Abstract
Patients having transcatheter aortic valve implantation (TAVI) routinely undergo coronary angiography before the procedure to define the coronary anatomy and to evaluate the extend of coronary artery disease (CAD). Whether percutaneous coronary intervention (PCI) prior/concomitant with TAVI confers any additional clinical benefit in patients with CAD remains unclear. Literature search was performed using Medline, Embase, Google Scholar, and Scopus from inception of these databases till April 2019. Included outcomes were 30-day all-cause mortality, stroke, myocardial infarction (MI), acute kidney injury, and 1-year mortality. The main summary estimate was random effects odds ratio (OR) with 95% confidence intervals (CIs). Eleven cohort studies enrolling 5,580 patients (mean age 82.4 years and 52.6% females) were included. Our study found no difference in effect estimates for 30-day all-cause mortality (OR 1.30 [0.85 to 1.98], p = 0.22, I2 = 37.5%), stroke (OR 0.7 (0.36 to 1.45), p = 0.36, I2 = 32.8%), MI (OR 2.71 [0.55 to 12.23], p = 0.22, I2 = 41.3%), acute kidney injury (OR 0.7 [0.46 to 1.06], p = 0.08, I2 = 14.4%) and 1-year all-cause mortality (OR 1.19 [0.92 to 1.52], p = 0.18, I2 = 0.0%) in patients who underwent TAVI with and without PCI. In conclusion, our analysis indicates that PCI with TAVI in patients with severe aortic stenosis and concomitant CAD grants no additional clinical advantage in terms of patient important clinical outcomes. Further randomized studies are needed to better delineate the clinical practice for myocardial revascularization in patients receiving transcatheter therapy for aortic valve disease.
Transcatheter aortic valve implantation (TAVI) has revolutionized the management of patients with severe aortic stenosis (AS). The procedure was initially approved in patients deemed inoperable or at a high risk for surgical aortic valve replacement.1 However, with the recent publication of the PARTNER 3 and Evolut low-risk trials, the procedure is expected to be approved even in patients at low risk, further increasing the number of patients eligible for TAVI.2–4 Coronary artery disease (CAD) is prevalent in patients who underwent TAVI in part because of old age and co-morbidities in this patient population which can predispose to atherogenesis. Patients having TAVI routinely undergo coronary angiography before the procedure to define the coronary anatomy and to evaluate for the extent of CAD. Such an approach can also help facilitate planning for coronary artery bypass grafting if the patient is deemed a surgical candidate.5,6 Percutaneous coronary intervention (PCI) is also performed in patients before TAVI, although the practice patterns are heterogeneous. Herein, we investigate whether PCI before TAVI is associated with any improvements in the hard clinical endpoints.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Two authors (NL and SUK) devised the search strategy and performed literature search using Medline (via PubMed), Embase, Google Scholar, and Scopus databases from inception to April 2019. Following key search words were used: “transcatheter aortic valve replacement” or “transcatheter aortic valve implantation,” “coronary artery disease,” “percutaneous coronary intervention” or “coronary revascularization.” We applied restrictions on humans’ studies. No restrictions were applied on publication year, language, or text availability. We also searched for “meta-analysis” as the article type and hand searched the reference lists of the selected systematic reviews to identify further studies. The citations were downloaded to Endnote X7 (Thompson ISI Research Soft, Philadelphia, PA) and duplicates were identified and removed. Two authors (NL and SUK) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First citations were evaluated on title and abstract level, followed by full-text screening of the final list of articles. Any disagreements were resolved by discussion or third-party review.
The priori inclusion criteria were: (1) retrospective or prospective studies that included adult (age >18) patients who underwent TAVI for severe symptomatic aortic stenosis; (2) patients had previous history of CAD with studies providing definition for anatomically significant CAD; (3) the prespecified intervention groups were (a) TAVI with PCI versus (b) TAVI alone; (4) sample size >100 patients with any duration of follow-up.
Two authors (NL and SUK) independently abstracted data on study characteristics and baseline characteristics of participants in both treatment groups including information on study design, valve type, vascular approach, timing of PCI, mean age, gender, structural parameters (LVEF, Euro-Score) and co-morbidities (diabetes mellitus, hypertension, previous stroke, chronic kidney disease, peripheral vascular disease). Disagreements related to data were resolved by discussion, referring back to the original article or opinion of the third author (MSK). Risk of bias of the included cohort studies was assessed using the Newcastle-Ottawa scale (NOS).8
Included outcomes were: all-cause 30-day mortality, 1-year all-cause mortality, stroke, acute kidney injury (AKI), and myocardial infarction (MI). The end points were defined as reported in individual studies. Outcomes were combined using DerSimonian and Laird random effects model. The principal summary statistic was either crude events in each group or any risk ratio (RR) or odds ratio (OR) estimates with 95% confidence interval (CI). Heterogeneity was assessed using Cochrane Q statistics and was quantified via I2 with values 25% to 50%, 50% to 75%, and >75% consistent with low, moderate, and high degree of heterogeneity, respectively. Subgroup analysis was also performed to estimate whether the treatment effect was influenced by prevalence of CAD by segregating studies with 100% prevalence of CAD from studies reporting <100% prevalence in the TAVI alone group. Publication bias was assessed using funnel plot and Egger’s regression test. For all analyses, statistical significance was set as p ≤0.05. A study level analysis was done using Review Manager (RevMan, Version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
A total of 11 studies including 5,188 patients (1,271 in TAVI with PCI group and 3,917 in TAVI alone group) were included in the analysis (Figure 1).9–19 Out of these, 9 were retrospective and 2 were prospective cohort studies. Coronary revascularization was performed before TAVI in 5 studies, concomitant with TAVI in 1 study and concomitant and before TAVI in 5 studies. Mean age and percentage of male patients were 82.7/82.0 years and 47.1%/47.6% in TAVI with PCI and without PCI groups, respectively. Further details on study and participant characteristics are summarized in Tables 1 and 2. No evidence of publication bias was found (Supplementary Figure 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram illustrating study selection process.
Table 1.
Study characteristics including in our meta-analysis.
| Study | Publication year | Design | Country | Valve type | Vascular approach | Timing of staged PCI (days) | NOS score |
|---|---|---|---|---|---|---|---|
| Guedeney et al | 2018 | Prospective cohort study | Europe,United States | Medtronic CoreValve, Sapien XT, Others | Transfemoral: 288 (88%) | 30 mean | 8 |
| Huczek et al | 2017 | Prospective cohort study | Poland | Not reported | Transfemoral: 363 (79%) | 28 mean | 8 |
| Singh et al | 2016 | Retrospective cohort study | United States | Not reported | Transfemoral/Transaortic: (85%) Transapical: (15%) | Not reported | 7 |
| Snow et al | 2015 | Retrospective cohort study | United Kingdom | Medtronic CoreValve 1243 (48%0, Edwards SAPIEN/Sapien XT: 1345 (52%) | Transfemoral: (68%) | Not applicable | 7 |
| Khawaja et al | 2015 | Retrospective cohort study | United Kingdom | Edwards Sapien | Transfemoral: 47 (50%), Transaortic: 29 (31%), Transapical 17 (18%) | 50 (25–127) median | 8 |
| Griese et al | 2014 | Prospective cohort study | Germany | Medtronic CoreValve, Sapien XT | Transfemoral: 190 (46%), Transaortic: 221 (54%) | 36 mean | 7 |
| Penkalla et al | 2014 | Retrospective cohort study | Germany | Edwards SAPIEN (100%) | Transaortic: (100%) | Not applicable | 8 |
| Abramowitz et al | 2013 | Retrospective cohort study | Israel | Medtronic CoreValve, Edwards SAPIEN | Transfemoral, Transaortic | 57 ± 29 mean | 9 |
| Abdel-Wahab et al | 2012 | Retrospective cohort study | Germany | Medtronic CoreValve | Transfemoral: 124 (99%), Transaortic: 1 (1%) | 10 median | 7 |
| Wenaweser et al | 2011 | Retrospective cohort study | Switzerland | Medtronic CoreValve, Edwards SAPIEN | Transfemoral, Transaortic, Transapical | 34 ± 26 mean | 7 |
| Masson et al | 2010 | Retrospective cohort study | Canada | Edwards SAPIEN (100%) | Transfemoral: 68 (59%) | 26 (3–180) median | 7 |
Table 2.
Baseline patient characteristics. Data are presented as numbers (percentages), and mean § standard deviation.
| Study | Groups | Mean age (years) | Male | Logistic EuroScore | CAD | LVEF | HTN | DM | Stroke | PVD | CKD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Guedeney et al | TAVI with PCI | 83 ± 7 | NA | 17.7 ± 9.2 | 81 (100) | 55 | 69 (86) | 21 (26) | 6(8) | 6(8) | 23 (29) |
| TAVI Alone | 82.5 ± 7 | 19 ± 11.6 | 247 (100) | 55 | 199 (82) | 81 (33) | 19(8) | 33 (13) | 79 (32) | ||
| Huczek et al | TAVI with PCI | 80.3 ± 6 | 86 (51) | NA | 169 (100) | 52 ± 13 | 130 (77) | 72 (43) | 20 (12) | NA | 11 (6) |
| TAVI Alone | 79.4 ± 8 | 155(53) | 293 (100) | 53 ± 12 | 200 (68) | 100 (34) | 38 (13) | 22 (8) | |||
| Singh et al | TAVI with PCI | 83 ± 0.6 | 279 (47) | NA | 493 (84) | NA | (72) | (26) | NA | 189 (32) | NA |
| TAVI Alone | 82.9 ± 0.4 | 812(56) | 1125 (64) | (78) | (34) | 526 (30) | |||||
| Snow et al | TAVI with PCI | NA | NA | NA | 172 (100) | NA | NA | NA | NA | NA | NA |
| TAVI Alone | 1167 (100) | ||||||||||
| Khawaja et al | TAVI with PCI | NA | NA | NA | 25 (100) | NA | NA | NA | NA | NA | NA |
| TAVI Alone | 68 (100) | ||||||||||
| Griese et al | TAVI with PCI | 82 ± 6 | 24 (37) | 21.7 ± 13.9 | 65 (100) | 52 ± 15 | NA | 19 (29) | 8(12) | NA | 36 (55) |
| TAVI Alone | 82 ± 5 | 129 (37) | 20.3 ± 14.6 | 346 (100) | 54 ± 14 | 126 (36) | 35 (10) | 177 (51) | |||
| Penkalla et al | TAVI with PCI | 83 | 21 (28) | 32.1 | 76 (100) | 55 | NA | 16(21) | 15 (20) | 50 (66) | NA |
| TAVI Alone | 81 | 88 (38) | 28.5 | 232 (100) | 50 | 83 (36) | 59 (25) | 160 (69) | |||
| Abramowitz et al | TAVI with PCI | 83.6 ± 6 | 33 (51) | 31.3 ± 13.8 | 61 (100) | 55 ± 9 | 55 (90) | 15 (25) | 5 (8) | 10(16) | NA |
| TAVI Alone | 83 ± 5 | 40 (48) | 29.2 ± 13.8 | 83 (100) | 55 ± 8 | 67 (81) | 29 (35) | 7 (8) | 14(17) | ||
| Abdel-Wahab et al | TAVI with PCI | 81 ± 7 | 26 (47) | 25.08 ± 12.6 | 55 (100) | 47 ± 14 | 46 (84) | 18 (33) | 4(7) | 11 (20) | NA |
| TAVI Alone | 81 ± 6 | 34 (49) | 23.62 ± 15.1 | 36(51.4) | 48 ± 15 | 56 (80) | 14 (20) | 9(13) | 10 (14) | ||
| Wenaweser et al | TAVI with PCI | 83.6 ± 5 | 29 (49) | 26.8 ± 16.3 | 59 (100) | 51 ± 12 | 48 (81) | 10(17) | 6 (10) | 16 (27) | NA |
| TAVI Alone | 81.7 ± 6 | 83 (42) | 24.2 ± 14.4 | 108 (54.8) | 51 ± 15 | 152 (77) | 52 (26) | 17(9) | 48 (24) | ||
| Masson et al | TAVI with PCI | 85.7 | 10 (67) | 24.5 | 15 (100) | 45 | NA | 7 (47) | NA | 3 (20) | 0 (0) |
| TAVI Alone | 85 | 60 (58) | 31.05 | 104 (100) | 58 | 31 (30) | 42 (40) | 93 (89) |
CAD = coronary artery disease; CKD = chronic kidney disease; DM = diabetes mellitus; HTN = hypertension; LVEF = left ventricular ejection fraction; PVD = peripheral vascular disease.
The prevalence of CAD was reported in all studies. Eight studies reported 100% prevalence in both the groups, whereas 2 studies reported 51.4% and 54.8% prevalence in TAVI alone group.17,18 Study by Singh et al represented the largest study and noted a prevalence of 84% in TAVI with PCI and 64% in TAVI alone group.11 The definition of significant CAD varied between studies and included ≥50% stenosis of the luminal diameter of the 3 main coronary arteries or their major epicardial branches in 6 studies,9,12,16–18 ≥70% stenosis in 2 studies,10,13 and ≥90% stenosis in 1 study.15 When left main coronary artery was involved, significant stenosis was defined as ≥50% stenosis in 3 studies.12,13,15 None of the studies provided any details on fractional flow reserve or other forms of functional assessment of coronary stenosis.
Ten studies reported 334 cases of 30-day all-cause mortality with 103 events out of 1,194 occurring in TAVI with PCI group and 231 out of 3,386 occurring in TAVI alone group. There was no significant difference (OR 1.30 [0.85 to 1.98], p = 0.22, I2 = 37.5%) between the 2 groups. Two subgroup analyses were also performed, first with regard to prevalence of CAD which showed no difference when studies were separated on the basis of 100% CAD (OR 1.22 [0.66 to 2.25]) and <100% CAD (OR 1.46 [0.80 to 2.69; Figure 2). Second, in congruence with current guidelines which recommend PCI in patients with critical (≥70%) stenosis in proximal coronary arteries, we performed a subgroup analysis for 30-day all-cause mortality which contained 3 studies with significant CAD defined as ≥70% stenosis in both the treatment groups; however, no difference in outcome between TAVI with PCI and TAVI alone group was observed (OR 0.83 [0.37 to 1.47; Supplementary Figure 2).
Figure 2.
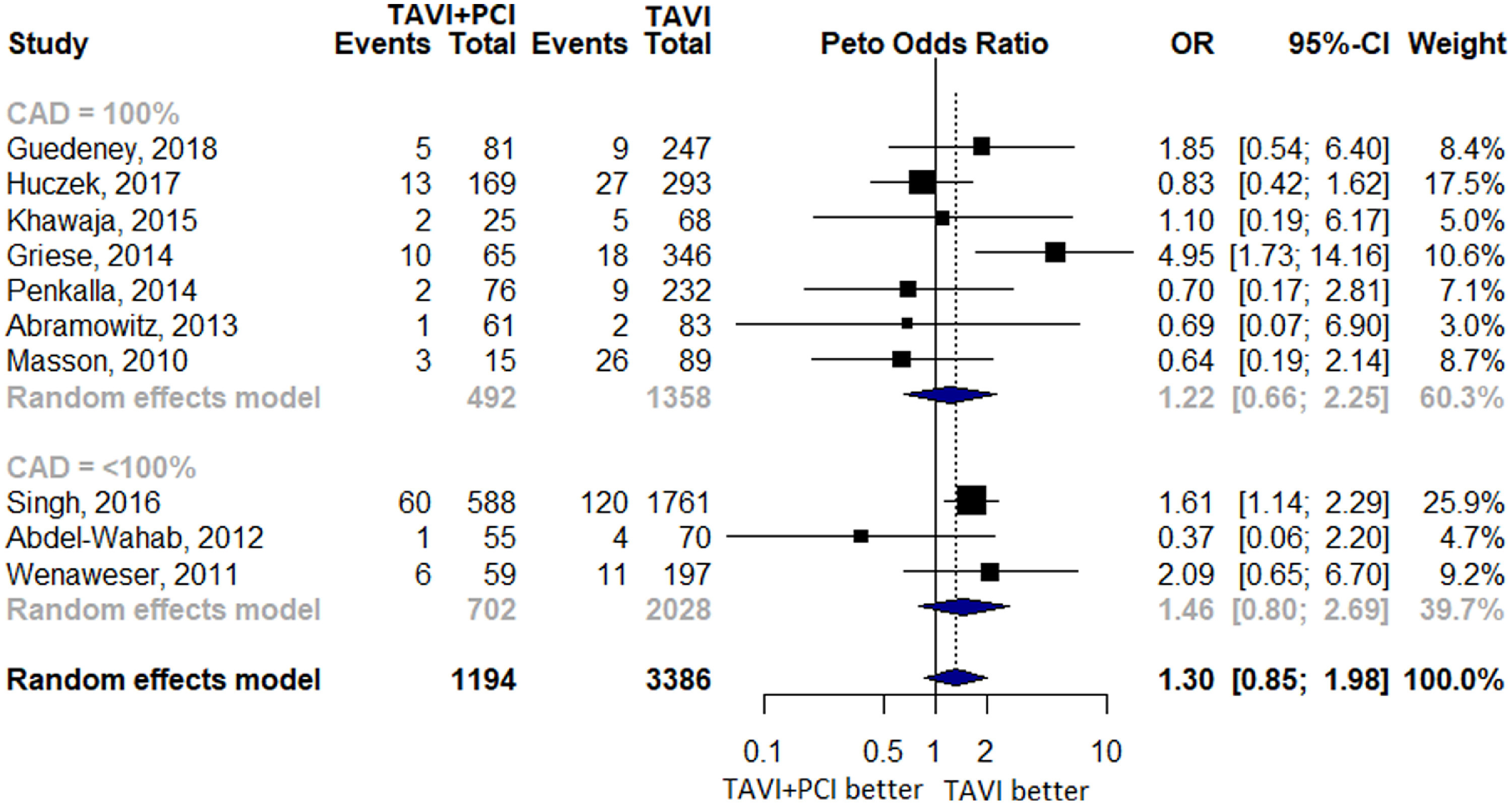
Forest Plot evaluating the cumulative risk of 30-day all-cause mortality in patients with TAVI and PCI versus TAVI Alone. Other annotations as in Figure 3. Squares represent the risk ratio of the individual studies; Horizontal lines represent the 95% confidence intervals (CI) of the risk ratio. The size of the squares reflects the weight that the corresponding study contributes in the meta-analysis. The diamonds represent the pooled risk ratio or the overall effect. PCI = percutaneous coronary intervention; TAVI = transcatheter aortic valve implantation.
Six studies reported 799 cases of 1-year all-cause mortality of which 110 events occurred in 434 patients in TAVI with PCI group and 689 events occurred in 3,398 patients in TAVI alone group. No difference with an OR of 1.19 (0.92 to 1.52, p = 0.18, I2 = 0.0%) was noted between the 2 groups (Figure 3). A total of 5 studies reported MI with 12 events in TAVI with PCI group and 23 events in TAVI alone group. There was no significant difference in effect estimate when the 2 groups were compared (OR 2.71 [0.55 to 12.23], p = 0.22, I2 = 41.3%; Figure 4).
Figure 3.
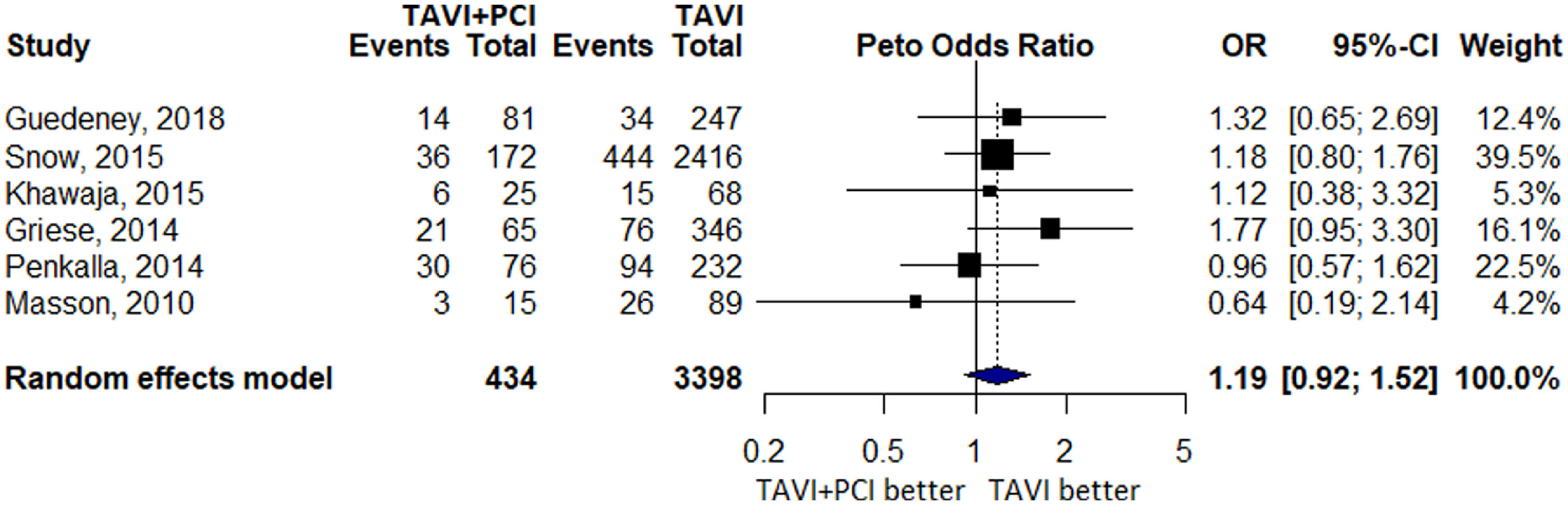
Forest Plot evaluating the cumulative risk of 1-year all-cause mortality in patients with TAVI and PCI versus TAVI Alone. Other annotations as in Figure 2.
Figure 4.
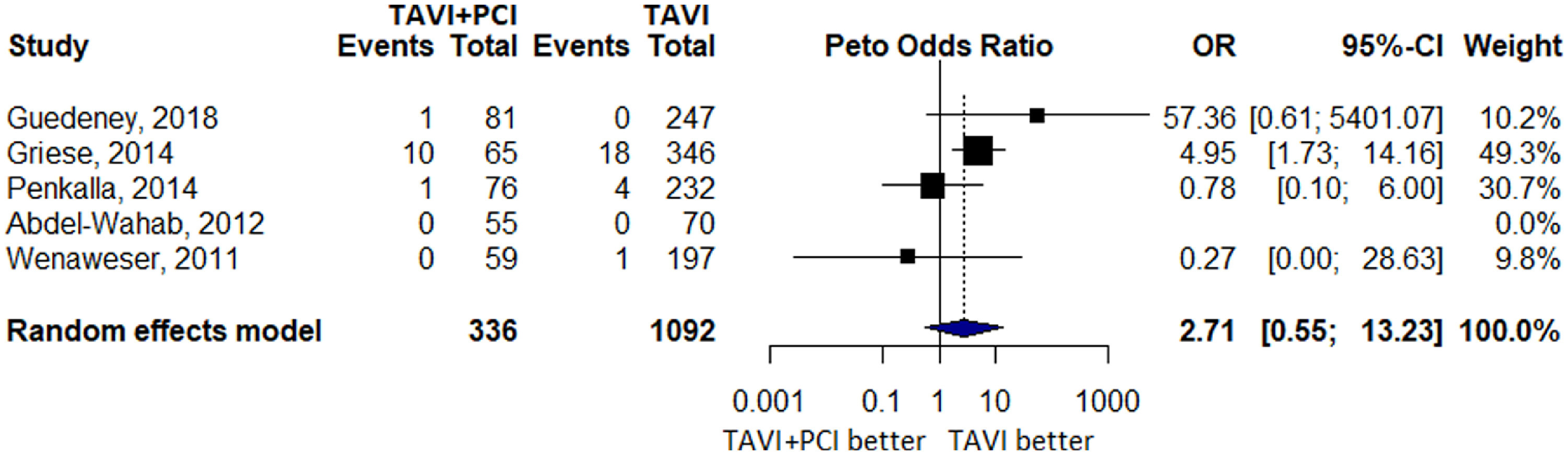
Forest Plot evaluating the cumulative risk of myocardial infarction (MI) in patients with TAVI and PCI versus TAVI Alone. Other annotations as in Figure 2.
Six studies reported 178 events of stroke of which 28 events occurred in 909 patients in TAVI with PCI and 150 events occurred in 2,704 patients in TAVI alone group. Five studies reported 179 events of AKI with 32 cases seen in 985 patients in TAVI with PCI group and 147 cases in 2,936 patients in TAVI alone group. No difference was observed for overall estimate of stroke (OR 0.7 [0.36 to 1.45], p = 0.36, I2 = 32.8%) and AKI (OR 0.7 [0.46 to 1.06], p = 0.08, I2 = 14.4%) between the 2 arms. Subgroup analyses including studies with 100% prevalence of CAD noted an OR of 1.47 (0.26 to 8.32) for stroke and 1.01 (0.58 to 1.76) for AKI, whereas a lower risk of these outcomes was observed in studies with <100% prevalence of CAD (stroke, OR 0.53 [0.37 to 0.76]; AKI, OR 0.53 [0.32 to 0.87]; Figure 5 and Supplementary Figure 3).
Figure 5.
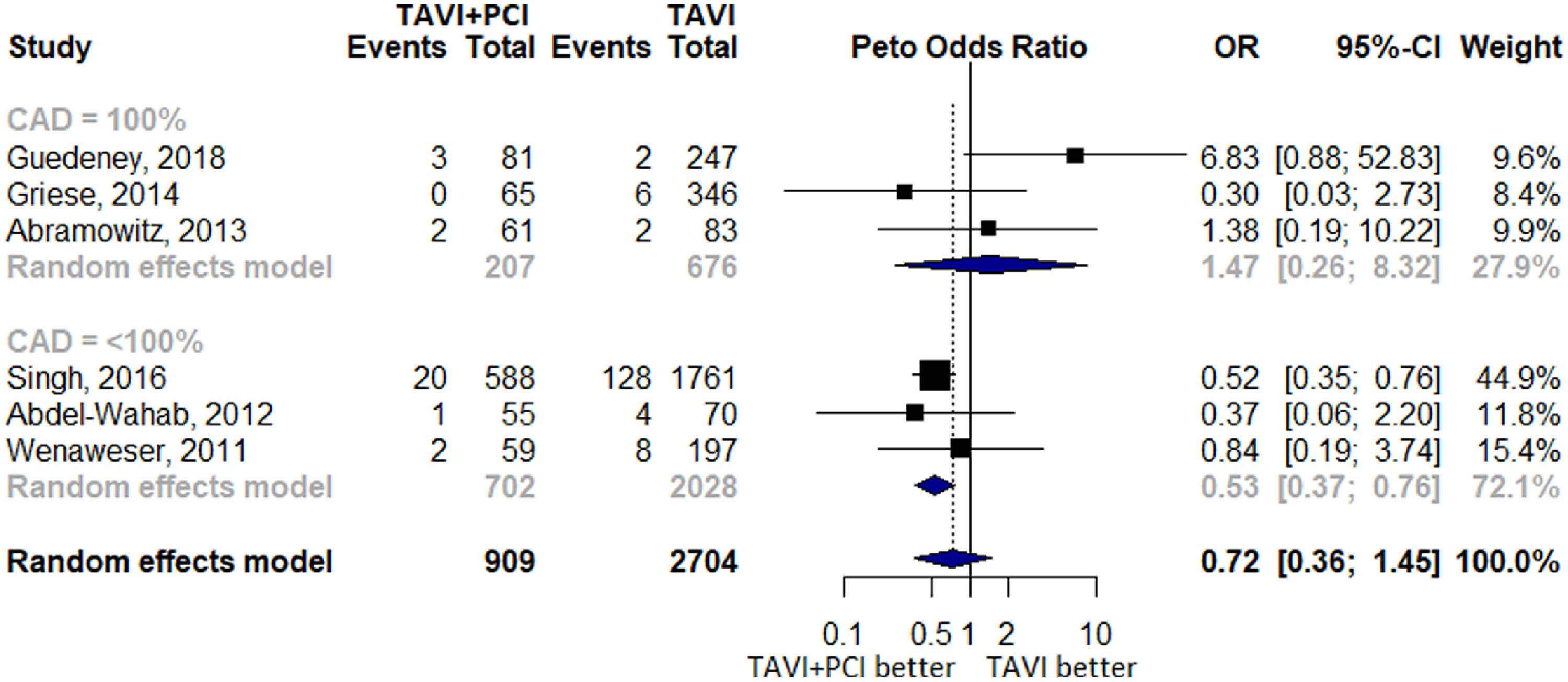
Forest Plot evaluating the cumulative risk of stroke in patients with TAVI and PCI versus TAVI alone.
Discussion
Our systematic review and meta-analysis with pooled evidence from more than 5,100 patients revealed that coronary revascularization in form of PCI either before or at the time of TAVI does not improve any relevant cardiovascular outcomes. We believe that these findings have direct clinical relevance.
Patients with TAVI frequently have preponderance of co-morbidities that predispose them to coronary artery disease.20 With increasing number of TAVI procedures being performed in the United States and worldwide,21 it is imperative to devise consistent strategies with regard to the management of CAD diagnosed as part of TAVI workup. The practice at the time of surgical aortic valve replacement revolves around performing coronary artery bypass grafting for vessel(s) deemed obstructive by a preoperative coronary angiogram.6 This practice emanates not from randomized data but based on convenience of treating the obstructive disease when an open heart surgery is contemplated.22 However, current percutaneous revascularization trends around the time of TAVI tend to be heterogeneous, leading to variable clinical practice.23
We believe that the findings of our analysis are consistent with the existent CAD literature that illustrates no improved outcomes after PCI in stable CAD, except for improved quality of life.24,25 Patients with stable CAD tend to have a different plaque morphology than the vulnerable plaque in patients presenting with acute coronary syndrome (ACS). For instance, patients with stable CAD have a large lipid core stabilized with a fibrous core, whereas patients with ACS have an ulcerated core and an inflammatory milieu. Moreover, most cases of ACS do not present in patients with obstructive CAD, rather they present in non-obstructive CAD with unstable plaques.26,27
Randomized controlled trials in patients with stable CAD and the ensuing meta-analysis of such trials also endorse the importance of medical management for stable CAD with PCI reserved only for patients who have refractory symptoms despite optimal medical management.28 For instance, Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial enrolled 2,287 patients and followed them for a cumulative of 4.6 years. All key primary and secondary end points were negative except for angina relief. Similarly, the occluded artery trial investigators found that PCI done in patients presenting after the duration of myocardial salvage did not reduce key hazardous end points in patients at 4 years of follow-up.29 Current guidelines recommend considering PCI only in patients with >70% stenosis of proximal epicardial vessels or the left main coronary artery.30 Subgroup analysis on the basis of above criterion was performed for 30-day all-cause mortality which showed no difference in outcomes between the 2 groups and further resonates with our recommendation. However, an important consideration is limited data provided by the studies which restricted analyses of other outcomes. Therefore, based on the available evidence from stable CAD and our systematic review, we recommend against routine revascularization of patients around the time of TAVI, with PCI reserved only for patients presenting with ACS or the ones in which angina could not be mitigated with medical management.
Further limitations of our study are as follows: first, this is a trial-level meta-analysis as we did not have access to the individual patient data. Second, we do not know the symptom status of patients who underwent PCI. Third, most of the included studies were from 2005 to 2015 and did not include patients with low or medium surgical risk. Fourth, this is an analysis of observational studies, and an RCT is needed to definitively address this question. However, there is no physiological reason why the findings from the stable CAD literature cannot be extrapolated to the TAVI patient population.
In conclusion, coronary revascularization in the form of PCI does not lead to any improvement in any key end points at the time of TAVI and should be reserved for symptomatic patients or patients presenting with ACS.
Supplementary Material
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2019.08.024.
References
- 1.Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochelliére R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multi-center Canadian experience. J Am Coll Cardiol 2010;55:1080–1090. 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR, PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 3.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ, Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–1715. 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 4.Culler SD, Cohen DJ, Brown PP, Kugelmass AD, Reynolds MR, Ambrose K, Schlosser ML, Simon AW, Katz MR. Trends in aortic valve replacement procedures between 2009 and 2015: has transcatheter aortic valve replacement made a difference? Ann Thorac Surg 2018;105:1137–1143. 10.1016/j.athoracsur.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 5.Voudris KV, Petropulos P, Karyofillis P, Charitakis K. Timing and outcomes of PCI in the TAVR era. Curr Treat Options Cardiovasc Med 2018;20:22 10.1007/s11936-018-0619-x. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–2488. 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tug-well P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses 2009. [http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp]
- 9.Guedeney P, Tchétché D, Petronio AS, Mehilli J, Sartori S, Lefèvre T, Presbitero P, Capranzano P, Iadanza A, Sardella G, Van Mieghem NM, Sorrentino S, Claessen BEPM, Chandrasekhar J, Vogel B, Kalkman DN, Meliga E, Dumonteil N, Fraccaro C, Trabattoni D, Mikhail G, Ferrer-Grazia MC, Naber C, Kievit P, Baber U, Sharma S, Morice MC, Chieffo A, Mehran R, WIN TAVI Investigators. Impact of coronary artery disease and percutaneous coronary intervention in women undergoing transcatheter aortic valve replacement: from the WINTAVI registry. Catheter Cardiovasc Interv 2019;93:1124–1131. 10.1002/ccd.28012. [DOI] [PubMed] [Google Scholar]
- 10.Huczek Z, Zbroński K, Grodecki K, Scisło P, Rymuza B, Kochman J, Dąbrowski M, Witkowski A, Wojakowski W, Parma R, Ochała A, Grygier M, Olasińska-Wiśniewska A, Araszkiewicz A, Jagielak D, Ciećwierz D, Puchta D, Paczwa K, Filipiak KJ, Wilimski R, Zembala M, Opolski G. Concomitant coronary artery disease and its management in patients referred to transcatheter aortic valve implantation: insights from the POL-TAVI Registry. Catheter Cardiovasc Interv 2018;91:115–123. 10.1002/ccd.27251. [DOI] [PubMed] [Google Scholar]
- 11.Singh V, Rodriguez A, Thakkar B, Patel N, Ghatak A, Badheka A, Alfonso C, de Marchena E, Sakhuja R, Inglessis-Azuaje I, Palacios I, Cohen M, Elmariah S, O’Neill W. Comparison of outcomes of transcatheter aortic valve replacement plus percutaneous coronary intervention versus transcatheter aortic valve replacement alone in the United States. Am J Cardiol 2016;118:1698–1704. 10.1016/j.amjcard.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Snow TM, Ludman P, Banya W, DeBelder M, MacCarthy PM, Davies SW, Di Mario C, Moat NE. Management of concomitant coronary artery disease in patients undergoing transcatheter aortic valve implantation: the United Kingdom TAVI Registry. Int J Cardiol 2015; 199:253–260. 10.1016/j.ijcard.2015.06.166. [DOI] [PubMed] [Google Scholar]
- 13.Khawaja M, Haran H, Nadra I, Wilson K, Clack L, Macgillivray K, Hancock J, Young CP, Bapat V, Thomas M, Redwood S. The effect of coronary artery disease defined by quantitative coronary angiography and SYNTAX score upon outcome after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention 2015;11:450–455. 10.4244/EIJY14M05_09. [DOI] [PubMed] [Google Scholar]
- 14.Griese DP, Reents W, Toth A, Kerber S, Diegeler A, Babin-Ebell J. Concomitant coronary intervention is associated with poorer early and late clinical outcomes in selected elderly patients receiving transcatheter aortic valve implantation. Eur J Cardiothorac Surg 2014;46:e1–e7. 10.1093/ejcts/ezu187. [DOI] [PubMed] [Google Scholar]
- 15.Penkalla A, Pasic M, Drews T, Buz S, Dreysse S, Kukucka M, Mladenow A, Hetzer R, Unbehaun A. Transcatheter aortic valve implantation combined with elective coronary artery stenting: a simultaneous approach. Eur J Cardiothorac Surg 2015;47:1083–1089. 10.1093/icvts/ivr144. [DOI] [PubMed] [Google Scholar]
- 16.Abramowitz Y, Banai S, Katz G, Steinvil A, Arbel Y, Havakuk O, Halkin A, Ben-Gal Y, Keren G, Finkelstein A. Comparison of early and late outcomes of TAVI alone compared to TAVI plus PCI in aortic stenosis patients with and without coronary artery disease. Catheter Cardiovasc Interv 2014;83:649–654. 10.1002/ccd.25233. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Wahab M, Mostafa AE, Geist V, St€ocker B, Gordian K, Merten C, Richardt D, Toelg R, Richardt. Comparison of outcomes in patients having isolated transcatheter aortic valve implantation versus combined with preprocedural percutaneous coronary intervention. Am J Cardiol 2012;109:581–586. 10.1016/j.amjcard.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 18.Wenaweser P, Pilgrim T, Guerios E, Stortecky S, Huber C, Khattab AA, Kadner A, Buellesfeld L, Gloekler S, Meier B, Carrel T, Windecker S. Impact of coronary artery disease and percutaneous coronary intervention on outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention 2011;7:541–548. 10.4244/EIJV7I5A89. [DOI] [PubMed] [Google Scholar]
- 19.Masson J-B, Lee M, Boone RH, Al Ali A, Al Bugami S, Hamburger J, John Mancini GB, Ye J, Cheung A, Humphries KH, Wood D, Nietlispach F, Webb JG. Impact of coronary artery disease on outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2010;76:165–173. 10.1002/ccd.22501. [DOI] [PubMed] [Google Scholar]
- 20.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142–147. 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 21.Landes U, Barsheshet A, Finkelstein A, Guetta V, Assali A, Halkin A, Vaknin-Assa H, Segev A, Bental T, Ben-Shoshan J, Bar-bash IM, Kornowski R. Temporal trends in transcatheter aortic valve implantation, 2008–2014: patient characteristics, procedural issues, and clinical outcome. Clin Cardiol 2017;40:82–88. 10.1002/clc.22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Sabbagh A, Nishimura RA. Clinical conundrum of coronary artery disease and aortic valve stenosis. J Am Heart Assoc 2017;206: e005593 10.1161/JAHA.117.005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Ascenzo F, Conrotto F, Giordana F, Moretti C, D’Amico M, Salizzoni S, Omede P, La Torre M, Thomas M, Khawaja Z, Hildick-Smith D, Ussia G, Barbanti M, Tamburino C, Webb J, Schnabel RB, Seiffert M, Wilde S, Treede H, Gasparetto V, Napodano M, Tarantini G, Presbitero P, Mennuni M, Rossi ML, Gasparini M, Biondi Zoccai G, Lupo M, Rinaldi M, Gaita F, Marra S. Mid-term prognostic value of coronary artery disease in patients undergoing transcatheter aortic valve implantation: a meta-analysis of adjusted observational results. Int J Cardiol 2013;168:2528–2532. 10.1016/j.ijcard.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 24.Cecil WT, Kasteridis P, Barnes JW Jr, Mathis RS, Patric K, Martin S. A meta-analysis update: percutaneous coronary interventions. Am J Manag Care 2008;14:521–528 https://www.ajmc.com/journals/issue/2008/2008-08-vol14-n8/aug08-3509p521-528. [PubMed] [Google Scholar]
- 25.Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005;111:2906–2912 10.1161/CIRCULATIONAHA.104.521864. [DOI] [PubMed] [Google Scholar]
- 26.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1988;12:56–62 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 27.Little WC, Constantinescu M, Applegate RJ, Kutcher MA, Burrows MT, Kahl FR, Santamore WP. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 1988;78:1157–1166 10.1161/01.CIR.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 28.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB III, Kligfield PD, Krumholz HM, Kwong RYK, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44–164. 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–1516. 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 30.Ramee S, Anwaruddin S, Kumar G, Piana RN, Babaliaros V, Rab T, Klein LW, Aortic Stenosis AUC Writing Group, Interventional Section of the Leadership Council of the American College of Cardiology. The rationale for performance of coronary angiography and stenting before transcatheter aortic valve replacement: from the Interventional Section Leadership Council of the American College of Cardiology. JACC Cardiovasc Interv 2016;9:2371–2375. 10.1016/j.jcin.2016.09.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


