Abstract
Temporal and surgical risk dependent associations with clinical outcomes in patients receiving transcatheter versus surgical aortic valve implantation (TAVI vs SAVI) are uncertain. In this meta-analysis, 7 randomized controlled trials (7,771 patients) were included to investigate trends in outcomes in TAVI versus SAVI up to 5 years, and variation in outcomes with respect to low-, intermediate-, and high-surgical risk of the patients up to 1 year. Estimates were calculated as random effects hazard ratios (HRs) with 95% confidence intervals (CI). All-cause mortality was similar in TAVI and SAVI at 30 days (HR 0.81, 95% CI 0.55 to 1.21, p = 0.31), 1 year (HR 0.97, 95% CI 0.89 to 1.06, p = 0.49), 2 years (HR 0.96, 95 CI 0.85 to 1.09, p = 0.54), and 5 years (HR 1.04, 95% CI 0.89 to 1.21, p = 0.62). Cardiac mortality, myocardial infarction and stroke were similar in both interventions up to 5 years. TAVI was associated with lower risk of atrial fibrillation, but higher risk of vascular complications, pacemaker implantation, and paravalvular leak up to 5 years. The lower risks of major bleeding and acute kidney injury with TAVI versus SAVI were limited to 1 and 2 years, respectively. Compared with SAVI, TAVI was superior in reducing all-cause mortality in low surgical risk patients at 30 days only, whereas TAVI was noninferior to SAVI in intermediate- and high-risk patients at 30 days and across all risks at 1 year. In conclusion, TAVI was noninferior to SAVI in terms of mortality, myocardial infarction, and stroke up to 5 years. TAVI improved survival versus SAVI in low-risk patients at 30 days.
Transcatheter aortic valve implantation (TAVI) has revolutionized the management of patients with severe aortic stenosis (AS).1 Given the increasing number of patients who are potentially eligible to undergo TAVI, it is critical to understand the temporal association between implantation of transcatheter versus surgical aortic prosthesis and clinically relevant complications. In this framework, while TAVI was initially approved for patients considered to be at prohibitive or high risk for surgical aortic valve replacement (SAVI), more recent data have shown the safety and efficacy of TAVI in patients with intermediate risk.2,3 The recent publication of PARTNER 3 (Placement of Aortic Transcatheter Valves) and Evolut Low Risk (Evolut Surgical Replacement and Transcatheter Aortic Valve Implantation in Low Risk Patients) trials have shifted the paradigm even further for patients at low surgical risk.4,5 Herein, we performed a meta-analysis of clinical trials comparing TAVI versus SAVI to investigate 2 key issues (1) whether the mortality and clinical outcomes in patients with AS undergoing TAVI versus SAVI vary up to 5 years, and (2) whether mortality and hard cardiovascular end points vary in different surgical risk groups (low, intermediate, and high risk) up to 1 year.
Methods
This meta-analysis was performed according to the Cochrane Collaboration guidelines and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis).6,7
Two independent researchers (MUK and MSZ) performed the literature search using Medline, Embase, and CENTRAL (inception-April 2019). Additional online resources included Clinical Trial Results (http://www.clinicaltrialresults.org), TCTMD (https://www.tctmd.com/) and ClinicalTrials.gov. The search strategy is reported in Table Supplement S1. Two investigators (MUK and MSZ) screened the remaining articles at the title and abstract level and then at full text level based on a prespecified inclusion criteria.
The inclusion criteria were (1) randomized controlled trials comparing TAVI versus SAVI in patients with AS, (2) trials reporting all-cause mortality and clinical outcomes of interest and, (3) trials having sample size of at least 100 patients in each arm.8 Although trials reporting clinical outcomes at different time lengths were included, post hoc analyses of trials and observational studies were excluded. There was no restriction on the language or follow-up duration.
Two investigators (MUK and MSK) independently extracted the data on standard data collection forms, adjudicated the data, and resolved any disagreements with discussion or opinion of third investigator (SUK). Data was abstracted on intention to treat principle and following information was extracted—characteristics of the trials and participants, crude point estimates, number of events and sample sizes in each groups, and follow-up duration. The surgical risk classification was performed at trial level according to STS (Society of Thoracic Surgery) predicted risk of mortality score: mean STS score <4% defined as low risk, 4% to 8% as intermediate risk and >8% as high risk, or if the trial had clearly categorized the patients based on combination of risk estimates (STS score or Logistic EuroScore).9–11 Data was available at 30 days, 1 year, 2 years, 3 years, and 5 years, however since there was limited data (<2 trials) at 3 years, we omitted this time point from meta-analyses.12 The risk of bias assessment was performed at trial level using the Cochrane Risk of Bias Tool (Table S2).13
The primary outcome was all-cause mortality. The secondary end points were cardiac mortality, myocardial infarction (MI), stroke, major bleeding, new onset of atrial fibrillation (NOAF), acute kidney injury (AKI), vascular complications, permanent pacemaker (PPM) implantation, paravalvular leak, endocarditis, and repeat hospitalization. Outcomes were defined as reported in selected trials.
Estimates were calculated as random effects hazard ratios (HR) with 95% confidence intervals (CI). For effect size calculation, HRs with 95% CI for each outcome of the trials was extracted when reported. For trials in which HRs were not given, we estimated log (HR) and its variance using a previously validated method: Log-HR = 2 × [(# observed events Group 1) − (# observed events Group 2)]/[(# observed events Group 1) + (# observed events Group 2)] and Variance (log-HR) = 4/[(# observed events Group 1) + (# observed events Group 2)].14,15 The SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation)16 and Evolut Low Risk4 trials reported outcomes using Bayesian methods. We extracted raw event rates and sample sizes from these trials to estimate log HRs and variances. Heterogeneity was evaluated via Q statistics with I2 >75% being consistent with a high degree of heterogeneity (Table S3).17
Meta-analyses were performed at 30 days, 1 year, 2 years, and 5 years. For examining outcomes based on surgical risk, analyses were performed at 30 days and 1 year as there was paucity of data on other time points. Publication bias was not performed due to a smaller number of studies (<10). Statistical significance threshold was 5%. Statistical analyses were conducted using Comprehensive Meta-Analysis Software 3.0 (Biostat, Englewood, New Jersey).
Results
Of 294 identified records, 7 trials (7,771 patients) met inclusion criteria (Table S1). Two trials were of high surgical risk,11,18–22 2 trials were of intermediate risk,16,23 and 3 trials recruited patients with low surgical risk patients.4,5,24–26 The mean STS score varied from 1.9% to 11.8% and the Log Euro SCORE varied from 1.5% to 29.3% across the trials. Table 1 and Tables S4–S5 show the characteristics of the trials and participants.
Table 1.
Characteristics of randomized trials
| Characteristics | U.S CoreValve11,18,19 | PARTNER20–22 | PARTNER 2A23 | SURTAVI16 | NOTION24–26 | EVOLUT1 | PARTNER 35 |
|---|---|---|---|---|---|---|---|
| End of enrolment | 2012 | 2009 | 2013 | 2012 | 2013 | 2016 | 2016 |
| Year of publication | 2014 | 2011 | 2016 | 2017 | 2015 | 2019 | 2019 |
| No. of patient | 795 | 699 | 2031 | 1660 | 280 | 1468 | 950 |
| No. of countries | 1 | 3 | 2 | 9 | 2 | 7 | 5 |
| Valve type | CoreValve (Medtronic) | Sapien (Edwards) | Sapien XT (Edwards) | CoreValve and Evolut R. (Metronic) | CoreValve (Medtronic) | CoreValve, Evolut R, Evolut PRO (Medtronic) | Sapien 3 (Edwards) |
| Design | Noninferiority and superiority | Noninferiority | Noninferiority | Noninferiority | Superiority | Noninferiority | Noninferiority and Superiority |
| Primary end point | All-cause mortality | All-cause mortality | All-cause mortality or disabling stroke | All-cause mortality or disabling stroke | All-cause mortality, stroke or Myocardial Infarction | All-cause mortality or disabling stroke | All-cause mortality, stroke, or re-hospital ization |
| Length of study (years) | 1 | 1 | 2 | 2 | 1 | 2 | 1 |
| Result | Noninferiority and superiority shown. | Noninferiority shown | Noninferiority shown | Noninferiority | Superiority not shown. | Noninferiority shown | Superiority shown. |
EVOLUT, Transcatheter Aortic Valve Replacement With the Medtronic Transcatheter Aortic Valve Replacement System In Patients at Low Risk for Surgical Aortic Valve Replacement Trial; NOTION, Nordic Aortic Valve Intervention Trial; PARTNER, Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve Trial; SURTAVI, the Surgical Replacement and Transcatheter Aortic Valve Implantation trial; U.S. CoreValve, Medtronic CoreValve® U.S. Pivotal Trial.
TAVI was noninferior to SAVI in terms of all-cause mortality at 30 days (HR 0.81, 95% CI 0.55 to 1.21, p = 0.31), 1 year (HR 0.97, 95% CI 0.89 to 1.06, p = 0.49), 2 years (HR 0.96, 95% CI 0.85 to 1.09, p = 0.54), and 5 years (HR 1.04, 95% CI 0.89 to 1.21, p = 0.62; Figure 1). TAVI was noninferior to SAVI in terms of cardiac mortality at 30 days (HR 1.01, 95% CI 0.76 to 1.32, p = 0.97), 1 year (HR 0.93, 95% CI 0.83 to 1.04, p = 0.22), 2 years (HR 1.03, 95% CI 0.93 to 1.13, p = 0.60), and 5 years (HR 1.07, 95% CI 0.91 to 1.26, p = 0.42; Figure 2). Similarly, TAVI was noninferior to SAVI in terms of MI, and stroke up to 5 years (Figure S2–S3). Compared with SAVI, TAVI was associated with lower risk of NOAF up to 5 years, and lower risk of AKI up to 2 years (Figure S4–S5). TAVI was associated with lower risk of major bleeding compared with SAVI up to 1 year (Figure S6). TAVI had higher risk of vascular complications, paravalvular leak, PPM implantation compared with SAVI up to 5 years (Figure S7–S9). Although the risk of repeat hospitalization was comparable between both strategies up to 1 year, TAVI had higher risk at 2 years and 5 years (Figure S10). There were no differences between both interventions in terms of risk of endocarditis up to 5 years (Figure S11).
Figure 1.
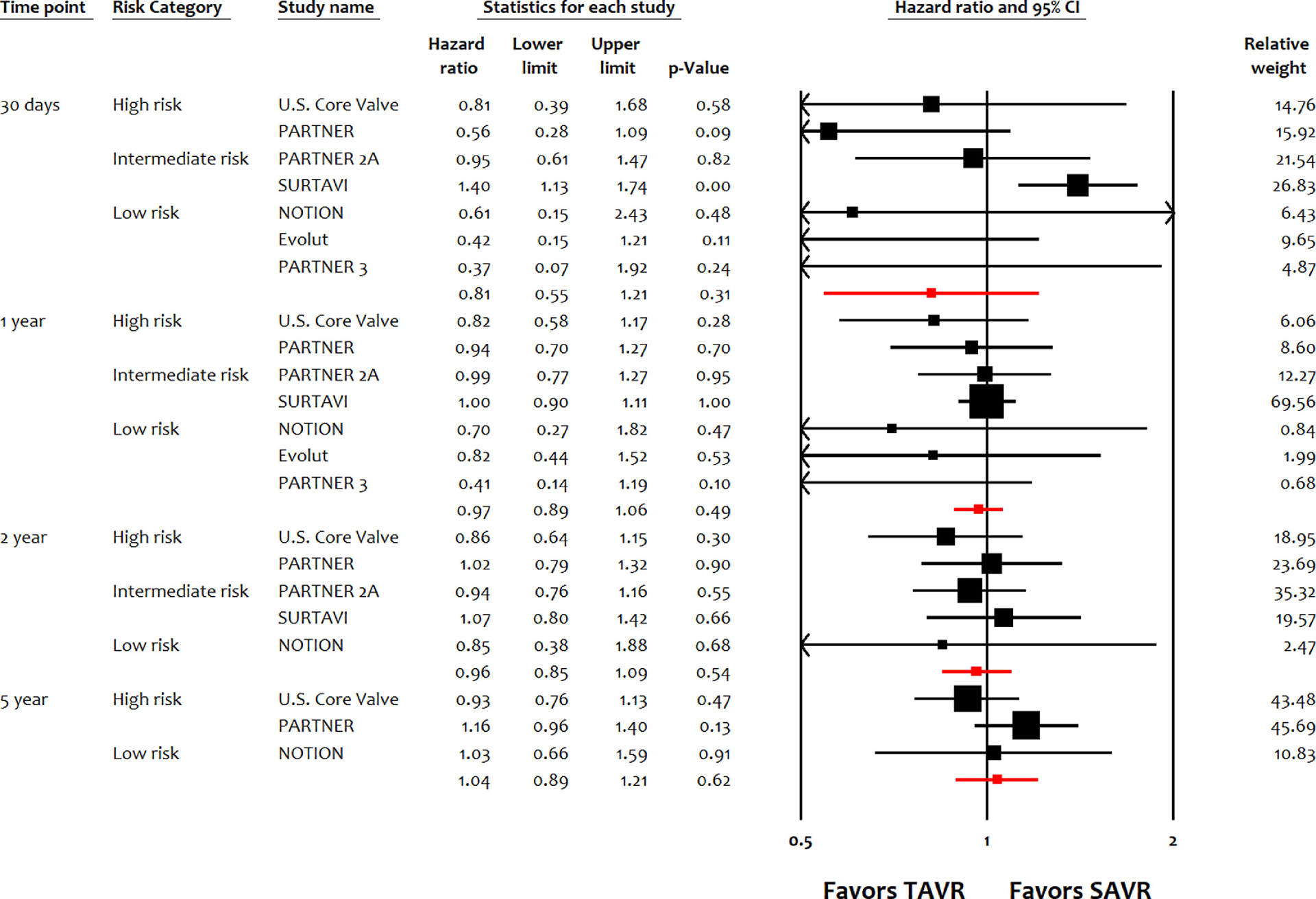
Meta-analysis for all-cause mortality up to 5 years. EVOLUT, Transcatheter Aortic Valve Replacement With the Medtronic Transcatheter Aortic Valve Replacement System In Patients at Low Risk for Surgical Aortic Valve Replacement Trial; NOTION, Nordic Aortic Valve Intervention Trial; PARTNER, Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve Trial; SURTAVI, the Surgical Replacement and Transcatheter Aortic Valve Implantation trial; U.S. CoreValve, Medtronic CoreValve U.S. Pivotal Trial.
Figure 2.
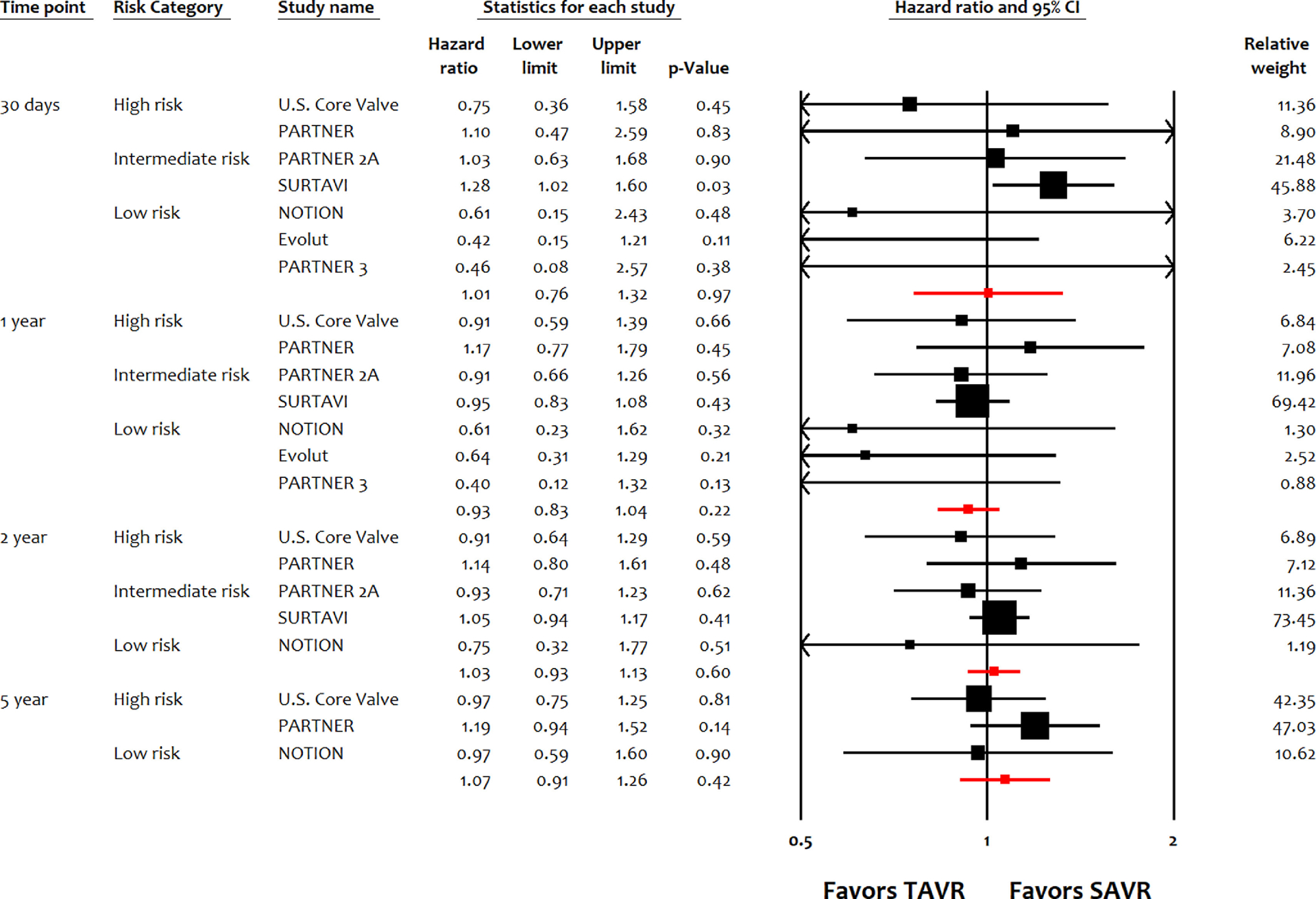
Meta-analysis for cardiac mortality up to 5 years. EVOLUT, Transcatheter Aortic Valve Replacement With the Medtronic Transcatheter Aortic Valve Replacement System In Patients at Low Risk for Surgical Aortic Valve Replacement Trial; NOTION, Nordic Aortic Valve Intervention Trial; PARTNER, Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve Trial; SURTAVI, the Surgical Replacement and Transcatheter Aortic Valve Implantation trial; U.S. CoreValve, Medtronic CoreValve U.S. Pivotal Trial.
At 30 days, TAVI reduced the risk of all-cause mortality in low-risk patients (HR 0.46, 95% CI 0.22 to 0.96) compared with SAVI, while TAVI was noninferior to SAVI in high-risk patients (HR 0.66, 95% CI 0.40 to 1.08) and intermediate-risk patients (HR 1.21, 95% CI 0.84 to 1.75) (P-interaction = 0.03; Figure 3). In terms of cardiac mortality, there was a favorable trend toward reduced mortality with TAVI in low-risk candidates, compared with intermediate-risk patients or high-risk patients (P-interaction = 0.04; Figure S12). There was also favorable trend toward TAVI for MI in low-risk patients versus intermediate-risk or high-risk patients, however subgroup interaction was not significant (P-interaction = 0.27; Figure S13). For stroke, risk remained consistently similar in the treatment arms in low-risk, intermediate-risk or high-risk patients (Figure S14). At 1 year, there were no significant differences in terms of all-cause mortality, cardiac mortality, MI, or stroke across different surgical risks between TAVI versus SAVI.
Figure 3.
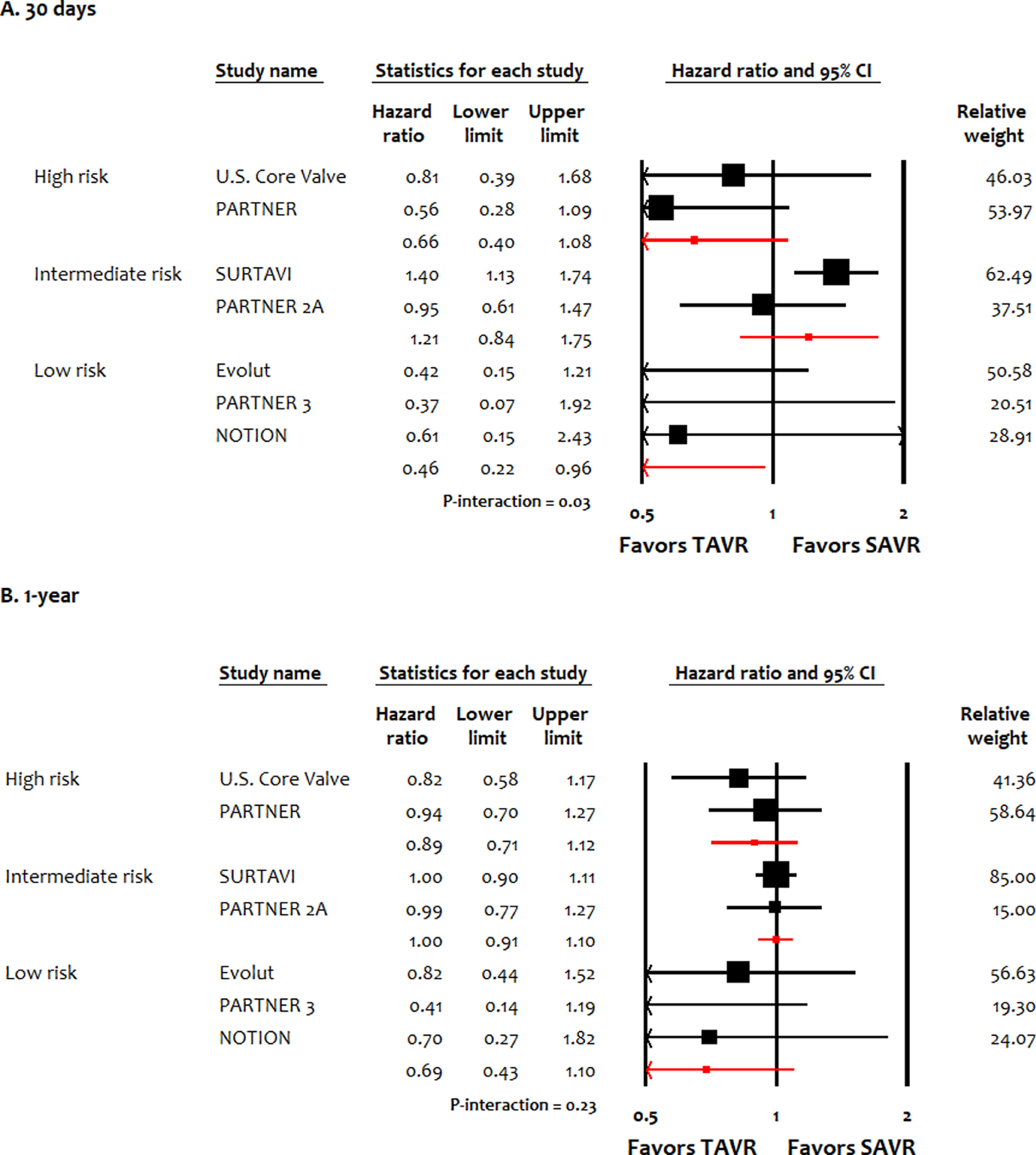
Meta-analysis for all-cause mortality stratified by surgical risk of patients at 30 days and 1 year. EVOLUT, Transcatheter Aortic Valve Replacement With the Medtronic Transcatheter Aortic Valve Replacement System In Patients at Low Risk for Surgical Aortic Valve Replacement Trial; NOTION, Nordic Aortic Valve Intervention Trial; PARTNER, Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve Trial; SURTAVI, the Surgical Replacement and Transcatheter Aortic Valve Implantation trial; U.S. CoreValve, Medtronic CoreValve U.S. Pivotal Trial.
Discussion
In these analyses, TAVI was noninferior to SAVI in terms of all-cause mortality, cardiac mortality, MI, and stroke up to 5 years. TAVI was consistently associated with lower risk of NOAF, but higher risk of vascular complications, paravalvular leak, and PPM implantation compared with SAVI. The changing trends between TAVI versus SAVI were noticed only in cases of major bleeding and AKI, where lower risks with TAVI versus SAVI were limited up to 2 years. Overall, these findings show that the comparative outcomes data in both interventions are likely to persist during longer term follow up. Whether these observations persist during an even longer term follow up (e.g., 10 years), or whether it would extend to younger and even lower risk populations remain to be seen.
Gargiulo et al suggested nonsignificant differences between TAVI and SAVI for early, mid-term or long-term all-cause mortality.2 This meta-analysis lacked contemporary clinical trials4,5,16 and predominantly relied on observational studies, hence was confounded by degree of selection and attrition biases. Khan et al had similar limitations, which showed numerically higher short-term mortality rates with TAVI compared with SAVI in intermediate to low-surgical risk patients,3 however this analysis included the STACCATO trial (A Prospective, Randomized Trial of Transapical TAVI versus Surgical Aortic Valve Replacement in Operable Elderly Patients with AS) which was prematurely terminated because of higher adverse event rates with TAVI.27 The STACCATO trial included transapical access, which is rarely used in contemporary practice.
The survival benefit with TAVI versus SAVI at 30 days was most likely secondary to reduction in cardiac mortality and MI in low risk participants. Due to previously limited data and concerns about valve durability,28 the role of TAVI in low surgical risk patients has been intensely debated, and current guidelines have not yet endorsed it.1 However, previous comparative studies in this area were mostly observational, or had modest sample sizes precluding the synthesis of high-quality evidence.5,29 Nonetheless, given the significant implications of these data and the large number of candidates; long-term data on durability, efficacy, and cost-effectiveness of TAVI in low surgical risk patient will likely remain necessary before a wider application of this technology in these patients. Short-term mortality benefit in low risk TAVI recipients also reflects current practice patterns in the United States.30 A recent analysis of Transcatheter Valve Therapy Registry reports a major improvement in 30-day mortality with TAVI, from 7.5% in 2012 to 2.5%–3.0% in transfemoral TAVI.30 These results most likely reflect the use of sophisticated delivery devices which have reduced the incidence of complications such as paravalvular leak.30
Current report is profoundly affected by the changing trends occurring in TAVI practice between the timing of different risk trials spanning over the last 10 years. The major changes that occurred included reduction of valve and sheath profile and improved delivery systems, considerable dominance of transfemoral approach and practical elimination of transapical approach, repositioning and recapture options in self-expandable valves, sealing skirts to mitigate paravalvular regurgitation, and considerable reduction in pre-TAVI balloon dilatation. Hence, first and second generation TAVI valves matched up to SAVI third generation valves and promoted TAVI superiority in the low risk trials. Similarly, the trial population is biased by excluding considerable proportions of patients in all risk categories that are deemed not suitable for SAVI (frailty or special exclusive conditions) but amenable for TAVI, whereas it is quite unusual to see the opposite scenario. The design of most clinical trials also mandated a single TAVI device but allowed the surgeon to use the SAVI device and procedure versatility. TAVI clinical practice fortunately is less restrictive and allows the operator to tailor the most suitable device for his individual patient based on a series of measurements and clinical and anatomical considerations.
This meta-analysis has several limitations. First, this is a trial level meta-analysis and we lacked access to individual patient data. Second, the summary estimates at different time points were derived from clinical trials having patients with different TAVI devices, delivery systems, and device access. Third, we could not assess the association of surgical risk with hard outcomes beyond 1 year due to limited data. Fourth, we could not perform subgroup analysis with respect to balloon expandable versus self-expandable valves, access (such as transfemoral, subclavian, and apical) or age due to smaller number of studies. Finally, since our focus was to evaluate hard end points, this analysis did not evaluate some important outcomes, such as length of hospital-stay, quality of life indicators, procedural and post-procedural cost, incidence of prosthetic mismatch, residual gradient, and effective valvular orifice.
In summary, TAVI was noninferior to SAVI in terms of all-cause mortality, cardiac mortality, MI, and stroke up to 5 years. TAVI was associated with survival benefit compared with SAVI in low surgical risk patients at 30 days. Overall, both TAVI and SAVI were associated with their own array of adverse events.
Supplementary Material
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2019.07.066.
References
- 1.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 2.Gargiulo G, Sannino A, Capodanno D, Barbanti M, Buccheri S, Perrino C, Capranzano P, Indolfi C, Trimarco B, Tamburino C, Esposito G. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a systematic review and Meta-analysis. Ann Intern Med 2016;165:334–344. [DOI] [PubMed] [Google Scholar]
- 3.Khan SU, Lone AN, Saleem MA, Kaluski E. Transcatheter vs surgical aortic-valve replacement in low- to intermediate-surgical-risk candidates: a meta-analysis and systematic review. Clin Cardiol 2017;40: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tch etch e D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 5.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 6.Van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine 2003;28:1290–1299. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarzer G, Carpenter JR, Rücker G. Small-Study Effects in Meta-Analysis. Meta-Analysis with R Cham: Springer International Publishing; 2015:107–141. [Google Scholar]
- 9.O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SLT, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: part 2-isolated valve surgery. Ann Thorac Surg 2009;88:S23–S42. [DOI] [PubMed] [Google Scholar]
- 10.Piazza N, Wenaweser P, van Gameren M, Pilgrim T, Tzikas A, Otten A, Nuis R, Onuma Y, Cheng JM, Kappetein AP, Boersma E, Juni P, de Jaegere P, Windecker S, Serruys PW. Relationship between the logistic EuroSCORE and the Society of Thoracic Surgeons Predicted Risk of Mortality score in patients implanted with the CoreValve ReValving system–a Bern-Rotterdam Study. Am Heart J 2010;159:323–329. [DOI] [PubMed] [Google Scholar]
- 11.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK. U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis.. N Engl J Med 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 12.Deeb GM, Reardon MJ, Chetcuti S, Patel HJ, Grossman PM, Yakubov SJ, Kleiman NS, Coselli JS, Gleason TG, Lee JS, Hermiller JB, Heiser J, Merhi W, Zorn GL, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Maini B, Mumtaz M, Conte J, Resar J, Aharonian V, Pfeffer T, Oh JK, Qiao H, Adams DH, Popma JJ. 3-year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol 2016;67:2565–2574. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 15.Song X, Wang C-Y. Proportional hazards model with covariate measurement error and instrumental variables. J Am Stat Assoc 2014;109: 1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Lee JS, Hermiller JB, Chetcuti S, Heiser J, Merhi W, Zorn GL, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Maini B, Mumtaz M, Conte JV, Resar JR, Aharonian V, Pfeffer T, Oh JK, Qiao H, Popma JJ. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol 2015;66:113–121. [DOI] [PubMed] [Google Scholar]
- 19.Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, Kleiman NS, Chetcuti S, Hermiller JB, Heiser J, Merhi W, Zorn GL, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte JV, Mumtaz M, Oh JK, Huang J, Adams DH. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol 2018;72:2687–2696. [DOI] [PubMed] [Google Scholar]
- 20.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 21.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686–1695. [DOI] [PubMed] [Google Scholar]
- 22.Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Davidson MJ, Svensson LG, Akin J. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477–2484. [DOI] [PubMed] [Google Scholar]
- 23.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 24.Thyregod HGH, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Søndergaard L. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 25.Lars S, Andreas SD, Nikolaj I, Henrik N, Juel KB, Petur P, Thuc NA, Thue ON, Yanping C, Walter FO, Thomas E, Peter C, Skov OP, Hørsted THG. Two-year outcomes in patients with severe aortic valve stenosis randomized to transcatheter versus surgical aortic valve replacement. Circ Cardiovasc Interv 2016;9:e003665. [DOI] [PubMed] [Google Scholar]
- 26.Hørsted THG, Nikolaj I, Højsgaard JT, Henrik N, Juel KB, Petur P, Yanping C, Walter FO, Thomas E, Peter C, Bo HP, Willy AL, Andreas SD, Skov OP, Lars S. Five-year clinical and echocardiographic outcomes from the NOTION randomized clinical trial in patients at lower surgical risk. Circulation 2019;139:2714–2723. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen HHM, Klaaborg K-E, Nissen H, Terp KA, Mortensen PE, Kjeldsen BJ, Jakobsen C-J, Andersen HR, Egeblad H, Krusell LR, Thuesen L, Hjortdal VE. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention 2012;8:383–389. [DOI] [PubMed] [Google Scholar]
- 28.Alkhouli M The Unrelenting Search for Bioprosthetic Aortic Valve Durability. JACC Cardiovasc Imaging 2019. 10.1016/j.jcmg.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Waksman R, Corso PJ, Torguson R, Gordon P, Ehsan A, Wilson SR, Goncalves J, Levitt R, Hahn C, Parikh P, Bilfinger T, Butzel D, Buchanan S, Hanna N, Garrett R, Buchbinder M, Asch F, Weissman G, Ben-Dor I, Shults C, Bastian R, Craig PE, Ali S, Garcia-Garcia HM, Kolm P, Zou Q, Satler LF, Rogers T. Transcatheter aortic valve replacement in low-risk patients: one-year results from the LRT trial. JACC Cardiovasc Interv 2019:4353. [DOI] [PubMed] [Google Scholar]
- 30.Vemulapalli S, Carroll JD, Mack MJ, Li Z, Dai D, Kosinski AS, Kumbhani DJ, Ruiz CE, Thourani VH, Hanzel G, Gleason TG, Herrmann HC, Brindis RG, Bavaria JE. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med 2019; 380:2541–2550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


