placebo-controlled, double-blinded, multicenter study. Clin Cardiol 2018;41:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Nicholls SJ, Kastelein JJ, Schwartz GG, Bash D, Rosenson RS, Cavender MA, Brennan DM, Koenig W, Jukema JW, Nambi V, Wright RS, Menon V, Lincoff AM, Nissen SE; VISTA-16 Investigators. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA 2014;311:252–262. [DOI] [PubMed] [Google Scholar]
- 148. Tardif JC, L'Allier PL, Gregoire J, Ibrahim R, McFadden G, Kostuk W, Knudtson M, Labinaz M, Waksman R, Pepine CJ, Macaulay C, Guertin MC, Lucas A.. A randomized controlled, phase 2 trial of the viral serpin Serp-1 in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2010;3:543–548. [DOI] [PubMed] [Google Scholar]
- 149. Tardif J-C, L'Allier PL, Ibrahim R, Grégoire JC, Nozza A, Cossette M, Kouz S, Lavoie M-A, Paquin J, Brotz TM, Taub R, Pressacco J.. Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ Cardiovasc Imaging 2010;3:298–307. [DOI] [PubMed] [Google Scholar]
- 150. Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M.. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004;428:198–202. [DOI] [PubMed] [Google Scholar]
- 151. Perico N, Ostermann D, Bontempeill M, Morigi M, Amuchastegui CS, Zoja C, Akalin E, Sayegh MH, Remuzzi G.. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J Am Soc Nephrol 1996;7:594–601. [DOI] [PubMed] [Google Scholar]
- 152. Pope RM, Tschopp J.. The role of interleukin-1 and the inflammasome in gout: implications for therapy. Arthritis Rheum 2007;56:3183–3188. [DOI] [PubMed] [Google Scholar]
- 153. Cerquaglia C, Diaco M, Nucera G, Regina M, Montalto M, Manna R.. Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: an update. Curr Drug Targets Inflamm Allergy 2005;4:117–124. [DOI] [PubMed] [Google Scholar]
- 154. Wesley RB, Meng X, Godin D, Galis ZS.. Extracellular matrix modulates macrophage functions characteristic to atheroma: collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro. Arterioscler Thromb Vasc Biol 1998;18:432–440. [DOI] [PubMed] [Google Scholar]
- 155. Chao FC, Shepro D, Tullis JL, Belamarich FA, Curby WA.. Similarities between platelet contraction and cellular motility during mitosis: role of platelet microtubules in clot retraction. J Cell Sci 1976;20:569–588. [DOI] [PubMed] [Google Scholar]
- 156. Sneddon JM. Effect of mitosis inhibitors on blood platelet microtubules and aggregation. J Physiol 1971;214:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, Moratti M, Gaschino G, Giammaria M, Ghisio A, Belli R, Trinchero R.. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005;112:2012–2016. [DOI] [PubMed] [Google Scholar]
- 158. Imazio M, Bobbio M, Cecchi E, Demarie D, Pomari F, Moratti M, Ghisio A, Belli R, Trinchero R.. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med 2005;165:1987–1991. [DOI] [PubMed] [Google Scholar]
- 159. Imazio M, Brucato A, Cemin R, Ferrua S, Belli R, Maestroni S, Trinchero R, Spodick DH, Adler Y; CORP (COlchicine for Recurrent Pericarditis) Investigators. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med 2011;155:409–414. [DOI] [PubMed] [Google Scholar]
- 160. Imazio M, Brucato A, Rovere ME, Gandino A, Cemin R, Ferrua S, Maestroni S, Zingarelli E, Barosi A, Simon C, Sansone F, Patrini D, Vitali E, Belli R, Ferrazzi P, Trinchero R, Spodick DH, Adler Y.. Colchicine prevents early postoperative pericardial and pleural effusions. Am Heart J 2011;162:527–532.e1. [DOI] [PubMed] [Google Scholar]
- 161. Deftereos S, Giannopoulos G, Kossyvakis C, Efremidis M, Panagopoulou V, Kaoukis A, Raisakis K, Bouras G, Angelidis C, Theodorakis A, Driva M, Doudoumis K, Pyrgakis V, Stefanadis C.. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol 2012;60:1790–1796. [DOI] [PubMed] [Google Scholar]
- 162. Crittenden DB, Lehmann RA, Schneck L, Keenan RT, Shah B, Greenberg JD, Cronstein BN, Sedlis SP, Pillinger MH.. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol 2012;39:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hemkens LG, Ewald H, Gloy VL, Arpagaus A, Olu KK, Nidorf M, Glinz D, Nordmann AJ, Briel M.. Colchicine for prevention of cardiovascular events. Cochrane Database Syst Rev 2016;1:CD011047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Aviv A, Levy D.. Hemothelium, clonal hematopoiesis of indeterminate potential, and atherosclerosis. Circulation 2019;139:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL.. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, Zeiher A, Jaiswal S, Schulz C, Blankstein R, Bolton KL, Steensma D, Levine RL, Ebert BL.. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J Am Coll Cardiol 2019;74:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL.. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL.. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K.. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, Schmid T, Brune B, Wagner S, Serve H, Hoffmann J, Seeger F, Dimmeler S, Zeiher AM, Rieger MA.. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol 2019;4:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Libby P, Rocha VZ.. All roads lead to IL-6: a central hub of cardiometabolic signaling. Int J Cardiol 2018;259:213–215. [DOI] [PubMed] [Google Scholar]
- 172. Liu T, Zhang L, Joo D, Sun SC.. NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2017;2. pii: 17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Mariette X, Tubach F, Bagheri H, Bardet M, Berthelot JM, Gaudin P, Heresbach D, Martin A, Schaeverbeke T, Salmon D, Lemann M, Hermine O, Raphael M, Ravaud P.. Lymphoma in patients treated with anti-TNF: results of the 3-year prospective French RATIO registry. Ann Rheum Dis 2010;69:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ait-Oufella H, Libby P, Tedgui A.. Anticytokine immune therapy and atherothrombotic cardiovascular risk. Arterioscler Thromb Vasc Biol 2019;39:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. O’Donoghue ML, Glaser R, Cavender MA, Aylward PE, Bonaca MP, Budaj A, Davies RY, Dellborg M, Fox KAA, Gutierrez JAT, Hamm C, Kiss RG, Kovar F, Kuder JF, Im KA, Lepore JJ, Lopez-Sendon JL, Ophuis TO, Parkhomenko A, Shannon JB, Spinar J, Tanguay J-F, Ruda M, Steg PG, Theroux P, Wiviott SD, Laws I, Sabatine MS, Morrow DA; LATITUDE-TIMI 60 Investigators. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA 2016;315:1591–1599. [DOI] [PubMed] [Google Scholar]
- 176. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 177. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B.. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, Shah RC, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Johnston CI, Ryan J, Radziszewska B, Jelinek M, Malik M, Eaton CB, Brauer D, Cloud G, Wood EM, Mahady SE, Satterfield S, Grimm R, Murray AM; ASPREE Investigator Group. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, Tendera M, Tognoni G; ARRIVE Executive Committee. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018;392:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.The ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J.. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 181. Gargiulo G, Windecker S, Vranckx P, Gibson CM, Mehran R, Valgimigli M.. A critical appraisal of aspirin in secondary prevention: is less more? Circulation 2016;134:1881–1906. [DOI] [PubMed] [Google Scholar]
- 182. White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension 2007;49:408–418. [DOI] [PubMed] [Google Scholar]
- 183. Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H.. Fish oil and omega-3 fatty acids in cardiovascular disease: do they really work? Eur Heart J 2012;33:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]