Abstract
Data on the efficacy of left atrial appendage occlusion (LAAO) in clinical practice are limited. We performed a systematic review and meta-analysis of observational studies that reported observed versus expected rates of ischemic strokes and/or major bleeding following LAAO. Our primary end points were the pooled relative risk reduction (RRR) in ischemic stroke and major bleeding with corresponding 95% confidence intervals compared with what was expected by the CHA2DS2-VASc and HASBLED scores, respectively. Twenty-nine studies including 11,071 patients (age 74.0 ± 8.7 years, 60% males) met the inclusion criteria. The mean CHA2DS2-VASc score was 4.22 ± 1.48, and the mean HASBLED score was 3.04 ± 1.16. During 19,567 patient-year follow-up, 290 of 11,071 patients (2.62%) suffered an acute ischemic stroke. This represented a 73.6% (95% confidence interval 68.9–78.2%) RRR in ischemic strokes compared with what was expected based on the CHA2DS2-VASc score. A total of 26 studies reported observed versus expected major bleeding (10,056 patients; age 74.0 ± 8.7, 60% males). During 16,967 patient-year follow-up, 404 of 10,056 patients (4.0%) suffered a major bleeding event. This represented a 55% (95% confidence interval 44.2% to −65.9%) RRR in major bleeding compared with what was expected based on the HASBLED score. These estimates were consistent across subgroups stratified according to age, CHADS2VASc, HASBLED scores and type of LAAO device used. In conclusion, LAAO is associated with a favorable observed/expected ratio with regards to ischemic stroke and major bleeding in clinical practice. Future clinical trials remain essential to further assess the efficacy of LAAO via a direct comparison with oral anticoagulation.
Stroke prevention is a cornerstone in the management of nonvalvular atrial fibrillation (NVAF).1,2 Percutaneous Left atrial appendage occlusion (LAAO) has emerged as an effective alternative to oral anticoagulation for stroke prevention in patients with NVAF. The safety and efficacy of this approach were established in 2 pivotal randomized clinical trials that demonstrated non-inferiority of LAAO with the Watchman device (Boston Scientific, Marlborough, MA) compared with Warfarin. No other clinical trials on percutaneous LAAO have been published to date. However, many observational studies/registries sought to assess the effectiveness of LAAO in reducing stroke risk and bleeding events in patients with NVAF in clinical practice. Although these studies did not have a comparator anticoagulant arm, many consistently reported observed vs. expected event rates using validated and commonly used risk scores (CHA2DS2-VASc and HASBLED scores). To assess whether the benefits of LAAO observed in randomized trials extend to clinical practice, we performed a systematic review of observational studies observed versus expected ischemic and bleeding event rates following LAAO.
Methods
This study was conducted in accordance with the Cochrane Collaboration guidelines and reported as per the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guidelines (Supplementary Protocol). We conducted a literature search in PUBMED, EMBASE, EBSCO, CINAHL, Web of Science, and Cochrane (December 1st, 2019) to identify eligible studies using the key search terms: “Watchman”; “Amplatzer”; “Amulet”; “appendage”; “percutaneous occlusion”; “percutaneous closure” (Appendix Table-1). We manually searched reference lists of relevant studies for additional publications. After removal of duplicates, articles were screened at title and abstract level followed by full text screening based on the pre-specified selection criteria: (1) single and multi-institutional observational registries reporting both the actual rates of ischemic stroke and/or major bleeding and those that were expected by the CHA2DS2-VASc and HASBLED scores, respectively, AND (2) sample size of at least 100 subjects and follow-up of at least 6 months to minimize small study effects. We excluded randomized controlled trials (to avoid heterogeneity based on study design) and studies not reporting outcomes of interest (Figure 1). The process of study search and selection was performed 2 of the authors (T.B. and M.A). Any disagreements were resolved through consensus and arbitration by the senior author (M.A).
Figure 1.
Study flow chart.
The data abstraction was carried on a standard data collection form by 2 independent investigators (T.B. and M.A) who were not involved in any of the included studies, and was adjudicated under supervision of the senior author(M.A). Following information was abstracted: baseline characteristics of study and participants, number of patients, point estimates, follow up duration (calculated as patient-year [PY]), procedural success rate, post-discharge medications, and major adverse events (stroke, transient ischemic attack, major bleeding, device related thrombus). When multiple publications from the same study population were found, data from the most inclusive report were used. The primary outcomes of interests were ischemic stroke and major bleeding, wherein we abstracted relative risk reductions (RRR) for stroke and major compared with what was expected by the CHA2DS2-VASc and HASBLED scores, respectively from individual studies. Major bleeding was defined per the individual studies but mostly included intracerebral hemorrhage, and gastrointestinal and other bleeding requiring intervention or blood transfusion. The quality assessment at study level was performed as per Newcastle-Ottawa Scale criteria (Appendix Table-2).
Estimates were pooled using random-effects model. The DerSimonian and Laird method was used for estimation of τ.2 Outcomes were reported as RRR with 95% confidence intervals (CI). We used I2 statistics to measure the extent of unexplained statistical heterogeneity: I2 greater than 50% was considered a high degree of between-study statistical heterogeneity. Sensitivity analyses were performed by sequential removal of one study at a time. Subgroup analyses were performed according to age (≤, >75 years), CHADS2-VASc score (≤, >4), HASBLED score (≤, >3), and device type. Publication bias was assessed using Egger’s regression test. Statistical significance was set at 5%. Comprehensive meta-analysis V 3.0 (Biostat, Englewood, NJ) was used.
Results
Twenty-nine studies including 11,071 patients met the inclusion criteria.3–29 All 29 studies reported observed vs. expected ischemic stroke events, while 26 studies among them including 10,056 patients reported observed versus expected bleeding events. Among the 11,071 enrolled patients, 6,597 (60%) were males and the mean age was 74.0 ± 8.7 years. Majority of patients (84%) had nonparoxysmal atrial fibrillation. The mean CHA2DS2-VASc score was 4.22 ± 1.48, and the mean HASBLED score was 3.04 ± 1.16. Majority of patients were discharged on antiplatlets therapy rather than oral anticoagulation (Table 1). Appendix Table-3 reports baseline characteristics of individual studies and participants. Follow up ranged among the individual studies from 91 to 2293 PY (pooled follow up 19,567 PY). During follow up, 290 of 11,071 patients (2.62%) suffered an acute ischemic stroke. All 29 studies reported significantly lower ischemic stroke rates compared with what was expected based on the CHA2DS2-VASc score (RRR range: 40.8% to 100%). The pooled RRR for ischemic stroke compared with what was expected based on the CHA2DS2-VASc score among all studies was 73.6% (95% CI, 68.9% to 78.3%) (Figure 2).
Table 1.
Baseline characteristics of patients enrolled in observational studies on left atrial appendage occlusion that reported observed versus expected stroke events
| Baseline characteristics | n (%), Mean ± SD |
|---|---|
| Total number of studies | 29 |
| Total number of patients | 11,071 |
| Age (years) | 74.0 ± 8.7 |
| Men | 6,597 (60%) |
| Type of atrial fibrillation | |
| Paroxysmal | 1,804(16%) |
| Non-paroxysmal | 9,267 (84%) |
| Hypertension | 7,659 (69%) |
| Diabetes mellitus | 2,681 (24%) |
| Prior stroke/transient ischemic attack | 3,395 (31%) |
| Peripheral vascular disease | 1,199(11%) |
| Congestive heart failure | 1,845 (17%) |
| Mean CHA2DS2-VASc Score | 4.22±1.48 |
| Mean HASBLED Score | 3.04±1.16 |
| Left appendage occlusion device | |
| Watchman | 5,579 (50%) |
| Amplatzer cardiac plug | 2,332(21%) |
| Amulet | 1,724(16%) |
| Amplatzer cardiac plug /amulet | 1,436(13%) |
| Procedural success | 10,775 (97%) |
| Antithrombotic therapy at discharge | |
| Oran anticoagulant | 2,610 (23%) |
| Warfarin | 1,512(58%) |
| Nonvitamin K antagonist oral anticoagulant | 450 (17%) |
| Not specified (Warfarin/NOAC) | 648 (25%) |
| Antiplatelet | 7,144(65%) |
| Dual antiplatlets | 5,097 (72%) |
| Single antiplatelet | 1,151 (16%) |
| Not specified (SAPT/DAPT) | 896 (12%) |
| Not reported/none/other | 1,317(12%) |
DAPT = dual anti-platelet therapy; NOAC = nonvitamin K antagonist oral anticoagulant; SAPT = single anti-platelet therapy.
Figure 2.
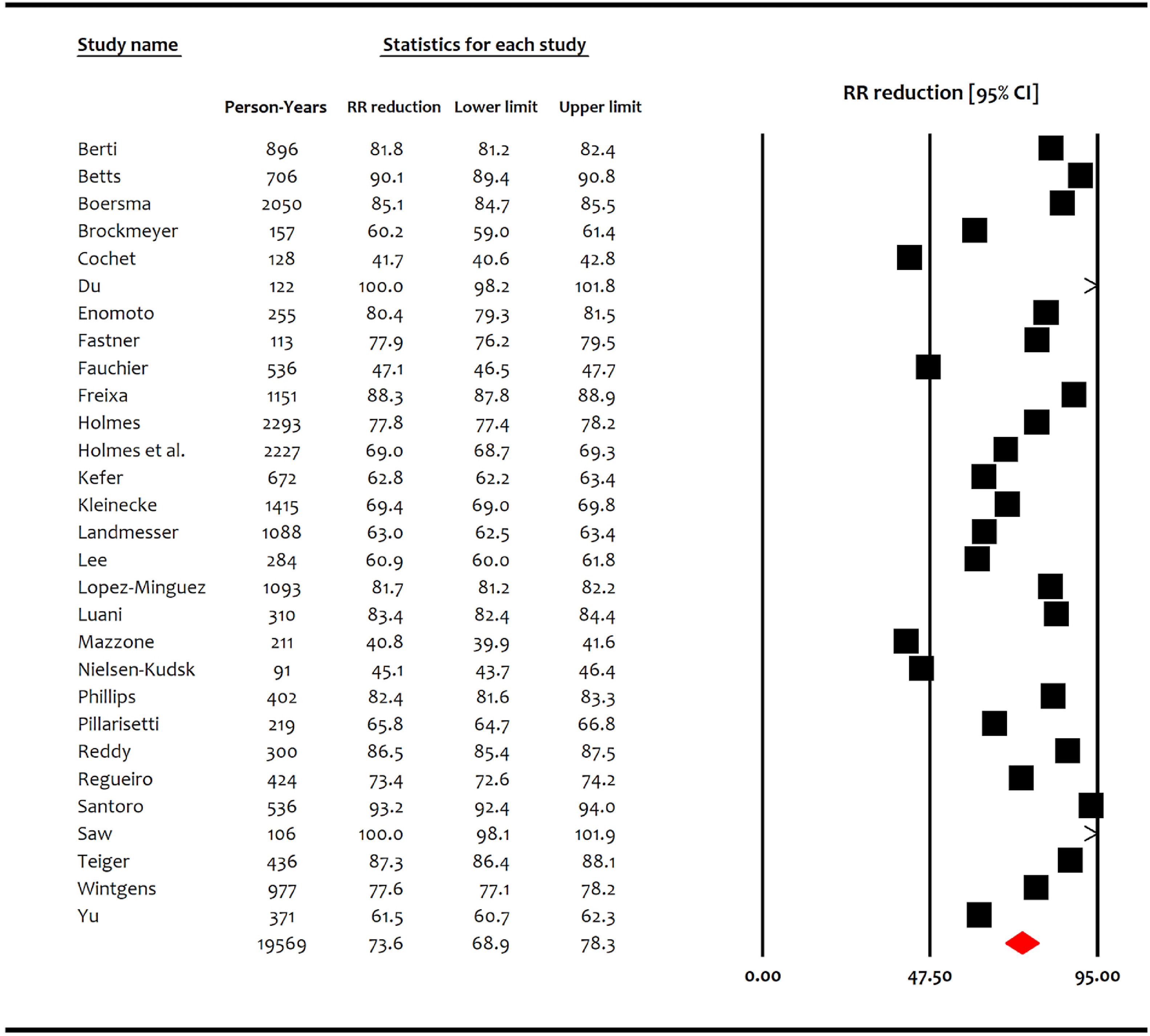
Forest plot showing the pooled relative risk reduction of ischemic stroke with left atrial appendage occlusion compared with the expected based on CHA2DS2-VASc score. CI = confidence interval; RRR = relative risk reduction.
Among 10,056 patients enrolled in studies reporting observed versus expected major bleeding, 6,029 (60%) were males and the mean age was 74.0 ± 8.7. Majority of patients (85%) had nonparoxysmal atrial fibrillation. The mean CHA2DS2-VASc score was 4.21 ± 1.48, and the mean HASBLED score was 3.08 ± 1.14. Majority of patients were discharged on antiplatlets therapy rather than oral anticoagulation (Table 2). Appendix Table-4 reports baseline characteristics of individual studies and participants. Follow up ranged among the individual studies from 113 to 2293 PY (pooled follow up 16,967 PY). During follow up, 404 of 10,056 patients (4.0%) suffered a major bleeding event. All studies but one (Landmesser et al) reported significantly lower major bleeding rates compared with what was expected based on the HASBLED score. The RRR of major bleeding based on the observed versus expected rates according the HASBLED score ranged from −19.3% to 100%. The pooled RRR for major bleeding compared with what was expected based on the HASBLED score among all studies was 55.0% (95% CI, 44.2% to 65.9%) (Figure 3).
Table 2.
Baseline characteristics of patients enrolled in observational studies on left atrial appendage occlusion that reported observed versus expected bleeding events
| Baseline characteristics | n (%), Mean ± SD |
|---|---|
| Total number of studies | 26 |
| Total number of patients | 10,056 |
| Age (years) | 74.0 ± 8.7 |
| Men | 6,029 (60%) |
| Type of atrial fibrillation | |
| Paroxysmal | 1,495 (15%) |
| Nonparoxysmal | 8,561 (85%) |
| Hypertension | 6,825 (68%) |
| Diabetes mellitus | 2,397 (24%) |
| Prior stroke/transient ischemic attack | 3,135 (31%) |
| Peripheral vascular disease | 868 (9%) |
| Congestive heart failure | 1,628(16%) |
| Mean CHA2DS2-VASc Score | 4.21±1.48 |
| Mean HASBLED Score | 3.08±1.14 |
| Left appendage occlusion device | |
| Watchman | 4,634 (46%) |
| Amplatzer cardiac plug | 2,350 (23%) |
| Amulet | 1,787(18%) |
| Amplatzer cardiac plug /amulet | 1,285 (13%) |
| Procedural success | 9,801 (97%) |
| Antithrombotic therapy at discharge | |
| Oral anticoagulant | 1,805 (18%) |
| Warfarin | 717 (40%) |
| Nonvitamin K antagonist oral anticoagulant | 450 (25%) |
| Not specified (Warfarin/NOAC) | 638 (35%) |
| Antiplatelet | 6,942 (69%) |
| Dual antiplatlets | 4,955 (71%) |
| Single antiplatelet | 1,091 (16%) |
| Not specified (SAPT/DAPT) | 896 (13%) |
| Not reported/none/other | 1,317(13%) |
DAPT = dual anti-platelet therapy; NOAC = nonvitamin K antagonist oral anticoagulant; SAPT = single anti-platelet therapy.
Figure 3.
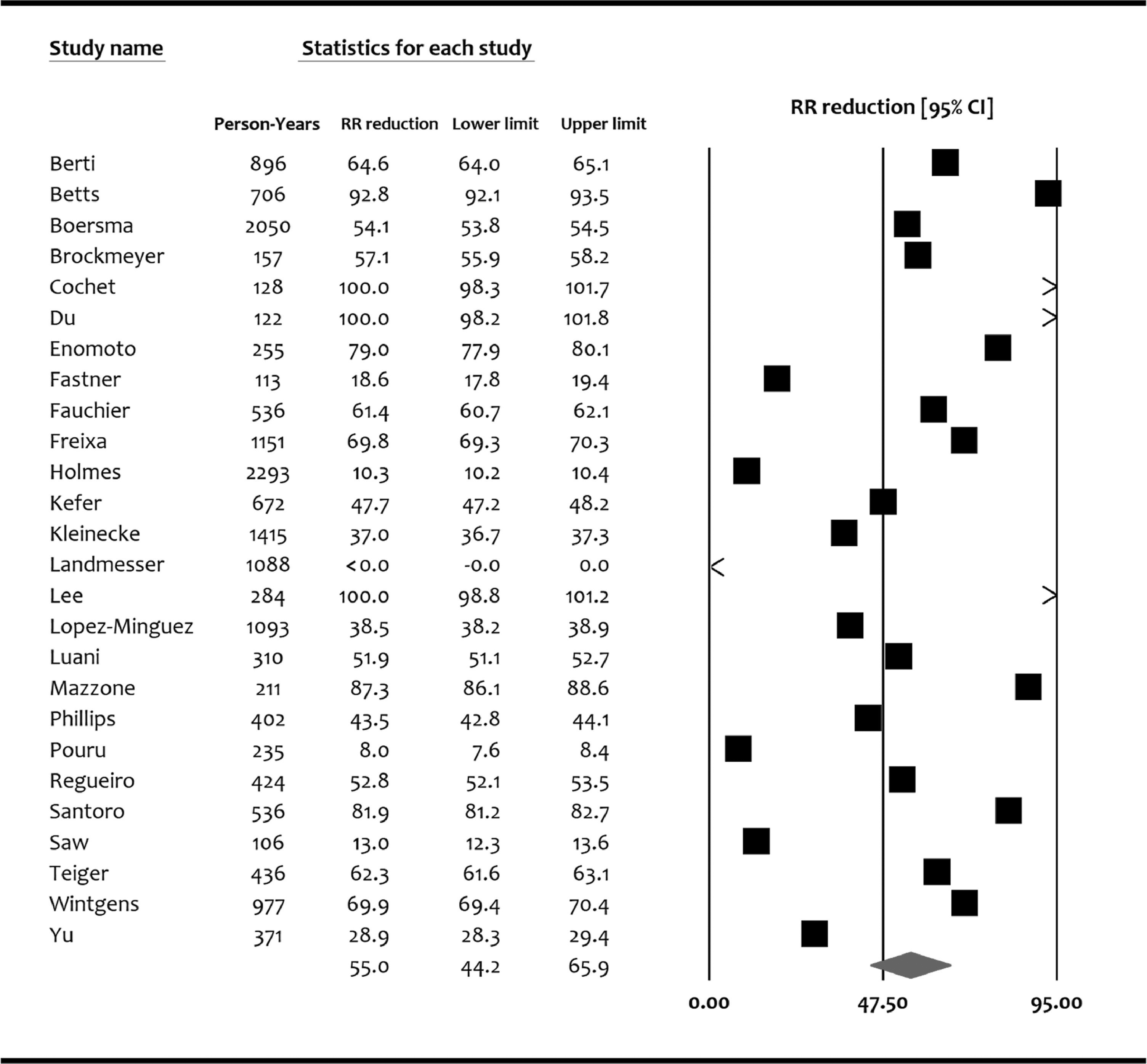
Forest Plot showing the pooled relative risk reduction of major bleeding with left atrial appendage occlusion compared with the expected based on HASBLED score. CI = confidence interval; RRR = relative risk reduction.
Sensitivity analyses by removal of one study at a time showed consistent >70% RRR in case of stroke and >50% RRR in case of major bleeding (Appendix Figures 1–2). Subgroup analyses showed that these estimates did not vary based on age, CHADS2-Vasc score, HASBLED score and type of LAAO device (Figure 4; Appendix Table-5). Egger’s regression test did not detect publication bias (p value [2 tailed] = 0.83).
Figure 4.
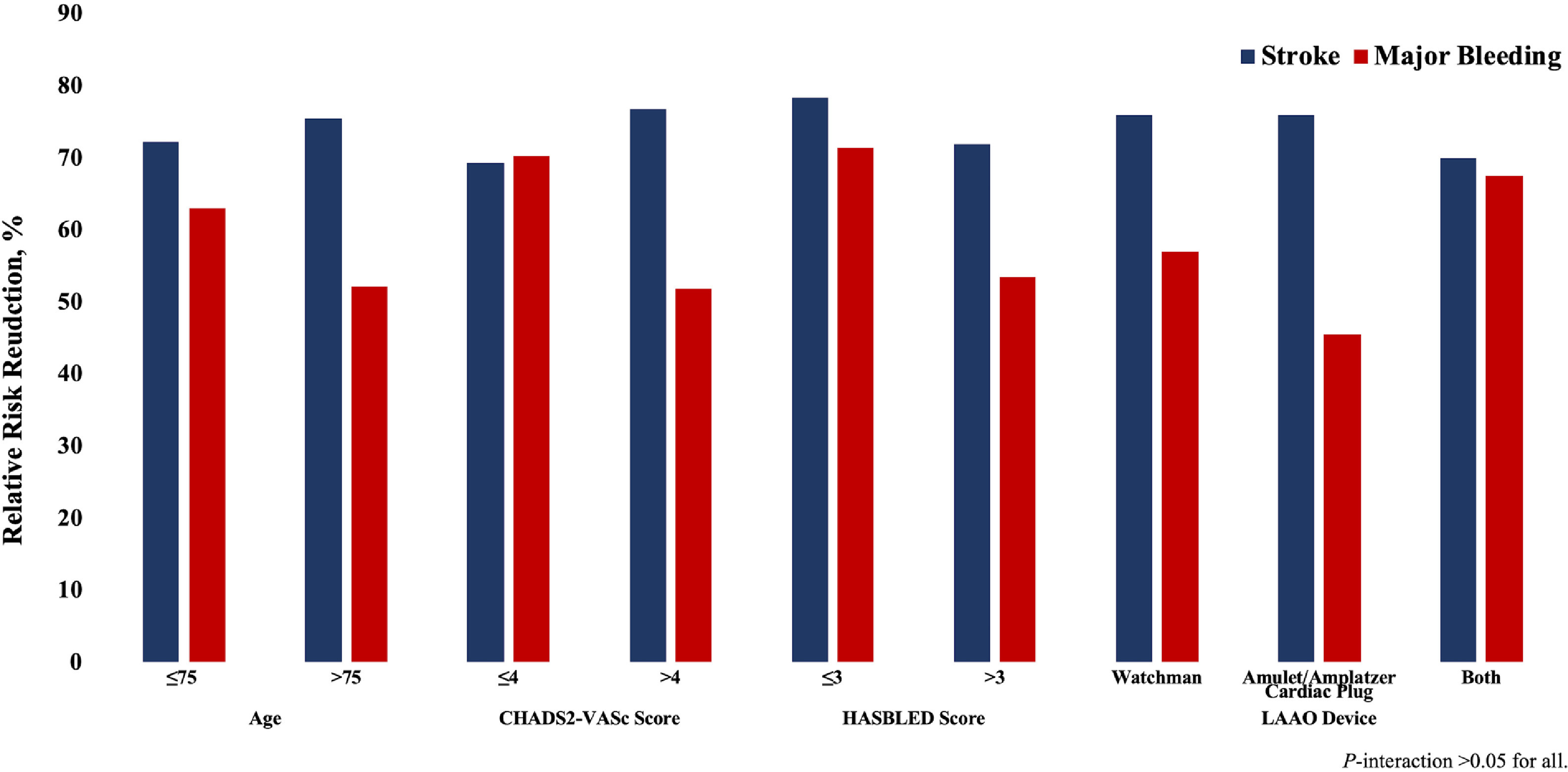
Subgroup analyses based on age, CHA2DS2-VASc Score, HASBLED Score, or Device Type. LAAO = left atrial appendage occlusion.
Discussion
Data supporting the efficacy of LAAO has been limited to a single randomized clinical trial. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) study randomized 707 patients in 2:1 ratio to undergo LAAO with the Watchman device versus Warfarin. The primary efficacy end point was a composite of stroke, cardiovascular death, or systemic embolization. At 1065 patient-years of follow-up, the primary efficacy event rate was 3.0 per 100 PY (95% credible interval 1.9 to 4.5) in the intervention group and 4.9 per 100 PY (2.8 to 7.1) in the control group (rate ratio 0.62, 95% credible interval 0.35 to 1.25),12 establishing the efficacy of LAAO for stroke prevention in patients with NVAF.
The only other randomized trial published to date is PREVAIL (Prospective Randomized Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation versus Long Term Warfarin Therapy).30 This study, however, was not powered to assess the efficacy of LAAO but rather to address the safety concerns raised by the Food and Drug Administration due to the high complication rates in the device arm in PROTECT AF. Although PREVAIL demonstrated the improved safety of LAAO, paving the way to the approval of the Watchman device in the United States, concerns arose about the higher rates of ischemic stroke in device arm (1.9% vs 0.7% in the Warfarin arm). Hence, the question about efficacy of LAAO remained open. Given that a large number of LAAO procedures have been performed worldwide, attempts were made to validate the initial efficacy observed in PROTECT AF in real world cohorts. Many observational studies have then compared short- and mid-term outcomes following LAAO to those that were expected by historical data. Our study aimed to provide a systematic review of those data and provide a pooled estimated of RRR of ischemic and bleeding events with LAAO compared to the predicted rates by CHA2DS2-VASc and HASBLED scores.
Our findings suggested >70% and >50% RRR in predicted ischemic stroke and bleeding events following LAAO compared to what is predicted by CHA2DS2-VASc and HASBLED scores, respectively. The reduction in stroke and major bleeding rates were consistent across sensitivity analyses and subgroup analyses. These findings may be reassuring given the paucity of randomized data demonstrating LAAO efficacy, and the growing concerns about some issues related to the LAAO procedure (device related thrombus, and peridevice leaks). However, the interpretation of those findings deserves more scrutiny.
First, the use of expected event rates derived from risk prediction models to assess the efficacy of a new treatment modality has not been well studied. This may be especially problematic if the risk prediction model has been found to have modest prognostic value in validation cohorts (e.g., CHA2DS2-VASc). This is illustrated in the PREVAIL study where only 1 ischemic stroke occurred during a 207 patient-year in the control arm. Patients in this arm had a CHA2DS2-VASc of ~4 which would have predicted 14 ischemic events during follow up without Warfarin, and 6 to 7 ischemic events with Warfarin. Nonetheless, these data remain important to inform practicing physicians about the efficacy of LAAO as there are currently no other comparative trials of LAAO versus anticoagulation. Although 2 such trials are planned for 2020, data from those trials are several years away and hence, and till then the use of observed versus expected event rates provide a crude ‘indirect’ method to confirm the efficacy of LAAO in patients with NVAF. Second, it is estimated that more than 100,000 LAAO procedures have been performed world-wide. However, follow up data are only available in <12,000 patients. Hence, significant publication bias might exist and has to be considered while interpreting this study. Third, the magnitude of RRR was higher for ischemic stroke than for major bleeding. This is contrary to what was observed in PROTECT AF where the main benefit of LAAO was in the reduction of hemorrhagic strokes and major bleeding. This, however, could represent differences in the predictive value and accuracy of the CHA2DS2-VASc and HASBLED scores.
Our study has a number of limitations. (1) this systematic review included only observational studies. Hence, its findings have to be interpreted in the context of the known limitations of nonrandomized data including selection and publication bias, lack of ascertainment of clinical events, and the variability in definitions of end-points and in follow up duration. (2) The risk scores used to predict event rates in those studies have shown variable and modest predictability in various cohorts. The RRR demonstrated in this study could be partially related to the over-estimation of predicted events by these scores. (3) We are unable to control for some important variables that could have impacted the event rates across the studies (post-LAAO anti-platelets and anti-anticoagulation regimens, device selection, learning curve, quality of follow up, etc.). Nonetheless, despite these limitations, these data represent the first pooled analysis attempting to assess the efficacy of LAAO outside of the clinical trial setting.
Supplementary Material
Footnotes
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2020.02.041.
References
- 1.Alkhouli M, Alqahtani F, Aljohani S, Alvi M, Holmes DR. Burden of atrial fibrillation-associated ischemic stroke in the United States. JACC Clin Electrophysiol 2018;4:618–625. [DOI] [PubMed] [Google Scholar]
- 2.Alkhouli M, Noseworthy PA, Rihal CS, Holmes DR Jr.. Stroke prevention in nonvalvular atrial fibrillation: a stakeholder perspective. J Am Coll Cardiol 2018;71:2790–2801. [DOI] [PubMed] [Google Scholar]
- 3.Berti S, Santoro G, Brscic E, Montorfano M, Vignali L, Danna P, Tondo C, D’Amico G, Stabile A, Sacca S, Patti G, Rapacciuolo A, Poli A, Golino P, Magnavacchi P, De Caterina A, Meucci F, Pezzulich B, Rezzaghi M, Stolcova M, Tarantini G. Left atrial appendage closure using AMPLATZER devices: a large, multicenter, Italian registry. Int J Cardiol 2017;248:103–107. [DOI] [PubMed] [Google Scholar]
- 4.Betts TR, Leo M, Panikker S, Kanagaratnam P, Koa-Wing M, Davies DW, Hildick-Smith D, Wynne DG, Ormerod O, Segal OR, Chow AW, Todd D, Cabrera Gomez S, Kirkwood GJ, Fox D, Pepper C, Foran J, Wong T. Percutaneous left atrial appendage occlusion using different technologies in the United Kingdom: a multicenter registry. Catheter Cardiovasc Interv 2017;89:484–492. [DOI] [PubMed] [Google Scholar]
- 5.Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, Gori T, Meincke F, Protopopov AV, Betts T, Mazzone P, Foley D, Grygier M, Sievert H, De Potter T, Vireca E, Stein K, Bergmann MW, following i, institutions participated in the Es. Evaluating real-world clinical outcomes in trial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology. Circ Arrhythm Electrophysiol 2019;12:e006841. [DOI] [PubMed] [Google Scholar]
- 6.Brockmeyer M, Wolff G, Krieger T, Lin Y, Karathanos A, Afzal S, Zeus T, Westenfeld R, Polzin A, Heinen Y, Perings S, Kelm M, Schulze V. Kidney function stratified outcomes of percutaneous left atrial appendage occlusion in patients with atrial fibrillation and high bleeding risk. Acta Cardiol 2019;14:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Du X, Chu H, Ye P, He B, Xu H, Jiang S, Lin M, Lin R, Liu J, Wang B, Feng M, Yu Y, Chen X. Combination of left atrial appendage closure and catheter ablation in a single procedure for patients with atrial fibrillation: multicenter experience. J Formos Med Assoc 2019;118: 891–897. [DOI] [PubMed] [Google Scholar]
- 8.Fastner C, Hoffmann L, Aboukoura M, Behnes M, Lang S, Borggrefe M, Akin I, Nienaber CA. Real-world experience comparing two common left atrial appendage closure devices. BMC Cardiovasc Disord 2018;18:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Gras D, Mansourati J, Deharo JC, Montalescot G, Defaye P. Major adverse events with percutaneous left atrial appendage closure in patients with atrial fibrillation. J Am Coll Cardiol 2019;73:2638–2640. [DOI] [PubMed] [Google Scholar]
- 10.Freixa X, Llull L, Gafoor S, Cruz-Gonzalez I, Shakir S, Omran H, Berti S, Santoro G, Kefer J, Landmesser U, Nielsen-Kudsk JE, Kanagaratnam P, Nietlispach F, Gloekler S, Aminian A, Danna P, Rezzaghi M, Stock F, Stolcova M, Paiva L, Costa M, Millan X, Ibrahim R, Tichelbacker T, Schillinger W, Park JW, Sievert H, Meier B, Tzikas A. Characterization of cerebrovascular events after left atrial appendage occlusion. Am J Cardiol 2016;118:1836–1841. [DOI] [PubMed] [Google Scholar]
- 11.Holmes DR Jr., Reddy VY, Gordon NT, Delurgio D, Doshi SK, Desai AJ, Stone JE Jr., Kar S Long-term safety and efficacy in continued access left atrial appendage closure registries. J Am Coll Cardiol 2019;74:2878–2889. [DOI] [PubMed] [Google Scholar]
- 12.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P, Investigators PA. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 13.Kefer J, Aminian A, Vermeersch P, de Potter T, Stammen F, Benit E, Budts W, Missault L, Drieghe B, Buysschaert I, Cornelis K, Herzet JM, Guedes A, Debbas N, Rivero M, Lempereur M, Lochy S, CasadoArroyo R, Laruelle C, Debruyne P, Ledent T. Transcatheter left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation: results from the Belgian registry. EuroIntervention 2018;13: 1603–1611. [DOI] [PubMed] [Google Scholar]
- 14.Kleinecke C, Cheikh-Ibrahim M, Schnupp S, Fankhauser M, Nietlispach F, Park JW, Brachmann J, Windecker S, Meier B, Gloekler S. Long-term clinical outcomes of Amplatzer cardiac plug versus Amulet occluders for left atrial appendage closure. Catheter Cardiovasc Interv 2019. 10.1002/ccd.28530. [DOI] [PubMed]
- 15.Landmesser U, Tondo C, Camm J, Diener HC, Paul V, Schmidt B, Settergren M, Teiger E, Nielsen-Kudsk JE, Hildick-Smith D. Left atrial appendage occlusion with the AMPLATZER Amulet device: one-year follow-up from the prospective global Amulet observational registry. EuroIntervention 2018;14:e590–e597. [DOI] [PubMed] [Google Scholar]
- 16.Lee OH, Kim JS, Pak HN, Hong GR, Shim CY, Uhm JS, Cho IJ, Joung B, Yu CW, Lee HJ, Kang WC, Shin ES, Choi RK, Lim DS, Jang Y. Feasibility of left atrial appendage occlusion for left atrial appendage thrombus in patients with persistent atrial fibrillation. Am J Cardiol 2018;121:1534–1539. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Minguez JR, Nogales-Asensio JM, Infante De Oliveira E, De Gama Ribeiro V, Ruiz-Salmeron R, Arzamendi-Aizpurua D, Costa M, Gutierrez-Garcia H, Fernandez-Diaz JA, Martin-Yuste V, Rama-Merchan JC, Moreno-Gomez R, Benedicto-Buendia A, Iniguez-Romo A. Long-term event reduction after left atrial appendage closure. Results of the Iberian Registry II. Rev Esp Cardiol (Engl Ed) 2019;72:449–455. [DOI] [PubMed] [Google Scholar]
- 18.Luani B, Genz C, Herold J, Mitrasch A, Mitusch J, Wiemer M, Schmeisser A, Braun-Dullaeus RC, Rauwolf T. Cerebrovascular events, bleeding complications and device related thrombi in atrial fibrillation patients with chronic kidney disease and left atrial appendage closure with the WATCHMAN device. BMC Cardiovasc Disord 2019;19:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzone P, D’Angelo G, Regazzoli D, Molon G, Senatore G, Sacca S, Canali G, Amellone C, Turri R, Bella PD. Percutaneous left atrial appendage closure with WATCHMAN device: peri-procedural and mid-term outcomes from the TRAPS registry. J Interv Card Electrophysiol 2018;52:47–52. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen-Kudsk JE, Johnsen SP, Wester P, Damgaard D, Airaksinen J, Lund J, De Backer O, Pakarinen S, Odenstedt J, Vikman S, Settergren M, Kongstad O, Rosenqvist M, Krieger DW. Left atrial appendage occlusion versus standard medical care in patients with atrial fibrillation and intracerebral haemorrhage: a propensity score-matched follow-up study. EuroIntervention 2017;13:371–378. [DOI] [PubMed] [Google Scholar]
- 21.Phillips KP, Santoso T, Sanders P, Alison J, Chan JLK, Pak HN, Chandavimol M, Stein KM, Gordon N, Razali OB. Left atrial appendage closure with WATCHMAN in Asian patients: 2year outcomes from the WASP registry. Int J Cardiol Heart Vasc 2019;23:100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillarisetti J, Reddy YM, Gunda S, Swarup V, Lee R, Rasekh A, Horton R, Massumi A, Cheng J, Bartus K, Badhwar N, Han F, Atkins D, Bommana S, Earnest M, Nath J, Ferrell R, Bormann S, Dawn B, Di Biase L, Mansour M, Natale A, Lakkireddy D. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm 2015;12:1501–1507. [DOI] [PubMed] [Google Scholar]
- 23.Pouru JP, Jaakkola S, Lund J, Biancari F, Saraste A, Airaksinen KEJ. Effectiveness of only aspirin or clopidogrel following percutaneous left atrial appendage closure. Am J Cardiol 2019;124:1894–1899. [DOI] [PubMed] [Google Scholar]
- 24.Reddy VY, Mobius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, Sick P, Sievert H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013;61:2551–2556. [DOI] [PubMed] [Google Scholar]
- 25.Regueiro A, Cruz-Gonzalez I, Bethencourt A, Nombela-Franco L, Champagne J, Asmarats L, Jimenez-Quevedo P, Rodriguez-Gabella T, Rama-Merchan JC, Puri R, O’Hara G, Rodes-Cabau J. Long-term outcomes following percutaneous left atrial appendage closure in patients with atrial fibrillation and contraindications to anticoagulation. J Interv Card Electrophysiol 2018;52:53–59. [DOI] [PubMed] [Google Scholar]
- 26.Santoro G, Meucci F, Stolcova M, Rezzaghi M, Mori F, Palmieri C, Paradossi U, Pastormerlo LE, Rosso G, Berti S. Percutaneous left atrial appendage occlusion in patients with non-valvular atrial fibrillation: implantation and up to four years follow-up of the AMPLATZER Cardiac Plug. EuroIntervention 2016;11:1188–1194. [DOI] [PubMed] [Google Scholar]
- 27.Saw J, Fahmy P, Azzalini L, Marquis JF, Hibbert B, Morillo C, Carrizo A, Ibrahim R. Early Canadian multicenter experience with WATCHMAN for percutaneous left atrial appendage closure. J Cardiovasc Electrophysiol 2017;28:396–401. [DOI] [PubMed] [Google Scholar]
- 28.Teiger E, Thambo JB, Defaye P, Hermida JS, Abbey S, Klug D, Juliard JM, Pasquie JL, Rioufol G, Lepillier A, Elbaz M, Horvilleur J, Brenot P, Pierre B, Le Corvoisier P, French National Left Atrial Appendage Closure Registry I. Percutaneous left atrial appendage closure is a reasonable option for patients with atrial fibrillation at high risk for cerebrovascular events. Circ Cardiovasc Interv 2018;11: e005841. [DOI] [PubMed] [Google Scholar]
- 29.Wintgens L, Romanov A, Phillips K, Ballesteros G, Swaans M, Folkeringa R, Garcia-Bolao I, Pokushalov E, Boersma L. Combined atrial fibrillation ablation and left atrial appendage closure: long-term follow-up from a large multicentre registry. Europace 2018;20:1783–1789. [DOI] [PubMed] [Google Scholar]
- 30.Holmes DR Jr., Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


