Abstract
Inflammation is a hallmark and potent driver of pathological vascular remodelling in atherosclerosis. However, current anti-inflammatory therapeutic strategies have shown mixed results. As an alternative perspective on the conundrum of chronic inflammation emerging evidence points towards a small subset of senescent cells as a critical player and central node driving atherosclerosis. Senescent cells belonging to various cell types are a dominant and chronic source of a large array of pro-inflammatory cytokines and various additional plaque destabilizing factors, being involved with various aspects of atherosclerosis pathogenesis. Antagonizing these key agitators of local chronic inflammation and plaque instability may provide a causative and multi-purpose therapeutic strategy to treat atherosclerosis. Anti-senescence treatment options with translational potential are currently in development. However, several questions and challenges remain to be addressed before these novel treatment approaches may enter the clinical setting.
Keywords: Atherosclerosis, Inflammation, Ageing, Cardiovascular disease, Vascular disease
Introduction
Inflammation is a critical driver of atherosclerosis and an independent risk factor for myocardial infarction and cardiovascular death.1 The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) showed that targeting inflammation can provide significant, clinically relevant cardiovascular benefit.2 In another attempt to target inflammation, the Cardiovascular Inflammation Reduction Trial (CIRT)3 yielded negative, but highly educative results. These trials highlighted the potential, but also the limitations of current anti-inflammatory approaches, including immune suppression and the associated risk of sepsis.4 Furthermore, targeting individual cytokines of the pro-inflammatory cascade may not fully address the effects of various other pro-inflammatory elements.5 Strategies to target upstream effectors in the inflammatory signalling cascade may provide a solution for these issues.5
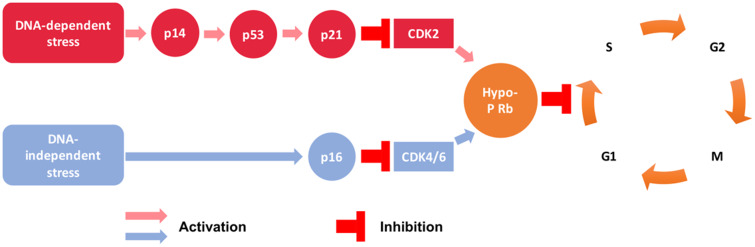
A strong suspect for the central upstream source of inflammatory factors is ageing, being the strongest independent risk factor for cardiovascular disease (CVD).6 On the cellular level, ageing is driven by the accumulation of senescent cells.7 Cellular senescence is defined as an irreversible cell cycle arrest (mechanism outlined in Figure 1)8 and is accompanied by a pro-inflammatory phenotype. The phenotype is referred to as the senescence-associated secretory phenotype (SASP), producing pro-inflammatory cytokines [interleukin (IL)-1α, IL-1β, IL-6, IL-8, IL-18, CCL-2, tumour necrosis factor α (TNF-α)], metalloproteinases (MMP-1, -2, -3, -7, -8, -9, -10, -12, -13, and -14), and other factors.9,10 Emerging experimental evidence indicates that, in contrast to an acute inflammatory response, the SASP is a protracted and chronic source of inflammatory and other plaque-destabilizing factors,9,10 gradually contributing to atherosclerosis pathogenesis.
Figure 1.
Cell cycle arrest mechanisms in senescence. CDK, cyclin-dependent kinase; hypo-p-Rb, hypo-phosphorylated retinoblastoma protein. G1, S, G2, and M are cell cycle phases; p16, p53, and p21 are cell cycle inhibitors.
Intriguingly, cellular senescence and atherosclerosis share multiple causative stimuli1,11,12: oxidized and electronegative low-density lipoprotein (LDL),13,14 inflammation,15 reactive oxygen species,8 cigarette smoke,16 hypertension,17 hyperglycaemia,18 cytomegalovirus (CMV) infection,19 telomere attrition,20 mitochondrial failure,21 dysfunctional autophagy,22 and genomic damage.8 Senescent cells are more than simple bystanders, as multiple molecules involved in the SASP act as promoters of atherosclerosis and are biomarkers of CVD risk.23 Furthermore, effects of experimental overexpression of many molecules drives both atherosclerosis and senescence. On the other hand, antagonizing cellular senescence and ageing processes has anti-atherosclerotic effects. These overlapping features of senescence and atherosclerosis are shown in Table 1. For a better understanding of the cell-specific roles of these molecules refer to Figure 2, which explains expression patterns on a cellular level of senescence and their roles in atherogenesis.
Table 1.
Clinical and experimental expression of molecules in atherosclerosis and senescence
| Molecules | Effect on CVD risk | Over-expression effect on atherosclerosis | Over-expression effect on senescence | Down-regulation effect on atherosclerosis | Down-regulation effect on senescence | References |
|---|---|---|---|---|---|---|
| Ang II |

|

|

|

|

|
24–26 |
| AMPK |

|

|

|

|

|
27–29 |
| CCL2 |

|

|

|

|

|
30–32 |
| FOXO3 |

|

|

|

|

|
33–35 |
| GATA4 |

|

|

|

|

|
36–38 |
| HMGB1 |

|

|

|

|

|
39–41 |
| HSP90 |

|

|

|

|

|
42–44 |
| IL1α/β |

|

|

|

|

|
1 , 30 , 45 |
| IL6 |

|

|

|

|

|
1 , 30 , 45 |
| IL18 |

|

|

|

|

|
46–48 |
| MMP9 |

|

|

|

|

|
45 , 48–50 |
| MMP12 |

|

|

|

|

|
51–53 |
| mTOR |

|

|

|

|

|
27 , 54 |
| NF-κB |

|

|

|


|

|
55 , 56 |
| NLRP3 |

|

|

|

|

|
57–60 |
| NR3C2 |

|

|

|

|

|
25 , 61 |
| OPG |

|

|

|

|

|
62–65 |
| PI3K |

|

|

|

|

|
27 , 54 , 66 , 67 |
| p66Shc |

|

|

|

|

|
68–71 |
| SIRT1 |

|

|

|

|

|
27 , 72–74 |
| STAT3 |

|

|

|

|

|
75 , 76 |
| TRAF6 |

|

|

|

|

|
77–79 |
| TRF2 |

|

|

|

|

|
80 |
 , promoting effect;
, promoting effect;  , inhibitory effect;
, inhibitory effect;  , unknown/unclear evidence; Ang II, angiotensin II; AMPK, AMP-activated protein kinase; CVD, cardiovascular risk; FOXO, forkhead box class O; HMGB1, high-mobility group box 1; IL, interleukin; MMP, matrix metalloproteinase; mTOR, mechanistic target of rapamycin; NF-κB, nuclear factor kappa B; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; NR3C-2, mineralocorticoid receptor; PI3K, phosphoinositide 3-kinase; OPG, osteoprotegerin; SIRT, sirtuin; STAT3, signal transducer and activator of transcription 3; TRAF6, tumour necrosis factor receptor-associated factor 6; TRF2, telomeric repeat-binding factor 2.
, unknown/unclear evidence; Ang II, angiotensin II; AMPK, AMP-activated protein kinase; CVD, cardiovascular risk; FOXO, forkhead box class O; HMGB1, high-mobility group box 1; IL, interleukin; MMP, matrix metalloproteinase; mTOR, mechanistic target of rapamycin; NF-κB, nuclear factor kappa B; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; NR3C-2, mineralocorticoid receptor; PI3K, phosphoinositide 3-kinase; OPG, osteoprotegerin; SIRT, sirtuin; STAT3, signal transducer and activator of transcription 3; TRAF6, tumour necrosis factor receptor-associated factor 6; TRF2, telomeric repeat-binding factor 2.
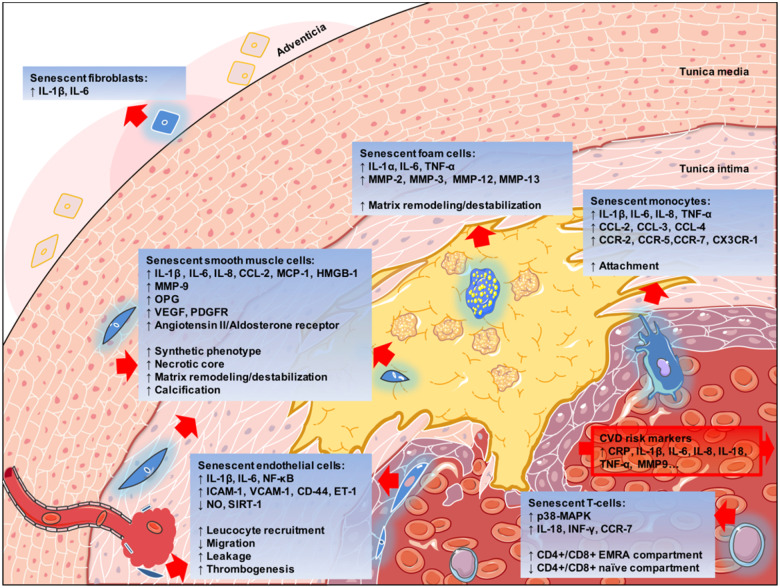
Figure 2.
Roles of different senescent cell types in atherosclerosis, represented in blue. CRP, C-reactive protein; CVD, cardiovascular disease; EC, endothelial cell; ET-1, endothelin-1; ICAM, intercellular adhesion molecule; IL, interleukin; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NO, nitric oxide; NF-κB, nuclear factor κB; TNF-α, tumour necrosis factor α; VCAM, vascular cell adhesion molecule; VSMC, vascular smooth muscle cell.
These observations suggest that senescent cells are an important source of local, chronic, low-grade inflammation and plaque destabilization, and thus, a promising upstream therapeutic target. Targeting of senescent cells and ageing processes therefore holds unexplored potential to ameliorate atherosclerosis as a causal approach to decrease inflammation and provide plaque stabilization, possibly without overt immunosuppression.
In this review, we present the current experimental knowledge close ties of senescence with many aspects of atherosclerosis pathophysiology. Further on, we present clinical evidence connecting cellular senescence and atherosclerosis. We elaborate on how targeting senescence can be utilized to develop novel therapeutic strategies for atherosclerosis. Finally, we discuss the challenges and unmet needs that remain to be addressed before anti-senescence therapies can be introduced in the clinical setting.
Evidence on the cellular level of the contribution of senescence to atherosclerosis
Cellular senescence evolves with ageing and in sites of disease7 in response to sub-lethal exposure to various stimuli,81 which are outlined in the Introduction section. Senescent cells, within their physiological roles, facilitate tissue repair in response to acute damage,82 but are promptly cleared by the innate immune system after injury resolution.83 However, some senescent cells remain and gradually accumulate in tissues.84 These persisting senescent cells have been recently tied to multiple chronic diseases other than atherosclerosis, including diabetes mellitus,85 obesity,85 Alzheimer’s disease,86 heart failure,10,87 idiopathic pulmonary fibrosis,88,89 chronic obstructive pulmonary disease,90 chronic kidney disease,91 cancer,92 and osteoarthritis.93 It is thus not surprising that senescent cell burden is a good predictor of animal lifespan.94,95
Cellular senescence is observed in early stages of atherosclerosis,9 affecting different cell types in the arterial wall that have distinct roles in the pathogenesis of this disease: endothelial cells (ECs),96–98 vascular smooth muscle cells (VSMCs),45,99–101 macrophages, foam cells,9,102 monocytes,103 fibroblasts,104–106 and T cells.107,108
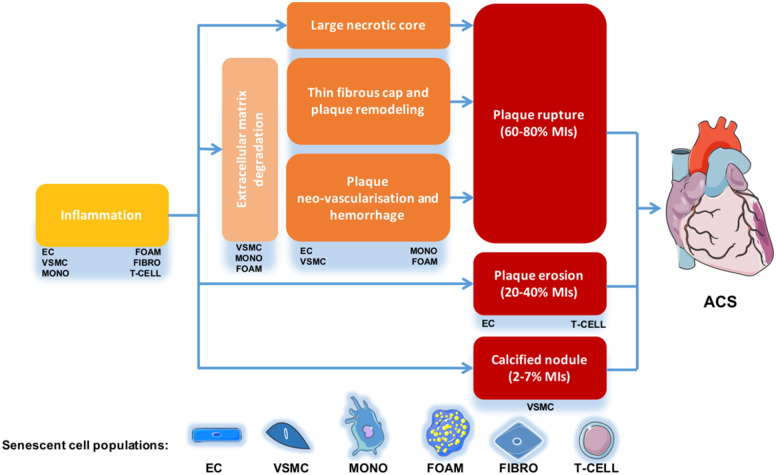
Originating from these different cell types, senescent cells directly facilitate multiple key pathophysiological processes in atherosclerosis, which are displayed in Figure 2 (necrotic core enlargement, extracellular matrix degeneration and cap thinning, erosion, calcification, intra-plaque angiogenesis).109Take home figure details the complex mechanisms leading to plaque destabilizing outcomes. Take home figure also exemplifies that some features of cellular senescence are shared by different senescent cell types, while some are cell population specific.
Take home figure.
Senescent cell contributions to the mechanisms of atherosclerotic plaque rupture.
All senescent cells contribute to plaque inflammation. Consistent SASP elements in the majority of senescent cells are IL-1α, IL-1β, IL-6, IL-8, IL-18, and TNF-α,15,105,110 all of which are clinically validated CVD risk factors.1,5 These inflammatory cytokines promote senescence locally in a paracrine manner30 and perhaps, at a systemic level.111 Other features depend on the cell type.
Endothelial cell senescence directly compromised the endothelial barrier through disruption of cellular proliferation, permeability, and motility,112–114 possibly contributing to endothelial erosion and intra-plaque haemorrhage. As seen in a retinopathy model, the overall effect of the SASP leads to pathological neo-angiogenesis.115
Vascular smooth muscle cell senescence was associated with necrotic core enlargement80 and plaque calcification62 in human atherosclerosis. Senescent VSMCs acquire an osteoblastic secretory phenotype and activate several osteogenic pathways [e.g. Runt-related transcription factor 2 (RUNX-2), bone morphogenetic protein 2 (BMP-2), alkaline phosphatase (ALP), osteopontin (OPN), and osteoprotegerin (OPG)], thus contributing to plaque calcification.62 OPG, a soluble factor and key element of the SASP,63 is a CVD risk factor.64 Plaque destabilization through cap thinning is promoted by various MMPs,49 such as MMP-1, -2, -3, -7, -8, -9, -10, -12, -13, and -14,45,49,51 which are secreted as part of the SASP of senescent VSMCs, monocytes, macrophages, and foam cells.
Furthermore, CD4+, CD28-null, CD45 re-expressing senescent T-cells (also known as CD4+ T-EMRA cells) were found to be highly active subpopulations, expressing increased amounts of TNF-α, INF-γ, CXCR-3 (C-X-C chemokine receptor), CCR-6, CCR-7 (C-C motif chemokine receptor 6 and 7), and the anti-apoptotic Bcl-2 family proteins.19,116 In addition to the pro-inflammatory phenotype, T-EMRA cells showed atypical cytotoxic activity towards the plaque endothelium,117 likely contributing to plaque erosion.
Together, these findings indicate that different types of senescent cells in the vasculature exhibit features that are considered highly unfavourable in the setting of atherosclerosis.
Clinical evidence linking ageing and senescence processes to atherosclerosis
Ageing is well-documented as a risk factor for CVD118 and age is considered in various cardiovascular risk scoring systems (Framingham, Reynolds, PROCAM, ESC, and Diamond Forrester). In addition, ageing-associated morphological and haemodynamical arterial disturbances are known.119,120 The majority of individuals older than 70 years of age (57.1% male and 68.7% female) have atherosclerotic lesions.121 The trend progressively worsens, as 87.4% of male and 88.9% of female patients older than 85 were affected by subclinical or clinical forms of vascular disease.122 However, ageing has a strong residual effect that cannot be simply explained by longer exposure to classical risk factors. Only 11.9% and 40.3% of the effect of age can be attributed to longer exposure to risk factors in men and women, respectively.123
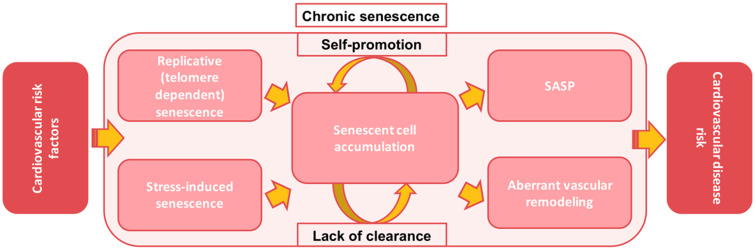
Cellular senescence may account for a part of this void, acting a hub between various cardiovascular risk factors. As depicted in Figure 3, cellular senescence can be a consequence of two processes which may run in parallel and are intertwined. The first is replicative exhaustion resulting from chronological age or intense proliferation (telomere dependent)20,124,125 and the latter is exposure to cardiovascular risk factors (stress-induced).13–18 Therefore, cellular senescence burden is a phenomenon only partly correlated with age from birth; it is rather a consequence of a combined effect of chronological ageing and risk factor exposure. Being a shared consequence of the effect of all of these various factors, senescence is an important upstream effector that promotes atherogenesis.
Figure 3.
Putative model of the role of cellular senescence in inflammation-associated cardiovascular disease risk.
In clinical practice, the capability of cellular senescence to drive atherosclerosis independently of chronological ageing and classical risk factor exposure is seen in drastic examples of human progeria syndromes, such as Hutchinson–Gilford progeria (HGS), Werner syndrome, and other laminopathies. Patients with these syndromes are characterized by a strong accumulation of senescent cells early in life and suffer from strikingly increased CVD risk due to a large atherosclerotic plaque burden.101,126,127 Patients with HGS suffer from myocardial infarction or stroke in the absence of classical risk factors at the mean age of 13 years.128
Furthermore, cancer therapies that induce cellular senescence, such as cisplatin, doxorubicin and radiation,129 accelerate atherosclerosis.130
Additional clinical evidence of the involvement of cellular senescence in the atherosclerotic vessel wall in the general population comes from post-mortem histological analysis,96,131–134 that showed that senescent EC and VSMC accumulate substantially more in atherosclerotic than in physiologically aged healthy arteries.96,131–134 Expression of senescence marker p16 INK4a in the diseased human coronary arteries positively coincided with unstable plaques and correlated with intra-plaque TNFα levels.135 Furthermore, coronary vessels from ischaemic heart disease patients showed significant endothelial senescent cell burden, while the mostly plaque-free internal mammary arteries from the same donors had no evidence of senescence.96 In human carotid artery atherosclerosis, senescent VSMCs were associated with phenotypical features of plaque instability80,134,136,137 and accounted for 18% of all plaque cells.134 Senescent T-EMRA cells were also found inside unstable plaques.117 Additional studies with detailed quantification of senescent cells, plaque features, and correlation to clinical data are necessary to strengthen the causal link between arterial wall cellular senescence and atherosclerosis.
As specific and safe imaging methods to evaluate senescent cell burden in the arteries of patients still do not exist, tracking senescent cell populations in the blood appears to be the best option to evaluate the contribution of cellular senescence to CVD risk.
Senescence of blood–borne immune cells has been tied to CVD risk. Bulk analysis of leucocyte populations showed that short telomeres predict atherosclerosis development and CVD.124,125 The ageing pro-oxidative marker p66Shc68 is overexpressed in leucocytes from patients with acute coronary syndromes, but not stable coronary artery disease.69 As these results from bulk leucocytes may be affected by telomere length and expression profiles of short-lived myeloid cells, the stable lymphoid populations attracted significant attention, specifically senescent T-EMRA cells. Accumulation of T-EMRA was associated with increased acute mortality and re-occurrence of MI, particularly among diabetic patients.19 Increased numbers of these cells were found in hypertension and rheumatoid arthritis, predicting worsened CV outcomes within these high-risk conditions.19 Both CD4+ and CD8+ T-EMRA cells are strong independent predictors of cardiovascular mortality in the elderly.107 Cytomegalovirus infection activated senescence mostly in CD4+ EMRA cells,19,107 providing an interesting connection between CMV infection, CD4+ EMRA senescence, and atherosclerosis.
In line herewith, the role of age-dependent accumulation of pro-inflammatory lymphocytes with somatic mutations (clonal haematopoiesis of indeterminate potential), mainly in TET2 and DNMT3a, is emerging.138 Senescence is associated wide perturbations in DNA methylation.139 Given its critical functions in this process, it is possible that DMNT3a plays a role in the immuno-senescence programme. Silencing of DMNT3a reportedly activated senescence in cancer and skin experimental models.140,141 However, it is unclear whether these mutations are involved in haematopoietic cellular senescence or SASP activation.
In summary, chronic senescent cells accumulate over time as a result of repeated tissue damage. Through the SASP, cellular senescence exerts many pro-atherogenic effects and it possibly is a key aetiologic driver of aberrant vascular remodelling, forming a perpetual loop that chronically amplifies the effects of risk factor exposure (Figure 3). Therefore, senescence appears to be a therapeutic target worth exploring for the prevention or treatment of atherosclerosis.
Treatment options for targeting senescence
Multiple clinically approved treatment strategies for CVD have an anti-senescent effect upon closer analysis. Aldosterone and Angiotensin II drove VSMC senescence independently and synergistically, this being prevented by mineralocorticoid receptor and angiotensin II receptor blockers.24,25 Pioglitazone ameliorated endothelial cell senescence by telomerase activation.142 Statins were shown to delay endothelial and T-cell senescence, through p38-mediated SASP inhibition, telomerase and cell cycle regulation.143 Metformin has anti-senescent and anti-atherosclerotic effects through SIRT-1 agonism.27 Rivaroxaban attenuates VSMC senescence, by inhibiting the signalling cascade between Factor Xa and Insulin-like growth factor-binding protein 5.137 The cardiovascular benefits of exercise in adulthood can be explained by a decrease of immuno-senescence.144
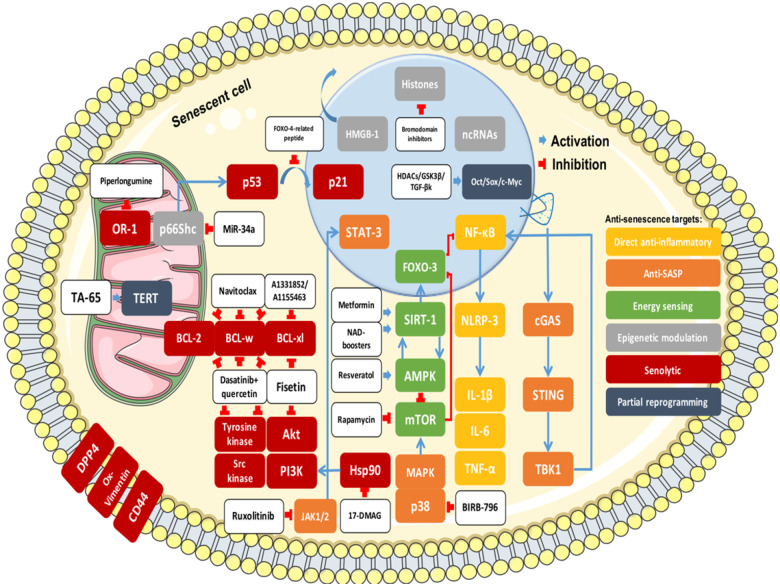
In a direct senescence-specific approach, several inflammatory and energy-sensing pathways and complex epigenetic modulators provide valid entry points to prevent, stabilize, or reverse atherosclerosis. Given the status quo of the clinical application of genome-based therapies in the CVD field, pharmacological approaches are discussed in detail below because of their immediate translational potential. Figure 4 provides an overview of the known molecular therapeutic targets in senescence and divides them into six most promising therapeutic strategies: direct anti-inflammatory, anti-SASP, energy sensing, epigenetic modulation, senolytics, and reprogramming. These approaches and the complex relationships between various molecular targets are explained in further detail below.
Figure 4.
Anti-senescence strategies. Akt, protein kinase B; AMPK, AMP-activated protein kinase; cGAS, cyclic GMP-AMP synthase; DPP4, dipeptidyl peptidase 4; FOXO, Forkhead box class O; HMGB-1, high-mobility group box 1; IL, interleukin; MAPK, mitogen-activated protein kinase; mTOR, mechanistic target of rapamycin; NF-κB, nuclear factor kappa B; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; ox-vimentin, oxidized vimentin; PI3K, phosphoinositide 3-kinase; SASP, senescence-associated secretory phenotype; SIRT, sirtuin; STING, stimulator of interferon genes; TBK, serine/threonine-protein kinase TBK1; TNFα, tumour necrosis factor α.
Targeting intracellular signalling molecules at the crossroads of inflammation and senescence
The idea of antagonizing pro-inflammatory molecules upstream of the IL-1-IL-6/TNFα-CRP cascade or other immune regulators145 is viable, not only for a direct anti-inflammatory effect but also to ameliorate senescence. Inhibition of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 inflammasome (NLRP3) suppressed T-cell senescence57 and atherosclerosis.58 Even more upstream, inhibition of nuclear factor κB (NF-κB) abrogated p53-mediated senescence.55 However, while inhibiting NF-κB in ECs was beneficial in atherosclerosis, in macrophages, this had a pro-atherogenic effect.56 Immunosuppression and the inhibition of cellular debris traffic are strong reasons not to target these and other major inflammatory pathways.
A related concept is the inhibition of the SASP. Recent data suggest DNA leakage from the senescent nucleus into the cytoplasm and consequent activation of the GMP-AMP Synthase-Stimulator of Interferon Genes (cGAS-STING) anti-viral pathway as a mechanism of SASP onset.146,147 This pathway is an attractive target to antagonize the SASP, but may come at the cost of lowering anti-viral defences. Alternatively, it is possible to modulate individual components of the SASP by inhibiting Janus kinase (JAK) 1/2148 and JAK2/signal transducer and activator of transcription 3 (STAT3).149 TNF receptor-associated factor 6 (TRAF6) inhibition in macrophages is another anti-inflammatory strategy to counter atherosclerosis development.77 The ATM-TRAF6-TAK1 axis is a driver of the SASP,78 and an Serine-protein kinase ATM inhibitor was found to have an anti-senescence effect.79 p38 Mitogen-activated protein kinase (MAPK) inhibition has been suggested as an anti-SASP strategy.150 Independently of the senescence context, blockade of this pathway failed to improve cardiovascular outcomes 12 h to 2 weeks post-myocardial infarction.151 However, anti-SASP strategies may not be effective in acute or subacute disease (see Future directions section).
Energy-sensing pathways, like those regulated by mammalian target of rapamycin (mTOR), sirtuin (SIRT)-1, Forkhead box class O 3 (FOXO3), and AMP-activated protein kinase (AMPK), have been shown to be largely responsible for the anti-ageing benefits of caloric restriction.27 Inhibition of mTOR complex 1 by rapamycin prolonged the healthy lifespan of mice and exerted anti-senescence and anti-atherosclerotic effects.152,153 However, mTOR inhibition can cause muscular atrophy.154 The SIRT-1 agonist resveratrol ameliorated ageing pathology, in part by activating FOXO3.33,155 Clinical application of resveratrol and related SIRT agonists is challenging due to stability and bioavailability issues.156 Direct FOXO3 agonism is pharmacologically possible.33 Importantly, more than 12.5-fold overexpression of FOXO3 has been shown to be harmful, possibly because of nicotinamide adenine dinucleotide (NAD) depletion155; thus, a careful dose-finding study is imperative. Recently, fine-tuning of FOXO3 levels has been achieved via a miRNA-132 antagonistic approach,157 that is currently clinically tested (NCT04045405). NADP boosters (nicotinamide mononucleotide,158 nicotinamide riboside,159 P7C3,160 apigenin161) can be used resolve the NAD depletion issue and are able to prevent senescence in ECs158 and VSMCs.159 The small scale Metformin in Longevity Study (MILES, NCT02432287) trial studied AMPK activation for anti-ageing purposes and the results are currently analysed. A larger scale study with a similar design is planned [Targeting Aging with Metformin (TAME) trial].
Epigenetic manipulation through long non-coding RNAs, miRNA modulators, and other oligonucleotides has yielded promising outcomes in experimental settings.162–165 Some of these molecules are currently clinically evaluated in CVD.166 Epigenetic modification through histone modification via bromodomain inhibitors could suppress atherosclerosis,167 senescence,168 and the SASP,169 indicating high potency for such interventions. The non-histone DNA-binding protein high mobility group protein B1 (HMGB1) is a major pro-inflammatory SASP factor, when externalized from the nucleus into the extracellular environment.39 Soluble HMGB1 promoted atherosclerosis40,41 and CVD risk.170 It remains to be seen if HMGB1 nuclear retention ameliorates the epigenetic landscape of senescent cells and provides benefits in atherosclerosis. Another promising strategy is the epigenetic blockade of p66Shc, since pharmacological inhibitors are not currently available. p66Shc is a promoter of oxidative stress70 and is highly expressed in senescent cells68 and atherosclerosis.171 Inhibiting p66Shc its key regulator MiR-34a is promising,172 as p66Shc knock-out led to senescence prevention,68 30% animal lifespan extension,173 and strong inhibition of atherogenesis.70,71
Targeting senescent cells as the root of inflammation: senolytic approaches or cellular reprogramming
Senolytic approaches
Safe removal of chronic senescent cells is an attractive approach to blocking ageing pathology and atherosclerosis. It is possible to specifically delete senescent cells through activation of apoptosis by compounds that selectively target senescent cells, called senolytics. INK-ATTACK transgenic mice contain an inducible suicide gene in the CDKNA2 locus, which encodes p16 INK4a, a key molecule in senescent cells. This elegant transgenic model has been used to selectively eliminate senescent cells in vivo, leading to reversal of age-related characteristics without noticeable side effects.94,95 The positive effect of senescent cell removal in atherosclerosis in LDL receptor-knockout mice has been replicated in INK-ATTACK, p16-3MR, and INK-nitroreductase mouse models using the senolytic navitoclax (ABT-263).9 Senescent cell elimination diminished plaque size, inflammation, and instability features.9 These data provide a rational basis for the use of senolytics in the treatment of atherosclerosis.
Recently, multiple senolytic compounds have been developed and have been tested for the treatment of CVD (Figure 4).42,174–185 Navitoclax, a Bcl-2 inhibitor, has beneficial effects in atherosclerosis,9 and may be a good candidate for the targeting of T-EMRA cells, which overexpress Bcl-2 family proteins.116 Combination therapy with dasatinib and quercetin (D+Q) removed senescent cells from the tunica media, but not the intima, improving vascular function parameters, but not arterial compliance and plaque burden.33 Senolytic drugs were known to be more effective against particular senescent cell types in vitro, and less effective against others, for unclear reasons.42,174–185 It is otherwise unknown how the cell type specificity of senolytics affects cells of the arterial wall, but it may explain why D+Q removed senescent cells only within the tunica media. Further studies are needed to corroborate the senolytic effect within plaque.
Another option for using senolytics may with the goal of improving outcomes of autologous transplantation therapies of bone marrow-derived angiogenic cells in ischaemic heart failure. In ischaemic heart failure patients, these cells are severely impaired186,187 and display markers of senescence,188,189 disabling their use for patient-derived cellular therapy. Senolytics may be used to remove senescent cells, either in vivo or ex vivo, leading to a rejuvenated population and more effective therapeutic outcomes.186,189
In the context of drug repurposing, some senolytic compounds are already clinically approved (dasatinib) or in clinical trials (navitoclax, HSP90 inhibitors) for indications in oncology. The results of the first human trial of dasatinib and quercetin in idiopathic pulmonary fibrosis (IPF) have been recently published.88 This small scale single-arm study reported improvements of 6-min walk distance, 4 m gait speed, chair-stands, and Short Physical Performance Battery (SPPB) score, showing the promise of this therapy for IPF. An ongoing trial is evaluating dasatinib and quercetin for their senolytic ability in chronic kidney disease (NCT02848131).
Currently available senolytics drugs are limited by adverse effects. When used for cancer treatment, these drugs often exhibit adverse effects such as nausea, vomiting diarrhoea, and skin rashes; notably, Bcl-xl inhibitors induce severe thrombocytopenia.190 To avoid these effects, localized delivery via percutaneous intervention may be required to target senescence in a CVD setting. This could be combined with senescent cell-specific delivery methods, such as the galactose-encapsulation of drugs that reduced thrombocytopenia after navitoclax application in an experimental setting.191 Alternatively, the drugs could be applied periodically, with long off-drug periods.175
Cellular reprogramming
The induction or reprogramming of mesenchymal cells into a pluripotent state is possible via octamer-binding transcription factor (Oct)3/4, Sox2, c-Myc, and Krüppel-like factor (KLF4).192 Cells can potentially be reverted to a non-senescent state, reversing their cellular biological clock. Short-term cyclic expression of Oct4, Sox2, KLF4, and c-Myc in LAKI 4F mice suppressed the ageing phenotype, prevented VSMC degeneration, and reversed bradycardia without observed tumorigenesis.193 These results suggest an intriguing possibility that low-dose short-term reprogramming may be beneficial for atherosclerosis. A drug cocktail of HDACs, GSK-3β, and TGF-β kinase enabled cell reprogramming in vitro, thus improving liver regeneration and function after acute injuries in mice.194 The authors attributed this effect to the up-regulation of pluripotency genes. Extensive reprogramming may be tumourigenic, as it leads to up-regulation of strong oncogenes, such as c-Myc.195 Extremely precise and reliable control of this anti-senescence strategy is an absolute must to avoid malignant transformation. Of note, senescent cells from elderly people could be reverted to a pluripotent state only by the additional over-expression of NANOG and LIN2 in addition to Oct3/4, Sox2, c-Myc, and KLF4.196
Another intriguing strategy is direct reprogramming or trans-differentiation, which enables a phenotypic change from one differentiated cell type into another without achieving pluripotency.197 Hypothetically, senescent secretory VSMCs could thereby regain a contractile phenotype.
Future directions towards clinical application of anti-senescence strategies
Research regarding ageing mechanisms and cellular senescence is confronted with pre-clinical and clinical challenges that need to be addressed in order to pinpoint the role of cellular senescence in atherosclerosis and define optimal therapeutic strategies. Firstly, in pre-clinical research, the detection of senescent cells is hampered by the lack of specific markers. In vitro, the gold standard senescence-associated β-galactosidase staining and p16 INK4a expression detection can yield false-positive results in macrophages.198In vivo, the best currently available models for senescent cell detection are reporter-harbouring transgenic mice (p16-LUC, p16 tdTom)199,200 and transgenic mouse strains used for selective senescent cell removal (INK-ATTAC, p16-3MR).201
Secondly, a better understanding of the role of cellular senescence in vascular disease development is needed. Studies in cell cycle inhibitor knock-out models have raised questions about the complex relationships between senescence, proliferation, and apoptosis in different phases of atherosclerosis. p16 INK4a, p14 ARF (p19 ARF in mice), p21, and p53 enable cell cycle arrest in senescence. However, polymorphisms that hinder their activity promote atherosclerosis in humans.202 Knock-out of p16 INK4a, p19 ARF, p21, and p53 had a pro-atherosclerotic effect in mice.203–205 On the other hand, cellular senescence promotes atherosclerosis in each developmental phase.9,101 Selective removal of p16 INK4a-positive senescent cells prevented atherosclerosis, while stabilizing plaques and decreasing plaque inflammation.9 This discrepancy can be explained by other critical roles of cell cycle inhibitors in cell physiology and disease development, all of which are afflicted by the abovementioned genetic polymorphisms and knock-outs in a nondiscriminatory way.
Thirdly, special care must be taken in selecting patients for possible anti-senescence clinical trials. Experimental evidence shows that cellular senescence has roles in limiting myocardial206 and liver207 fibrosis, promoting skin healing,82 as well as in embryonal development,208 which have to be considered. However, these studies focused on the function of senescence in acute organ injury, not in chronic processes such as atherosclerosis. The aim of anti-senescent therapies would be to clear chronic senescent cells or suppress their noxious effects, while not permanently abrogating the senescence programme. Nevertheless, these findings may imply that initial anti-senescence/anti-SASP therapeutic trials need to be applied to patients with a clinically stable phase of vascular disease, with high residual CVD risk,2 and not in a setting of acute injury. Another concern would be that inducing senescence in the short term initially slows cancer growth due to the cell cycle arrest induction,81 which raises questions about oncogenesis as an adverse effect. However, chronic senescence promotes cancer.92 These issues remain to be carefully addressed and analysed in future studies exploring anti-senescence strategies.
To track the effect of anti-senescence therapies in a clinical setting, it would be important to specifically detect total or localized senescent cell burden.7 Using a matrix-biomarker approach of 48 SASP-associated cytokines and miRNAs88 seemed not to be an effective strategy in evaluating the effects of senolytic therapy in IPF. The possible explanation is that immune cells can also produce these factors, making senescence-specific detection problematic. An alternative to the matrix approach may be the discovery of senescence-specific soluble antigens, such as oxidized vimentin.209
Anti-senescence approaches may be a crucial component of precision medicine therapy. The development and decreasing cost of –omics methods or imaging can lead to a clinically applicable biomarker set, which combines senescence-associated soluble biomarkers and cellular compartments to identify patients with a high senescence burden, and thus the best candidates for anti-senescence therapies. For example, patients with a high burden of Bcl-2 overexpressing senescent T-EMRA cells may be good candidates for therapy with a Bcl-2-targeting senolytic, navitoclax. On the other hand, if there is a dominance of endothelial senescence, SASP inhibitors may be more beneficial than senolytics to avoid endothelial erosion. Decision-making can be further tailored with individual genetic profiling. Speculatively, a patient with a down-regulating rs2802292 FOXO3 T; T polymorphism210 may benefit more from FOXO/SIRT-1 agonists and NAD boosters than from other forms of anti-senescence therapy. Ultimately, developing next-generation biomarkers and imaging methods seems essential to translate anti-senescence therapeutics to the clinic.
Conclusions
Current therapeutic strategies targeting known risk factors for CVD, such as hypertension, diabetes mellitus, and cholesterol levels lower CVD risk. However, the disease risk remains high and will increase in the ageing western population. Targeting specific upstream inflammatory processes involved in deleterious local plaque pathophysiology has emerged as a novel approach to limit atherosclerosis. Accumulation of senescent cells promotes inflammation and negatively affects plaque remodelling. Translating novel anti-senescence strategies into the clinic has potential to causally and effectively prevent and treat atherosclerosis and/or CVD.
Acknowledgements
Graphical illustrations were drawn using the open source public database Servier Medical Art (http://www.servier.com/Powerpoint-image-bank, https://creativecommons.org/licenses/by/3.0/).<https://creativecommons.org/licenses/by/3.0/>).
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (KFO FOR311-2, TH903/20-1 to T.T.), Cluster of Excellence REBIRTH (from Regenerative Biology to Reconstructive Therapy EXC 62 to S.D.S., J.B., and T.T.), H2020 Project 777111-REPO-TRIAL (to J.B), German Academic Exchange Service Graduate School Scholarship Programme (DAAD-GSSP) (57320205, to S.D.S), Foundation Leducq (to T.T.) and the ERA Network grant EXPERT (to T.T.).
Conflict of interest: T.T. has filed and licensed patents regarding noncoding RNAs in CVD. T.T. is founder and shareholder of Cardior Pharmaceuticals GmbH.
References
- 1. Libby P, Ridker PM, Hansson GK.. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ.. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Libby P, Everett BM.. Novel antiatherosclerotic therapies. Arterioscler Thromb Vasc Biol 2019;39:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ridker PM. From CRP to IL-6 to IL-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. North BJ, Sinclair DA.. The intersection between aging and cardiovascular disease. Circ Res 2012;110:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkland JL, Tchkonia T.. Cellular senescence: a translational perspective. EBioMedicine 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McHugh D, Gil J.. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol 2018;217:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM.. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016;354:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Childs BG, Li H, van Deursen JM.. Senescent cells: a therapeutic target for cardiovascular disease. J Clin Invest 2018;128:1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah A, Gray K, Figg N, Finigan A, Starks L, Bennett M.. Defective base excision repair of oxidative DNA damage in vascular smooth muscle cells promotes atherosclerosis. Circulation 2018;138:1446–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grootaert MOJ, Roth L, Schrijvers DM, De Meyer GRY, Martinet W.. Defective autophagy in atherosclerosis: to die or to senesce? Oxid Med Cell Longev 2018;2018:7687083.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang J, Bai Y, Zhao X, Ru J, Kang N, Tian T, Tang L, An Y, Li P.. oxLDL-mediated cellular senescence is associated with increased NADPH oxidase p47phox recruitment to caveolae. Biosci Rep 2018;38:BSR20180283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang YC, Lee AS, Lu LS, Ke LY, Chen WY, Dong JW, Lu J, Chen Z, Chu CS, Chan HC, Kuzan TY, Tsai MH, Hsu WL, Dixon RAF, Sawamura T, Chang KC, Chen CH.. Human electronegative LDL induces mitochondrial dysfunction and premature senescence of vascular cells in vivo. Aging Cell 2018;17:e12792.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freund A, Orjalo AV, Desprez PY, Campisi J.. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 2010;16:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW.. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol 2006;35:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Westhoff JH, Hilgers KF, Steinbach MP, Hartner A, Klanke B, Amann K, Melk A.. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 2008;52:123–129. [DOI] [PubMed] [Google Scholar]
- 18. Kitada K, Nakano D, Ohsaki H, Hitomi H, Minamino T, Yatabe J, Felder RA, Mori H, Masaki T, Kobori H, Nishiyama A.. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications 2014;28:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Broadley I, Pera A, Morrow G, Davies KA, Kern F.. Expansions of cytotoxic CD4(+)CD28(-) T cells drive excess cardiovascular mortality in rheumatoid arthritis and other chronic inflammatory conditions and are triggered by CMV infection. Front Immunol 2017;8:195.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Victorelli S, Passos JF.. Telomeres and cell senescence—size matters not. EBioMedicine 2017;21:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun N, Youle RJ, Finkel T.. The mitochondrial basis of aging. Mol Cell 2016;61:654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang C, Elledge SJ.. How autophagy both activates and inhibits cellular senescence. Autophagy 2016;12:898–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM.. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 2017;16:718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kunieda T, Minamino T, Nishi J-I, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I.. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 2006;114:953.. [DOI] [PubMed] [Google Scholar]
- 25. Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, Tsukuda K, Iwai M, Horiuchi M.. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res 2007;76:506–516. [DOI] [PubMed] [Google Scholar]
- 26. Gavras I, Gavras H.. Angiotensin II as a cardiovascular risk factor. J Hum Hypertens 2002;16(Suppl 2):S2–S6. [DOI] [PubMed] [Google Scholar]
- 27. Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L.. Interventions to slow aging in humans: are we ready? Aging Cell 2015;14:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma A, Wang J, Yang L, An Y, Zhu H.. AMPK activation enhances the anti-atherogenic effects of high density lipoproteins in apoE(-/-) mice. J Lipid Res 2017;58:1536–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salt IP, Hardie DG.. AMP-activated protein kinase: an ubiquitous signaling pathway with key roles in the cardiovascular system. Circ Res 2017;120:1825–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tasdemir N, Lowe SW.. Senescent cells spread the word: non-cell autonomous propagation of cellular senescence. EMBO J 2013;32:1975–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E.. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 2003;107:690–695. [DOI] [PubMed] [Google Scholar]
- 32. Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Russo HM, Eitzman DT.. Monocyte chemoattractant protein-1 deficiency protects against visceral fat-induced atherosclerosis. Arterioscler Thromb Vasc Biol 2010;30:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morris BJ, Willcox DC, Donlon TA, Willcox BJ.. FOXO3: a major gene for human longevity—a mini-review. Gerontology 2015;61:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sedding DG. FoxO transcription factors in oxidative stress response and ageing—a new fork on the way to longevity? Biol Chem 2008;389:279–283. [DOI] [PubMed] [Google Scholar]
- 35. Yu H, Fellows A, Foote K, Yang Z, Figg N, Littlewood T, Bennett M.. FOXO3a (Forkhead Transcription Factor O Subfamily Member 3a) links vascular smooth muscle cell apoptosis, matrix breakdown, atherosclerosis, and vascular remodeling through a novel pathway involving MMP13 (Matrix Metalloproteinase 13). Arterioscler Thromb Vasc Biol 2018;38:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahmoud M, Souilhol C, Serbanovic-Canic J, Evans P.. GATA4-Twist1 signalling in disturbed flow-induced atherosclerosis. Cardiovasc Drugs Ther 2019;33:231–237. [DOI] [PubMed] [Google Scholar]
- 37. Pan X, Bradfield CA, Hussain MM.. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat Commun 2016;7:13011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, Lu T, Yankner BA, Campisi J, Elledge SJ.. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015;349:aaa5612.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersson U, Tracey KJ.. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 2011;29:139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu M, Yu Y, Jiang H, Zhang L, Zhang PP, Yu P, Jia JG, Chen RZ, Zou YZ, Ge JB.. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(-/-) mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol Sin 2013;34:830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu X, Jiang H, Bai Q, Zhou X, Xu C, Lu Z, Cui B, Wen H.. Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin Chim Acta 2009;406:139–142. [DOI] [PubMed] [Google Scholar]
- 42. Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL, Dorronsoro A, Corbo L, Tang P, Bukata C, Ring N, Giacca M, Li X, Tchkonia T, Kirkland JL, Niedernhofer LJ, Robbins PD.. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 2017;8:422.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Madrigal-Matute J, López-Franco O, Blanco-Colio LM, Muñoz-García B, Ramos-Mozo P, Ortega L, Egido J, Martín-Ventura JL.. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc Res 2010;86:330–337. [DOI] [PubMed] [Google Scholar]
- 44. Lazaro I, Oguiza A, Recio C, Lopez-Sanz L, Bernal S, Egido J, Gomez-Guerrero C.. Interplay between HSP90 and Nrf2 pathways in diabetes-associated atherosclerosis. Clin Investig Arterioscler 2017;29:51–59. [DOI] [PubMed] [Google Scholar]
- 45. Gardner SE, Humphry M, Bennett MR, Clarke M.. Senescent vascular smooth muscle cells drive inflammation through an interleukin-1α-dependent senescence-associated secretory phenotype. Arterioscler Thromb Vasc Biol 2015;35:1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang J, Sun C, Gerdes N, Liu C, Liao M, Liu J, Shi MA, He A, Zhou Y, Sukhova GK, Chen H, Cheng XW, Kuzuya M, Murohara T, Zhang J, Cheng X, Jiang M, Shull GE, Rogers S, Yang CL, Ke Q, Jelen S, Bindels R, Ellison DH, Jarolim P, Libby P, Shi GP.. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat Med 2015;21:820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang LM, Zhang J, Zhang Y, Fei C, Wang L, Yi ZW, Zhang ZQ.. Interleukin-18 promotes fibroblast senescence in pulmonary fibrosis through down-regulating Klotho expression. Biomed Pharmacother 2019;113:108756.. [DOI] [PubMed] [Google Scholar]
- 48. Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD, Jorgensen T, Danesh J.. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014;35:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang M, Kim SH, Monticone RE, Lakatta EG.. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 2015;65:698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ma Y, Chiao YA, Clark R, Flynn ER, Yabluchanskiy A, Ghasemi O, Zouein F, Lindsey ML, Jin YF.. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res 2015;106:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hudgins AD, Tazearslan C, Tare A, Zhu Y, Huffman D, Suh Y.. Age- and tissue-specific expression of senescence biomarkers in mice. Front Genet 2018;9:59.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goncalves I, Bengtsson E, Colhoun HM, Shore AC, Palombo C, Natali A, Edsfeldt A, Duner P, Fredrikson GN, Bjorkbacka H, Ostling G, Aizawa K, Casanova F, Persson M, Gooding K, Strain D, Khan F, Looker HC, Adams F, Belch J, Pinnoli S, Venturi E, Kozakova M, Gan LM, Schnecke V, Nilsson J.. Elevated plasma levels of MMP-12 are associated with atherosclerotic burden and symptomatic cardiovascular disease in subjects with type 2 diabetes. Arterioscler Thromb Vasc Biol 2015;35:1723–1731. [DOI] [PubMed] [Google Scholar]
- 53. Yamada S, Wang KY, Tanimoto A, Fan J, Shimajiri S, Kitajima S, Morimoto M, Tsutsui M, Watanabe T, Yasumoto K, Sasaguri Y.. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol 2008;172:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kurdi A, Martinet W, De Meyer G.. mTOR inhibition and cardiovascular diseases: dyslipidemia and atherosclerosis. Transplantation 2018;102:S44–S46. [DOI] [PubMed] [Google Scholar]
- 55. Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW.. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 2011;25:2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cheng HS, Njock MS, Khyzha N, Dang LT, Fish JE.. Noncoding RNAs regulate NF-kappaB signaling to modulate blood vessel inflammation. Front Genet 2014;5:422.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, Owen JS, Thomas MJ, Francis J, Parks JS, Dixit VD.. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep 2012;1:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slütter B, Foks AC, Bot I, Kuiper J.. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler Thromb Vasc Biol 2017;37:1457–1461. [DOI] [PubMed] [Google Scholar]
- 59. Wang R, Wang Y, Mu N, Lou X, Li W, Chen Y, Fan D, Tan H.. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Invest 2017;97:922–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bullon P, Cano-Garcia FJ, Alcocer-Gomez E, Varela-Lopez A, Roman-Malo L, Ruiz-Salmeron RJ, Quiles JL, Navarro-Pando JM, Battino M, Ruiz-Cabello J, Jimenez-Borreguero LJ, Cordero MD.. Could NLRP3-inflammasome be a cardiovascular risk biomarker in acute myocardial infarction patients? Antioxid Redox Signal 2017;27:269–275. [DOI] [PubMed] [Google Scholar]
- 61. Dutzmann J, Bauersachs J, Sedding DG.. Evidence for the use of mineralocorticoid receptor antagonists in the treatment of coronary artery disease and post-angioplasty restenosis. Vascul Pharmacol 2017;107:20–26. [DOI] [PubMed] [Google Scholar]
- 62. Burton DG, Matsubara H, Ikeda K.. Pathophysiology of vascular calcification: pivotal role of cellular senescence in vascular smooth muscle cells. Exp Gerontol 2010;45:819–824. [DOI] [PubMed] [Google Scholar]
- 63. Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J.. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008;6:2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tschiderer L, Willeit J, Schett G, Kiechl S, Willeit P.. Osteoprotegerin concentration and risk of cardiovascular outcomes in nine general population studies: literature-based meta-analysis involving 26,442 participants. PLoS One 2017;12:e0183910.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Van Campenhout A, Golledge J.. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 2009;204:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun G, Li Y, Ji Z.. Up-regulation of MIAT aggravates the atherosclerotic damage in atherosclerosis mice through the activation of PI3K/Akt signaling pathway. Drug Deliv 2019;26:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bent EH, Gilbert LA, Hemann MT.. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev 2016;30:1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suski JM, Karkucinska-Wieckowska A, Lebiedzinska M, Giorgi C, Szczepanowska J, Szabadkai G, Duszynski J, Pronicki M, Pinton P, Wieckowski MR.. p66Shc aging protein in control of fibroblasts cell fate. Int J Mol Sci 2011;12:5373–5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Franzeck FC, Hof D, Spescha RD, Hasun M, Akhmedov A, Steffel J, Shi Y, Cosentino F, Tanner FC, von Eckardstein A, Maier W, Luscher TF, Wyss CA, Camici GG.. Expression of the aging gene p66Shc is increased in peripheral blood monocytes of patients with acute coronary syndrome but not with stable coronary artery disease. Atherosclerosis 2012;220:282–286. [DOI] [PubMed] [Google Scholar]
- 70. Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P.. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA 2003;100:2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martin-Padura I, de Nigris F, Migliaccio E, Mansueto G, Minardi S, Rienzo M, Lerman LO, Stendardo M, Giorgio M, De Rosa G, Pelicci PG, Napoli C.. p66Shc deletion confers vascular protection in advanced atherosclerosis in hypercholesterolemic apolipoprotein E knockout mice. Endothelium 2008;15:276–287. [DOI] [PubMed] [Google Scholar]
- 72. de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Man CD, Cobelli C, Fadini GP, Avogaro A.. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes 2010;59:1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ridker PM, Luscher TF.. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J 2014;35:1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. D'Onofrio N, Servillo L, Balestrieri ML.. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal 2018;28:711–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Adnot S, Lipskaia L, Bernard D.. The STATus of STAT3 in lung cell senescence? Am J Respir Cell Mol Biol 2019;61:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dutzmann J, Daniel JM, Bauersachs J, Hilfiker-Kleiner D, Sedding DG.. Emerging translational approaches to target STAT3 signalling and its impact on vascular disease. Cardiovasc Res 2015;106:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Seijkens TTP, van Tiel CM, Kusters PJH, Atzler D, Soehnlein O, Zarzycka B, Aarts SABM, Lameijer M, Gijbels MJ, Beckers L, den Toom M, Slütter B, Kuiper J, Duchene J, Aslani M, Megens RTA, van ‘T Veer C, Kooij G, Schrijver R, Hoeksema MA, Boon L, Fay F, Tang J, Baxter S, Jongejan A, Moerland PD, Vriend G, Bleijlevens B, Fisher EA, Duivenvoorden R, Gerdes N, de Winther MPJ, Nicolaes GA, Mulder WJM, Weber C, Lutgens E.. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J Am Coll Cardiol 2018;71:527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang B, Fu D, Xu Q, Cong X, Wu C, Zhong X, Ma Y, Lv Z, Chen F, Han L, Qian M, Chin YE, Lam EW, Chiao P, Sun Y.. The senescence-associated secretory phenotype is potentiated by feedforward regulatory mechanisms involving Zscan4 and TAK1. Nat Commun 2018;9:1723.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kang HT, Park JT, Choi K, Kim Y, Choi HJC, Jung CW, Lee YS, Park SC.. Chemical screening identifies ATM as a target for alleviating senescence. Nat Chem Biol 2017;13:616–623. [DOI] [PubMed] [Google Scholar]
- 80. Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M.. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 2015;132:1909–1919. [DOI] [PubMed] [Google Scholar]
- 81. Childs BG, Baker DJ, Kirkland JL, Campisi J, Deursen JM.. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 2014;15:1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J.. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 2014;31:722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V.. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 2013;32:1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, Windheim J, Tsoory M, Schirmbeck R, Amit I, Geiger H, Krizhanovsky V.. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 2018;9:5435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shakeri H, Lemmens K, Gevaert AB, Meyer G, Segers V.. Cellular senescence links aging and diabetes in cardiovascular disease. Am J Physiol Heart Circ Physiol 2018;315:H448–H462. [DOI] [PubMed] [Google Scholar]
- 86. Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ.. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018;562:578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gevaert AB, Shakeri H, Leloup AJ, Van Hove CE, De Meyer GRY, Vrints CJ, Lemmens K, Van Craenenbroeck EM.. Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ Heart Fail 2017;10:e003806. [DOI] [PubMed] [Google Scholar]
- 88. Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL.. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 2019;40:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK.. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 2017;8:14532.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rashid K, Sundar IK, Gerloff J, Li D, Rahman I.. Lung cellular senescence is independent of aging in a mouse model of COPD/emphysema. Sci Rep 2018;8:9023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Valentijn FA, Falke LL, Nguyen TQ, Goldschmeding R.. Cellular senescence in the aging and diseased kidney. J Cell Commun Signal 2018;12:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Demaria M, Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, Alston S, Academia EC, Kilmarx S, Valdovinos A, Wang B, de Bruin A, Kennedy BK, Melov S, Zhou D, Sharpless NE, Muss H, Campisi J.. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 2017;7:165.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH.. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 2017;23:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM.. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011;479:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, A. Saltness R, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM.. Naturally occurring p16Ink4a -positive cells shorten healthy lifespan. Nature 2016;530:184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I.. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002;105:1541–1544. [DOI] [PubMed] [Google Scholar]
- 97. Silva GC, Abbas M, Khemais-Benkhiat S, Burban M, Ribeiro TP, Toti F, Idris-Khodja N, Côrtes SF, Schini-Kerth VB.. Replicative senescence promotes prothrombotic responses in endothelial cells: role of NADPH oxidase- and cyclooxygenase-derived oxidative stress. Exp Gerontol 2017;93:7–15. [DOI] [PubMed] [Google Scholar]
- 98. Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol (1985) 2009;106:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bennett MR, Sinha S, Owens GK.. Vascular smooth muscle cells in atherosclerosis. Circ Res 2016;118:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Uryga AK, Bennett MR.. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J Physiol 2016;594:2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hamczyk MR, Villa-Bellosta R, Gonzalo P, Andrés-Manzano MJ, Nogales P, Bentzon JF, López-Otín C, Andrés V.. Vascular smooth muscle-specific progerin expression accelerates atherosclerosis and death in a mouse model of Hutchinson-Gilford progeria syndrome. Circulation 2018;138:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Prattichizzo F, Bonafè M, Olivieri F, Franceschi C. Senescence associated macrophages and “macroph-aging”: are they pieces of the same puzzle? Aging (Albany NY) 2016;8:3159–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shaw AC, Goldstein DR, Montgomery RR.. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 2013;13:875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dutzmann J, Koch A, Weisheit S, Sonnenschein K, Korte L, Haertle M, Thum T, Bauersachs J, Sedding DG, Daniel JM.. Sonic hedgehog-dependent activation of adventitial fibroblasts promotes neointima formation. Cardiovasc Res 2017;113:1653–1663. [DOI] [PubMed] [Google Scholar]
- 105. Coppe JP, Desprez PY, Krtolica A, Campisi J.. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jazbutyte V, Fiedler J, Kneitz S, Galuppo P, Just A, Holzmann A, Bauersachs J, Thum T.. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age (Dordr) 2013;35:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Spyridopoulos I, Martin-Ruiz C, Hilkens C, Yadegarfar ME, Isaacs J, Jagger C, Kirkwood T, von Zglinicki T.. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell 2016;15:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN, Henson SM.. Human CD8(+) EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 2018;17:e12675.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bentzon JF, Otsuka F, Virmani R, Falk E.. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- 110. Prattichizzo F, De Nigris V, La Sala L, Procopio AD, Olivieri F, Ceriello A.. “Inflammaging” as a druggable target: a senescence-associated secretory phenotype-centered view of type 2 diabetes. Oxid Med Cell Longev 2016;2016:1810327.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL.. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013;123:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lahteenvuo J, Rosenzweig A.. Effects of aging on angiogenesis. Circ Res 2012;110:1252–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sedding DG, Boyle EC, Demandt JAF, Sluimer JC, Dutzmann J, Haverich A, Bauersachs J.. Vasa vasorum angiogenesis: key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front Immunol 2018;9:706.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Boyle EC, Sedding DG, Haverich A.. Targeting vasa vasorum dysfunction to prevent atherosclerosis. Vascul Pharmacol 2017;96–98:5–10. [DOI] [PubMed] [Google Scholar]
- 115. Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain MA, Bourdel G, Popovic N, Rezende FA, Kaufman RJ, Mallette FA, Sapieha P.. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med 2016;8:362ra144.. [DOI] [PubMed] [Google Scholar]
- 116. Bullenkamp J, Dinkla S, Kaski JC, Dumitriu IE.. Targeting T cells to treat atherosclerosis: odyssey from bench to bedside. Eur Heart J Cardiovasc Pharmacother 2016;2:194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM.. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation 2002;105:570–575. [DOI] [PubMed] [Google Scholar]
- 118. Lakatta EG. So! What’s aging? Is cardiovascular aging a disease? J Mol Cell Cardiol 2015;83:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Terentes-Printzios D, Vlachopoulos C, Xaplanteris P, Ioakeimidis N, Aznaouridis K, Baou K, Kardara D, Georgiopoulos G, Georgakopoulos C, Tousoulis D.. Cardiovascular risk factors accelerate progression of vascular aging in the general population: results from the CRAVE study (Cardiovascular Risk Factors Affecting Vascular Age). Hypertension 2017;70:1057–1064. [DOI] [PubMed] [Google Scholar]
- 120. Andreou I, Antoniadis AP, Shishido K, Papafaklis MI, Koskinas KC, Chatzizisis YS, Coskun AU, Edelman ER, Feldman CL, Stone PH.. How do we prevent the vulnerable atherosclerotic plaque from rupturing? Insights from in vivo assessments of plaque, vascular remodeling, and local endothelial shear stress. J Cardiovasc Pharmacol Ther 2015;20:261–275. [DOI] [PubMed] [Google Scholar]
- 121. Jaffer FA, O’Donnell CJ, Larson MG, Chan SK, Kissinger KV, Kupka MJ, Salton C, Botnar RM, Levy D, Manning WJ.. Age and sex distribution of subclinical aortic atherosclerosis: a magnetic resonance imaging examination of the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2002;22:849–854. [DOI] [PubMed] [Google Scholar]
- 122. Kuller L, Borthani N, Furberg C, Gardin J, Manolio T, O'Leary D, Psaty B, Robbins J.. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol 1994;139:1164–1179. [DOI] [PubMed] [Google Scholar]
- 123. Kannel WB, Vasan RS.. Is age really a non-modifiable cardiovascular risk factor? Am J Cardiol 2009;104:1307–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Benetos A, Toupance S, Gautier S, Labat C, Kimura M, Rossi PM, Settembre N, Hubert J, Frimat L, Bertrand B, Boufi M, Flecher X, Sadoul N, Eschwege P, Kessler M, Tzanetakou IP, Doulamis IP, Konstantopoulos P, Tzani A, Korou M, Gkogkos A, Perreas K, Menenakos E, Samanidis G, Vasiloglou-Gkanis M, Kark JD, Malikov S, Verhulst S, Aviv A.. Short leukocyte telomere length precedes clinical expression of atherosclerosis the blood-and-muscle model. Circ Res 2018;122:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P.. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2014;349:g4227.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Capell BC, Collins FS, Nabel EG.. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res 2007;101:13–26. [DOI] [PubMed] [Google Scholar]
- 127. Wang JC, Bennett M.. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 2012;111:245–259. [DOI] [PubMed] [Google Scholar]
- 128. Prakash A, Gordon LB, Kleinman ME, Gurary EB, Massaro J, D’Agostino R, Kieran MW, Gerhard-Herman M, Smoot L.. Cardiac abnormalities in patients with Hutchinson-Gilford progeria syndrome. JAMA Cardiol 2018;3:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ghebre YT, Yakubov E, Wong WT, Krishnamurthy P, Sayed N, Sikora AG, Bonnen MD.. Vascular aging: implications for cardiovascular disease and therapy. Transl Med (Sunnyvale) 2016;6:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Parry AJ, Narita M.. Old cells, new tricks: chromatin structure in senescence. Mamm Genome 2016;27:320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Minamino T, Komuro I.. Vascular cell senescence: contribution to atherosclerosis. Circ Res 2007;100:15–26. [DOI] [PubMed] [Google Scholar]
- 132. Katsuumi G, Shimizu I, Yoshida Y, Minamino T.. Vascular senescence in cardiovascular and metabolic diseases. Front Cardiovasc Med 2018;5:18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF.. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J 2001;15:458–466. [DOI] [PubMed] [Google Scholar]
- 134. Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M.. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 2006;99:156–164. [DOI] [PubMed] [Google Scholar]
- 135. Holdt LM, Sass K, Gabel G, Bergert H, Thiery J, Teupser D.. Expression of Chr9p21 genes CDKN2B (p15(INK4b)), CDKN2A (p16(INK4a), p14(ARF)) and MTAP in human atherosclerotic plaque. Atherosclerosis 2011;214:264–270. [DOI] [PubMed] [Google Scholar]
- 136. Alloza I, Goikuria H, Idro JL, Triviño JC, Fernández Velasco JM, Elizagaray E, García-Barcina M, Montoya-Murillo G, Sarasola E, Vega Manrique R, Freijo MDM, Vandenbroeck K.. RNAseq based transcriptomics study of SMCs from carotid atherosclerotic plaque: BMP2 and IDs proteins are crucial regulators of plaque stability. Sci Rep 2017;7:3470.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sanada F, Muratsu J, Otsu R, Shimizu H, Koibuchi N, Uchida K, Taniyama Y, Yoshimura S, Rakugi H, Morishita R.. Local production of activated factor X in atherosclerotic plaque induced vascular smooth muscle cell senescence. Sci Rep 2017;7:17172.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Libby P, Ebert BL.. CHIP (Clonal Hematopoiesis of Indeterminate Potential). Circulation 2018;138:666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xie W, Baylin SB, Easwaran H.. DNA methylation in senescence, aging and cancer. Oncoscience 2019;6:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang Y, Gao Y, Zhang G, Huang S, Dong Z, Kong C, Su D, Du J, Zhu S, Liang Q, Zhang J, Lu J, Huang B.. DNMT3a plays a role in switches between doxorubicin-induced senescence and apoptosis of colorectal cancer cells. Int J Cancer 2011;128:551–561. [DOI] [PubMed] [Google Scholar]
- 141. Xie HF, Liu YZ, Du R, Wang B, Chen MT, Zhang YY, Deng ZL, Li J.. miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1. Cell Death Dis 2017;8:e2663.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Werner C, Gensch C, Poss J, Haendeler J, Bohm M, Laufs U.. Pioglitazone activates aortic telomerase and prevents stress-induced endothelial apoptosis. Atherosclerosis 2011;216:23–34. [DOI] [PubMed] [Google Scholar]
- 143. Bennaceur K, Atwill M, Al Zhrany N, Hoffmann J, Keavney B, Breault D, Richardson G, von Zglinicki T, Saretzki G, Spyridopoulos I.. Atorvastatin induces T cell proliferation by a telomerase reverse transcriptase (TERT) mediated mechanism. Atherosclerosis 2014;236:312–320. [DOI] [PubMed] [Google Scholar]
- 144. Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM.. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018;17:e12750.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lutgens E, Atzler D, Doring Y, Duchene J, Steffens S, Weber C.. Immunotherapy for cardiovascular disease. Eur Heart J 2019;40:3937–3946. [DOI] [PubMed] [Google Scholar]
- 146. Yang H, Wang H, Ren J, Chen Q, Chen ZJ.. cGAS is essential for cellular senescence. Proc Natl Acad Sci USA 2017;114:E4612–E4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gluck S, Guey B, Gulen MF, Wolter K, Kang TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A.. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 2017;19:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL.. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA 2015;112:E6301–E6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Toso A, Revandkar A, Di Mitri D, Guccini I, Proietti M, Sarti M, Pinton S, Zhang J, Kalathur M, Civenni G, Jarrossay D, Montani E, Marini C, Garcia-Escudero R, Scanziani E, Grassi F, Pandolfi PP, Catapano CV, Alimonti A.. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep 2014;9:75–89. [DOI] [PubMed] [Google Scholar]
- 150. Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM.. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med 2014;20:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. O’Donoghue ML, Glaser R, Cavender MA, Aylward PE, Bonaca MP, Budaj A, Davies RY, Dellborg M, Fox KAA, Gutierrez JAT, Hamm C, Kiss RG, Kovar F, Kuder JF, Im KA, Lepore JJ, Lopez-Sendon JL, Ophuis TO, Parkhomenko A, Shannon JB, Spinar J, Tanguay J-F, Ruda M, Steg PG, Theroux P, Wiviott SD, Laws I, Sabatine MS, Morrow DA.. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA 2016;315:1591–1599. [DOI] [PubMed] [Google Scholar]
- 152. Walters HE, Deneka-Hannemann S, Cox LS.. Reversal of phenotypes of cellular senescence by pan-mTOR inhibition. Aging (Albany NY) 2016;8:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Evangelisti C, Cenni V, Lattanzi G.. Potential therapeutic effects of the MTOR inhibitors for preventing ageing and progeria-related disorders. Br J Clin Pharmacol 2016;82:1229–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yoon MS. mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol 2017;8:788.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sedding D, Haendeler J.. Do we age on Sirt1 expression? Circ Res 2007;100:1396–1398. [DOI] [PubMed] [Google Scholar]
- 156. Baur JA, Sinclair DA.. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 2006;5:493–506. [DOI] [PubMed] [Google Scholar]
- 157. Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T.. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 2012;3:1078.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA.. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell 2018;173:74–89.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Watson A, Nong Z, Yin H, O’Neil C, Fox S, Balint B, Guo L, Leo O, Chu MWA, Gros R, Pickering JG.. Nicotinamide phosphoribosyltransferase in smooth muscle cells maintains genome integrity, resists aortic medial degeneration, and is suppressed in human thoracic aortic aneurysm disease. Circ Res 2017;120:1889–1902. [DOI] [PubMed] [Google Scholar]
- 160. Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL.. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 2014;158:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O'Neil L, White TA, Sinclair DA, Chini EN.. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 2013;62:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S.. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980–984. [DOI] [PubMed] [Google Scholar]
- 163. Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, Just A, Fendrich J, Scherf K, Bolesani E, Schambach A, Weidemann F, Zweigerdt R, de Windt LJ, Engelhardt S, Dandekar T, Batkai S, Thum T.. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med 2016;8:326ra22.. [DOI] [PubMed] [Google Scholar]
- 164. Thum T. Non-coding RNAs in ageing. Ageing Res Rev 2014;17:1–2. [DOI] [PubMed] [Google Scholar]
- 165. Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, Hein L, Batkai S, Pinet F, Thum T.. Preclinical development of a MicroRNA-based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol 2016;68:1557–1571. [DOI] [PubMed] [Google Scholar]
- 166. Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol Med 2012;4:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Jahagirdar R, Zhang H, Azhar S, Tobin J, Attwell S, Yu R, Wu J, McLure KG, Hansen HC, Wagner GS, Young PR, Srivastava RA, Wong NC, Johansson J.. A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis 2014;236:91–100. [DOI] [PubMed] [Google Scholar]
- 168. Heo JI, Kim W, Choi KJ, Bae S, Jeong JH, Kim KS.. XIAP-associating factor 1, a transcriptional target of BRD7, contributes to endothelial cell senescence. Oncotarget 2016;7:5118–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM.. Cellular senescence in renal ageing and disease. Nat Rev Nephrol 2017;13:77–89. [DOI] [PubMed] [Google Scholar]
- 170. Wang JS, Sheu WH, Lee WJ, Lee IT, Lin SY, Lee WL, Liang KW, Lin SJ.. Levels of serum high mobility group box 1 were independently associated with cardiovascular risk in patients undergoing coronary angiography. Clin Chim Acta 2018;483:130–134. [DOI] [PubMed] [Google Scholar]
- 171. Shahzad K, Gadi I, Nazir S, Al-Dabet MM, Kohli S, Bock F, Breitenstein L, Ranjan S, Fuchs T, Halloul Z, Nawroth PP, Pelicci PG, Braun-Dullaeus RC, Camerer E, Esmon CT, Isermann B.. Activated protein C reverses epigenetically sustained p66(Shc) expression in plaque-associated macrophages in diabetes. Commun Biol 2018;1:104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Tong N, Jin R, Zhou Z, Wu X.. Involvement of microRNA-34a in age-related susceptibility to oxidative stress in ARPE-19 cells by targeting the silent mating type information regulation 2 homolog 1/p66shc pathway: implications for age-related macular degeneration. Front Aging Neurosci 2019;11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG.. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999;402:309–313. [DOI] [PubMed] [Google Scholar]
- 174. Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD.. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016;15:973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL.. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015;14:644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL.. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL.. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016;15:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D.. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016;22:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Zhang X, Zhang S, Liu X, Wang Y, Chang J, Zhang X, Mackintosh SG, Tackett AJ, He Y, Lv D, Laberge RM, Campisi J, Wang J, Zheng G, Zhou D.. Oxidation resistance 1 is a novel senolytic target. Aging Cell 2018;17:e12780.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, Zhou D, Zheng G.. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY) 2016;8:2915–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IWF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, de Keizer P.. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017;169:132–147.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, McGuckian C, Wade EA, Kato JI, Grassi D, Wentworth M, Burd CE, Arriaga EA, Ladiges WL, Tchkonia T, Kirkland JL, Robbins PD, Niedernhofer LJ.. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018;36:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I, Krizhanovsky V.. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 2016;7:11190.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL.. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018;24:1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kim KM, Noh JH, Bodogai M, Martindale JL, Yang X, Indig FE, Basu SK, Ohnuma K, Morimoto C, Johnson PF, Biragyn A, Abdelmohsen K, Gorospe M.. Identification of senescent cell surface targetable protein DPP4. Genes Dev 2017;31:1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Dimmeler S, Leri A.. Aging and disease as modifiers of efficacy of cell therapy. Circ Res 2008;102:1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Nollet E, Hoymans VY, Rodrigus IR, De Bock D, Dom M, Vanassche B, Van Hoof VO, Cools N, Van Ackeren K, Wouters K, Vermeulen K, Vrints CJ, Van Craenenbroeck EM.. Bone marrow-derived progenitor cells are functionally impaired in ischemic heart disease. J Cardiovasc Transl Res 2016;9:266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Angelini F, Pagano F, Bordin A, Picchio V, De Falco E, Chimenti I.. Getting old through the blood: circulating molecules in aging and senescence of cardiovascular regenerative cells. Front Cardiovasc Med 2017;4:62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Nollet E, Hoymans VY, Rodrigus IR, De Bock D, Dom M, Van Hoof VOM, Vrints CJ, Van Craenenbroeck EM.. Accelerated cellular senescence as underlying mechanism for functionally impaired bone marrow-derived progenitor cells in ischemic heart disease. Atherosclerosis 2017;260:138–146. [DOI] [PubMed] [Google Scholar]
- 190. Debrincat MA, Pleines I, Lebois M, Lane RM, Holmes ML, Corbin J, Vandenberg CJ, Alexander WS, Ng AP, Strasser A, Bouillet P, Sola-Visner M, Kile BT, Josefsson EC.. BCL-2 is dispensable for thrombopoiesis and platelet survival. Cell Death Dis 2015;6:e1721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Munoz-Espin D, Rovira M, Galiana I, Gimenez C, Lozano-Torres B, Paez-Ribes M, Llanos S, Chaib S, Munoz-Martin M, Ucero AC, Garaulet G, Mulero F, Dann SG, VanArsdale T, Shields DJ, Bernardos A, Murguia JR, Martinez-Manez R, Serrano M.. A versatile drug delivery system targeting senescent cells. EMBO Mol Med 2018;10:e9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 193. Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JL, Xu J, Rodriguez Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua Belmonte JC.. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 2016;167:1719–1733 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tang Y, Cheng L.. Cocktail of chemical compounds robustly promoting cell reprogramming protects liver against acute injury. Protein Cell 2017;8:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Milanovic M, Fan DNY, Belenki D, Dabritz JHM, Zhao Z, Yu Y, Dorr JR, Dimitrova L, Lenze D, Monteiro Barbosa IA, Mendoza-Parra MA, Kanashova T, Metzner M, Pardon K, Reimann M, Trumpp A, Dorken B, Zuber J, Gronemeyer H, Hummel M, Dittmar G, Lee S, Schmitt CA.. Senescence-associated reprogramming promotes cancer stemness. Nature 2018;553:96–100. [DOI] [PubMed] [Google Scholar]
- 196. Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM.. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 2011;25:2248–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kelaini S, Cochrane A, Margariti A.. Direct reprogramming of adult cells: avoiding the pluripotent state. Stem Cells Cloning 2014;7:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin II, Leonova KI, Consiglio CR, Gollnick SO, Chernova OB, Gudkov AV.. p16(Ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY) 2017;9:1867–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Liu JY, Souroullas GP, Diekman BO, Krishnamurthy J, Hall BM, Sorrentino JA, Parker JS, Sessions GA, Gudkov AV, Sharpless NE.. Cells exhibiting strong p16 (INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci USA 2019;116:2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, Bardeesy N, Castrillon DH, Beach DH, Sharpless NE.. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 2013;152:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Childs BG, Durik M, Baker DJ, van Deursen JM.. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 2015;21:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Dechamethakun S, Ikeda S, Arai T, Sato N, Sawabe M, Muramatsu M.. Associations between the CDKN2A/B, ADTRP and PDGFD Polymorphisms and the Development of Coronary Atherosclerosis in Japanese Patients. J Atheroscler Thromb 2014;21:680–690. [DOI] [PubMed] [Google Scholar]
- 203. Kuo CL, Murphy AJ, Sayers S, Li R, Yvan-Charvet L, Davis JZ, Krishnamurthy J, Liu Y, Puig O, Sharpless NE, Tall AR, Welch CL.. Cdkn2a is an atherosclerosis modifier locus that regulates monocyte/macrophage proliferation. Arterioscler Thromb Vasc Biol 2011;31:2483–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Mohlke KL, Ibrahim JG, Thomas NE, Sharpless NE.. INK4/ARF transcript expression is associated with chromosome 9p21 variants linked to atherosclerosis. PLoS One 2009;4:e5027.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cao RY, Eves R, Jia L, Funk CD, Jia Z, Mak AS.. Effects of p53-knockout in vascular smooth muscle cells on atherosclerosis in mice. PLoS One 2017;12:e0175061.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A.. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol 2016;67:2018–2028. [DOI] [PubMed] [Google Scholar]
- 207. Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW.. Senescence of activated stellate cells limits liver fibrosis. Cell 2008;134:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM.. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013;155:1119–1130. [DOI] [PubMed] [Google Scholar]
- 209. Frescas D, Roux CM, Aygun-Sunar S, Gleiberman AS, Krasnov P, Kurnasov OV, Strom E, Virtuoso LP, Wrobel M, Osterman AL, Antoch MP, Mett V, Chernova OB, Gudkov AV.. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc Natl Acad Sci USA 2017;114:E1668–E1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Willcox BJ, Tranah GJ, Chen R, Morris BJ, Masaki KH, He Q, Willcox DC, Allsopp RC, Moisyadi S, Poon LW, Rodriguez B, Newman AB, Harris TB, Cummings SR, Liu Y, Parimi N, Evans DS, Davy P, Gerschenson M, Donlon TA.. The FoxO3 gene and cause-specific mortality. Aging Cell 2016;15:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]