Abstract
Cardiogenic shock (CS) carries high mortality and morbidity. Early revascularization is an important strategy in management of these patients. We sought to determine the outcomes and predictors of revascularization among patients with CS. Patients with CS and acute myocardial infarction were identified using the National Inpatient Sample (NIS) data from January 2002 to December 2014 using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. Subsequently, patients who underwent revascularization were then selected. A total of 118,618 patients with CS were identified. Out of these, about 55,735 (47%) patients underwent revascularization. Mean age of patients who underwent revascularization was lower when compared with patients not who underwent revascularization (66.40 vs 72.24 years, p < 0.01). Patients who underwent revascularization had lower mortality when compared with patients not who underwent revascularization (25.1% vs 52.2%, p < 0.01). Extracorporeal membrane oxygenation and mechanical circulatory support devices were often utilized more in patients who underwent revascularization. Overall, we found modest increased trend of revascularization over our study years with decline in mortality. Female gender, weekend admission, drug abuse, pulmonary hypertension, anemia, renal failure, neurological disorders, malignancy were associated with lower odds of revascularization. In conclusion, in this large nationally represented US population sample of CS patients, we found revascularization rate of about 47% with improvement in overall mortality over our study years.
Cardiogenic shock (CS) complicates clinical trajectory of approximately 5% to 10% patients admitted with acute myocardial infarction (AMI).1,2 Moreover, CS is associated with worsened mortality despite advances in utilization of mechanical support devices.3 The landmark SHOCK trial showed improved outcomes with revascularization by utilizing either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery in these patients.4 The rate of CABG in CS patients is about 5% to 6% and relatively stable over the years.5 Few observational studies have assessed the trends and outcomes of PCI in AMI patients complicated by CS from national databases.6,7 American Heart Association guidelines recommend coronary revascularization in all eligible CS patients, which encompasses utility of both PCI and CABG.8 No earlier studies have reported cumulative trends and outcomes after PCI and CABG in CS patients from national database. The purpose of this study was to assess trends, outcomes, and predictors of coronary revascularization (both PCI and CABG) in patients admitted with CS from a nationally represented United States (US) population sample.
Methods
Data were derived from National Inpatient Sample (NIS). NIS is part of Healthcare Cost and Utilization Project (HCUP) databases and is a Federal-State-Industry partnership sponsored by the Agency for Healthcare Research and Quality (AHRQ). The NIS is derived from all States participating in HCUP, representing more than 97 percent of the US population. Since NIS is compiled annually, the data can be used for analysis of disease trends overtime. Institutional review board approval and informed consents were not required for this study given the de-identified nature of the NIS database.
We analyzed NIS data from January 2002 to December 2014 using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. Patients ≥18 years of age were included. To identify patients with CS, the diagnosis code of 785.51 was used in all diagnosis fields. This code has been used in earlier studies and has a specificity of 99.3%, a sensitivity of 60%, a positive predictive value of 78.8%, and a negative predictive value of 98.1% for CS.9 Subsequently AMI patients were identified using relevant ICD-9 codes. A total of 118,618 patients with CS (ICD-9-CM code of 785.51) and AMI (ICD-9-CM code of 410.01–92, excluding 410.7) were identified. ICD-9-CM of 37.61 was used to identify intraaortic balloon pump (IABP) and 37.68 for percutaneous ventricular assist devices (PVADs). To identify the population of patients who underwent revascularization [PCI and CABG], ICD-9-CM codes of 36.01–36.07, 36.09–17, and 36.19 in all procedure fields were used (see Figure 1).
Figure 1.
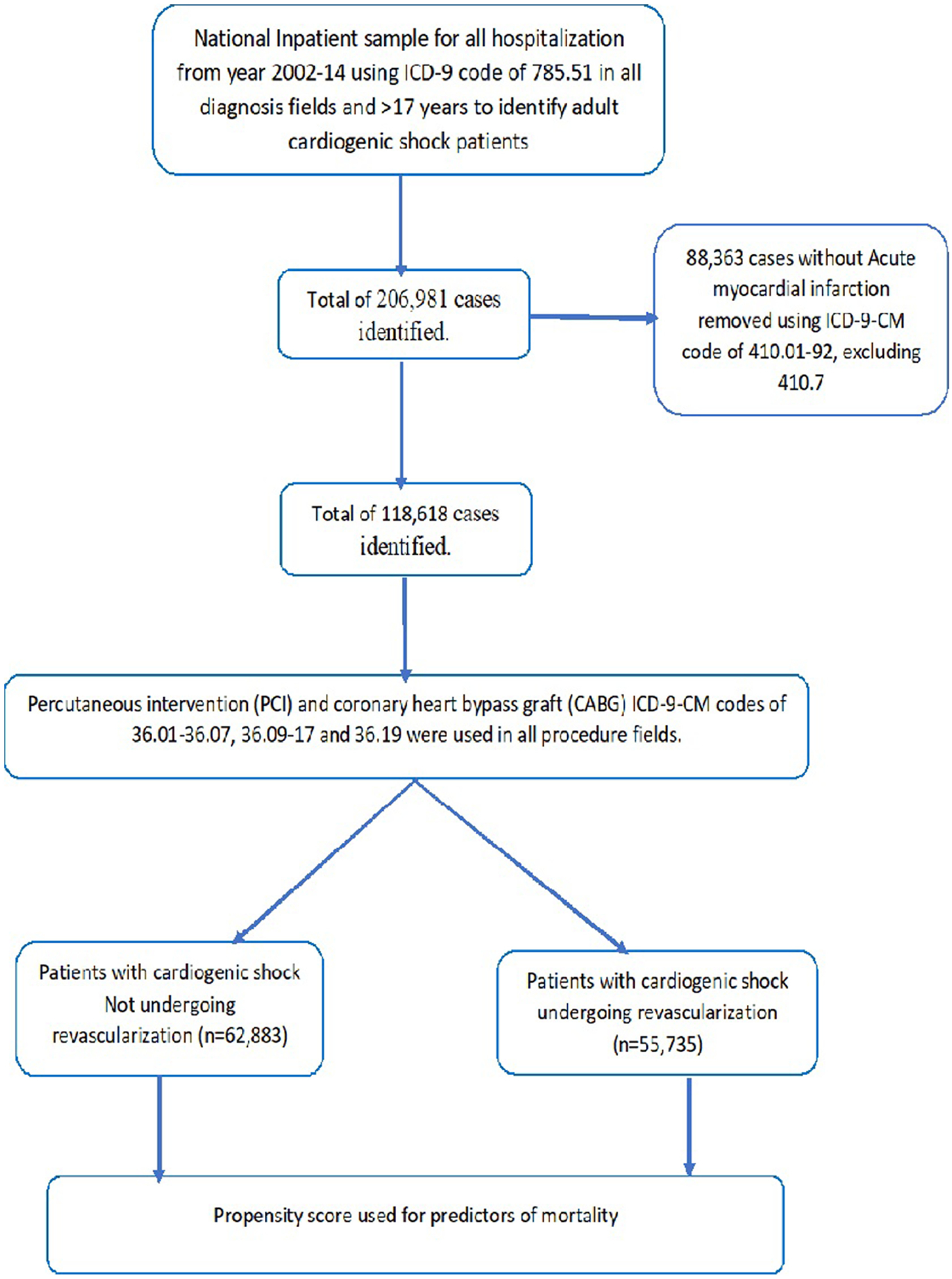
Flow sheet of the paper
AHRQ co-morbidities were generated as binary variables for analysis.10 Baseline characteristics of patients with AMI-CS who underwent revascularization and patients who didn’t undergo revascularization were compared. We studied in-hospital mortality as the primary outcome. Descriptive statistics were presented as frequencies with percentages for categorical variables and as means with standard deviations for continuous variables. Baseline characteristics were compared using a Pearson χ2 test and Fisher’s exact test for categorical variables and independent samples t test for continuous variables.
A binomial logistic regression model was used to identify variables from demographic data (Table 1) that were associated with revascularization with a p value <0.10. Then, these variables were subsequently utilized in a multiple logistic regression model to determine predictors of revascularization. Propensity score was calculated using logistic regression. The predicted probability of revascularization (as derived from propensity score) along with relevant significant variables were used in logistic regression to estimate odds ratios (ORs) with 95% confidence intervals (CIs) to determine predictors of in-patient mortality for patients with AMI-CS. Statistical analyses were performed using statistical package for social science version 24 (IBM Corp) and R for propensity Matching.
Table 1.
Baseline characteristics of the study population
| Variables | AMI-CS† Not undergoing revascularization (n = 62,883) | AMI-CS undergoing revascularization (n = 55,735) | All patients of AMI-CS (n= 118,618) | p value |
|---|---|---|---|---|
| Age (mean [SD]) (years) | 72.24 (13.3) | 66.40 (12.4) | 69.49 (13.2) | <0.01 |
| Women | 27,782 (44.2%) | 19,685 (35.3%) | 47,467 (40%) | <0.01 |
| Race | ||||
| White | 39,819 (75.6%) | 35,334 (77.2%) | 75,153 (76.4%) | <0.01 |
| Black | 4,967 (9.4%) | 3,103 (6.8%) | 8,070 (8.2%) | |
| Hispanic | 4,176 (7.9%) | 3,630 (7.9%) | 7,806 (7.9%) | |
| Asian or Pacific Islander | 1,591 (3%) | 1,432 (3.1%) | 3,023 (3.1%) | |
| Native American | 286 (0.5%) | 268 (0.6%) | 554 (0.6%) | |
| AHRQ‡ co-morbidities | ||||
| Deficiency anemia | 12,129 (19.5%) | 9,277 (16.8%) | 21,406 (18.2%) | <0.01 |
| Collagen vascular diseases | 1,239 (2%) | 968 (1.8%) | 2,207 (1.9%) | <0.01 |
| Congestive heart failure | 13,127 (21.1%) | 3,295 (6%) | 16,422 (14%) | <0.01 |
| Chronic pulmonary disease | 15,100 (24.2%) | 12,229 (22.1%) | 27,329 (23.3%) | <0.01 |
| Coagulopathy | 8,150 (13.1%) | 9,136 (16.5%) | 17,286 (14.7%) | <0.01 |
| Diabetes, uncomplicated | 15,926 (25.6%) | 13,952 (25.3%) | 29,878 (25.4%) | <0.01 |
| Diabetes with chronic complications | 4,424 (7.1%) | 3,198 (5.8%) | 7,622 (6.5%) | <0.01 |
| Hypertension | 31,320 (50.3%) | 28,374 (51.4%) | 59,694 (50.8%) | <0.01 |
| Hypothyroidism | 5,469 (8.8%) | 3,746 (6.8%) | 9,215 (7.8%) | <0.01 |
| Liver disease | 1,496 (2.4%) | 698 (1.3%) | 2,194 (1.9%) | <0.01 |
| Fluid and electrolyte disorders | 31,016 (49.8%) | 22,197 (40.2%) | 53,213 (45.3%) | <0.01 |
| Obesity | 4,481 (7.2%) | 5,825 (10.5%) | 10,306 (8.8%) | <0.01 |
| Peripheral vascular disorders | 7,967 (12.8%) | 6,209 (11.2%) | 14,176 (12.1%) | <0.01 |
| Pulmonary circulation disorders | 1,893 (3%) | 323 (0.6%) | 2,216 (1.9%) | <0.01 |
| Renal failure | 16,255 (26.1%) | 8,894 (16.1%) | 25,149 (21.4%) | <0.01 |
| Valvular disease | 3,564 (5.7%) | 898 (1.6%) | 4,462 (3.8%) | <0.01 |
| Concurrent diagnosis | ||||
| Atrial Fibrillation | 23,409 (37.2%) | 19,305 (34.6%) | 42,714 (36%) | <0.01 |
| Risk factors | ||||
| familial hyperlipidemia | 2,958 (4.7%) | 4,010 (7.2%) | 6,968 (5.9%) | <0.01 |
| Smoking history | 4,111 (6.5%) | 4,414 (7.9%) | 8,525 (7.2%) | <0.01 |
| Family history sudden death or Myocardial infarction | 1,036 (1.6%) | 2,634 (4.7%) | 3,670 (3.1%) | <0.01 |
| Region | ||||
| Northeast | 13,180 (21%) | 8,543 (15.3%) | 21,723 (18.3%) | <0.01 |
| Midwest | 13,127 (20.9%) | 13,349 (24%) | 26,476 (22.3%) | |
| South | 23,865 (38%) | 21,704 (38.9%) | 45,569 (38.4%) | |
| West | 12,711 (20.2%) | 12,139 (21.8%) | 24,850 (20.9%) | |
| Hospital Location | ||||
| Rural | 5,995 (9.5%) | 2,572 (4.6%) | 8,567 (7.2%) | <0.01 |
| Urban Non-teaching | 25,219 (40.1%) | 21,751 (39%) | 46,970 (39.6%) | |
| Urban Teaching | 31,669 (50.4%) | 31,412 (56.4%) | 63,081 (53.2%) | |
| Bed size of the hospital | ||||
| small | 6,588 (10.5%) | 3,758 (6.7%) | 10,346 (8.7%) | <0.01 |
| medium | 15,214 (24.2%) | 11,310 (20.3) | 26,524 (22.4%) | |
| large | 41,081 (65.3%) | 40,667 (73%) | 81,748 (68.9%) | |
| Primary payer | ||||
| Medicare | 44,886 (71.5%) | 30,443 (54.7%) | 75,329 (63.6%) | <0.01 |
| Medicaid | 3,911 (6.2%) | 3,935 (7.1%) | 7,846 (6.6%) | |
| Private insurance | 10,226 (16.3%) | 15,908 (28.6%) | 26,134 (22.1%) | |
| Self-pay | 2,259 (3.6%) | 3,373 (6.1%) | 5,632 (4.8%) | |
| No charge | 170 (0.3%) | 296 (0.5%) | 466 (0.4%) | |
| Median household income (percentile) | ||||
| 0–25th | 7,975 (27.2%) | 10,297 (25.3%) | 18,272 (26.1%) | <0.01 |
| 26–50th | 7,795 (26.6%) | 10,648 (26.2%) | 18,443 (26.4%) | |
| 51–75t | 6,977 (23.8%) | 10,266 (25.3%) | 17,243 (24.7%) | |
| 76–100th | 6,558 (22.4%) | 9,420 (23.2%) | 15,978 (22.8%) |
Acute myocardial infarction-Cardiogenic shock.
Agency for Healthcare Research and Quality.
Results
A total of 118,618 patients with AMI-CS were identified from January 2002 to December 2014. Out of these 55,735 (47%) patients underwent revascularization. Mean age was 69.49 (±13.17) years. Mean age of patients who underwent revascularization was lower when compared with patients who didn’t undergo revascularization (66.40 v. 72.24 years, p < 0.01). Total cohort consisted of 47,467 (40%) females. About 19,685 (35.3%) female patients underwent revascularization. A higher proportion of Caucasians underwent revascularization when compared with African Americans. Table 1 shows the baseline characteristics of the study population.
Over the study period the proportion of patients who underwent revascularization increased from 46% in 2002 to 47% in 2014. Surgical revascularization was performed in 16.10% and percutaneous revascularization was performed in 32.4% of AMI-CS patients. The trends of revascularization (PCI vs CABG) are shown in Figure 2. Heart assist devices (IABP and PVADs) were commonly used in patients who underwent revascularization and there was an increased trend noticed in PVAD utilization with subsequent reduced trend of IABP application over our study years (see Figure 3).
Figure 2.
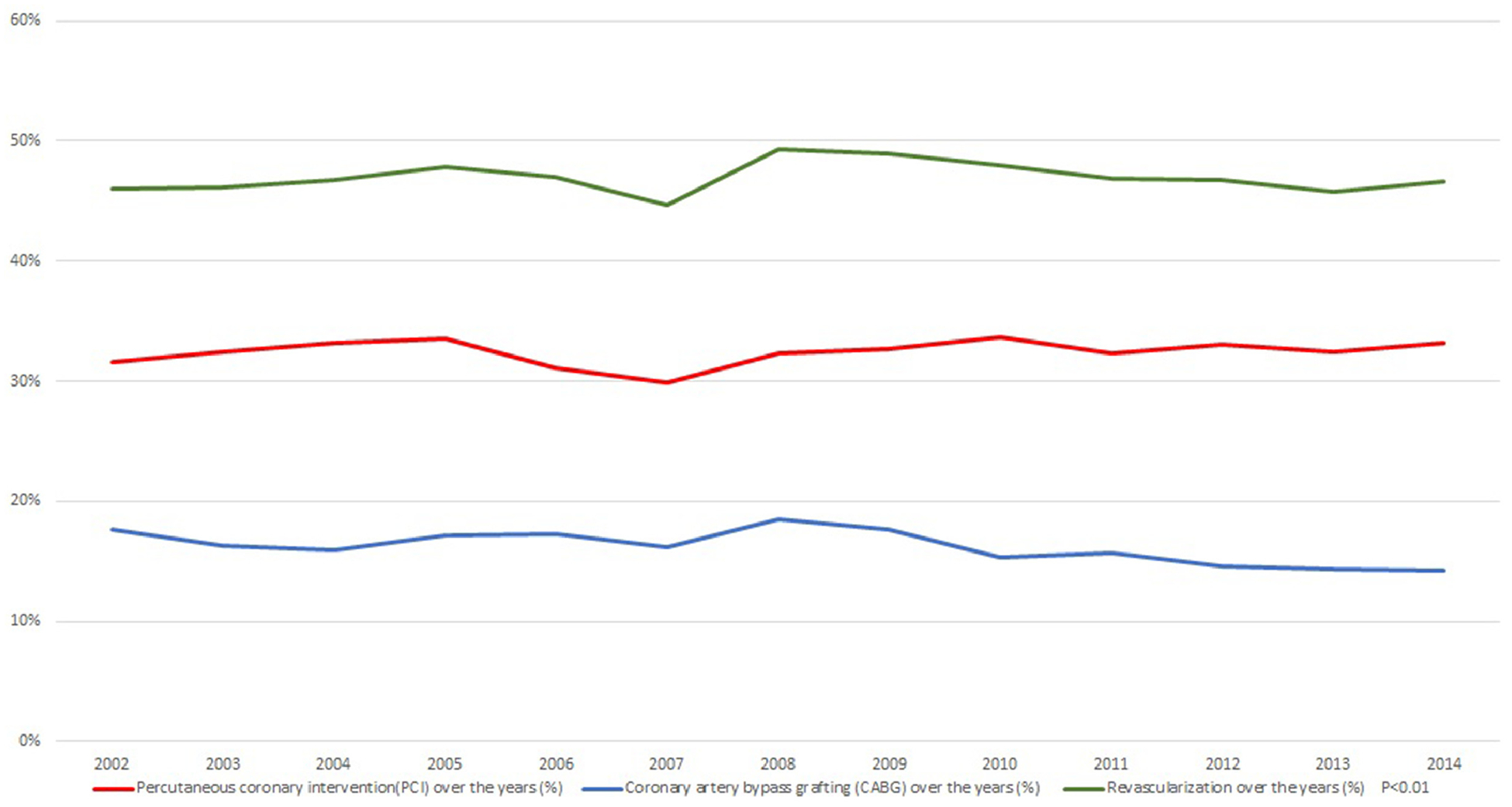
Revascularization trends over the years in cardiogenic shock patients
Figure 3.
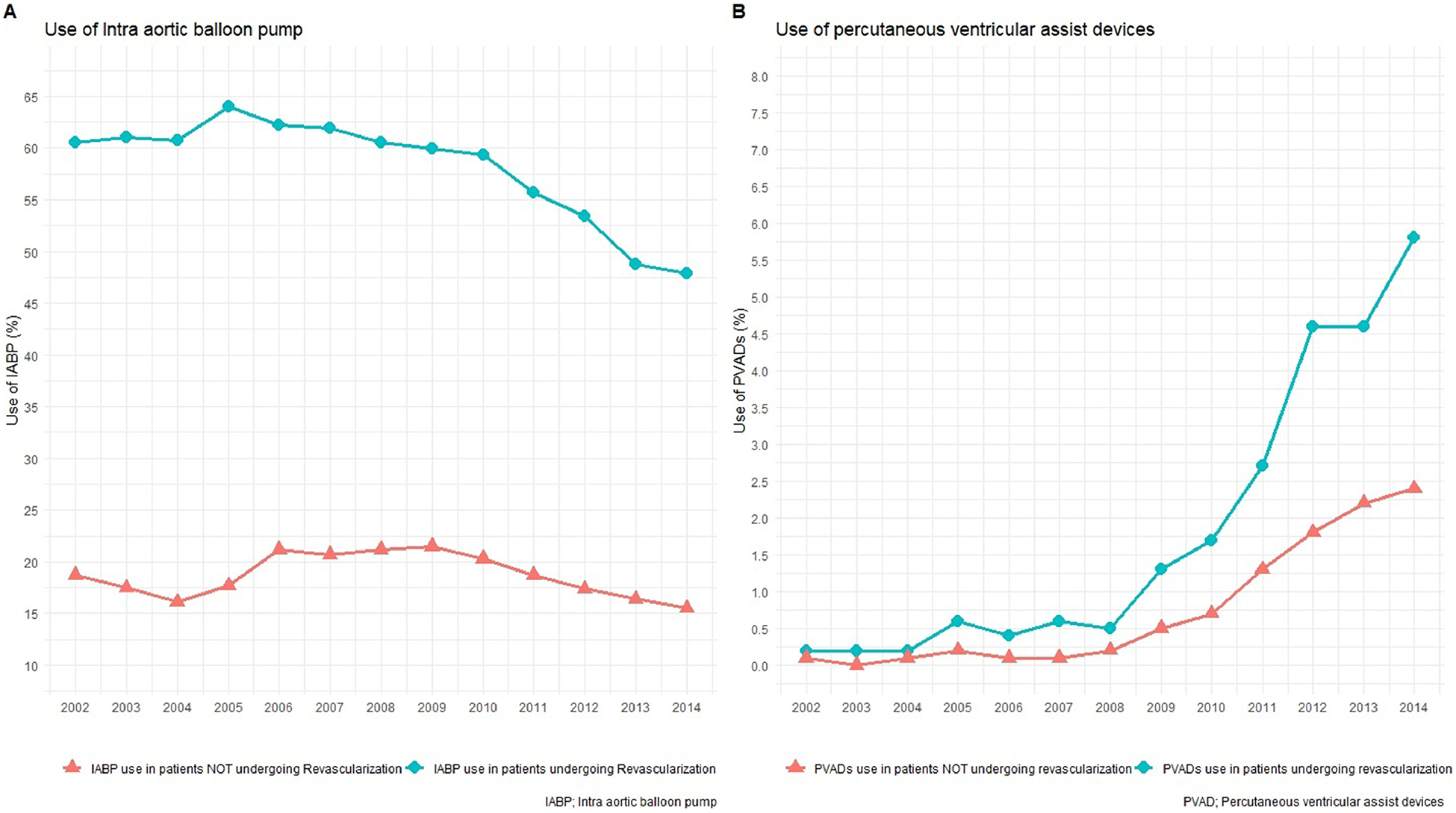
Trends in use of balloon pump and percutaneous left ventricular devices over years
Hospital outcomes and resource utilization are shown in Table 2. There was a gradual reduction in in-patient mortality over our study period that was uniform across both genders (see Figure 4). Patients who underwent revascularization had lower mortality when compared with patients not who underwent revascularization (25.1% vs 52.2%, p <0.01). Procedures such as extracorporeal membrane oxygenation and mechanical circulatory assist devices were more often utilized in patients who underwent revascularization. However, cardiopulmonary resuscitation and vasopressors were utilized more in AMI-CS patients not who underwent revascularization.
Table 2.
Hospital encounter outcomes and resource utilization in cardiogenic shock patients
| Hospital Outcomes no. (%) | AMI-CS† Not undergoing revascularization (n = 62,883) | AMI-CS undergoing revascularization (n = 55,735) | All patients of AMI-CS (n = 118,618) | p value |
|---|---|---|---|---|
| Died at discharge | 32816 (52.2%) | 14017 (25.1%) | 46833 (39.5%) | <0.01 |
| Died at discharge males | 18138 (51.7%) | 8378 (23.2%) | 26516 (37.3%) | <0.01 |
| Died at discharge females | 14677 (52.80%) | 5637 (28.60%) | 20314 (42.80%) | <0.01 |
| Discharge Disposition of surviving patients | ||||
| Routine/self-care | 7344 (24.40%) | 19529 (46.80%) | 26873 (37.40%) | <0.01 |
| Short-term hospital | 5605 (18.60%) | 2487 (6.00%) | 8092 (11.30%) | |
| Another type of facility | 12131 (40.30%) | 11832 (28.40%) | 23963 (33.40%) | |
| Home Health Care | 4712 (15.70%) | 7652 (18.30%) | 12364 (17.20%) | |
| Resource utilization | ||||
| PCI‡ | 0 | 384829 (69.0%) | 384829 (32.4%) | <0.01 |
| CABG§ | 0 | 19128 (34.30%) | 19128 (16.10%) | <0.01 |
| Vasopressors | 4342 (6.9%) | 2765 (5.0%) | 7107 (6.0%) | <0.01 |
| CPR¶ | 8139 (12.90%) | 4373 (7.80%) | 12512 (10.50%) | <0.01 |
| ECMO# | 220 (0.30%) | 18625 (33.40%) | 18845 (15.90%) | <0.01 |
| Intra-aortic balloon pump | 12056 (18.6%) | 30993 (57.5%) | 43049 (36.3%) | <0.01 |
| PVADs†† | 548 (0.8%) | 1096 (2%) | 1644 (1.4%) | <0.01 |
| Mechanical ventilation | 34808 (55.4%) | 21464 (38.5%) | 56272 (47.4%) | <0.01 |
| Tracheostomy | 2341 (3.70%) | 2624 (4.70%) | 4965 (4.20%) | <0.01 |
| Gastrostomy | 1682 (2.70%) | 1141 (2.00%) | 2823 (2.40%) | <0.01 |
| Length of stay, mean (SD), days | 8.75 (12.071) | 11.49 (12.075) | 10.04 (12.15) | <0.01 |
| Cost of hospitalization-mean (SD), $ | 101,123 (156,007) | 176,131 (168,821) | 136,541 (166,450) | <0.01 |
Acute myocardial infarction-cardiogenic shock.
Per Cutaneous coronary intervention.
Coronary artery bypass graft.
cardiopulmonary resuscitation.
Extracorporeal membrane oxygenation.
Percutaneous ventricular assist devices.
Figure 4.
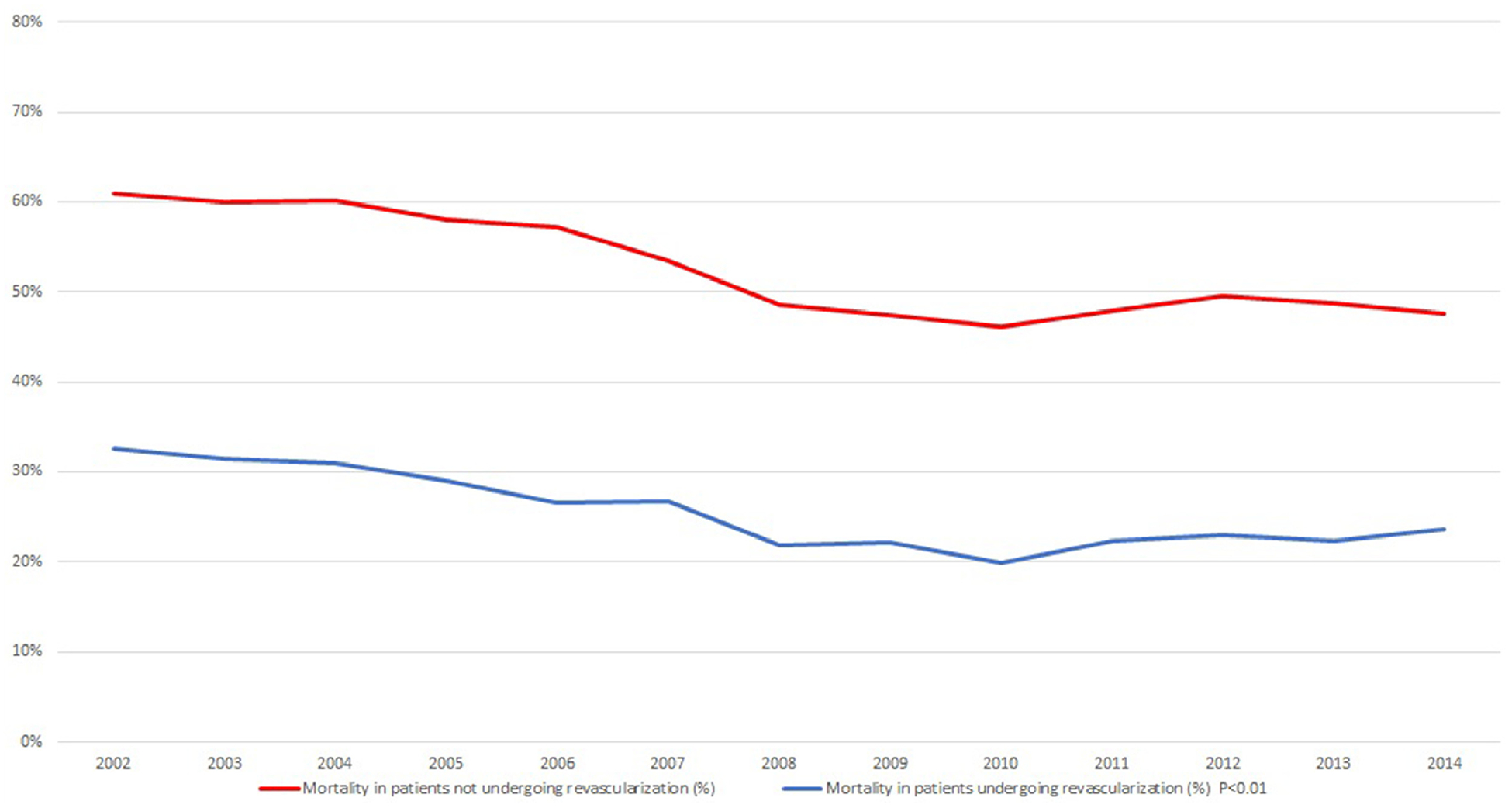
Mortality trend over the years in cardiogenic shock patients
Increased age, weekend admission, female gender, and racial minorities were associated with lower odds of revascularization. Co-morbidities like chronic pulmonary disease (OR = 1.079, C.I. [1.035 to 1.422] p <0.01), diabetes mellitus (OR = 1.081, C.I. [1.008 to 1.159] p <0.01), peripheral vascular disease (OR = 1.075, C.I. [1.02 to 1.133] p <0.01), obesity (OR = 1.454, C.I. [1.37 to 1.544] p <0.01), smoking history (OR = 1.089, C.I. [1.02 to 1.161] p <0.01), hyperlipidemia (OR = 1.75, C.I. [1.585 to 1.933] p <0.01), and hypertension (OR = 1.221, C.I. [1.176 to 1.268] p <0.01) were associated with higher odds of who underwent revascularization. In addition, liver disease, pulmonary hypertension, anemia, renal failure, neurological disorders, sepsis, malignant tumors including lymphoma and metastatic cancers were associated with lower odds of revascularization. The predictors of revascularization and mortality are shown in Figures 5 and 6, respectively. Of note, the probability of who underwent coronary revascularization as constructed using propensity score was significantly associated with improved mortality in CS patients.
Figure 5.
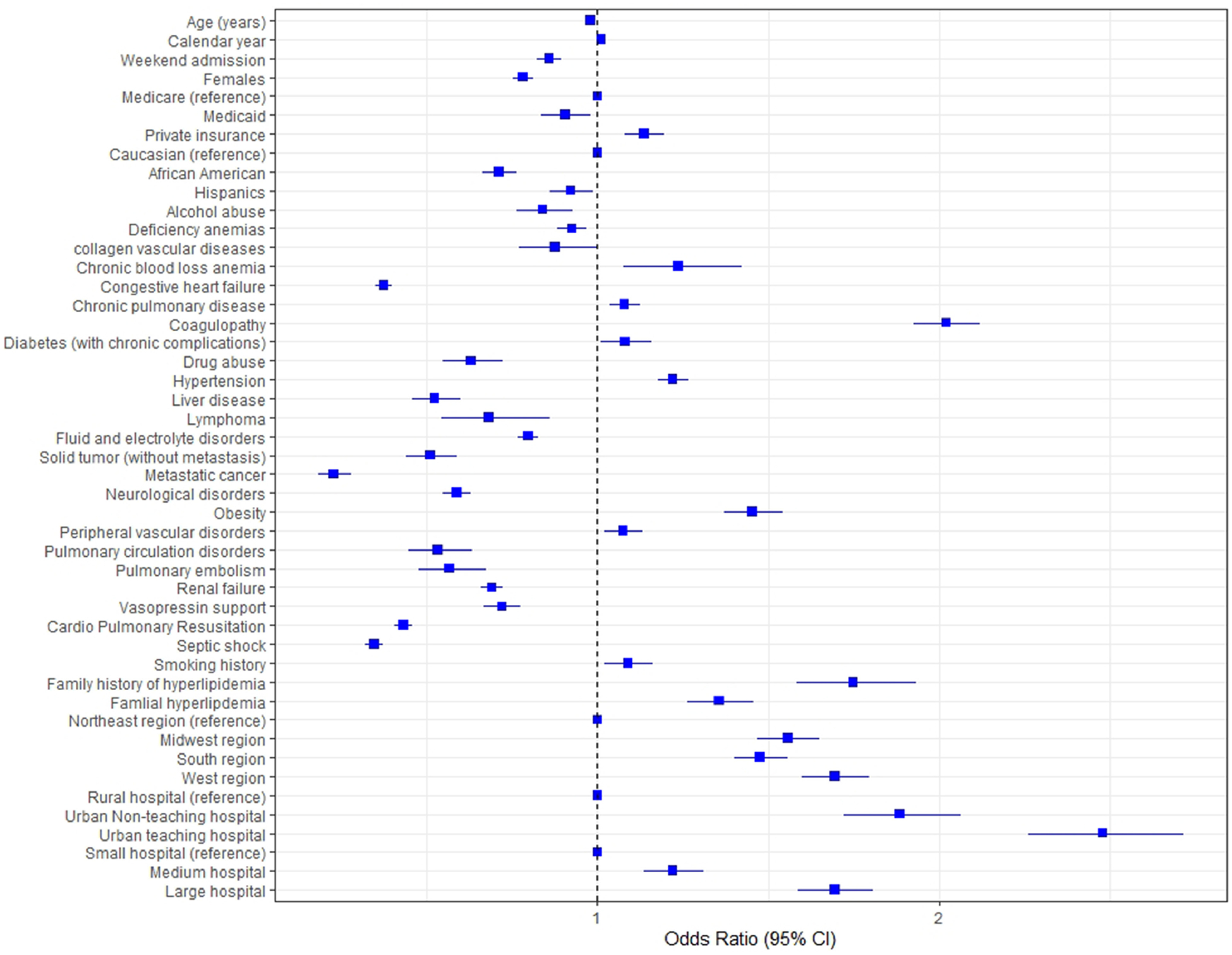
Predictors of revascularization in cardiogenic shock
Figure 6.
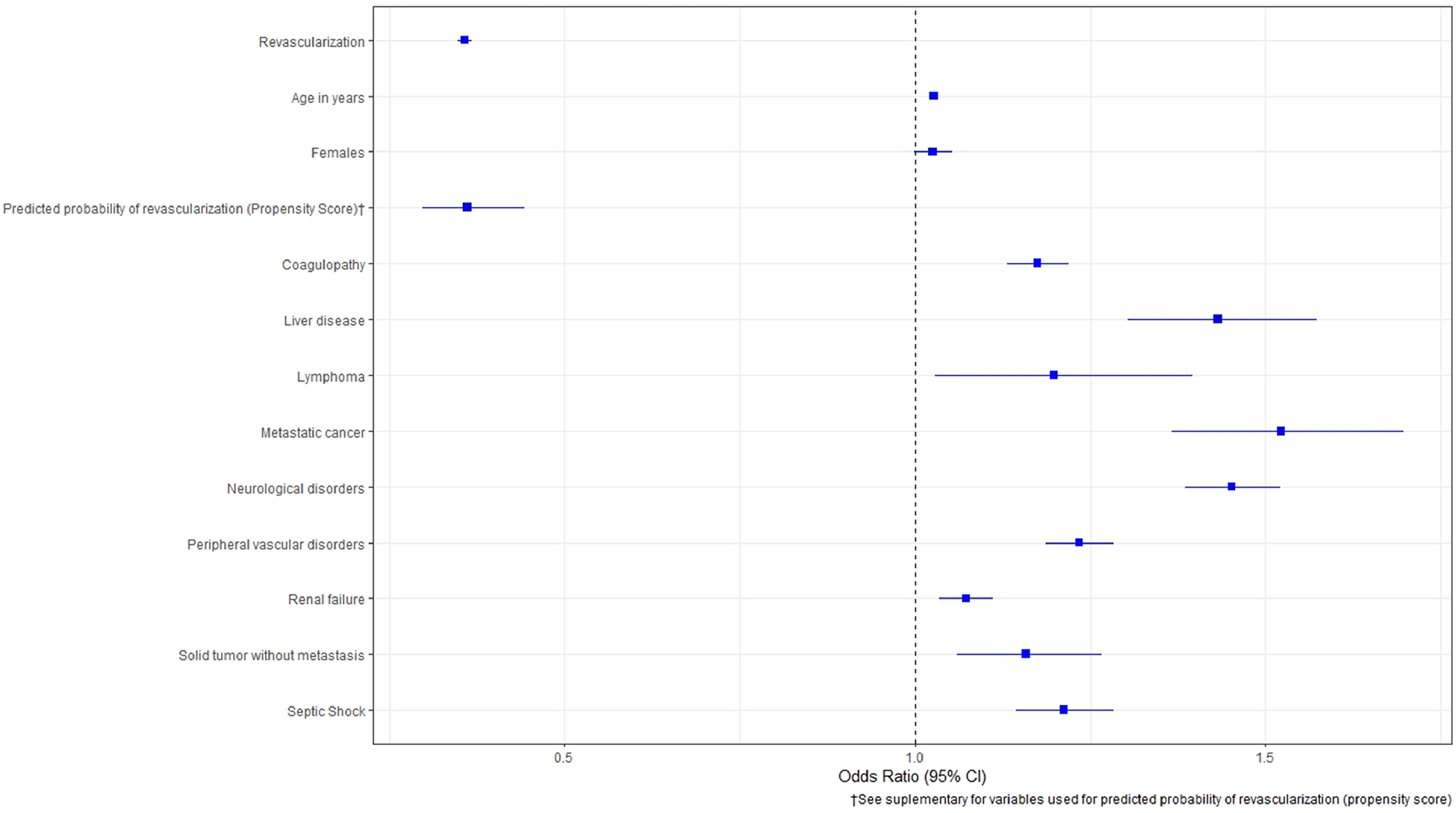
Predictors of mortality in cardiogenic shock
Discussion
The main findings of our current study are: (1) in patients admitted with AMI-CS, about 47% underwent coronary revascularization and there is a modest increased trend of revascularization seen over study years. (2) Coronary revascularization is associated with reduced mortality and better discharge outcomes. More patients in this group received extracorporeal membrane oxygenation and heart assist devices. (3) Gender and ethnic disparities were noted with respect to coronary revascularization in AMI-CS patients. Female gender and certain ethnic minorities such as African Americans had low utilization of revascularization in our cohort. (4) Overall mortality continues to be high in patients admitted with AMI-CS with a downward trend witnessed over the study years (5) In CS cohort, the use of IABP has been on the decline while the use of PVADs is increasing over our study years.
CS is the most dreaded complication after AMI and seen in 5% to 10% of such patients.1,2 Moreover, CS is associated with worsened mortality and morbidity and constitutes a significant burden on health care resources. In the landmark SHOCK trial, about 56% of patients with CS who did not undergo revascularization died at 30-day follow-up.4 Similarly, in another landmark trial IABP-SHOCK II, about 40% of patients with CS did not survive to hospital discharge.11 Our current study shows that in patients in whom revascularization was not attempted; the inpatient mortality was significantly high around 52% when compared with 25% with revascularization. We also demonstrated that revascularization is an independent predictor of low mortality in CS patients. Two early-randomized trials have studied the role of early revascularization in patients admitted with CS. The SMASH trial only enrolled 55 CS patients and found no significant 30-day death rate in patients with and without revascularization.12 Subsequently, the SHOCK trial found reduced mortality at 30 days in the invasive arm compared with medical stabilization, but this difference did not reach statistical significance.4 However, further follow up of these patients at 6 and 12 months showed statistically significant reduction in mortality if revascularization was attempted at index CS hospitalization (13% absolute difference, p = 0.03).4 The trial also showed successful PCI to be associated with lower mortality when compared with unsuccessful PCI (35% vs 80%). It should be noted that the PCI intervention that was employed primarily in SHOCK trial was balloon angioplasty and only 34% of patients received a stent (bare metal).4 The likelihood of successful PCI was more in the SHOCK trial if stents were used. Our study showed mortality of about 25% in patients receiving revascularization compared with over 46% in the SHOCK trial. The difference in mortality seems to be related to advancements in stent design, improved operator experience, and frequent utilization of mechanical circulatory support devices in this patient population.
In our cohort, mechanical circulatory support devices supported significant number of patients who underwent revascularization. Previous studies have also shown increased likelihood of invasive therapy and lower in-patient mortality with utilization of these devices in patients admitted with CS. In a study utilizing Nationwide Readmissions Database, Enezate et al. showed that rate of invasive treatment (coronary angiogram with or without PCI) was about 76% in patients with mechanical circulatory support devices when compared with only 26% in patients without these support devices.13 They also found reduced mortality in CS patients who were assisted with mechanical devices compared with patients who were not (33% vs 39.7%, p <0.01). Several potential mechanisms have been proposed by which these devices affect favorable outcomes in CS patients. These devices are known to reduce left ventricular filling pressure and wall stress with subsequent improvement in coronary perfusion that potentially reduces infarct size and myocardial cell death.14,15 These devices also enable complete coronary revascularization with subsequent greater improvements in myocardial function and better patient outcomes.16
Our study has several limitations which need to be highlighted. First, NIS is an administrative claim-based database that uses ICD-9-CM codes, which are prone to errors, however, the hard clinical end points used in this study such as revascularization, death and discharge disposition are less prone to diagnostic errors. Second, NIS collects data on in-patient discharges and each admission is registered as an independent event, it is therefore possible that one patient may have more than one admission in the same or subsequent years which may lead to duplicate registration of patients. Third, patients are not followed longitudinally in NIS so long-term outcomes could not be assessed from present dataset. Additionally, the whole premise of the study was to assess revascularization outcomes after CS and it is possible few patients will have developed CS as complication of revascularization in our cohort rather than other way around. Unfortunately, our data set is not designed to exclude those patients; however, due to limited real-life occurrence of this complication, we believe that this cohort is not sufficient to affect our study results.
In conclusion, in this large nationally represented US population sample of CS patients, we found revascularization rate of about 47% in patients admitted with CS with improvement in overall mortality over our study years.
Supplementary Material
Footnotes
Disclosures
No disclosures by any authors.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2019.10.040.
References
- 1.Becker RC, Gore JM, Lambrew C, Weaver WD, Rubison RM, French WJ, Tiefenbrunn AJ, Bowlby LJ, Rogers WJ. A composite view of cardiac rupture in the United States national registry of myocardial infarction. J Am Coll Cardiol 1996;7:1321–1326. [DOI] [PubMed] [Google Scholar]
- 2.Jeger RV, Radovanovic D, Hunziker PR, Pfisterer ME, Stauffer JC, Erne P, Urban P. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med 2008;149:618–626. [DOI] [PubMed] [Google Scholar]
- 3.Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448–454. [DOI] [PubMed] [Google Scholar]
- 4.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock: SHOCK Investigators: should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999;341:625–634. [DOI] [PubMed] [Google Scholar]
- 5.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST elevation myocardial infarction in the United States. J Am Heart Assoc 2014;3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolte D, Khera S, Dabhadkar KC, Agarwal S, Aronow WS, Timmermans R, Jain D, Cooper HA, Frishman WH, Menon V, Bhatt DL, Abbott JD, Fonarow GC, Panza JA. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating Non-ST-elevation myocardial infarction. Am J Cardiol 2016;117:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR Jr, Prasad A. Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol 2019;124: 491–498. [DOI] [PubMed] [Google Scholar]
- 8.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG. Contemporary management of cardiogenic shock: a scientific statement from the american heart association. Circulation 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 9.Lambert L, Blais C, Hamel D, Brown K, Rinfret S, Cartier R, Giguère M, Carroll C, Beauchamp C, Bogaty P. Evaluation of care and surveillance of cardiovascular disease: can we trust medico-administrative hospital data? Can J Cardiol 2012;28:162–168. [DOI] [PubMed] [Google Scholar]
- 10.ICD 9 codes in National In-patient sample. https://www.hcup-us.ahrq.gov/db/tools/I9_Formats.TXT. Accessed on September 25, 2019.
- 11.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–1296. [DOI] [PubMed] [Google Scholar]
- 12.Urban P, Stauffer JC, Bleed D, Khatchatrian N, Amann W, Bertel O, vanden Brand M, Danchin N, Kaufmann U, Meier B, Machecourt J, Pfisterer M. A randomized evaluation of early revascularization to treat shock complicating acute myocardial infarction: the (Swiss) Multicenter Trial of Angioplasty for Shock-(S)MASH. Eur Heart J 1999;20:1030–1038. [DOI] [PubMed] [Google Scholar]
- 13.Enezate T, Eniezat M, Thomas J. Utilization and outcomes of temporary mechanical circulatory support devices in cardiogenic shock. Am J Cardiol 2019;124:505–510. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Wahab M, Saad M, Kynast J, Geist V, Sherif MA, Richardt G, Toelg R. Comparison of hospital mortality with intra-aortic balloon counterpulsation insertion before versus after primary percutaneous coronary intervention for cardiogenic shock complicating acute myocardial infarction. Am J Cardiol 2010;105:967–971. [DOI] [PubMed] [Google Scholar]
- 15.Basir MB, Schreiber T, Dixon S, Alaswad K, Patel K, Almany S, Khandelwal A, Hanson I, George A, Ashbrook M, Blank N, Abdelsalam M, Sareen N, Timmis SBH, O’Neill WW. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv 2018;91:454–461. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal B, Aman W, Jeroudi O, Kleiman NS. Mechanical circulatory support in high-risk percutaneous coronary intervention. Methodist Debakey Cardiovasc J 2018;14:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


