Abstract
Aims
In epidemiologic cohorts initiated >30 years ago, inflammatory biomarkers, such as interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hsCRP) were shown to independently predict future cardiovascular events with a magnitude of effect comparable to that of low-density lipoprotein cholesterol (LDLC). Whether aggressive contemporary therapy for atherosclerosis has altered these relationships is unknown yet has major implications for future drug development.
Methods and results
Interleukin-6, hsCRP, and LDLC were measured at baseline in up to 4168 North American patients enrolled in the contemporary Cardiovascular Inflammation Reduction Trial with prior myocardial infarction or multivessel coronary disease who additionally had diabetes or metabolic syndrome and were followed for a period of up to 5 years for incident major recurrent cardiovascular events and all-cause mortality. Three-quarters of the cohort were previously revascularized and the great majority was taking statins, angiotensin blocking agents, beta-blockers, and antithrombotic agents. Participants were randomly allocated to low-dose methotrexate 15 mg weekly or to placebo. Randomized use of methotrexate had no effect on event rates nor plasma levels of IL-6, hsCRP, or LDL over time. Yet, baseline levels of IL-6, hsCRP, and LDLC were all predictors of major recurrent cardiovascular events; adjusted hazard ratios [HR; 95% confidence interval (CI)] for the lowest to highest baseline quartiles of IL-6 were 1.0 (referent), 1.66 (1.18–2.35), 1.92 (1.36–2.70), and 2.11 (1.49–2.99; P < 0.0001), while adjusted HRs for increasing quartiles of hsCRP were 1.0 (referent), 1.28 (0.92–1.79), 1.73 (1.25–2.38), and 1.79 (1.28–2.50; P < 0.0001) and adjusted HRs for increasing quartiles of LDLC were 1.0 (referent), 1.12 (0.78–1.62), 1.25 (0.87–1.79), and 2.38 (1.72–3.30; P < 0.0001). Effect estimates were not statistically different in these analyses for comparisons between IL-6, hsCRP, or LDLC, although IL-6 was the strongest predictor of all-cause mortality. The highest absolute risks were observed among those with elevated levels of both cholesterol and inflammation [HR 6.4 (95% CI 2.9–14.1) for those in the top quartiles of baseline IL-6 and LDLC, HR 4.9 (95% CI 2.6–9.4) for those in the top quartiles of baseline hsCRP and LDLC, both P < 0.0001].
Conclusion
Despite aggressive contemporary secondary prevention efforts, the relationships between inflammation, cholesterol, and cardiovascular risk are largely unchanged from those described two decades ago. These data are consistent with the hypothesis that future treatments for atherosclerosis may require a combination of inflammation inhibition and additional cholesterol reduction.

Clinical trial
ClinicalTrials.gov NCT01594333.
Keywords: Interleukin-6, C-reactive protein, Cholesterol, Coronary heart disease, Mortality
See page 2962 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa186)
Introduction
In primary prevention cohorts initiated >30 years ago, the inflammatory biomarkers C-reactive protein (CRP) and interleukin-6 (IL-6) were found to independently predict future cardiovascular events with a magnitude similar to that of low-density lipoprotein cholesterol (LDLC).1–5 Since that time, high-sensitivity C-reactive protein (hsCRP) has emerged as a validated biomarker commonly used in North American clinical practice to measure inflammatory risk in primary prevention and residual inflammatory risk in secondary prevention following statin therapy.6 However, use of hsCRP is limited in European practice.
C-reactive protein itself, however, is a downstream hepatic acute phase reactant unlikely to play a direct role in atherothrombosis. By contrast, IL-6, the primary cytokine driving hepatic CRP production, sits at a crucial juncture in a proven pathway linking inflammation to vascular events.7 , 8 Mendelian randomization studies have found polymorphism in the IL-6 signalling pathway to associate with lifelong hsCRP levels and lifelong vascular risk.9 , 10 The large-scale CANTOS inflammation inhibition trial has shown that the magnitude of anti-cytokine benefit in atherothrombosis relates directly to the magnitude of inflammation reduction achieved.11–13 Interleukin-6 is thus a prominent inflammatory target for future cardiovascular clinical trials.14–17 Very recently, the COLCOT trial has shown that anti-inflammatory therapy with colchicine can also reduce vascular events, a finding of interest as this microtubule inhibitor may also have impact on the NLRP3 inflammasome and hence downstream production of both IL-6 and hsCRP.18
From a diagnostic perspective, as IL-6 is situated directly in a central causal pathway, its measurement in contemporary clinical practice might yield an improved signal to noise ratio compared with hsCRP. Yet, translational evidence comparing the predictive utility of IL-6, hsCRP, and LDLC is sparse and little comparative data are available among secondary prevention patients taking statins, a lipid-lowering therapy with concomitant anti-inflammatory effects.19 Further, whether aggressive adjunctive therapies beyond statins have altered contemporary relationships between inflammation, lipids, and vascular risk is unknown, yet is a critical issue for drug development.
We addressed these issues in a contemporary prospective cohort of 4168 aggressively treated North American participants in the Cardiovascular Inflammation Reduction Trial (CIRT)20 with atherosclerotic disease who had IL-6, hsCRP, and LDLC measured at baseline and were then followed over a period of up to 5 years for major incident cardiovascular events and all-cause mortality.
Methods
The study population derived from participants in the National Heart Lung and Blood sponsored CIRT, a randomized double-blind placebo-controlled evaluation of low-dose methotrexate in the prevention of recurrent cardiovascular events that was conducted exclusively in the U SA and Canada.20
Participants were eligible for CIRT if they had a history of myocardial infarction or multivessel coronary disease and either Type 2 diabetes or the metabolic syndrome. All participants were medically stable and had completed any planned revascularization procedures prior to enrolment. Those with a history of chronic infection, tuberculosis, pulmonary fibrosis, interstitial pneumonitis, hepatic or renal dysfunction, alcohol abuse, or Class IV heart failure were excluded, as were individuals taking oral glucocorticoids or other immunosuppressive agents.
The current analysis includes data from up to 4168 CIRT participants (88 % of the trial) who underwent evaluation of IL-6, hsCRP, and lipid levels at baseline. Levels of hsCRP and LDLC were measured in a commercial central laboratory as part of the main trial procedures. Levels of IL-6 assays were measured after trial conclusion in frozen plasma samples using human high-sensitivity ELISA from R&D Systems ( Minneapolis, MN, USA) in the Department of Laboratory Medicine, Boston Children’s Hospital, the U S reference laboratory for inflammatory biomarkers. In blinded split samples derived from the CIRT biobank, intra-assay, and inter-assay coefficients of variation for all three analytes were <8 % across the reference range.
Participants were followed prospectively over a maximal period of 5 years for incident major adverse cardiovascular events inclusive of nonfatal myocardial infarction, non-fatal stroke, hospitalization for unstable angina requiring urgent revascularization, cardiovascular death, and all-cause mortality. All of these events were adjudicated by endpoints committee unaware of treatment assignment.
Spearmen correlation coefficients were used to address relationships between IL-6, hsCRP, and LDLC. Cox proportional hazard models were used to estimate relative hazards for major adverse cardiovascular events according to increasing quartiles of baseline IL-6, hsCRP, and LDLC. Multivariable models adjusted for age, gender, smoking status, diabetes, blood pressure, and body mass index were evaluated. The multivariate analyses were further stratified by type of index event, time since index event, and presence of diabetes or metabolic syndrome. For IL-6 and hsCRP, we additionally performed analyses adjusting for lipid levels, while for LDLC, we additionally performed models adjusting for inflammatory biomarkers. We tested for trend across quartiles of IL-6, hsCRP, of LDLC by entering a single ordinal term for the quartile in the Cox regression model. In sensitivity analyses, we additionally calculated the risk per standard deviation increase for each biomarker. To evaluate joint inflammatory effects, we repeated our analyses after classifying all study participants into one of four groups on the basis of whether their IL-6 and CRP levels were above or below the respective study medians. To evaluate joint effects between inflammation and lipids, we repeated our analyses after classifying all study participants into 1 of 16 groups on the basis of baseline quartile of both IL-6 and LDLC (or hsCRP and LDLC). Kaplan–Meier survival curves were constructed to visually express relationships between each biomarker and subsequent risk over time. All P-values are two-tailed and all confidence intervals (CIs) computed at the 95% level.
Results
Supplementary material online, Table S1 presents the baseline clinical characteristics of the study population according to increasing quartiles of IL-6, while Supplementary material online, Tables S2 and S3 present similar data for hsCRP and LDLC. While most factors were balanced, the prevalence of obesity, elevated triglycerides, and female gender were all higher among those with elevated levels of IL-6, CRP, and LDLC, whereas the prevalence of diabetes was lower in those with higher LDLC levels.
As would be anticipated, baseline plasma levels of IL-6 and hsCRP were correlated (Spearman rho = 0.47, P < 0.0001). By contrast, there was no significant correlation between IL-6 and LDLC (rho = 0.015, P = 0.33) and only a small correlation between hsCRP and LDLC (rho = 0.18, P < 0.001).
Interleukin-6, high-sensitivity C-reactive protein, and low-density lipoprotein cholesterol as predictors of major adverse cardiovascular events
During the follow-up period, 341 first major adverse cardiovascular events accrued and there were 154 deaths from any cause. With regard to individual endpoints, for which participants could have suffered more than one, there were 186 non-fatal myocardial infarctions, 54 non-fatal strokes, 77 hospitalizations for unstable angina requiring urgent revascularization, and 77 cardiovascular deaths.
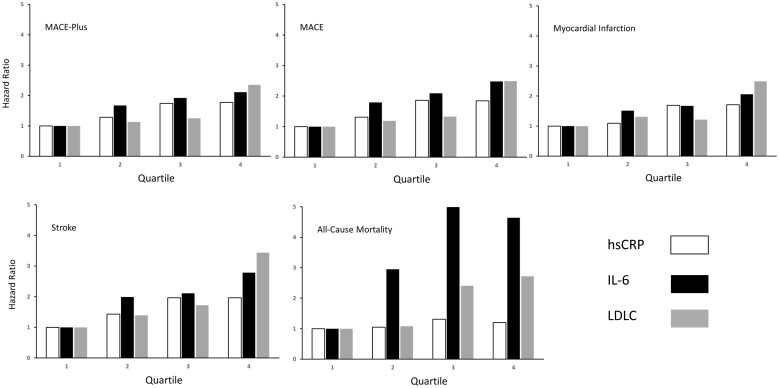
Baseline levels of IL-6, hsCRP, and LDLC were all predictive of future major adverse cardiovascular events (Tables 1– 3). For example, for the CIRT primary endpoint of non-fatal myocardial infarction, non-fatal stroke, hospitalization for unstable angina requiring urgent revascularization, or cardiovascular death [major adverse cardiovascular events (MACE)-plus], multivariable hazard ratios (HR; 95% CI) adjusted for age, gender, smoking status, blood pressure, body mass index, and diabetes or metabolic syndrome for the lowest to highest baseline quartiles of IL-6 were 1.0 (referent), 1.66 (1.18–2.35), 1.92 (1.36–2.70), and 2.11 (1.49–2.99; P < 0.0001), while adjusted HRs for increasing quartiles of hsCRP were 1.0 (referent), 1.28 (0.92–1.79), 1.73 (1.25–2.38), and 1.79 (1.28–2.50; P < 0.0001) and adjusted HRs for increasing quartiles of LDLC were 1.0 (referent), 1.12 (0.78–1.62), 1.25 (0.87–1.79), and 2.38 (1.72–3.30; P < 0.0001) (Tables 1– 3 and Figure 1).
Table 1.
Incidence rates and adjusted hazard ratios for future cardiovascular events and all-cause mortality associated with baseline interleukin-6 levels in the Cardiovascular Inflammation Reduction Trial
| Interleukin-6 (baseline) |
Multivariable-adjusted risk (per quartile) |
|||||
|---|---|---|---|---|---|---|
| IL-6 (pg/mL) | Quartile 1a ≤1.69 | Quartile 2a 1.70–2.50 | Quartile 3a 2.51–3.86 | Quartile 4a ≥3.87 | Excluding lipidsa | Including lipidsb |
| MACE-plus | ||||||
| IR (N) | 2.28 (53) | 4.12 (90) | 4.66 (96) | 5.30 (102) | — | — |
| HR | 1.0 | 1.66 | 1.92 | 2.11 | 1.24 | 1.20 |
| 95% CI | — | 1.18–2.35 | 1.36–2.70 | 1.49–2.99 | 1.12–1.38 | 1.08–1.33 |
| P | — | 0.004 | 0.0002 | <0.0001 | <0.0001 | 0.0008 |
| MACE | ||||||
| IR (N) | 1.70 (40) | 3.29 (73) | 3.78 (79) | 4.62 (90) | — | — |
| HR | 1.0 | 1.72 | 2.01 | 2.35 | 1.28 | 1.23 |
| 95% CI | — | 1.16–2.54 | 1.36–2.96 | 1.59–3.47 | 1.14–1.44 | 1.10–1.38 |
| P | — | 0.007 | 0.0004 | <0.0001 | <0.0001 | 0.0005 |
| Myocardial infarction | ||||||
| IR (N) | 1.31 (31) | 2.15 (48) | 2.33 (49) | 2.95 (58) | — | — |
| HR | 1.0 | 1.49 | 1.67 | 2.08 | 1.25 | 1.20 |
| 95% CI | — | 0.94–2.35 | 1.05–2.64 | 1.32–3.29 | 1.09–1.43 | 1.04–1.38 |
| P | — | 0.09 | 0.03 | 0.002 | 0.002 | 0.01 |
| Stroke | ||||||
| IR (N) | 0.29 (7) | 0.61 (14) | 0.65 (14) | 0.94 (19) | — | — |
| HR | 1.0 | 1.83 | 1.88 | 2.35 | 1.26 | 1.17 |
| 95% CI | — | 0.73–4.57 | 0.75–4.73 | 0.95–5.80 | 0.97–1.63 | 0.90–1.53 |
| P | — | 0.20 | 0.18 | 0.06 | 0.08 | 0.24 |
| All-cause mortality | ||||||
| IR (N) | 0.47 (12) | 1.50 (36) | 2.47 (56) | 2.31 (50) | — | — |
| HR | 1.0 | 2.54 | 4.14 | 3.55 | 1.39 | 1.35 |
| 95% CI | — | 1.31–4.90 | 2.21–7.77 | 1.85–6.78 | 1.19–1.63 | 1.15–1.59 |
| P | — | 0.006 | <0.0001 | 0.0001 | <0.0001 | 0.0002 |
CI, confidence interval; HR, hazard ratio; IR, incidence rate (per 100 person-years); MACE, major adverse cardiovascular events (non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death); MACE-plus, MACE plus hospitalization for unstable angina requiring urgent revascularization.
Adjusted for age, gender, smoking status, body mass index, and blood pressure and stratified on diabetes and or metabolic syndrome.
Additionally adjusted for total cholesterol and high-density lipoprotein cholesterol.
Table 3.
Incidence rates and adjusted hazard ratios for future cardiovascular events and all-cause mortality associated with baseline low-density lipoprotein cholesterol in the Cardiovascular Inflammation Reduction Trial
| LDLC (baseline) |
Multivariable-adjusted risk (per quartile) |
|||||
|---|---|---|---|---|---|---|
| LDLC (mg/dL) | Quartile 1a≤53.9 | Quartile 2a 54.0–68.9 | Quartile 3a 69.0–87.0 | Quartile 4a ≥87.0 | Excluding IL-6 and hsCRPa | Including IL-6 and hsCRPb |
| MACE-plus | ||||||
| IR (N) | 2.82 (53) | 3.12 (66) | 3.30 (72) | 6.06 (124) | — | — |
| HR | 1.0 | 1.12 | 1.25 | 2.38 | 1.35 | 1.33 |
| 95% CI | — | 0.78–1.62 | 0.87–1.79 | 1.72–3.30 | 1.22–1.50 | 1.20–1.48 |
| P | — | 0.53 | 0.22 | <0.0001 | <0.0001 | <0.001 |
| MACE | ||||||
| IR (N) | 2.21 (42) | 2.57 (55) | 2.73 (60) | 5.04 (105) | — | — |
| HR | 1.0 | 1.18 | 1.33 | 2.59 | 1.39 | 1.37 |
| 95% CI | — | 0.79–1.77 | 0.90–1.98 | 1.80–3.72 | 1.24–1.56 | 1.21–1.54 |
| P | — | 0.42 | 0.16 | <0.0001 | <0.0001 | <0.0001 |
| Myocardial infarction | ||||||
| IR (N) | 1.47 (28) | 1.86 (40) | 1.63 (36) | 3.32 (70) | — | — |
| HR | 1.0 | 1.29 | 1.20 | 2.58 | 1.37 | 1.34 |
| 95% CI | — | 0.79–2.09 | 0.73–1.97 | 1.65–4.02 | 1.18–1.57 | 1.16–1.55 |
| P | — | 0.31 | 0.47 | <0.0001 | <0.0001 | <0.0001 |
| Stroke | ||||||
| IR (N) | 0.31 (6) | 0.41 (9) | 0.49 (11) | 0.97 (21) | — | — |
| HR | 1.0 | 1.39 | 1.71 | 3.56 | 1.55 | 1.52 |
| 95% CI | — | 0.49–3.92 | 0.63–4.66 | 1.42–8.92 | 1.17–2.06 | 1.14–2.02 |
| P | — | 0.53 | 0.29 | 0.007 | 0.003 | 0.005 |
| All-cause mortality | ||||||
| IR (N) | 1.57 (32) | 1.53 (35) | 1.55 (37) | 1.96 (46) | — | — |
| HR | 1.0 | 0.98 | 1.05 | 1.49 | 1.15 | 1.14 |
| 95% CI | — | 0.60–1.58 | 0.65–1.70 | 0.94–2.37 | 0.99–1.33 | 0.98–1.33 |
| P | — | 0.92 | 0.83 | 0.09 | 0.07 | 0.08 |
CI, confidence interval; HR, hazard ratio; IR, incidence rate (per 100 person-years); MACE, major adverse cardiovascular events (non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death); MACE-plus, MACE plus hospitalization for unstable angina requiring urgent revascularization.
Adjusted for age, gender, smoking status, body mass index, and blood pressure and stratified on diabetes and or metabolic syndrome.
Additionally adjusted for IL-6 and hsCRP.
Figure 1.
Adjusted hazard ratios for major adverse cardiovascular events-plus, major adverse cardiovascular events, myocardial infarction, stroke, and all-cause mortality according to baseline quartiles of interleukin-6, high-sensitivity C-reactive protein, and low-density lipoprotein cholesterol.
Table 2.
Incidence rates and adjusted hazard ratios for future cardiovascular events and all-cause mortality associated with baseline high-sensitivity C-reactive protein in the Cardiovascular Inflammation Reduction Trial
| hsCRP (baseline) |
Multivariable-adjusted risk (per quartile) |
|||||
|---|---|---|---|---|---|---|
| hsCRP (mg/L) | Quartile 1a ≤0.73 | Quartile 2a 0.74–1.50 | Quartile 3a 1.51–3.41 | Quartile 4a ≥3.42 | Excluding lipidsa | Including lipidsb |
| MACE-plus | ||||||
| IR (N) | 2.82 (63) | 3.58 (77) | 4.88 (100) | 4.90 (101) | — | — |
| HR | 1.0 | 1.28 | 1.73 | 1.79 | 1.22 | 1.11 |
| 95% CI | — | 0.92–1.79 | 1.25–2.38 | 1.28–2.50 | 1.10–1.35 | 1.00–1.24 |
| P | — | 0.15 | 0.0009 | 0.0006 | 0.0001 | 0.05 |
| MACE | ||||||
| IR (N) | 2.21 (50) | 2.89 (63) | 4.10 (85) | 4.02 (84) | — | — |
| HR | 1.0 | 1.31 | 1.83 | 1.88 | 1.24 | 1.12 |
| 95% CI | — | 0.90–1.91 | 1.28–2.61 | 1.30–2.73 | 1.11–1.39 | 1.00–1.26 |
| P | — | 0.15 | 0.0009 | 0.0008 | 0.0002 | 0.05 |
| Myocardial infarction | ||||||
| IR (N) | 1.58 (36) | 1.73 (38) | 2.68 (56) | 2.65 (56) | — | — |
| HR | 1.0 | 1.11 | 1.71 | 1.83 | 1.25 | 1.14 |
| 95% CI | — | 0.70–1.75 | 1.12–2.63 | 1.17–2.85 | 1.09–1.44 | 0.99–1.32 |
| P | — | 0.67 | 0.01 | 0.008 | 0.002 | 0.08 |
| Stroke | ||||||
| IR (N) | 0.39 (9) | 0.54 (12) | 0.75 (16) | 0.78 (17) | — | — |
| HR | 1.0 | 1.40 | 1.84 | 1.85 | 1.22 | 1.04 |
| 95% CI | — | 0.59–3.33 | 0.80–4.23 | 0.79–4.36 | 0.94–1.58 | 0.79–1.36 |
| P | — | 0.45 | 0.15 | 0.16 | 0.13 | 0.79 |
| All-cause mortality | ||||||
| IR (N) | 1.46 (35) | 1.49 (35) | 1.92 (44) | 1.72 (40) | — | — |
| HR | 1.0 | 0.99 | 1.19 | 1.14 | 1.06 | 1.00 |
| 95% CI | — | 0.61–1.58 | 0.76–1.88 | 0.71–1.85 | 0.91–1.24 | 0.85–1.17 |
| P | — | 0.99 | 0.44 | 0.59 | 0.44 | 0.95 |
CI, confidence interval; HR, hazard ratio; IR, incidence rate (per 100 person-years); MACE, major adverse cardiovascular events (non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death); MACE-plus, MACE plus hospitalization for unstable angina requiring urgent revascularization.
Adjusted for age, gender, smoking status, body mass index, and blood pressure and stratified on diabetes and or metabolic syndrome.
Additionally adjusted for total cholesterol and high-density lipoprotein cholesterol.
In analyses assuming a linear effect across quartiles, the multivariable-adjusted hazard associated with each increasing quartile of IL-6 was 24% (95% CI 12–38; P < 0.0001) as compared to an increase of 22% for each increasing quartile of hsCRP (95% CI 10–35; P = 0.0001) and an increase of 35% for each increasing quartile of LDLC (95% CI 22–50 ; P < 0.001).
After additional adjustment for lipids, the effect of IL-6 on residual inflammatory risk appeared to be somewhat greater than that of hsCRP although the difference between biomarkers was not statistically significant; specifically, after further adjustment for total cholesterol and high-density lipoprotein cholesterol, the HR for each increasing quartile of IL-6 increased by 20% (95% CI 8–33 ; P = 0.0008) as compared to an increase of 11% for each increasing quartile of hsCRP (95% CI 0–24; P = 0.05). In parallel, but after additional adjustment for IL-6 and hsCRP rather than for lipid levels, each increasing quartile of LDLC at baseline remained associated with a 35% increase in risk (95% CI 21–50, P < 0.001).
In sensitivity analyses using log-transformed data, the adjusted risks associated with each 1 SD increase in IL-6, hsCRP, and LDLC for the endpoint of MACE-plus were estimated to be 23%, 23%, and 55%, respectively (all P-values < 0.001). By contrast, for the endpoint of all-cause mortality, the magnitude of risk associated with IL-6 was greater in magnitude than that observed for either hsCRP or LDLC (Tables 1– 3 and Figure 1).
Joint effects of interleukin-6, high-sensitivity C-reactive protein, and low-density lipoprotein cholesterol
Despite substantive correlation between IL-6 and hsCRP, individuals with elevated levels of both inflammatory biomarkers had higher cardiovascular event rates; compared to those with below median levels of both IL-6 and hsCRP at baseline (incidence rate for MACE-plus 2.82 per 100 person-years, referent group), those with above median levels of both inflammatory biomarkers at baseline had an approximate doubling of risk (incidence rate for MACE-plus 5.40 per 100 person-years, adjusted HR 1.9, 95% CI 1.4–2.4; P < 0.0001; Figure 2, top). Similarly, compared to those with below median levels of both IL-6 and hsCRP at baseline (incidence rate 0.90 per 100 person-years, referent group), those with above median levels of both inflammatory biomarkers at baseline had a more than doubled risk of all-cause mortality (incidence rate 2.2 per 100 person-years, adjusted HR 2.41, 95% CI 1.56–3.72, P < 0.0001). Comparable findings for the endpoint of MACE-plus for those with IL-6 or hsCRP above or below the study median and LDLC above or below the study median are presented in Figure 2 (middle and bottom).
Figure 2.

Cumulative incidence of major adverse cardiovascular events-plus among individuals with interleukin-6 and high-sensitivity C-reactive protein levels above or below the study median (top); among individuals with interleukin-6 and low-density lipoprotein cholesterol above or below the study median (middle); and among individuals with high-sensitivity C-reactive protein and low-density lipoprotein cholesterol above or below the study median (bottom).
The very highest risks, however, were observed among cohort participants who, despite aggressive prevention therapy, nonetheless had the highest baseline levels of both cholesterol and inflammation. Compared to those with the lowest baseline quartiles of IL-6 and LDLC (incidence rate for MACE-plus 1.39 per 100 person-years, referent group), those with the highest quartiles of both biomarkers had a six-fold increase in risk (incidence rate for MACE-plus 9.49 per 100 person-years, adjusted HR 6.36, 95% CI 2.9–14.1; P < 0.0001; Take home figure, top). Similarly, compared to those with the lowest baseline quartiles of hsCRP and LDLC (incidence rate for MACE-plus 1.68 per 100 person-years, referent group), those with the highest quartiles of both biomarkers had a near five-fold increase in risk (incidence rate for MACE-plus 7.41 per 100 person-years, adjusted HR 4.90, 95% CI 2.6 –9.4; P < 0.0001; Take home figure, middle).
Take home figure.

Incidence rates for major adverse cardiovascular events-plus (per 100 person-years of exposure) according to increasing baseline levels of low-density lipoprotein cholesterol and interleukin-6 measured by quartiles (top); according to increasing baseline levels of low-density lipoprotein cholesterol and high-sensitivity C-reactive protein measured by quartiles (middle), and according to commonly used clinical cut-points for low-density lipoprotein cholesterol (<70, 70–100, and >100 mg/dL) and high-sensitivity C-reactive protein (<1, 1–3, and >3 mg/L) (bottom).
Similar additive effects were observed in analyses using traditional clinical cut-points for LDLC (>100, 100–70, <70 mg/dL) and hsCRP (<1, 1–3, and >3 mg/L) rather than quartiles; compared to those with the LDLC <70 mg/dL and hsCRP <1 mg/L (incidence rate for MACE-plus 2.23 per 100 person-years, referent group), those with LDLC >100 mg/dL and hsCRP >3 mg/L had a more than doubled risk (incidence rate for MACE-plus 7.06 per 100 person-years, HR 2.44, 95% CI 1.6–3.7; P < 0.0001; Take home figure, bottom).
Effect modification and subgroup analyses
We observed no significant effect modification in these data by gender, age, body mass index, blood pressure, or smoking pattern, all issues known to impact upon inflammatory biomarker levels. Observations were similar among those trial participants with a past history of myocardial infarction and among those without prior myocardial infarction but with known multivessel coronary disease, as well as among those with diabetes as compared to those with metabolic syndrome only.
In subgroup analyses limited to 3591 trial participants taking statin therapy at entry, we found similar overall effects for IL-6 and hsCRP. As might be anticipated given that those not taking statins had higher baseline LDLC levels, the effect across quartiles of LDLC was attenuated in this subgroup analysis. However, the magnitude of this attenuation was small and did not impact overall findings. For example, the fully adjusted increase in risk for the endpoint of MACE-plus per quartile of LDLC among those taking statins (33%, 95% CI 18 –49) was similar to that observed in the total cohort (35%, 95% CI 21–50).
Among those taking statin therapy, 17.8% had residual inflammatory risk only (hsCRP ≥ 2 mg/L, LDLC < 70 mg/dL), 25.6% had residual cholesterol risk only (hsCRP < 2 mg/L, LDL ≥ 70 mg/dL), 20.9% had both residual inflammation and residual hyperlipidaemia, and 35.7% had neither residual hyperlipidaemia nor residual inflammation.
As previously reported in the primary CIRT report,20 low-dose methotrexate had no effect on IL-6, hsCRP, or lipids during the trial nor any effect on cardiovascular event rates. In analyses stratified by randomized drug allocation, virtually identical relationships between baseline IL-6, hsCRP, and LDLC and incident cardiovascular events were observed in both treatment groups.
Discussion
In a contemporary cohort of 4168 North American atherosclerosis patients enrolled in the CIRT, we observed that LDL C, the central inflammatory cytokine IL-6, and the downstream inflammatory biomarker hsCRP all continue to predict high cardiovascular risk, despite aggressive contemporary care including statin therapy, angiotensin inhibitors, beta-blockers, antithrombotic therapy, and high rates of coronary revascularization. Moreover, these contemporary data demonstrate that treated atherosclerosis patients who nonetheless have both residual cholesterol risk and residual inflammatory risk have high event rates and thus, in theory, might benefit from further interventions that address hyperlipidaemia and inflammation, the key pathways that drive the development and progression of atherosclerotic cardiovascular disease. In contrast to recent data for canakinumab and colchicine, random allocation in CIRT to low-dose methotrexate neither lowered vascular event rates nor impacted on levels of IL-6, hsCRP, or LDL C over time.
Beyond implications for clinical care, we believe these data have potential importance for future treatment strategies for cardiovascular disease as well as the design of future clinical trials. The linear relationships of LDLC and inflammatory biomarkers with subsequent cardiovascular risk observed here in a contemporary secondary prevention cohort are almost identical in magnitude to those observed in the original reports that compared hsCRP and IL-6 to LDLC using data from primary prevention cohorts initiated >30 years ago.1–5 In those early cohorts, there was virtually no use of lipid-lowering drugs, minimal use of other proven therapies such as angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and all patients were free from revascularization procedures. By contrast, in the current secondary prevention cohort, two-thirds had previously undergone coronary revascularization and the great majority was being aggressively treated with moderate to high-intensity statins, angiotensin inhibitors, beta-blockers, and dual antithrombotic therapies. Yet, despite this aggressive contemporary care, the relationships observed between inflammation, lipids, and vascular risk are largely unchanged on a relative scale in both men and women.
As such, these data support the emerging hypothesis that combination therapies that go beyond statins to include further reductions in both LDLC and innate immune function may be effective for atherothrombosis. In recent trials of PCSK9 inhibition, aggressive lowering of LDLC-reduced vascular risk by 15–20% without concomitant effects on either IL-6 or hsCRP; indeed, following PCSK9 inhibition, on-treatment hsCRP levels remain a powerful predictor of risk.21 , 22 In parallel, in the recent CANTOS trial of canakinumab, aggressive lowering of IL-6 and CRP without concomitant effects on LDLC also lowered vascular risk by 15–20% , and on-treatment levels of LDLC remain a powerful predictor of risk.11–13
As lipids and inflammation adversely interact in multiple processes related to plaque development and rupture, combination therapies targeting both pathways might deliver clinical benefits that are more than additive, and thus in theory could lower relative risks by 40% or more.16 As suggested in the current data, such combination therapies could be targeted to those with high levels of inflammation and LDLC following statin therapy, a group with exceptionally high residual risk for cardiovascular events and all-cause mortality for whom few novel approaches are available or in development. The best method to formally test this hypothesis would be a 2 × 2 factorial trial simultaneously addressing aggressive lipid lowering and inflammation inhibiting therapies, the former targeting LDL production directly and the latter targeting either IL-1, downstream IL-6, or the upstream NLRP3 inflammasome.8 , 16 , 23 Such a trial would also help to resolve ongoing controversies related to the interconnected nature of LDLC and inflammation.24
Our data also have potential implications for patient care. Measurement of inflammatory biomarkers has been endorsed by guideline groups in the U SA, but not in Europe. Yet, as shown here, knowledge of residual inflammatory risk is relevant for prognosis as well as treatment at all levels of LDLC, including after use of high-intensity statins. Beyond pharmacologic interventions, diet, exercise, and smoking cessation all lower vascular inflammation, and thus information on inflammatory levels can be used to motivate lifestyle choices in high-risk individuals. The observation in these data that IL-6 levels appear to be superior to hsCRP for the endpoint of all-cause mortality requires external validation.
While our study evaluates in a contemporary prospective cohort of atherosclerosis patients the association of baseline IL-6, CRP, and LDLC and future vascular risk, it does so in the context of the CIRT where participants had to additionally have either metabolic syndrome or Type 2 diabetes. Further, all study participants were from North America. Thus, generalizability outside North America and to those without insulin resistance may be limited. An additional limitation of our analysis is that systematic evaluation of left ventricular ejection fraction, N-terminal pro-b-type natriuretic peptide, and high-sensitivity troponin are not available.
In sum, despite aggressive contemporary secondary prevention efforts, the relationships between inflammation, cholesterol, and cardiovascular risk are largely unchanged from those described two decades ago. Thus, in addition to clinical issues related to diagnosis and prognosis in the setting of secondary prevention, these contemporary data and those from other recent studies25 , 26 raise the hypothesis that current aggressive therapies for atherosclerosis may be insufficient to address the dual pathways that drive cardiovascular disease progression and residual risk. At the same time, these data, taken in the context of recent successful lipid-lowering and inflammation inhibiting trials, provide optimism that future combination therapies exploiting both pathways could improve patient care.
Funding
This article reports independent research funded by grants to P.M.R. and R.J.G. from the National Heart Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH), Bethesda, MD, USA.
Conflict of interest: P.M.R. has received investigator-initiated research support from the National Heart Lung and Blood Institute (NHLBI), Novartis, Pfizer, Astra-Zeneca, and Kowa; has served as a consultant to Agepha, Novartis, Pfizer, Corvidia, BioCivi, Amgen, Merck, Janssen, and Inflazome; and is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to AstraZeneca and Siemens but are no longer active. A.A.H. is an employee of the NHLBI; opinions, findings, and conclusions expressed in this article are those of the author and do not reflect the views of the NHLBI or the NIH. R.J.G. has received investigator-initiated research support from the NHLBI. The other authors have no conflict of interest to declare.
Supplementary Material
Contributor Information
Paul M Ridker, Division of Preventive Medicine, Center for Cardiovascular Disease Prevention, Brigham and Women’s Hospital, Harvard Medical School, 900 Commonwealth Avenue, Brigham and Women’s Hospital, Boston, MA 02215, USA; Cardiovascular Division, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Jean G MacFadyen, Division of Preventive Medicine, Center for Cardiovascular Disease Prevention, Brigham and Women’s Hospital, Harvard Medical School, 900 Commonwealth Avenue, Brigham and Women’s Hospital, Boston, MA 02215, USA.
Robert J Glynn, Division of Preventive Medicine, Center for Cardiovascular Disease Prevention, Brigham and Women’s Hospital, Harvard Medical School, 900 Commonwealth Avenue, Brigham and Women’s Hospital, Boston, MA 02215, USA.
Gary Bradwin, Department of Laboratory Medicine, Boston Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115, USA.
Ahmed A Hasan, National Heart Lung and Blood Institute, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Nader Rifai, Department of Laboratory Medicine, Boston Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115, USA.
References
- 1. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000;101:1767–1772. [DOI] [PubMed] [Google Scholar]
- 4. Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD, Jorgensen T, Danesh J. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014;35:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol 2016;67:712–723. [DOI] [PubMed] [Google Scholar]
- 7. Libby P, Rocha VZ. All roads lead to IL-6: a central hub of cardiometabolic signaling. Int J Cardiol 2018;259:213–215. [DOI] [PubMed] [Google Scholar]
- 8. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IL6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O’Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O., Danesh J. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012;379:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium, Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, Guo Y, Chung C, Peasey A, Pfister R, Mooijaart SP, Ireland HA, Leusink M, Langenberg C, Li KW, Palmen J, Howard P, Cooper JA, Drenos F, Hardy J, Nalls MA, Li YR, Lowe G, Stewart M, Bielinski SJ, Peto J, Timpson NJ, Gallacher J, Dunlop M, Houlston R, Tomlinson I, Tzoulaki I, Luan J, Boer JM, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Ferrucci L, Bandenelli S, Tanaka T, Meschia JF, Singleton A, Navis GM, Leach I, Bakker SJ, Gansevoort RT, Ford I, Epstein SE, Burnett MS, Devaney JM, Jukema JW, Westendorp RGJ, de Borst G, van der Graaf Y, de Jong PA, Mailand-van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Stephens JW, Eaton CB, Robinson JG, Manson JE, Fowkes FG, Frayling TM, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y, Redline S, Lange LA, Kumari M, Wareham NJ, Verschuren WM, Benjamin EJ, Whittaker JC, Hamsten A, Dudbridge F, Delaney JA, Wong A, Kuh D, Hardy R, Castillo BA, Connolly JJ, van der Harst P, Brunner EJ, Marmot MG, Wassel CL, Humphries SE, Talmud PJ, Kivimaki M, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Hakonarson H, Reiner AP, Keating BJ, Sattar N, Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet 2012;379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 12. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Kastelein J, Koenig W, Genest J, Lorenzatti A, Varigos J, Siostrzonek P, Sinnaeve P, Fonseca F, Nicolau J, Gotcheva N, Yong H, Urina-Triana M, Milicic D, Cifkova R, Vettus R, Anker SD, Manolis AJ, Wyss F, Forster T, Sigurdsson A, Pais P, Fucili A, Ogawa H, Shimokawa H, Veze I, Petrauskiene B, Salvador L, Cornel JH, Klemsdal TO, Medina F, Budaj A, Vida-Simiti L, Kobalava Z, Otasevic P, Pella D, Lainscak M, Seung K-B, Commerford P, Dellborg M, Donath M, Hwang J-J, Kultursay H, Flather M, Ballantyne C, Bilazarian S, Chang W, East C, Forgosh L, Harris B, Ligueros M. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomized controlled trial. Lancet 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 13. Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signaling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 2018;39:3499–3507. [DOI] [PubMed] [Google Scholar]
- 14. Zhao TX, Mallat Z. Targeting the immune system in atherosclerosis. JACC state-of-the art review. J Am Coll Cardiol 2019;73:1691–1706. [DOI] [PubMed] [Google Scholar]
- 15. Lutgens E, Atzler D, Doring Y, Duchene J, Steffens S, Weber C. Immunotherapy for cardiovascular disease. Eur Heart J 2019;40:3937–3946. [DOI] [PubMed] [Google Scholar]
- 16. Ridker PM. Anticytokine agents. Targeting interleukin signaling pathways for the treatment of athererothrombosis. Circ Res 2019;124:437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleveland O, Kunszt G, Bratlie M, Ueland T, Broch K, Holte E, Michelsen AE, Bendz B, Amundsen BH, Espevik T, Aakhus S, Damas JK, Aukrust P, Wiseth R, Gullestad L. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur Heart J 2016;37:2406–2413. [DOI] [PubMed] [Google Scholar]
- 18. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, Lopez-Sendon J, Ostadal P, Koenig W, Angoulvant D, Gregoire JC, Lavoie MA, Dube MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin MC, Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 19. Albert M, Danielson E, Rifai N, Ridker PM; PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 20. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bohula EA, Giugliano RP, Leiter LA, Verma S, Park J-G, Sever PS, Pineda AL, Honarpour N, Wang H, Murphy SA, Keech A, Pedersen TR, Sabatine MS. Inflammatory and cholesterol risk in the FOURIER trial (Further cardiovascular outcomes research with PCSK9 inhibition in patients with elevated risk). Circulation 2018;138:131–140. [DOI] [PubMed] [Google Scholar]
- 22. Pradhan A, Aday AW, Rose LM, Ridker PM. Residual inflammatory risk on treatment with PCSK9 inhibition and statin therapy. Circulation 2018;138:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ridker PM. Clinician’s guide to reducing inflammation to reduce atherothrombotic risk: JACC review topic of the week. J Am Coll Cardiol 2018;72:3320–3331. [DOI] [PubMed] [Google Scholar]
- 24. Tunon J, Badimon L, Bochaton-Piallat ML, Cariou B, Daemen MJ, Egido J, Evans PC, Hoefer IE, Ketelhuth DFJ, Lutgens E, Matter CM, Monaco C, Steffens S, Stroes E, Vindis C, Weber C, Back M. Identifying the anti-inflammatory response to lipid lowering therapy: a position paper from the working group on atherosclerosis and vascular biology of the European society of cardiology. Cardiovasc Res 2019;115:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, Weaver WD, Ostlund O, Wallentin L; STABILITY Investigators. Inflammatory biomarkers interleukin-6 and c-reactive protein and outcomes in stable coronary heart disease: experiences from the stability (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial. J Am Heart Assoc 2017;6:e005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fanola CL, Morrow DA, Cannon CP, Jarolim P, Lukas MA, Bode C, Hochman JS, Goodrich EL, Braunwald E, O'Donoghue ML. Interleukin-6 and the risk of adverse outcomes in patients after an acute coronary syndrome: observations from the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) trial. J Am Heart Assoc 2017;6:e005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.