Abstract
The slow progression of non-exudative age-related macular degeneration (dry AMD) presents challenges for drug discovery. The standard endpoint used for ophthalmic clinical trials, best-corrected visual acuity, is insensitive to the early stages and slow progression of dry AMD. Effective drug discovery for dry AMD treatments will therefore require novel applications of more effective visual function endpoints.
This review will present candidates for visual function endpoints for dry AMD clinical trials. The promising visual assessments include contrast sensitivity, reading speed, microperimetry, and dark adaptation. Their adoption as exploratory endpoints in future trials will be critical for determining their accuracy, precision, and applicability, and ultimately determine their value for drug discovery.
Introduction
The early stage of non-exudative age-related macular degeneration (dry AMD) is marked by changes in the retinal pigment epithelium: the accumulation of drusen, yellowish deposits of lipid and protein, and abnormal cellular pigmentation. Progression to the advanced form of dry AMD is defined by the localized degeneration of the retinal pigment epithelium in regions defined as geographic atrophy [1]. New imaging modalities that track this progression hold promise as anatomical endpoints for dry AMD trials, but visual function endpoints remain attractive for several reasons: early-stage drusen does not always cause vision loss nor progress to geographic atrophy [2], and drusen can resolve with no apparent functional effects [3]. The therapeutic goal of preserving vision is therefore best-judged by evaluating behavioral outcomes that directly assess subjective and objective visual function [4] [5] [6].
A primary challenge for dry AMD drug discovery is presented by the de facto standard endpoint in ophthalmic clinical trials, best-corrected visual acuity (BCVA). Despite the broad range of visual behavior affected by ocular disease, (e.g., walking, reading, driving, grasping, and eye movements), the BCVA endpoint only measures fine visual resolution [6]. The typical acuity loss in early dry AMD without geographic atrophy amounts to one or two letters [7], which is not clinically meaningful, given the variability of acuity assessment, (standard deviation ~ 4–6 letters; [8]). Even in advanced dry AMD, patterns of geographic atrophy that spare the central fovea can present normal acuity despite significant visual impairment [9]. This contrasts with the vision loss in wet AMD, which is more severe (10–20 letters; [10]), progresses rapidly, and thus provides larger treatment potential for short clinical trials. Therefore, for dry AMD drug discovery using acuity endpoints, the imprecise assessment, potential insensitivity to severe visual disability, and small treatment effect result in unfeasibly large or long clinical trials. This review will present alternative visual function endpoints, which are more sensitive to visual disability than acuity, and may enable clinical trials in earlier disease stages (i.e., before geographic atrophy) that provide better therapeutic targets.
Contrast Sensitivity
The phenomenon of contrast sensitivity is demonstrated in Figure 1A, which presents a sinusoidal pattern of black and white stripes that decrease in width (increase in spatial frequency) from left to right and increase in contrast (light-dark difference) from top to bottom. The contrast sensitivity function (CSF), reflected in the inverted ‘U’ shape boundary between the visible and invisible stripes, demonstrates that the visual system is not equally sensitive to all spatial frequencies. Relative to acuity, contrast sensitivity describes visual performance over broader conditions that better relate to real-world activities, which include mobility [11], and target and face identification [12]. Contrast sensitivity is better predictive of subtle disabilities in daily living activities such as driving, walking and reading [13].
Figure 1. Contrast sensitivity Function.
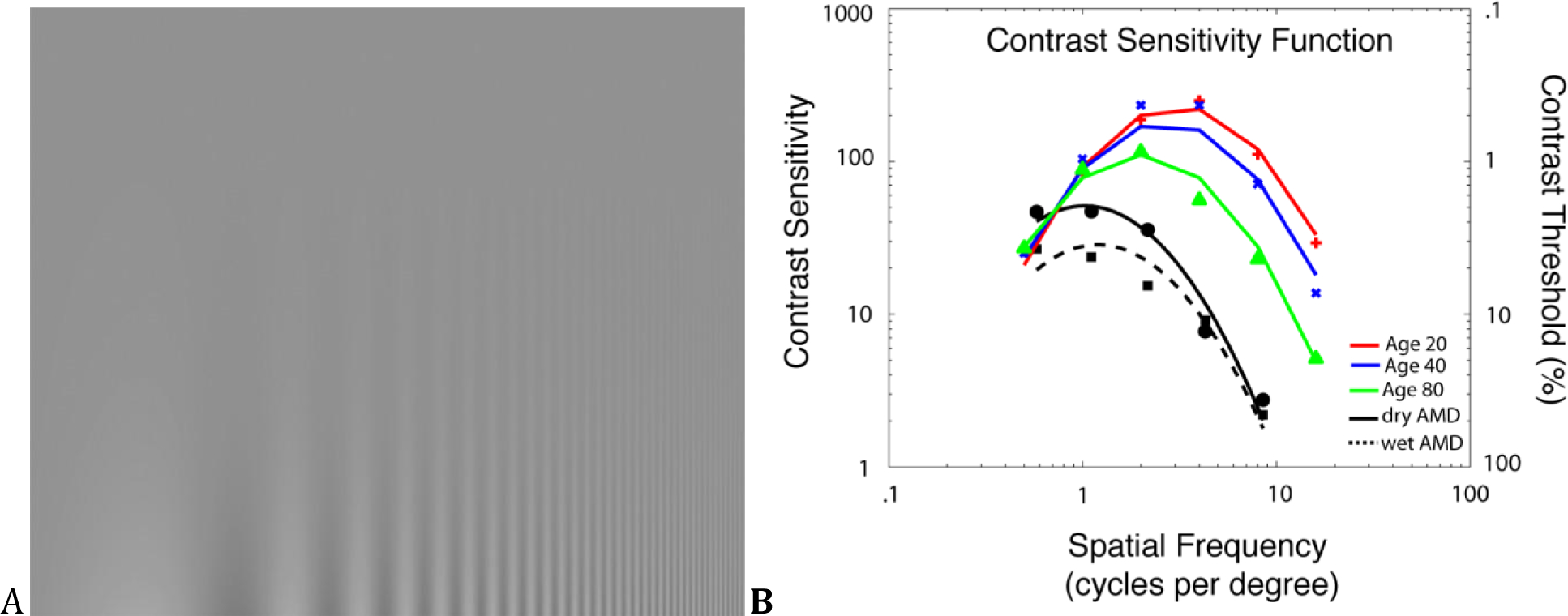
(A) This image demonstrates that the contrast at which stripes vanish depends on their width and therefore the visual system is not equally sensitive to contrast at all spatial frequencies. (B) Spatial Contrast sensitivity functions change systematically during normal aging; both maximal sensitivity and the frequency corresponding to maximal sensitivity decrease over time (after Owsley, Sekuler, & Siemsen, 1983). Deficits for dry (filled circles) and wet AMD (filled squares) are apparent (Mei and Leat, 2007). Curves show the best-fitting log Parabola.
Figure 1B shows contrast sensitivity (reciprocal threshold) as a function of spatial frequency for normal aging [14] and for patients with dry and wet AMD [15]. Consistent with examination of Figure 1A, contrast sensitivity is highest for intermediate spatial frequencies at all ages, but maximal sensitivity decreases, and the frequency corresponding to maximal sensitivity decreases to lower spatial frequencies with increasing age. Contrast sensitivity assessment is sensitive to visual changes in aging, as well as dry AMD pathology. It is correlated to perceived disability [16], reading speed [17], and OCT-derived retinal tissue parameters [18]. In dry AMD, contrast sensitivity deficits are present in AMD at low spatial frequencies [19] or at all spatial frequencies [20]. Such losses correlate with drusen density [21] and may be present even when visual acuity is near-normal in the early stages of AMD [22]. The AMD effects are large, relative to age-matched controls.
Contrast sensitivity can also be measured at different rates of motion and flicker (temporal frequencies), which may have more functional relevance for daily living. Several groups have demonstrated losses in sensitivity to mid temporal frequencies (4–14Hz) in AMD patients with uncompromised visual acuity [23]. The increase in metabolic demand needed to encode flicker makes this an intriguing endpoint to detect metabolic compromise of the outer retina [24].
Reading speed
Reading is a critical daily activity that is severely affected by central vision loss [25]. A large proportion of patients (86%) referred to low vision rehabilitation cite reading difficulty as a therapy priority [26]. The prevalence of reading problems and their relation to subjective quality of life [27] make reading behavior an attractive target for a visual function endpoint.
Normal reading involves the complex coordination of pattern recognition and eye movements [28], to integrate information from foveal and non-foveal areas. Multiple studies have demonstrated that reading speed in AMD is not correlated with age or acuity, but is correlated with the estimated size of the atrophic area [29] and neovascularization in wet AMD [30]. In dry AMD, reading speed is extraordinarily sensitive to geographic atrophy that affects the fovea [25], as perceptual crowding (identity confusions among adjacent letters and words; [31]) makes non-foveal reading especially difficult. While in some cases the acuity gain provided by ranibizumab treatment may increase reading speed [32], other studies have found that reading speed remains impaired [33].
Microperimetry (Macular or fundus-related perimetry)
Microperimetry, or macular or fundus-related perimetry, differs from typical perimetry because it implements real-time monitoring of the macula [34], which compensates for the differences in fixation behavior due to macular disease. Many patients with dry AMD can no longer fixate with their fovea, as do those with normal vision, but instead fixate with a peripheral retinal location [35]. Whereas foveal fixation is precisely focused, peripheral fixation is unstable [36] and largely invalidates the results from traditional automated perimetry. By tracking retinal features in real time, microperimetry can correct stimulus presentation to account for eye movement instability, isolate precisely prescribed retinal locations, and thereby provide accurate, fundus-oriented sensitivity maps of the central visual field (see Figure 2).
Figure 2. Microperimetry.
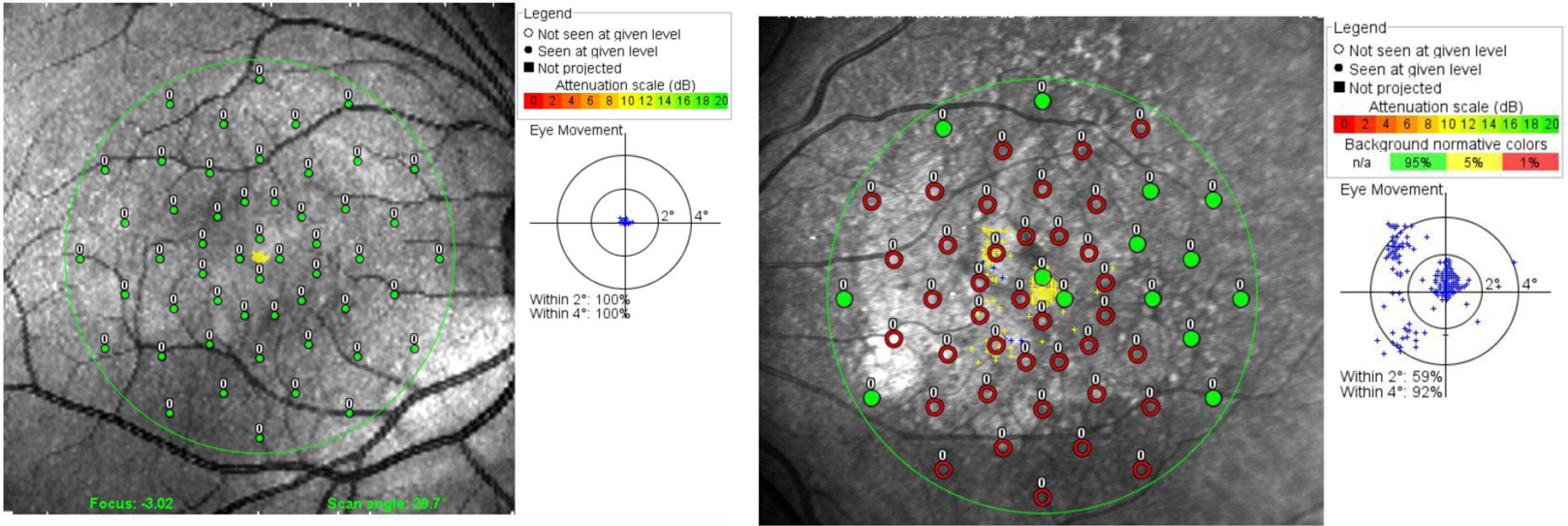
Suprathreshold testing results are presented for a healthy retina (left) and a dry AMD patient with a foveal-sparing central vision loss. Targets are presented in retinal co-ordinates - green points signify sighted areas, in which the patient detects even the faintest (−20dB) targets; red points signify areas of absolute vision loss where even the most intense targets were missed. The inset figures show the distribution of eye fixations (blue dots). With dry AMD, the fixation distribution demonstrates scattering and clustering that is not present in normal fixations.
Microperimetry provides a valuable correspondence between visual function and structural endpoints. The superposition of behavioral results on fundus and OCT retinal images (see Figure 2) demonstrates that visual sensitivity is reduced over drusen, areas with pigment abnormality, and altered autofluorescense [37].
Dark Adaptation
Adaptation is critical for the visual system to operate across the full range of day and night illumination levels. In dry AMD, dysfunctional adaptation is demonstrated by the visual impairment and subjective distress experienced during transitions from bright outdoors to dark indoors [38]. Visual deficits in low light predict later acuity losses from geographic atrophy [9] and flicker sensitivity at low light is impaired in clinically normal observers with high genetic risk for AMD [39].
A related adaptation phenomenon is the time-course of recovery following the visual insensitivity that follows an intense light flash, via inactivation (bleaching) of photoreceptor pigment. The dark adaptation curve (Figure 3) describes visual thresholds as a function of time after photopigment bleaching, and quantifies both low-light visual function and the dynamics of the retinoid cycle that underlie visual recovery [40]. For dry AMD, the deficit in the dark adaptation function is represented by the abnormally long period needed for adaptation recovery, for both rods and cones [41]. Clinical assessment of dark adaptation functions will soon be possible with the AdaptDx adaptometer, (www.maculogix.com), which has been used to demonstrate that impaired dark adaptation is correlated with macular thinning estimated from spectral OCT [42].
Figure 3. Dark Adaptation.
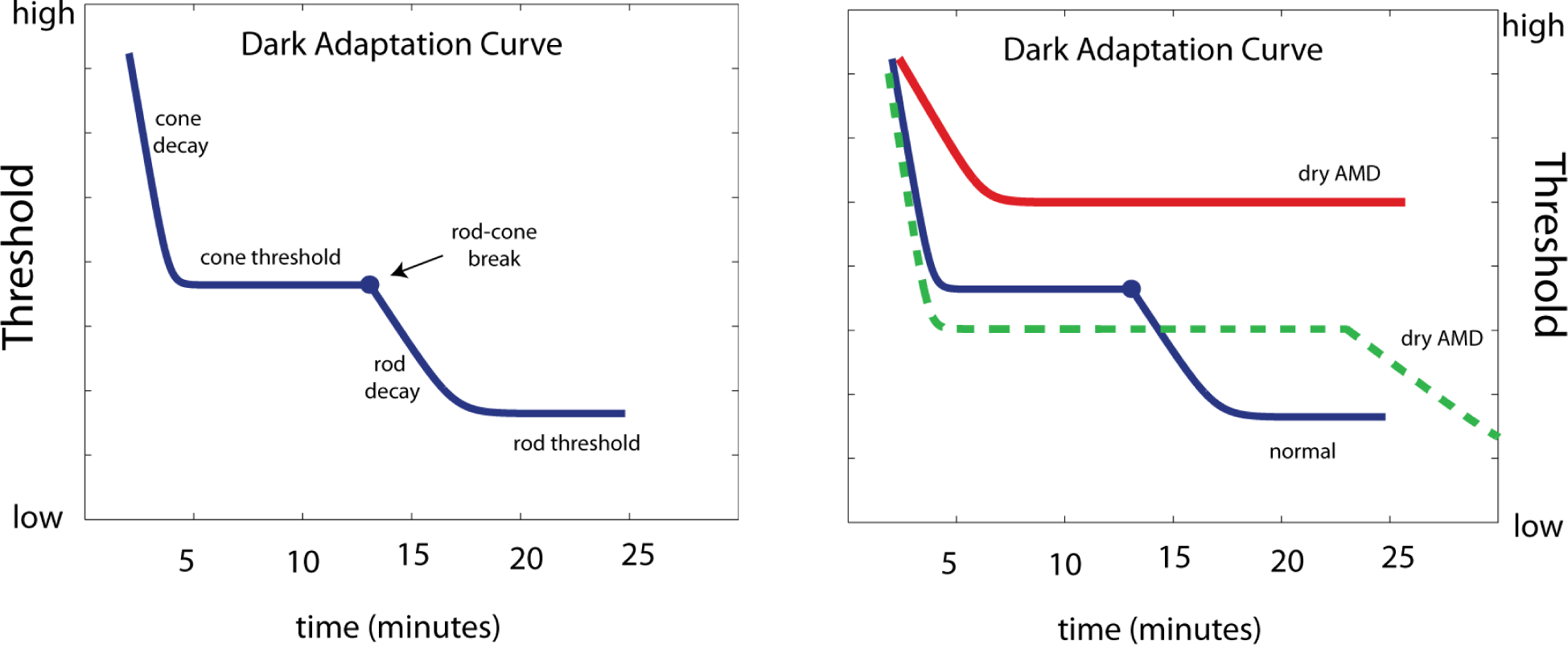
For one subject with normal vision, and three with AMD, visual sensitivity thresholds are measured for prolonged periods (40–90 min) following photopigment bleaching. The AMD patients show longer recovery times, which are attributed to rod dysfunction specific to AMD (after Dimitrov et al, 2008).
Strengths and Limitations
The strengths of these candidate endpoints include their relationships to subjective visual impairment and anatomical endpoints associated with dry AMD. Limitations that include endpoint imprecision or difficulty (impracticality) of test application are typical problems of these emerging technologies, which can potentially be addressed through further research and development.
Contrast sensitivity, reading speed, and dark adaptation are related with subjective visual experience and visual quality of life. The value of the connection between objective and subjective visual assessments is reinforced by the emerging importance of Patient Reported Outcomes [43]. Ultimately, patients seek clinical interventions that improve visual quality of life, e.g. through functional outcomes that maintain driving or reading. Both patients and regulatory bodies will favorably view any therapy that provides immediately tangible benefits for vision [4] [5].
The emerging importance of relating anatomical and functional endpoints [44], and the potential for anatomical endpoints as primary outcome measures [4], are important issues in clinical trials for ocular disease. Microperimetry, which can potentially relate anatomical and functional endpoints in individuals, therefore presents an especially interesting endpoint candidate. Despite microperimetry studies demonstrating that poor visual sensitivity corresponds to observable retinal atrophy, the converse demonstration, that functional sensitivity deficits emerge from apparently healthy retina, supports the regulatory viewpoint that functional endpoints provide more important assessments of visual quality of life. Until imaging technology improves the reliability for detecting dry AMD in its early stages, the greatest value of anatomical endpoints will likely come from their combination with functional endpoints, for clinical trial recruitment and patient stratification that will provide larger potential treatment effects and better sample homogeneity for clinical trials ([45]; [46]; see the next section, Impact on Clinical Trial Design).
Test precision and ease of application are critical to endpoint utility in clinical trials [6]. There is an unfortunate basic trade-off between test duration and precision: tests that are short and easy to apply tend to be imprecise, and precision requires longer testing times that are uncomfortable for patients. Long testing times and the need for pupil dilation are practical limitations of dark adaptation, which do not guarantee precise results [47]. Shortening longer protocols (from 90 to 20 min) maintains diagnostic accuracy [41], but clearer determination of the precision of dark adaptation parameter estimates will be important for clinical trial design.
Measuring the full CSF has likewise presented testing time problems. Consequently, charts measuring contrast sensitivity using either letter [48] or gratings [49] compromise by focusing on different parts of the static CSF [50] and precluding the assessment of temporal vision. These tests are easy to apply, but have demonstrable imprecision [51]. Recent advances in computerized adaptive testing -- intense computational strategies made possible by increasing computing power -- permit large testing time reductions without sacrificing much precision. For example, the quick CSF method applies an adaptive sampling algorithm, which uses knowledge of the CSF shape and a trial-to-trial information gain strategy, to reduce CSF testing times from 30 to <5 minutes [52] [53]. Importantly, despite the testing time reduction, variability of sensitivity estimates (std. dev. =.20 decimal log units) remains smaller than current contrast sensitivity charts [50], and comparable to the size of CSF deficits in dry AMD patients with normal acuity and early drusen [20][21].
Microperimetry is expensive, and though gaining ground in clinical research, it lack standards and remains underused in clinics [34]. Microperimetry lacks a testing algorithm that can reduce the testing time in a targeted way, as SITA does for visual field testing in glaucoma [54]. Expensive equipment makes it hard for the eventual incorporation of any effective endpoints into standard clinical care, which would provide a valuable connection to subjective visual health and patient monitoring and compliance. With time, wider adoption of this technology will determine the strengths and limitations of different imaging modalities, and better standards for testing protocols that ensure reliable, standardized assessment of visual function at diagnostic retinal locations, registration of different assessments and quantification and tracking of sensitivity changes over time.
Impact on Clinical Trial Design
The challenges presented by a dry AMD clinical trial, and the potential benefits provided by development and validation of novel endpoints, are demonstrated by a hypothetical trial using an acuity endpoint. To illustrate how endpoint properties critically affect trial design, (i.e., trial length and sample size), consider a study with a parallel group design and repeated measurements. Though the acuity endpoint has historically been defined as the group proportion experiencing more than three lines of vision loss, this endpoint faces obvious ceiling effects and can even introduces biases [10]. A better endpoint is defined by the mean letter difference between acuities measured at baseline and end-of-study. Alternatively, longitudinal design with endpoint assessment at more than two time-points would evaluate the rate of progressive vision loss (letters/yr) and its potential slowing by treatment [55]. With an assumption of linear progression of vision loss, a calculation for total required sample size, N, is:
where α is the significance level (p = 5%), β is the statistical power (e.g., 90%), and N includes the total sample of treatment and control groups. In this design, the statistical power for estimating differences in disease progression is constrained by: (1) the size of treatment effect, Δrate, (2) between-subjects variability σbetween, which reflects both population variability in the treatment effect and misfit of the linear progression assumption, (3) within-subjects variability σbetween, which reflects measurement error (i.e., endpoint standard deviation), and (4) the endpoint assessment schedule which is comprised of (a) the total number of assessments and (b) the time between assessments (see [55] for assessment notation details). The general effects of these factors on sample size are:
Put simply, sample sizes are reduced by either increasing the treatment effect, reducing variability, or increasing the number and spacing of endpoint assessments. These changes can be accomplished by stratifying patients using selection criteria [45], improving endpoint precision [43], or increasing trial length and adding intermediate endpoint assessments. Because Equation 1 contains squared terms for these endpoint properties, changes in these factors can rapidly and dramatically change sample size calculations (see Box 1).
Box 1. Impact of endpoint properties on clinical trial design.
When evaluating potential treatments for slowly progressing eye disease, the challenge is designing trials that are short in length and small in sample size. Consider a two-year dry AMD trial with two endpoint assessments (baseline and end-of-study), which applies selection criteria to recruit patients with relatively rapid progression (8.5 letters over 2 years; standard deviation = 14 letters; [44]). The assumption of a 50% treatment effect (4.25×.50=2.1 letters/yr), with within-and between- subjects standard deviations of 14 and 2 letters, yields a total sample size estimate of 1093, given an assumed statistical power of β=90% and one-tailed significance level of α = 5%.
To demonstrate how endpoint and clinical trial factors interact, Figure 4 presents how sample size can be reduced by a) increasing treatment effect increase, presented for three levels of standard deviation reduction,(b) reducing standard deviation, presented for three levels of treatment effect increase, (c) increasing trial length, presented for three levels of total assessments, and (d) increasing total assessments, presented for three levels of trial length. This presentation helps quantify intuition about clinical trial design. For example, increasing the clinical treatment effect by 10% – by using selection criteria to choose patients more likely to progress-- reduces sample size by 17%. Reducing the endpoint standard deviation by 10% —e.g., by applying longer or more rigorous assessments at each clinical visit--reduces sample size by 14%. The effects of reducing standard deviation are smaller because of the constraints of between-subjects variability. The effects are synergistic: endpoint development that concurrently increases the treatment effect by 10% and reduces standard deviation by 10% yields a sample size reduction of 29%. Panels (c) and (d) demonstrate the dramatic effects of changing the endpoint assessment schedule. Extending trial length from 2 to 4 years or adding 24 assessment points reduces sample size to 40% of baseline. These gains are not without potentially prohibitive practical costs. Longer trials do not accelerate the drug discovery cycle, and monthly visits to clinical sites increase patient burden and costs of specialized staff and equipment.
Home self-assessment may resolve the practical problems associated with aggressive endpoint assessment schedules, while still providing the sample size reduction benefits. For the reviewed endpoints, incorporation in future clinical trials as exploratory endpoints will be critical for determining their treatment effects and precision, and thus their ultimate impact on clinical trial designs for dry AMD.
Figure 4.
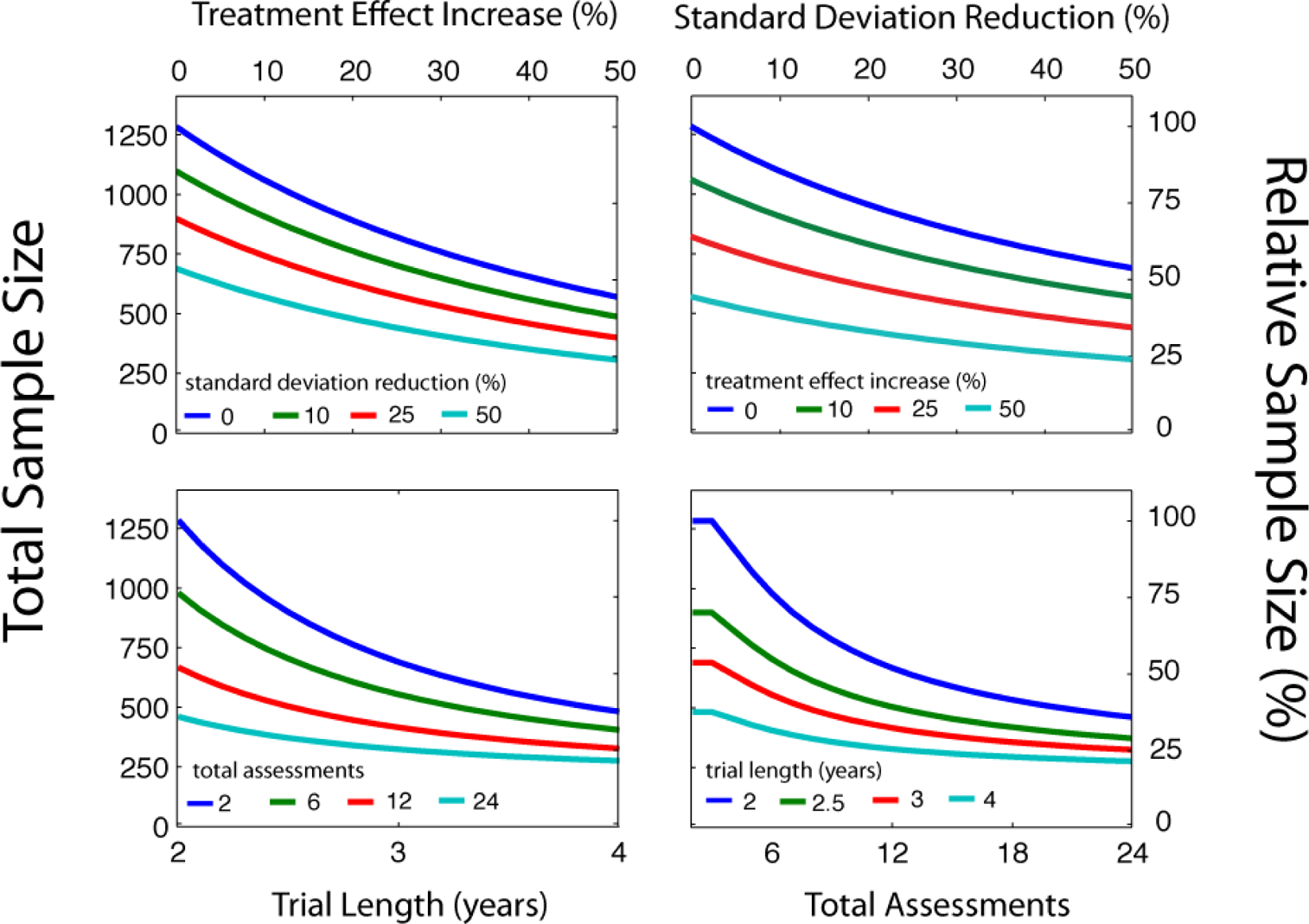
For the case of a dry AMD clinical trial, consider how sample size calculations might be affected by endpoint properties. Following an approach used for other slowly progressing visual diseases [44], Azuma et al (2010) propose recruiting dry AMD patients with grade 3 and 4 disease, who exhibit greater visual acuity loss -- 8.5 letters of BCVA over 2 years (standard deviation=14 letters)-- than grade 1 and 2 patients who show none [45]. Stratifying patients using these criteria provides a sample with larger potential treatment effects over shorter, more feasible trial lengths. A baseline clinical design, with high statistical power (90%), yields a total sample size of 1000–1100 subjects to be assessed over 2 years. Box 1 quantifies the impact on this design of several feasible endpoint developments. One notable effect is the great sample size reduction that results from improving endpoint reliability and reducing variability. Another striking effect is the reduction provided by an aggressive assessment schedule (e.g., 25–50% of baseline with 24 monthly exams). Given these potential benefits, home-based self-assessment [47] provides an intriguing platform for increasing patient assessment while minimizing the practical costs of medical equipment and personnel. Patient compliance for home vision-testing can be high - 85% of dry AMD patients complied with a once-weekly mobile-phone testing vision regimen [11], and results can compare favorably to clinical lab testing [57]. It should be noted that the potential sample-size benefits described above, provided by increasing the reliability or frequency of endpoint assessment, are fundamentally constrained by other factors typically ignored in many sample size calculations [55]. These include the variability in treatment effects between individuals, variability in disease progression, deviations from the assumption of linear progression, and non-uniformity of endpoint variability across vision loss levels. Careful studies that determine which constraints are specific to disease, and which are specific to endpoints, will be critical.
For the potential visual function endpoints reviewed here, one advantage is that deficits are apparent at early disease stages, relative to the acuity losses that are not detectable until advancing disease. For example, in patients with normal acuity, multiple studies, [20][21], have reported clinically meaningful CSF deficits (.15–.20 log units across multiple spatial frequencies), comparable in size to the variability of efficient laboratory contrast sensitivity testing [51], or even testing on a consumer mobile device [58].Though the relative size of this treatment effect makes the CSF an intriguing prospect, likewise unknown is the variability of disease progression. For funding agencies or trial sponsors, including these functional measures as exploratory endpoints in upcoming trials, establishing how these endpoints behave in control populations, would provide valuable and critical knowledge about their potential use for future trial decisions.
Endpoints with large treatment effects, which can be easily and continuously measured in early disease stages, and which precede the anatomical endpoint provided by geographic atrophy, will provide tremendous value. The most consistent benefits in clinical trial design will likely rely on concerted efforts of functional endpoint development and patient recruitment that uses both functional and anatomical endpoints.
CONCLUSION
Dry AMD presents significant ocular insult and consequently affects many different visual functions. This review considers functions that may serve as potential endpoints for dry AMD clinical trials. The ideal endpoint would provide a measure of both subjective and objective vision, which can flexibly describe the range of functional disability, and be measured in a rapid and relatively precise way. Further research, which evaluates potential endpoints on these criteria, will evaluate how new endpoints contribute to smaller, faster and more effective clinical trials and thereby improve drug discovery. This knowledge will be useful for endpoint development for other slowly progressing vision diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE, and Klein R, “An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group,” Surv Ophthalmol, vol. 39, no. 5, pp. 367–374, April 1995. [DOI] [PubMed] [Google Scholar]
- [2].Cook HL, Patel PJ, and Tufail A, “Age-related macular degeneration: diagnosis and management,” British Medical Bulletin, vol. 85, no. 1, pp. 127–149, March 2008. [DOI] [PubMed] [Google Scholar]
- [3].Sallo FB, Rechtman E, Peto T, Stanescu-Segall D, Vogt G, Bird AC, and Fitzke FW, “Functional aspects of drusen regression in age-related macular degeneration,” British Journal of Ophthalmology, vol. 93, no. 10, pp. 1345–1350, June 2009. [DOI] [PubMed] [Google Scholar]
- [4].Csaky KG, Richman EA, and Ferris FL III, “Report from the NEI/FDA ophthalmic clinical trial design and endpoints symposium,” Investigative ophthalmology & visual science, vol. 49, no. 2, pp. 479–489, 2008. [DOI] [PubMed] [Google Scholar]
- [5].Fleming TR and Powers JH, “Biomarkers and surrogate endpoints in clinical trials,” Statistics in Medicine, p. 10.1002/sim.5403, June 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Drum B, Calogero D, and Rorer E, “Assessment of visual performance in the evaluation of new medical products,” Drug Discovery Today: Technologies, vol. 4, no. 2, pp. 55–61, 2007. [DOI] [PubMed] [Google Scholar]
- [7].Klein R, Wang Q, Klein B, Moss SE, and Meuer SM, “The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity.,” Investigative ophthalmology & visual science, vol. 36, no. 1, pp. 182–191, 1995. [PubMed] [Google Scholar]
- [8].Patel PJ, Chen FK, Rubin GS, and Tufail A, “Intersession repeatability of visual acuity scores in age-related macular degeneration,” Investigative ophthalmology & visual science, vol. 49, no. 10, p. 4347, 2008. [DOI] [PubMed] [Google Scholar]
- [9].Sunness JS, Rubin GS, Applegate CA, Bressler NM, Marsh MJ, Hawkins BS, and Haselwood D, “Visual Function Abnormalities and Prognosis in Eyes with Age-related Geographic Atrophy of the Macula and Good Visual Acuity,” Ophthalmology, vol. 104, no. 10, pp. 1677–1691, October 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, and Ferris FL, “Visual Acuity as an Outcome Measure in Clinical Trials of Retinal Diseases,” Ophthalmology, vol. 114, no. 10, pp. 1804–1809, October 2007. [DOI] [PubMed] [Google Scholar]
- [11].Marron JA and Bailey IL, “Visual factors and orientation-mobility performance,” American Journal of Optometry and Physiological Optics, vol. 59, pp. 413–26, 1982. [DOI] [PubMed] [Google Scholar]
- [12].Owsley C and Sloane ME, “Contrast sensitivity, acuity, and the perception of ‘real-world’ targets.,” British Journal of Ophthalmology, vol. 71, no. 10, pp. 791–796, October 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Owsley C, “Contrast sensitivity,” Ophthalmology Clinics of North America, vol. 16, no. 2, pp. 171–178, 2003. [DOI] [PubMed] [Google Scholar]
- [14].Owsley C, Sekuler R, and Siemsen D, “Contrast sensitivity throughout adulthood,” Vision Research, vol. 23, no. 7, pp. 689–699, 1983. [DOI] [PubMed] [Google Scholar]
- [15].Mei M and Leat SJ, “Suprathreshold Contrast Matching in Maculopathy,” Investigative Ophthalmology & Visual Science, vol. 48, no. 7, pp. 3419–3424, July 2007. [DOI] [PubMed] [Google Scholar]
- [16].Lennerstrand G and Ahlström C, “Contrast sensitivity in macular degeneration and the relation to subjective visual impairment,” Acta Ophthalmologica, vol. 67, no. 3, pp. 225–233, June 1989. [DOI] [PubMed] [Google Scholar]
- [17].Brown B, “Reading performance in low vision patients: relation to contrast and contrast sensitivity,” Am J Optom Physiol Opt, vol. 58, no. 3, pp. 218–226, March 1981. [DOI] [PubMed] [Google Scholar]
- [18].Keane PA, Patel PJ, Ouyang Y, Chen FK, Ikeji F, Walsh AC, Tufail A, and Sadda SR, “Effects of retinal morphology on contrast sensitivity and reading ability in neovascular age-related macular degeneration,” Investigative ophthalmology & visual science, vol. 51, no. 11, pp. 5431–5437, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qiu F and Leat SJ, “Functional deficits in early stage age-related maculopathy,” Clinical and Experimental Optometry, vol. 92, no. 2, pp. 90–98, 2009. [DOI] [PubMed] [Google Scholar]
- [20].Loshin DS and White J, “Contrast Sensitivity - the Visual Rehabilitation of the Patient with Macular Degeneration,” Arch. Ophthalmol, vol. 102, no. 9, pp. 1303–1306, 1984. [DOI] [PubMed] [Google Scholar]
- [21].Kleiner RC, Enger C, Alexander MF, and Fine SL, “Contrast sensitivity in age-related macular degeneration,” Arch. Ophthalmol, vol. 106, no. 1, pp. 55–57, January 1988. [DOI] [PubMed] [Google Scholar]
- [22].Midena E, Degli Angeli C, Blarzino MC, Valenti M, and Segato T, “Macular function impairment in eyes with early age-related macular degeneration.,” Investigative ophthalmology & visual science, vol. 38, no. 2, pp. 469–477, 1997. [PubMed] [Google Scholar]
- [23].Mayer MJ, Spiegler SJ, Ward B, Glucs A, and Kim CB, “Mid-frequency loss of foveal flicker sensitivity in early stages of age-related maculopathy,” Invest. Ophthalmol. Vis. Sci, vol. 33, no. 11, pp. 3136–3142, October 1992. [PubMed] [Google Scholar]
- [24].Anderson AJ and Vingrys AJ, “Multiple processes mediate flicker sensitivity,” Vision Research, vol. 41, no. 19, pp. 2449–2455, September 2001. [DOI] [PubMed] [Google Scholar]
- [25].Legge GE, Rubin GS, Pelli DG, and Schleske MM, “Psychophysics of reading--II. Low vision,” Vision Research, vol. 25, no. 2, pp. 253–65, 1985. [DOI] [PubMed] [Google Scholar]
- [26].Owsley C, McGwin G Jr, Lee PP, Wasserman N, and Searcey K, “Characteristics of low-vision rehabilitation services in the United States,” Archives of ophthalmology, vol. 127, no. 5, p. 681, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mitchell J, Wolffsohn J, Woodcock A, Anderson SJ, Ffytche T, Rubinstein M, Amoaku W, and Bradley C, “The MacDQoL Individualized Measure of the Impact of Macular Degeneration on Quality of Life: Reliability and Responsiveness,” American Journal of Ophthalmology, vol. 146, no. 3, pp. 447–454.e2, September 2008. [DOI] [PubMed] [Google Scholar]
- [28].Rubin GS and Feely M, “The Role of Eye Movements During Reading in Patients with Age-Related Macular Degeneration (AMD),” Neuro-Ophthalmology, vol. 33, no. 3, pp. 120–126, January 2009. [Google Scholar]
- [29].Cacho I, Dickinson CM, Smith HJ, and Harper RA, “Clinical impairment measures and reading performance in a large age-related macular degeneration group,” Optometry & Vision Science, vol. 87, no. 5, p. 344, 2010. [DOI] [PubMed] [Google Scholar]
- [30].Richter-Mueksch S, Stur M, Stifter E, and Radner W, “Differences in reading performance of patients with Drusen maculopathy and subretinal fibrosis after CNV,” Graefe’s Archive for Clinical and Experimental Ophthalmology, vol. 244, no. 2, pp. 154–162, 2006. [DOI] [PubMed] [Google Scholar]
- [31].Whitney D and Levi DM, “Visual crowding: a fundamental limit on conscious perception and object recognition,” Trends in Cognitive Sciences, vol. 15, no. 4, pp. 160–168, April 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Frennesson C, Nilsson UL, Peebo BB, and Nilsson SEG, “Significant improvements in near vision, reading speed, central visual field and related quality of life after ranibizumab treatment of wet age-related macular degeneration,” Acta Ophthalmol, vol. 88, no. 4, pp. 420–425, June 2010. [DOI] [PubMed] [Google Scholar]
- [33].Källmark FP, “Increased Visual Acuity Will Not Necessarily Equal an Increased Reading Ability in Patients with Subfoveal Neovascular Macular Degeneration,” International Journal of Clinical Medicine, vol. 02, no. 04, pp. 404–410, 2011. [Google Scholar]
- [34].Crossland MD, Jackson M, and Seiple WH, “Microperimetry: a review of fundus related perimetry,” Optometry Reports, vol. 2, no. 1, pp. 11–15, 2012. [Google Scholar]
- [35].Schuchard RA and Raasch TW, “Retinal locus for fixation: Pericentral fixation targets,” Clinical Vision Science, vol. 7, no. 6, pp. 511–520, 1992. [Google Scholar]
- [36].Whittaker SG, Cummings RW, and Swieson LR, “Saccade control without a fovea,” Vision Research, vol. 31, no. 12, pp. 2209–2218, 1991. [DOI] [PubMed] [Google Scholar]
- [37].Midena E, Vujosevic S, Convento E, Manfre A’, Cavarzeran F, and Pilotto E, “Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration,” British Journal of Ophthalmology, vol. 91, no. 11, pp. 1499–1503, May 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Owsley C, McGwin G, Scilley K, and Kallies K, “Development of a Questionnaire to Assess Vision Problems under Low Luminance in Age-Related Maculopathy,” IOVS, vol. 47, no. 2, pp. 528–535, February 2006. [DOI] [PubMed] [Google Scholar]
- [39].Feigl B, Cao D, Morris CP, and Zele AJ, “Persons with Age-Related Maculopathy Risk Genotypes and Clinically Normal Eyes Have Reduced Mesopic Vision,” IOVS, vol. 52, no. 2, pp. 1145–1150, February 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lamb TD and Pugh EN Jr, “Dark adaptation and the retinoid cycle of vision,” Prog Retin Eye Res, vol. 23, no. 3, pp. 307–380, May 2004. [DOI] [PubMed] [Google Scholar]
- [41].Jackson GR and Edwards JG, “A short-duration dark adaptation protocol for assessment of age-related maculopathy,” Journal of Ocular Biology, Diseases, and Informatics, vol. 1, no. 1, pp. 7–11, May 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Clark ME, McGwin G, Neely D, Feist R, Mason JO, Thomley M, White MF, Ozaydin B, Girkin CA, and Owsley C, “Association between retinal thickness measured by spectral-domain optical coherence tomography (OCT) and rod-mediated dark adaptation in non-exudative age-related maculopathy,” British Journal of Ophthalmology, vol. 95, no. 10, pp. 1427–1432, February 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Varma R, Richman EA, Ferris FL, and Bressler NM, “Use of Patient-Reported Outcomes in Medical Product Development: A Report from the 2009 NEI/FDA Clinical Trial Endpoints Symposium,” IOVS, vol. 51, no. 12, pp. 6095–6103, December 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Quigley HA, “Clinical trials for glaucoma neuroprotection are not impossible,” Current Opinion in Ophthalmology, vol. 23, no. 2, p. 144, 2012. [DOI] [PubMed] [Google Scholar]
- [45].Azuma M, Chung K, Fujii A, and Shearer TR, “Patient Selection Criteria for Pilot Studies on Amelioration of Non-Neovascular Age-Related Macular Degeneration,” Journal of Ocular Pharmacology and Therapeutics, vol. 26, no. 4, pp. 367–371, August 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sunness JS, Applegate CA, Bressler NM, and Hawkins BS, “Designing Clinical Trials For Age-Related Geographic Atrophy Of The Macula,” Retina, vol. 27, no. 2, pp. 204–210, February 2007. [DOI] [PubMed] [Google Scholar]
- [47].Trevino R, “Recent progress in macular function self-assessment,” Ophthalmic and Physiological Optics, vol. 28, no. 3, pp. 183–192, 2008. [DOI] [PubMed] [Google Scholar]
- [48].Pelli DG, Robson JG, and Wilkins AJ, “The design of a new letter chart for measuring contrast sensitivity,” Clinical Vision Science, vol. 2, pp. 187–199, 1988. [Google Scholar]
- [49].Ginsburg A, “A new contrast sensitivity vision test chart,” American Journal of Optometry & Physiological Optics, vol. 61, pp. 403–407, 1984. [DOI] [PubMed] [Google Scholar]
- [50].Leat SJ and Woo GC, “The validity of current clinical tests of contrast sensitivity and their ability to predict reading speed in low vision,” Eye, vol. 11, no. 6, pp. 893–899, November 1997. [DOI] [PubMed] [Google Scholar]
- [51].Pesudovs K, Hazel CA, Doran RML, and Elliott DB, “The usefulness of Vistech and FACT contrast sensitivity charts for cataract and refractive surgery outcomes research,” Br J Ophthalmol, vol. 88, no. 1, pp. 11–16, January 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lesmes LA, Lu Z-L, Baek J, and Albright TD, “Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method,” J Vis, vol. 10, no. 3, pp. 17.1–21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lesmes LA, Wallis J, Jackson M, and Bex PJ, “Clinical Application Of A Novel Contrast Sensitivity Test To A Low Vision Population: The Quick CSF Method,” in Invesigative Ophthalmology and Visual Science, 2012. [Google Scholar]
- [54].Bengtsson B and Heijl A, “Evaluation of a new perimetric threshold strategy, SITA, in patients with manifest and suspect glaucoma,” Acta Ophthalmol Scand, vol. 76, no. 3, pp. 268–72, 1998. [DOI] [PubMed] [Google Scholar]
- [55].Peters SAE, Palmer MK, den Ruijter HM, Grobbee DE, Crouse III JR, O’Leary DH, Evans GW, Raichlen JS, and Bots ML, “Sample size requirements in trials using repeated measurements and the impact of trial design,” Current Medical Research and Opinion, vol. 28, no. 5, pp. 681–688, May 2012. [DOI] [PubMed] [Google Scholar]
- [56].Wang YZ, “Handheld Shape Discrimination Hyperacuity (hSDH) Test on a Mobile Device for Remote Monitoring of Visual Function in Maculopathy,” in ARVO, Fort Lauderdale, Florida, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bittner AK, Ibrahim MA, Haythornthwaite JA, Diener-West M, and Dagnelie G, “Vision Test Variability in Retinitis Pigmentosa and Psychosocial Factors,” Optometry and Vision Science, vol. 88, no. 12, pp. 1496–1506, September 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dorr M, Lesmes LA, Lu ZL, and Bex PJ, “Rapid and precise contrast sensitivity assessment on a tablet device,” Journal of Internet Medicine Research, no. in press, 2012. [Google Scholar]


