Abstract
Background:
Automated closed-loop control (CLC), known as the “artificial pancreas” is emerging as a treatment option for Type 1 Diabetes (T1D), generally superior to sensor-augmented insulin pump (SAP) treatment. It is postulated that evening-night (E-N) CLC may account for most of the benefits of 24–7 CLC; however, a direct comparison has not been done.
Methods:
In this trial (NCT02679287), adults with T1D were randomised 1:1 to two groups, which followed different sequences of four 8-week sessions, resulting in two crossover designs comparing SAP vs E-N CLC and E-N CLC vs 24–7 CLC, respectively. Eligibility: T1D for at least 1 year, using an insulin pump for at least six months, ages 18 years or older. Primary hypothesis: E-N CLC compared to SAP will decrease percent time <70mg/dL (3.9mmol/L) measured by continuous glucose monitoring (CGM) without deterioration in HbA1c. Secondary Hypotheses: 24–7 CLC compared to SAP will increase CGM-measured time in target range (TIR, 70–180mg/dL; 3.9–10mmol/L) and will reduce glucose variability during the day.
Findings:
Ninety-three participants were randomised and 80 were included in the analysis, ages 18–69 years; HbA1c levels 5.4–10.6%; 66% female. Compared to SAP, E-N CLC reduced overall time <70mg/dL from 4.0% to 2.2% () resulting in an absolute difference of 1.8% (95%CI: 1.2–2.4%), p<0.0001. This was accompanied by overall reduction in HbA1c from 7.4% at baseline to 7.1% at the end of study, resulting in an absolute difference of 0.3% (95% CI: 0.1–0.4%), p<0.0001. There were 5 severe hypoglycaemia adverse events attributed to user-directed boluses without malfunction of the investigational device, and no diabetic ketoacidosis events.
Interpretation:
In type 1 diabetes, evening-night closed-loop control was superior to sensor-augmented pump therapy, achieving most of the glycaemic benefits of 24–7 closed-loop.
Keywords: Continuous Glucose Monitoring (CGM), Continuous Subcutaneous Insulin Infusion (CSII), Closed-Loop Control (CLC), Artificial Pancreas
INTRODUCTION
It is well established that achieving tight glycaemic control remains a challenge in the management type 1 diabetes (T1D), particularly with the attendant increased risk of hypoglycaemia [1, 2]. Automated insulin delivery systems, known as the “artificial pancreas” or closed-loop control (CLC) of diabetes, have been developed in the past decade and offer the opportunity to minimize these glycaemic risks. The progress in the CLC field was reflected by a number of reviews and meta-analyses [3–8]. In the past three years: (i) a pivotal trial of the first commercial hybrid CLC system was completed – the Medtronic 670G which automatically modulates basal rates but not insulin boluses [9] – and the system was cleared by the FDA for clinical use; (ii) a six-month feasibility study reported improvement in glycemic control and reduction of hypoglycemia with long-term CLC use [10]; (iii) NIH/NIDDK funded 4 major research efforts to test advanced CLC systems; (iv) the first artificial pancreas ski camp was successfully completed showing that the use of CLC during winter sports is safe and effective for children with T1D [11], and a CLC system was successfully tested in high-risk patients, i.e. those who experience frequent hypoglycemia, or have history of severe hypoglycemia or hypoglycemia unawareness [12].
It is important to note that to date, virtually all CLC systems that have been tested in clinical trials are considered “Hybrid Closed Loop” in that the user is expected to announce meals to the system, leading to prandial boluses that resemble standard pump therapy. Furthermore, except for the Medtronic 670G system in which the CLC algorithm was embedded in the insulin pump, all other trials used an outboard controller, i.e. the algorithm was implemented in a different device, typically a smart phone. These systems became known as mobile CLC and are gaining popularity. The first mobile CLC was DiAs – the Diabetes Assistant developed at the University of Virginia by our team [13]. In 2012–2013, DiAs enabled the first outpatient trial of mobile CLC [14] and been used in a number of outpatient CLC studies [15–18]. More recently, two studies using different algorithms implemented in Android smart phones, reported mobile AP use of 12 weeks by significant number of participants – 86 [19] and 68 [20], respectively.
The International Diabetes Closed Loop (iDCL) Trial – a large-scale NIH-funded project (grant UC4 DK 108483) – evaluated CLC as a clinically accepted treatment for T1D, with two multi-centre clinical protocols testing a mobile (Protocol 1, NCT02985866) and an embedded (Protocol 3, NCT03563313) closed-loop system, both using the same control algorithm [21,22]. The new embedded CLC system – Control-IQ manufactured by Tandem Diabetes Care – uses a Dexcom G6 continuous glucose monitoring (CGM) sensor without fingerstick calibration and a t:slim X2 insulin pump. Protocol 3 was a pivotal trial aiming for regulatory clearance by enrolling 168 participants with T1D randomised 2:1 to CLC vs. sensor-augmented insulin pump (SAP) for 6 months. The results showed that CLC was superior to sensor-augmented pump therapy according to all accepted glycemic control criteria, including metrics reflecting hypoglycemia, hyperglycemia, average glucose, and glycemic variability [23].
While the most advanced CLC systems have consistently shown improved overnight glycaemic control, postprandial control remains a challenge [19,20,21,23]. Thus, in this study, we sought to compare different configurations of a CLC system with activation of automated insulin delivery during the evening-overnight hours (E-N CLC) compared to continuous 24–7 CLC, with both treatment modalities compared to SAP use. In addition, we compared mobile vs embedded CLC in exploratory analysis using the same two systems used by the iDCL Protocol 1 (NCT02985866) and Protocol 3 (NCT03563313) of the iDCL Trial described above. Since direct comparisons of E-N vs 24–7 have not been attempted before, the intent of this manuscript is to add this new dimension to the CLC literature.
METHODS
Study Conduct and Oversight:
This unblinded, parallel group randomised trial of approximately 10-months (four 8-week sessions with 2-week washouts) duration was conducted at the University of Virginia. The protocol was approved by an Institutional Review Board, and written informed consent was obtained as required. An Investigational Device Exemption was approved by the Food and Drug Administration. A Data and Safety Monitoring Board provided trial oversight.
Study Participants:
Participants were recruited from local clinics and an internet-based recruiting database of individuals who indicated an interest in studies at the UVA Center for Diabetes Technology. Elligibility criteria (see Supplemental Table 1) included: 18 to <70 years of age with a clinical diagnosis of type 1 diabetes for at least one year, using insulin for at least 1 year and an insulin pump for at least 6 months; currently using an insulin-to-carbohydrate ratio to calculate meal bolus sizes; and using or willing to use an insulin approved for use in the study pumps; and, for females, not currently pregnant. Exclusion criteria included: diabetic ketoacidosis in the previous 12 months; severe hypoglycaemia resulting in seizure or loss of consciousness in the previous 12 months; history of a seizure disorder; pregnancy; cystic fibrosis; coronary artery disease or heart failure; known medical condition that might interfere with the completion of the protocol; basal rates <0.1 units/hour or minimal total basal rates <2.40 units/day; and use of any medication the investigator believes is a contraindication to participation (e.g. liraglutide).
Study Design:
The trial consisted of a screening visit to determine eligibility, collect demographic and baseline questionnaire and glucose control data (e.g. HbA1c). The participants were randomised and proceeded with a run-in phase to train on study devices relevant to the study session. Each run-in phase was up to two-weeks long tailored to the participants experience with the devices; CGM and pump run-ins were allowed to overlap. A CGM run-in period was required for CGM naïve users, was optional for prior CGM users and was not required for current CGM users. For the mobile AP system, a run-in period using only the study pump for up to two weeks was required unless the participant’s personal pump was the same model. The mobile phone was then added and the system was used in open-loop mode for a minimum of 5 days. For the embedded system, an open-loop pump run-in was required for all participants for a minimum 5 day run-in period. The run-in periods were followed by four 8-week treatment sessions (Figure 1). Each session included one of the following therapies: Sensor-Augmented Pump (SAP – control condition); Evening-Night (E-N) CLC in which participants were instructed to initiate CLC every evening between dinner and bedtime and then discontinue CLC use at wakeup, and 24–7 CLC (continuous CLC use). The sessions were separated by 2-week washout periods in which participants resumed their usual care (personal insulin pump or a study-provided pump without CLC) and HbA1c was measured at a local laboratory (LabCorp, Burlington, NC). Questionnaires were collected and structured interviews were performed, not reported in this paper. Following run-in phases, study staff contacted participants at least once per month during SAP and daily during the initial week of CLC, followed by weekly to bi-weekly contact. Participants were asked to download study devices at least bi-weekly.
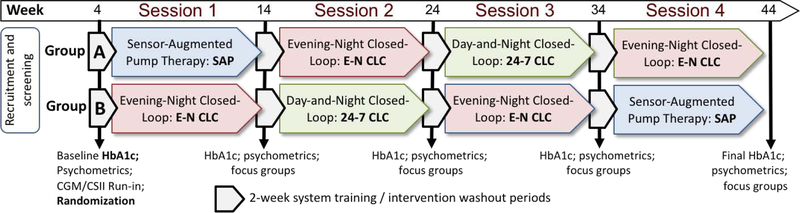
Figure 1.
Study design
Panel 1: Overall Design
Panel 1: Study participants were randomized into one of two groups: Group A following the sequence of sessions SAP → E-N CLC → 24–7 CLC → E-N CLC, and Group B following the sequence of sessions EN CLC → 24–7 CLC → E-N CLC → SAP. Each treatment session continued for 8 weeks; treatment sessions were separated by 2-week washout periods during which HbA1c was measured and questionnaires were collected.
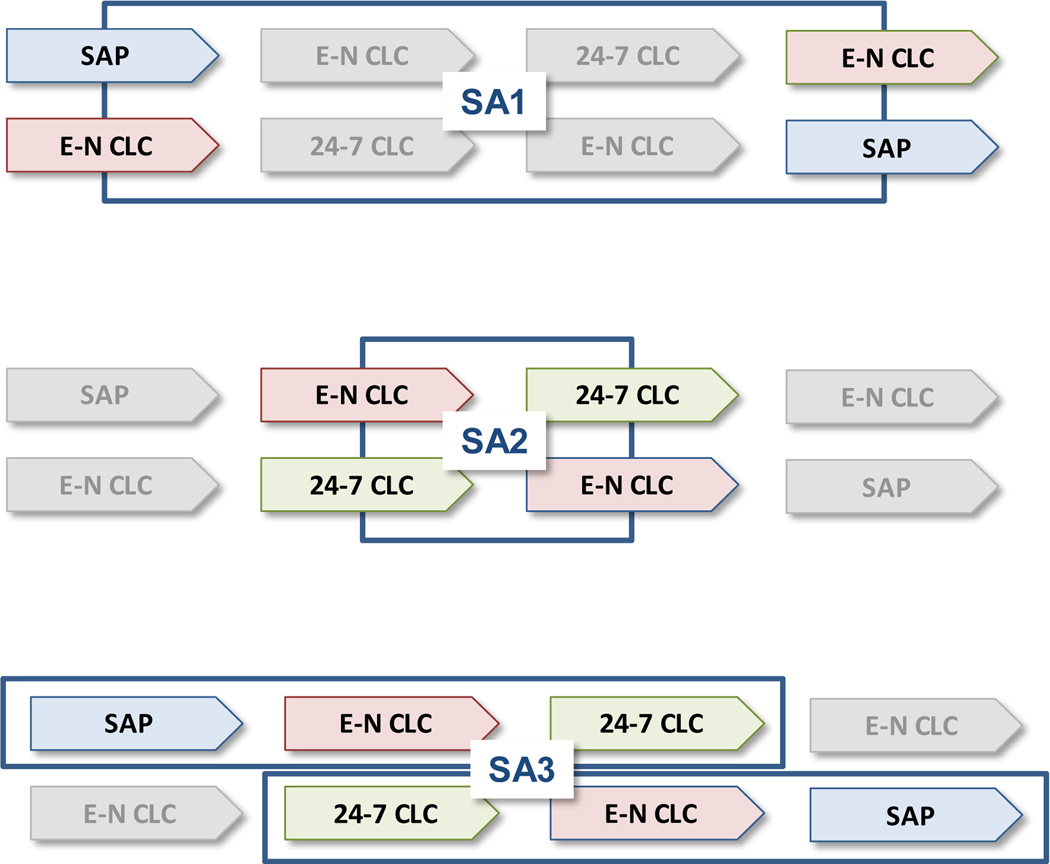
Panel 2: Sub-analyses (SAs) addressing the study objectives:
Panel 2: The overall design include two cross-over designs: SA1 comparing SAP vs. Evening-Night Closed-Loop Control and SAP during the day, and SA2 comparing Evening-Night Closed-Loop Control and SAP during the day vs 24–7 closed-loop control. A third sub-analysis, SA3, allowed the comparison of escalating vs. de-escalting treatments, SAP → E-N CLC → 24–7 CLC (Group A) vs. 24–7 CLC → E-N CLC → SAP (Group B).
Randomisation:
Randomisation was performed by non-clinical study staff using a computer-generated sequence of Bernoulli (binomial) trials resulting in 1:1 assignments to two groups: Group A following the sequence of treatment sessions SAP → E-N CLC → 24–7 CLC → E-N CLC and Group B following the sequence of sessions E-N CLC → 24–7 CLC → E-N CLC → SAP, after randomization.
Study Devices:
During closed-loop sessions, study participants used either a mobile version or an embedded version of a closed-loop system. When the embedded version became available for use (February 2018), all participants (N=41) who had not yet initiated a CLC session were transitioned over to the embedded version and participants currently on the mobile version were allowed to transition to the embedded version for their last CLC session (N=4). The CLC algorithm retained its essential elements between both versions. The components of the mobile version (inControl system, TypeZero Technologies) included a study-provided Roche Accu-Chek Spirit Combo insulin pump (Roche, Indianapolis, IN) and a Dexcom Platinum CGM system (Dexcom, San Diego, CA). Participants were instructed to calibrate the CGM through the inControl software approximately every 12 hours. Both the insulin pump and the CGM receiver were connected using Bluetooth to an N5X (Google, Mountain View, CA) Android phone that held the inControl software. inControl had a remote monitoring system used by the study team at defined timepoints during run-in to document appropriate use, and for glycaemic review and insulin parameter change per participant request at any point during the trial. The embedded system consisted of a t:slim X2 insulin pump with Control-IQ technology (Tandem Diabetes Care, San Diego, CA) wirelessly connected to a Dexcom G6 sensor (Dexcom, San Diego, CA). During SAP, all participants used their personal pump and a study-provided CGM (Dexcom G4). Participants calibrated the study CGM during SAP using their own glucometer or a study-provided glucometer (Aviva Plus, Roche Diagnostics).
Reporting of adverse events was solicited throughout the trial. Reportable events included serious adverse events, hypoglycemia if assistance was required by another person to actively administer carbohydrates, glucagon or other resuscitative methods and hyperglycemia if treatment was obtained by a health care provider or criteria for diabetes ketoacidosis (DKA) was met [24].
Outcomes:
The primary outcome of the study was percent time <3.9 mmol/L (70 mg/dL) during SAP compared to E-N CLC. Secondary outcomes include hemoglobin A1c, as well as metrics derived from CGM including percent time in the target range of 3.9–10 mmol/L (70–180 mg/dL), time <3 mmol/L (54 mg/dL), time >10 mmol/L (180 mg/dL), time >13.3 mmol/L (250 mg/dL), mean glucose, coefficient of variation (CV), low blood glucose index (LBGI) and high blood glucose index (HBGI) [25]. Outcomes were stratified by nighttime (11pm to 7am) vs. daytime (7am to 11pm).
Statistical analysis:
Sample size:
Sample Size Determination is based on pilot studies of overnight CLC. We conservatively estimate that the effect size of E-N CLC vs. SAP will be f≥0.2. Power calculations assuming α=0.01, 90% power, correlation of 0.4 between the repeated measures, and attrition of 31% yield a sample size of N=110 subjects to be randomised at baseline, with N=76 subjects completing the study.
Analytical Methods:
The overall analysis followed modified intention-to-treat approach with all available data for participants who had completed at least two study sessions included in the analysis regardless of actual use of CLC device. Per protocol analysis was done as well, excluding any periods of time in which the use of the experimental devices was stopped study-wide. Generalized Linear Models available in IBM SPSS were used, including fixed factors following the factorial designs presented in Figure 1 for the SAP vs E-N CLC and E-N vs 24–7 CLC comparisons. The comparison of SAP vs E-N CLC included as covariates the HbA1c differences pre-post Session 1 and pre-post Session 4. The comparison of E-N vs 24–7 CLC included as a covariate HbA1c prior to Session 2. The influence of the order of treatment progression was assessed by a linear contrast of escalated vs de-escalated treatment sequences. Exploratory analyses stratified the participants by baseline HbA1c cutoff of 7.5%, and compared mobile vs. embedded CLC. Descriptive statistics and confidence intervals were computed as necessary.
Role of the Funding Source:
The funder of the study and those providing material support had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The first and the corresponding authors had full access to all data and had final responsibility for the decision to submit.
RESULTS
Between April 2016 and June 2018, 103 participants signed informed consent, and 93 entered the randomised trial: 47 were randomly assigned to Group A and 46 to Group B (See CONSORT Flow Diagram in Supplemental Figure 1). The analysis included 80 participants who had completed at least 2 study phases (40 in Group A and 40 in Group B). Reasons for withdrawals after randomisation without completing at least two study sessions include personal/scheduling conflicts (6 participants), use of other automated insulin delivery systems (2 participants), dislike of study CLC system/study pump (3 participants), multiple basal profiles incompatible with use of inControl (1 participant) and severe hypoglycaemia event while on personal equipment prior to use of closed-loop (1 participant).
Mean (±SD) age was 42.2±11.9 years (range 18–69 years), duration of diabetes was 21.2±11.9 years (range 1–50 years), duration of insulin pump use was 11.6±7.5 years (range 6 months – 31 years), and mean HbA1c at screening was 7.4±1.0% (range 5.4–10.6%). Table 1 presents detailed characteristics of the participants, stratified by study group. There were no apparent baseline between-group differences in any of the demographic of diabetes-control characteristics.
Table 1.
Characteristics of the Study Participants
| All (N=80) | Group A (N=40) | Group B (N=40) | |
|---|---|---|---|
| Age at Randomization (yrs) | |||
| Mean (SD) | 42.3 (11.9) | 42.7 (13.5) | 41.9 (10.1) |
| Range | 18 – 69 | 18 – 69 | 20 – 62 |
| Diabetes Duration at Randomization (yrs) | |||
| Mean (SD) | 21 (12) | 20 (12) | 22 (12) |
| Range | 1 – 50 | 1 – 50 | 1 – 46 |
| Prior Continuous Glucose Monitor Use | |||
| Never | 10 | 4 | 6 |
| Past | 6 | 3 | 3 |
| Current | 64 | 33 | 31 |
| BMI at Enrollmennt (kg per m2) | |||
| Mean (SD) | 29.4 (5.7) | 29.2 (5.3) | 29.6 (6.1) |
| Range | 18.8 – 53.0 | 18.8 – 42.2 | 21.2 – 53.0 |
| Sex | |||
| Female N (%) | 53 (66%) | 27 (68%) | 26 (65%) |
| HbA1c at Randomization – Lab | |||
| Mean ± SD | 7.42 ± 1.03 | 7.60 ± 1.08 | 7.24 ± 0.97 |
| Range | 5.4 – 10.6 | 5.4 – 10.6 | 5.4 – 10.0 |
| HbA1c after Study Session (Mean ± SD) | |||
| Session 1 (A: SAP; B: E-N CLC) | 7.15 ± 0.80 | 7.30 ± 0.83 | 7.01 ± 0.76 |
| Session 2 (A: E-N CLC; B: 24–7 CLC) | 7.04 ±.0.74 | 7.12 ± 0.79 | 6.95 ± 0.68 |
| Session 3 (A: 24–7 CLC; B: E-N CLC) | 7.10 ± 0.88 | 7.13 ± 0.80 | 7.06 ± 0.95 |
| Session 4 (A: E-N CLC; B: SAP) | 7.11 ± 0.86 | 7.10 ± 0.73 | 7.12 ± 0.97 |
Primary Outcomes – Sub-Analysis SA1 in Figure 1:
Compared to the SAP sessions, the E-N CLC sessions reduced the overall (day and night) percent time <70mg/dL (3.9mmol/L) from 4.0% to 2.2% (p<0.0001), an absolute difference of 1.8% (95%CI: 1.2–2.4%). In the crossover design, Group A was first exposed to SAP and then to E-N CLC, which resulted in overall reduction in percent time <70mg/dL from 3.2% to 1.9%. Group B was first exposed to E-N CLC and then to SAP, which resulted in overall increase in percent time <70mg/dL from 2.5% to 4.9%, all p-levels <0.0001. These results were similar when stratified by baseline HbA1c of 7.5% or greater. The reduction in the CGM-measured incidence of hypoglycaemia was accompanied by overall reduction in HbA1c from 7.4% at baseline to 7.1% at the end of study (Table 1), an absolute difference of 0.3% (95% CI: 0.1–0.4%), and by improvement in the overall TIR from 58.9% to 67.3%, a difference of +8.4% (95% CI: 6.4–10.4%), all p levels <0.0001. Table 2 presents the primary outcome and additional CGM-based outcomes comparing SAP versus E-N CLC.
Table 2.
Primary outcome and additional CGM-based outcomes comparing SAP vs Evening-Overnight CLC corresponding to Sub-analysis SA1 in Figure 1, Panel 2.
| E-N CLC (N=78) | SAP (N=78) | |||||
|---|---|---|---|---|---|---|
| Session 1 (N=39) | Session 4 (N=39) | Session 1 (N=39) | Session 4 (N=39) | F-value† | P-value* | |
| Percent time <3.9mmol/L [%] | 2.5±2.0 | 1.9±1.5 | 3.2±2.2 | 4.9±3.9 | 41.33 | <0.0001 |
| Overall across sessions | 2.2±1.8 | 4.0±3.3 | ||||
| Difference E-N CLC - SAP | −1.8±2.6 | |||||
| Time in range 3.9–10mmol/L [%] | 69.2±11.4 | 65.4±11.5 | 59.0±13.1 | 58.8±16.4 | 72.13 | <0.0001 |
| Overall across sessions | 67.3±11.5 | 58.9±14.7 | ||||
| Difference E-N CLC - SAP | +8.4±9.0 | |||||
| Percent time <3mmol/L [%] | 0.61±0.63 | 0.35±0.37 | 0.70±0.54 | 1.52±1.72 | 26.93 | <0.0001 |
| Overall across sessions | 0.48±0.53 | 1.11±1.33 | ||||
| Difference E-N CLC - SAP | −0.63±1.12 | |||||
| Percent time >10mmol/L [%] | 28.3±12.3 | 32.6±11.8 | 37.9±14.1 | 36.3±18.8 | 34.87 | <0.0001 |
| Overall across sessions | 30.5±12.2 | 37.1±16.6 | ||||
| Difference E-N CLC - SAP | −6.6±10.0 | |||||
| Percent time >13.3mmol/L [%] | 7.2±6.1 | 9.4±7.5 | 12.2±9.3 | 12.8±11.7 | 29.2 | <0.0001 |
| Overall across sessions | 8.3±6.9 | 12.5±10.5 | ||||
| Difference E-N CLC - SAP | −4.2±6.9 | |||||
| MeanBG (mmol/L) | 8.65±1.08 | 9.02±1.06 | 9.36±1.38 | 9.18±1.78 | 14.7 | <0.0001 |
| Overall across sessions | 8.84±1.08 | 9.27±1.59 | ||||
| Difference E-N CLC - SAP | −0.43±0.98 | |||||
| Coefficient of Variation [%] | 34.9±3.6 | 35.6±4.8 | 37.2±3.9 | 38.5±5.3 | 25.8 | <0.0001 |
| Overall across sessions | 35.2±4.3 | 37.9±4.7 | ||||
| Difference E-N CLC - SAP | −2.6±4.6 | |||||
| Low Blood Glucose Index | 0.72±0.46 | 0.57±0.36 | 0.84±0.54 | 1.25±0.94 | 36.2 | <0.0001 |
| Overall across sessions | 0.64±0.42 | 1.04±0.79 | ||||
| Difference E-N CLC - SAP | −0.40±0.62 | |||||
| High Blood Glucose Index | 6.41±3.01 | 7.49±3.32 | 8.85±4.12 | 8.66±5.13 | 31.3 | <0.0001 |
| Overall across sessions | 6.95±3.20 | 8.75±4.62 | ||||
| Difference E-N CLC - SAP | −1.80±2.85 | |||||
Comparison of SAP vs E-N CLC.
All analyses include as covariates changes in HbA1c during the session.
Secondary Outcomes – Sub-Analysis SA2 in Figure 1:
During the day (7AM-11PM), 24–7 CLC compared to E-N CLC, improved TIR further, from 65.3% to 67.6% (p<0.001), and reduced the CGM-measured coefficient of variation from 35.9% to 34.6% (p<0.01). In the crossover design, Group A was first exposed to E-N CLC and then to 24–7 CLC, which resulted in minimal change in TIR during the day from 66.2% to 66.7%. Group B was first exposed to 24–7 CLC and then to E-N CLC, which resulted in overall decrease in TIR from 68.4% to 64.3%. This effect was accompanied by a small but statistically significant reduction of glycaemic variability during the day, measured by CGM-derived CV that in Group A decreased from 36.0% to 35.0% and in Group B increased from 34.2% to 35.8%. Similarly, in Group A the percent time <70mg/dL during the day decreased from 2.5% to 1.9% and in Group B increased from 2.2% to 3.1% (all p-levels <0.001). Supplemental Table 3 presents CGM-derived metrics comparing E-N CLC vs 24–7 CLC during the day corresponding to Sub-Analysis SA2 (see Figure 2, Panel 2)
Influence of the Order of Treatment Progression – Sub-Analysis SA3 in Figure 1:
In Group A, the treatment options intensified progressively (SAP → E-N CLC → 24–7 CLC) and in Group B the treatment options de-intensified in reverse order. Regardless of the order of treatment introduction, the effect of treatment intensification was progressively evident: from SAP to E-N CLC to 24–7CLC, the percent time <70md/dL decreased from 4.0% to 2.3% to 1.8% (p < 0.0001), and the percent time in the target range increased from 58.6% to 67.6% to 69.5% (p<0.0001). However, there was little interaction between the order of treatment and the effect of the intervention. In particular, for most variables, the initial outcome on SAP and the final outcome on 24–7 CLC were virtually identical, and some differences emerged in the intermediate E-N CLC stage and for hypoglycaemia (CGM time <70 and <54 mg/dL). Table 3 presents the overall intervention effect and the interactions between the order of treatment introduction and outcomes.
Table 3:
Overall outcomes on SAP, E-N CLC and 24–7CLC, and influence of the order of treatment introduction SAP → E-N CLC → 24–7 CLC (Group A N=39), or in reverse (Group B N=38).
| SAP N=77 | E-N N=77 | 24–7 N=77 | P-Value | ||
|---|---|---|---|---|---|
| Percent time <3.9mmol/L /dL | Overall | 4.0±3.3 | 2.3±1.7 | 1.8±1.4 | <0.0001* |
| Group A: | 3.3±2.2 | 2.1±1.6 | 1.7±1.4 | P=0.019† | |
| Group B: | 4.9±3.9 | 2.6±1.9 | 2.0±1.4 | ||
| Percent time in 3.9–10mmol/L | Overall | 58.6±14.6 | 67.6±12.9 | 69.5±10.9 | <0.0001* |
| Group A: | 59.0±13.1 | 69.3±10.4 | 69.4±9.8 | p=0.68† | |
| Group B: | 58.3±16.2 | 65.9±14.9 | 69.6±12.1 | ||
| Percent time <3mmol/L | Overall | 1.11±1.34 | 0.51±0.51 | 0.41±0.41 | <0.0001* |
| Group A: | 0.70±0.54 | 0.40±0.39 | 0.37±0.41 | p=0.0040† | |
| Group B: | 1.54±1.74 | 0.63±0.60 | 0.47±0.42 | ||
| Percent time >10mmol/L | Overall | 37.4±16.5 | 30.1±13.7 | 28.7±11.4 | <0.0001* |
| Group | 37.9±14.1 | 28.7±10.8 | 28.9±10.0 | p=0.78† | |
| Group B: | 36.9±18.7 | 31.5±16.2 | 28.5±12.8 | ||
| Percent time >13.3mmol/L | Overall | 12.6±10.5 | 8.6±7.5 | 7.3±5.9 | <0.0001* |
| Group A: | 12.2±9.3 | 7.6±5.9 | 7.3±5.5 | p=0.59† | |
| Group B: | 13.0±11.7 | 9.6±8.8 | 7.4±6.4 | ||
| Average CGM [mmol/L] | Overall | 9.30±1.58 | 8.82±1.22 | 8.74±0.99 | <0.0001* |
| Group A: | 9.36±1.38 | 8.70±0.96 | 8.75±0.88 | p=0.63† | |
| Group B: | 9.23±1.78 | 8.95±1.44 | 8.72±1.11 | ||
| Coefficient of Variation [%] | Overall | 37.8±4.5 | 35.3±4.2 | 34.3±4.0 | <0.0001* |
| Group A: | 37.2±4.0 | 35.3±4.4 | 34.6±4.5 | p=0.082† | |
| Group B: | 38.5±5.4 | 35.4±3.9 | 34.0±3.6 | ||
| Low Blood Glucose Index | Overall | 1.04±0.79 | 0.66±0.42 | 0.57±0.34 | <0.0001* |
| Group A: | 0.84±0.54 | 0.61±0.37 | 0.53±0.33 | p=0.020† | |
| Group B: | 1.24±0.95 | 0.72±0.48 | 0.60±0.35 | ||
| High Blood Glucose Index | Overall | 8.82±4.61 | 6.97±3.52 | 6.53±2.86 | <0.0001* |
| Group A: | 8.85±4.12 | 6.54±2.74 | 6.55±2.59 | p=0.99† | |
| Group B: | 8.80±5.12 | 7.41±4.16 | 6.51±3.16 |
Comparison of SAP vs E-N CLC vs 24–7 CLC
Linear contrast of treatment escalation SAP → E-N CLC → 24–7 CLC (Group A) vs treatment de-escalation 24–7 CLC → E-N CLC → SAP (Group B).
Supplemental Table 4 presents the study outcomes during the day and overnight on SAP, E-N CLC, and 24–7 CLC. It is evident that any effects of CLC were most prominent overnight, but a clear improvement of TIR during the day occur with 24–7 CLC (see Supplemental Figure 2). Supplemental Table 5 presents the differences between the mobile and embedded CLC systems used during the study. Despite the fact that the two systems used a CLC algorithm with the same essential elements, embedded CLC was superior to mobile CLC in terms of several glycaemic control metrics in post-hoc analysis. The median (inter-quartile range) percentage connectivity of the device to remain in closed-loop was 87% (59–97) for the mobile CLC and 93% (86–98) for the embedded system.
Device Issues:
There were two instances of study-wide temporary suspension of the use of study devices. In May 2017, the use of the mobile inControl software was temporarily suspended due to a software error in the use of the insulin parameters by the mobile system. This suspension affected 12 participants for approximately 3 weeks after which participants resumed use of the system to complete the phase. There were no identified serious adverse events associated with this software error. In March 2019, use of the embedded Control-IQ software was temporarily suspended due to a software error which could result in an erroneous discontinuation of insulin delivery for up to several hours or to an erroneous bolus being given when insulin delivery restarted. Participants continued to use the Control-IQ system in open-loop mode until a software update was available. This suspension affected 8 participants for approximately 4 weeks. The analyses presented included all available data recorded during this period, even if closed-loop was not in use. Per protocol analysis excluding these time periods did not change the study results.
Adverse events (Supplemental Table 2):
There were no cases of DKA. There were three adverse events with significantly elevated ketones that did not meet the criteria for DKA. There were five cases of severe hypoglycaemia attributed to user-directed boluses without malfunction of the investigational device (one during closed-loop use of a Control-IQ pump, one during open-loop use of a Control-IQ pump, one during open-loop use of inControl at the time of the event, and two during personal pump use). There were three serious adverse events that were not device related (Appendicitis, Hip Surgery, Renal Carcinoma).
DISCUSSION
This randomised trial of closed-loop control (CLC) in adults with Type 1 diabetes had a unique double cross-over design, which allowed for two independent cross-over comparisons of SAP vs overnight (E-N) CLC and E-N vs 24–7 CLC. Each of the SAP, E-N CLC, and 24–7 CLC session was of sufficient duration – 2 months – allowing the effect of the treatment to become established. Washout time (2 weeks) was included between the sessions as well. The first of the cross-over analyses (SAP vs E-N CLC) found that evening-night use of CLC decreased hypoglycaemia (time <70 mg/dL) without any deterioration in haemoglobin A1c when compared to sensor-augmented pump therapy. Generally, CLC in evening-night use and 24–7 use was superior to SAP in terms of all glycaemic outcomes, improving time in range and decreasing both hypoglycaemia and hyperglycaemia. The second cross-over analysis (E-N vs 24–7 CLC) found that evening-night use of CLC accounted for most of the glycaemic benefits of 24–7 CLC. Although 24–7 CLC time in range was superior during the day to evening-night use, these effects were of small magnitude making their clinical relevance uncertain. In addition to time in range effects, both evening-night CLC and 24–7 CLC decreased glycaemic variability as measured by coefficient of variation. The order of treatment did not make a difference in these results.
This trial enrolled adults with T1D with a wide range of baseline haemoglobin A1c values, although the study population overall was well controlled with a mean A1c of 7.4% (57.4 mmol/mol). There were 5 severe hypoglycaemic events that occurred during the trial. Only one of these events however occurred while the CLC system was active and none appeared attributable to device malfunction.
This trial is notable in its long-duration relative to other CLC trials and use of different hardware system configurations - a mobile version using a smart phone to run the CLC algorithm (inControl) and an embedded version in which the algorithm was implemented in the insulin pump (Control-IQ). In both systems the algorithm was the same, preserving all of its essential components. Both systems demonstrated improvement in all glycaemic outcomes compared to SAP and did not differ on any of the metrics reflecting frequency of hypoglycaemia. However, with the embedded system these improvements were greater at the hyperglycaemic end of the glucose scale in exploratory analysis, leading to larger improvement in time-in-range. This further improvement with the embedded system was most evident during the day and was attributed to the enhanced connectivity between the study devices, e.g. no smart phone needed to keep connection with both the sensor and the insulin pump. While this is intuitively clear, no other studies have made such a direct comparison, and these exploratory analysis may be of value with the increasing popularity of smart phone-based solutions.
Additional focus is the consistent results achieved with overnight control. This is likely a direct reflection of the algorithm sliding target range which, by design, intensifies insulin delivery and gradually tightens control overnight to achieve a consistent glucose of approximately 6–6.5 mmol/L at wake-up.
Limitations of this work include the use of a study CGM during SAP that was different from the study CGM used in the embedded version. This allowed the same comparator in all SAP periods but introduced a potential difference when the embedded version was used. Given the similarity in overall effect of the two systems, the differences in sensor accuracy are unlikely to have changed the overall conclusion. The effect of the short periods of study-wide suspension of device use in a small portion of participants were analysed and did not affect the results. Lastly, this study enrolled people with T1D without significant diabetes-related complications that may limit the generalizability.
In conclusion, this study has three major findings: (1) long-term use of closed-loop control resulted in lower hypoglycaemia and improved time in range, accompanied by gradual improvement in haemoglobin A1c; (2) evening-night closed-loop control accounted for most of the glycaemic improvements of 24–7 closed-loop control, and (3) a system with the control algorithm embedded in the insulin pump in post-hoc analysis was superior to a mobile system using essentially the same control algorithm on a smart phone, indicating that system connectivity is paramount.
Supplementary Material
RESEARCH IN CONTEXT
Evidence before this study
Between 2015 and 2018, the National Library of Medicine included over 120 publications per year related to the development of closed-loop control systems, known as the artificial pancreas. These publications reflected the state of the art in this rapidly developing field. In 2016, a pivotal trial of a commercial hybrid closed-loop system was reported in JAMA, but this study did not have a control group to allow assessment of its efficacy. Systematic reviews and meta-analyses were published by Lancet Diabetes Endocrinology, Nature Reviews Endocrinology, the British Medical Journal, and Bioelectronic Medicine. Most recently, we reported a large multi-centre randomised trial of a new closed-loop system in the New England Journal of Medicine. The sum of evidence to date sets the expectations for closed-loop control: reduced exposure to hypo- and hyperglycaemia, and improved time in a target range, most prominent overnight.
Added value of this study
The study reported here is the first long-term (four 8-week sessions) randomised controlled trial comparing, in crossover designs, sensor-augmented pump therapy vs evening-night closed-loop control, and evening-night vs 24–7 closed-loop control. These designs assess directly the glycaemic benefits of closing the loop for dinner and overnight, and any additional benefits of 24–7 automated control. In addition, this study compared two systems in exploratory analysis using the same control algorithm: mobile based on a smart phone and embedded, with the algorithm built on board of an insulin pump.
Implications of all the available evidence
Evening-night closed-loop control accounts for most of the glycaemic benefits of 24–7 closed-loop control, with the latter being statistically superior during the day but of a magnitude unlikely to be clinically relevant. This finding could offer certain flexibility, allowing 24–7 and dinner/bedtime solutions to coexist on the same system, and be used interchangeably over time. Furthermore, a system with the control algorithm embedded in the insulin pump had better connectivity compared to a mobile system using the same control algorithm on a smart phone, and it is possible this resulted in improved time in range.
Acknowledgements:
Study Funding: NIH/NIDDK grant RO1 DK 085623. The University of Virginia Strategic Investment Fund Project #88 provided institutional and regulatory support. Roche Diabetes Care provided insulin pumps for the mobile system, insulin pump-related supplies and pump trainers. Dexcom R&D Charlottesville (formerly TypeZero Technologies) created the inControl software used in the experimental mobile systems. Tandem Diabetes Care provided the experimental embedded closed-loop systems, system-related supplies, and technical expertise. Dexcom provided CGM sensors for the embedded version. Funding and support sources did not have any involvement in the study design, data analysis or manuscript preparation. A copy of the manuscript was provided to Roche Diagnostics, Tandem Diabetes Care, and Dexcom prior to submission. Portions of the data were presented at the International Conference for Advanced Technologies and Treatments for Diabetes 2019 and Diabetes Technology Meeting 2019.
Funding: NIH/NIDDK grant RO1 DK085623; Material Support: Roche, Tandem Diabetes Care, Dexcom.
Declaration of interests
BPK reports grants from National Institutes of Health, non-financial support from Roche Diagnostics, non-financial support from Tandem Diabetes Care, non-financial support from Dexcom, during the conduct of the study; personal fees and non-financial support from Dexcom, grants and personal fees from Sanofi, personal fees and non-financial support from Tandem Diabetes Care, other from TypeZero Technologies, outside the submitted work; In addition, BPK has a patent # 8,562,587 “CGM-based prevention of hypoglycemia via hypoglycemia risk assessment and smooth reduction of insulin delivery” with royalties paid to Dexcom, a patent # 9,750,438 B2 “CGM-based prevention of hypoglycemia via hypoglycemia risk assessment and smooth reduction of insulin delivery” with royalties paid to Dexcom, and a patent # 9,430,022 B2 “Method and apparatus for modular power management and protection of critical services in ambulatory medical devices” with royalties paid to Dexcom. SAB reports grants from National Institutes of Health, non-financial support from Tandem Diabetes Care, non-financial support from Dexcom, non-financial support from Roche Diagnostics, during the conduct of the study; grants and non-financial support from Tandem Diabetes Care, non-financial support from Dexcom, non-financial support from Roche Diagnostics, outside the submitted work. MDB reports grants from National Institutes of Health, non-financial support from Roche Diagnostics, non-financial support from Tandem Diabetes Care, non-financial support from Dexcom, during the conduct of the study; grants, personal fees and non-financial support from Dexcom, grants from Sanofi, grants, personal fees and non-financial support from Tandem, personal fees from Roche, personal fees from Ascencia, grants from Senseonics, personal fees from Air Liquide, other from TypeZero Technologies, outside the submitted work; In addition, MDB has a patent # 8,562,587 “CGM-based prevention of hypoglycemia via hypoglycemia risk assessment and smooth reduction of insulin delivery” with royalties paid to Dexcom, a patent # 9,750,438 B2 “CGM-based prevention of hypoglycemia via hypoglycemia risk assessment and smooth reduction of insulin delivery” with royalties paid to Dexcom, and a patent # 9,430,022 B2 “Method and apparatus for modular power management and protection of critical services in ambulatory medical devices” with royalties paid to Dexcom. SMA reports grants from National Institutes of Health, non-financial support from Roche Diagnostics, non-financial support from Tandem Diabetes Care, non-financial support from Dexcom, during the conduct of the study; grants from Medtronic, outside the submitted work. CAW reports grants from National Institutes of Health, non-financial support from Tandem Diabetes Care, non-financial support from Dexcom, Inc., non-financial support from Roche, during the conduct of the study; other from Dexcom, Inc., other from Tandem Diabetes Care, outside the submitted work. LK, CB, KC, RG, MCO report grants from National Institutes of Health, non-financial support from Roche Diagnostics, non-financial support from Tandem Diabetes Care, non-financial support from Dexcom, during the conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S61–S70. [DOI] [PubMed] [Google Scholar]
- 2.Foster NC, Beck RW, Miller KM, et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovatchev BP, Tamborlane WV, Cefalu WT, Cobelli C. The Artificial Pancreas in 2016: A Digital Treatment Ecosystem for Diabetes. Diabetes Care 2016; 39:1123–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovatchev BP. The Artificial Pancreas in 2017: The year of transition from research to clinical practice. Nature Reviews Endocrinology 2018; 14: 74–76. [DOI] [PubMed] [Google Scholar]
- 5.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karageorgiou V, Papaioannou TG, Bellos I, et al. Effectiveness of artificial pancreas in the non-adult population: A systematic review and network meta-analysis. Metabolism 2019;90:20–30. [DOI] [PubMed] [Google Scholar]
- 7.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–12. [DOI] [PubMed] [Google Scholar]
- 8.Kovatchev B. A Century of Diabetes Technology: Signals, Models, and Artificial Pancreas Control. Trends Endocrinol Metab 2019. [DOI] [PubMed] [Google Scholar]
- 9.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients with Type 1 Diabetes. JAMA, 2016; 316:1407–1408. [DOI] [PubMed] [Google Scholar]
- 10.Kovatchev BP, Cheng P, Anderson SM, et al. , for the Control to Range Study Group. Feasibility of Long-Term Closed-Loop Control: A Multicenter 6-Month Trial of 24/7 Automated Insulin Delivery. Diabetes Technol Ther 2017; 19:18–24. [DOI] [PubMed] [Google Scholar]
- 11.Breton MD, Cherñavvsky DR, DeBoer MD,et al. Closed Loop Control During Intense Prolonged Outdoor Exercise in Adolescents with Type 1 Diabetes: The Artificial Pancreas Ski Study. Diabetes Care 2017, 40:1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson SM, Buckingham BA, Breton MD, et al. Hybrid Closed-Loop Control is Safe and Effective for People with Type 1 Diabetes who are at Moderate to High Risk for Hypoglycemia. Diabetes Technol. Ther 2019. doi: 10.1089/dia.2019.0018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keith Hynes P, Guerlain S, Mize LB, et al. DiAs User Interface: A Patient-Centric Interface for Mobile Artificial Pancreas Systems. J Diabetes Sci Technol 2013; 7:1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobelli C, Renard E, Kovatchev BP, et al. Pilot Studies of Wearable Artificial Pancreas in Type 1 Diabetes. Diabetes Care 2012; 35:e65–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of Outpatient Fully Integrated Closed-Loop Control: First Studies of Wearable Artificial Pancreas. Diabetes Care 2013; 36:1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson SM, Raghinaru D, Pinsker JE, et al. Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016; 39:1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renard E, Farret A, Kropff J, et al. AP@home Consortium. Day and night closed loop glucose control in patients with type 1 diabetes under free-living conditions: comparison of a single-arm, 1-month experience to results of a previously reported feasibility study of evening and night at home. Diabetes Care 2016; 39:1151–60. [DOI] [PubMed] [Google Scholar]
- 18.Del Favero S, Boscari F, Messori M, et al. Randomized Summer Camp Crossover Trial in 5- to 9-Year-Old Children: Outpatient Wearable Artificial Pancreas Is Feasible and Safe. Diabetes Care. 2016; 39:1180–5. [DOI] [PubMed] [Google Scholar]
- 19.Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benhamou PY, Franc S, Reznik Y, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digital Health 2019;1:e17–25. [DOI] [PubMed] [Google Scholar]
- 21.Anderson SM. The International Diabetes Closed Loop Trial. Diabetes Technology Meeting, Bethesda, MD, 2018. [Google Scholar]
- 22.Brown S, Raghinaru D, Emory E, and Kovatchev BP. First Look at Control-IQ: A New-Generation Automated Insulin Delivery System. Diabetes Care 2018; 41:2634–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SA, Kovatchev BP, Raghinaru D, et al. for IDCL Trial Research Group Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med. 2019; 381:1707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- 25.Kovatchev BP. Metrics for Glycaemic Control: from HbA1c to Continuous Glucose Monitoring. Nature Reviews Endocrinology 2017; 13: 425–436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.