Abstract
Purpose:
To document the 5- and 10-year rates of late toxicity and vertebral compression fracture (VCF) in long-term survivors after stereotactic radiosurgery for spine metastases.
Methods and Materials:
A retrospective review was performed on 562 patients treated with SRS for spine metastases between April 2001 and July 2011. Selecting those with at least 5-year survival after SRS, included were 43 patients who collectively underwent 84 treatments at 54 spine sites. Most were treated with single-fraction stereotactic radiosurgery to a median dose of 16 Gy (range, 12-24 Gy), and 56% of sites had received prior external beam radiation therapy. Late toxicities and VCFs occurring in the absence of tumor progression were recorded. Binary logistic regression was used to identify predictors of late complications.
Results:
Nine patients (17% of treatment sites) developed grade ≥2 late toxicities at a median time of 12.8 months (range, 4.2-59.0 months). Actuarial 5- and 10-year rates of grade ≥2 late toxicity were 17% and 17%, respectively. On multivariate analysis, only cumulative biologically effective dose (BED3) > 200 Gy (or EQD22Gy [2-Gy equivalent dose calculated using an α/β ratio of 2] > 130 Gy) was associated with grade ≥2 late toxicity (P = .036). Maximum point BED3 > 110 Gy (or EQD22Gy > 70 Gy) to spinal cord or cauda equina was associated with grade ≥2 late neuropathy (P = .017). Nine VCFs (18%) occurred at a median time of 10.2 months (range, 3.2-57.2 months), with 5- and 10-year VCF rates of 17% and 17%, respectively.
Conclusion:
Stereotactic radiosurgery for primary treatment and reirradiation of spinal metastases is associated with a moderate risk of late toxicity with 10-year follow-up. Risk of late toxicity significantly increases with cumulative BED3 > 200 Gy and spinal cord or cauda equina point BED3 > 110 Gy. Patients remain at moderate risk of VCF up to 5 years after treatment, with a plateau in incidence thereafter up to 10 years.
Summary
Assuaging concerns of late effects being underestimated by short follow-up in previous studies, in a retrospective cohort study with a minimum of 5-year follow-up, stereotactic radiosurgery for reirradiation of spine metastases is associated with moderate 5- and 10-year grade ≥2 toxicity rates of 17%/17%, with a plateauing of toxicity and vertebral compression fractures after 5 years. Risk of late toxicity is significantly higher with cumulative BED3 ≥ 200 Gy and spinal cord or cauda equina BED3 ≥ 110 Gy.
Introduction
Made possible with the use of modern intensity modulated radiation and image guided radiation therapy techniques, stereotactic radiosurgery (SRS) is a novel treatment option for spinal metastases that allows precise delivery of high biologically equivalent doses with enhanced dose conformality. It is particularly useful in the treatment of previously irradiated tumors, where dose to the spinal cord is a limiting factor often precluding the use of conventional radiation therapy. In addition, because SRS delivers higher total doses to tumor compared with conventionally fractionated radiation therapy, SRS may result in improved local control and pain relief. A large body of data supports the safety and efficacy of SRS for the primary treatment and reirradiation of spine metastases (1), with local control rates ranging from 80% to 85% at 1 year and effective pain control lasting up to 12 months after treatment (2-6). However, concerns regarding late toxicity inherent to hypofractionation remain. Late effects, such as spinal cord myelopathy and vertebral compression fracture (VCF), may be underestimated and tumor control overestimated, because there are limited data beyond the expected 1- to 2-year survival of even the best selected cohorts (1). In the subset of long-term survivors after spine SRS, late toxicities are of paramount significance because they may detract from quality of life and ultimately negate the benefits of palliative SRS treatment. Thus, we aimed to document the 5- and 10-year rates of late toxicity and VCF in long-term survivors after SRS for spine metastases.
Methods and Materials
With institutional review board approval, a single-institution retrospective review was performed on 562 patients treated with spinal SRS between April 2001 and July 2011 at the University of Pittsburgh. Given the focus of this article on late effects and tumor control at 5 to 10 years, patients were selected for those with a minimum of 5-year survival after SRS. Stereotactic radiosurgery was delivered using either the Cyberknife (Accuray, Sunnyvale, CA), Synergy S (Elekta, Stockholm, Sweden), or TrueBeam/Trilogy (Varian Medical Systems, Palo Alto, CA) radiosurgical delivery platforms. Treatment localization was performed using either 6-D tracking, daily cone beam computed tomography, and/or ExacTrac (Brainlab, Novalis, Munich, Germany). For patients treated using linear accelerator platforms, Bodyfix (Elekta) immobilization was used whenever feasible. Single-fraction SRS to between 12 and 24 Gy was prescribed, most commonly to the 80% isodose line. Because the majority of patients included were treated before published guidelines for target definition, target volume was variable early in our experience (7, 8). For some patients, treatment was based on gross tumor volume only, whereas for more recently treated patients, a clinical target volume was delineated to include the involved vertebral segment. Planning target volume margin ranged from 0 to 2 mm, with editing to exclude the spinal cord or cauda equina when there was overlap. Delineation of the spinal cord and equina varied according to physician preference; spinal volumes consisted of either the entire spinal canal or the cord plus a 1-mm margin to create a planning risk volume. For the 58 treatments for which these data were available, the entire spinal canal was contoured in 22 (62.1%), and the spinal cord alone was contoured in 22 (37.9%). Nerve roots and plexuses were not separately contoured. Consistent with published international consensus recommendations, patients were generally recommended to have imaging follow-up, preferably with spine magnetic resonance imaging (MRI), 3 months after SRS, with further imaging protocols varying across the time periods examined, according to physician preference (9).
A single spine treatment site was defined as spanning 1 to 2 vertebral bodies. For the purposes of analysis, when patients were re-treated with SRS for local failure at the same vertebral level or within 1 adjacent vertebral level, this would be considered as having received multiple courses of SRS to the same treatment site. Single-fraction SRS was used for all repeat treatments to the same site.
Late toxicities occurring >3 months after treatment were graded using Common Terminology Criteria for Adverse Events version 4, based on retrospective review of follow-up records. Late toxicity was analyzed on a per-treatment-site basis, to better account for cumulative dose effects, given prior external beam irradiation and multiple re-treatments with SRS in a significant subset of patients. Univariate and multivariate binary logistic regression was used to identify potential predictors of late toxicity, such as age, sex, primary disease site, level treated (cervical, thoracic, lumbar, or sacral), cumulative biologically effective dose (BED3), and cumulative 2-Gy equivalent dose. The BED3 for each course of external beam radiation and SRS was calculated from the prescription dose, using the linear-quadratic model, with a normal tissue α/β ratio of 3. Owing to the wide range of published estimates of spinal cord α/β ratio with values varying between 0.9 and 4.0 (10, 11), we chose to use a uniform value of 3 for all levels of spine and cauda equina in our BED calculations. Cumulative BED3 was defined as the sum of the BED3 of all prior external beam treatments and SRS courses to a given treatment site, censored at the date of late toxicity. Because most late toxicities consisted of neuropathy, we performed a similar analysis to identify potential predictors of neuropathy, including cumulative point BED3 delivered to the spinal cord or cauda equina. Spinal cord and cauda equina point BED3 were calculated according to the prior external beam prescription dose and the maximum point dose delivered to the spinal cord (for lesions above L2) or cauda equina (for lesions below L2), for each SRS course delivered to a given treatment site. Finally, to make our results more comparable to other studies in which the 2-Gy equivalent dose was calculated using an α/β ratio of 2 (EQD22Gy) for the spinal cord (11, 12), we performed a similar analysis with the cumulative EQD22Gy delivered to each treatment site.
The incidence of de novo VCF or progression of pre-existing VCFs occurring in the absence of tumor progression was recorded, based on review of follow-up computed tomography or MRI spine imaging where available, in conjunction with review of clinic follow-up notes. In cases in which imaging was not available for review, such as with outside hospital films, the follow-up notes or radiology read describing the findings were used. Potential predictors of VCF, such as age, sex, primary tumor type, sclerotic versus lytic lesion type, presence of pre-existing compression fracture, SRS dose, cumulative BED3 of all prior treatments, and gross tumor volume, were analyzed using binary logistic regression. Local failure was defined as tumor progression at the treated level or within 1 vertebral body of the treated level, to conservatively account for marginal failures. The Kaplan-Meier method was used to estimate actuarial rates of local control, late toxicity, and VCF at 5 and 10 years. All endpoints were calculated from the date of first SRS treatment.
Results
Baseline patient characteristics
Of 84 treated lesions in 43 patients, 70 had a follow-up time of at least 5 years and were included in the analysis. There were 54 treatment sites. Table 1 summarizes baseline characteristics for included patients.
Table 1.
Baseline characteristics
| Characteristic | n | % or range | Grade ≥2 late toxicity |
Vertebral compression fracture |
||||
|---|---|---|---|---|---|---|---|---|
| No (%) | Yes (%) | HR (95% CI) | No (%) | Yes (%) | HR (95% CI) | |||
| Age (y) | ||||||||
| <60 | 32 | 59.3 | 84.4 | 15.6 | 81.3 | 18.8 | ||
| ≥60 | 22 | 40.7 | 81.8 | 18.2 | 1.2 (0.3–5.1) | 86.4 | 13.6 | 0.5 (0.1-2.6) |
| Sex | ||||||||
| Male | 23 | 42.6 | 82.6 | 17.4 | 87.0 | 13.0 | ||
| Female | 31 | 57.4 | 83.9 | 16.1 | 0.9 (0.2-3.9) | 80.6 | 19.4 | 1.3 (0.3-6.2) |
| Primary tumor histology | ||||||||
| Breast | 22 | 40.7 | 77.3 | 22.7 | 86.4 | 13.6 | ||
| Renal | 11 | 20.4 | 81.8 | 18.2 | 0.8 (0.1-4.7) | 81.8 | 18.2 | 1.4 (0.2-10.0) |
| NSCLC | 5 | 9.3 | 100.0 | 0.0 | - | 100.0 | 0.0 | - |
| Other | 16 | 29.6 | 87.5 | 12.5 | 0.5 (0.1-2.9) | 75.0 | 25.0 | 1.9 (0.3-11.2) |
| Level treated | ||||||||
| Cervical | 9 | 16.7 | 88.9 | 11.1 | 88.9 | 11.1 | ||
| Thoracic | 24 | 44.4 | 87.5 | 12.5 | 1.1 (0.1-12.7) | 83.3 | 16.7 | - |
| Lumbar | 13 | 24.1 | 100.0 | 0.0 | - | 76.9 | 23.1 | - |
| Sacral | 8 | 14.8 | 37.5 | 62.5 | 13.3 (1.1-166.4) | 87.5 | 12.5 | - |
| Prior EBRT | 30 | 55.6 | - | - | - | - | - | - |
| Median reirradiation interval after EBRT (mo) | 12.7 | 0.9-145.0 | - | - | - | - | - | - |
| Median prior EBRT BED3 (Gy) | 60 | 27-131 | - | - | - | - | - | - |
| Lesion type | ||||||||
| Sclerotic | 9 | 16.7 | - | - | - | 77.8 | 22.2 | |
| Lytic | 23 | 42.6 | - | - | - | 82.6 | 17.4 | 0.4 (0.1-2.2) |
| Mixed | 7 | 13.0 | 100.0 | 0.0 | ||||
| Pre-existing fracture | ||||||||
| No | 45 | 83.3 | - | - | - | 84.4 | 15.6 | |
| Yes | 9 | 16.7 | - | - | - | 77.8 | 22.2 | 1.7 (0.3-10.3) |
| Single-fraction SRS dose (Gy) | - | - | - | |||||
| ≤16 | 38 | 56.7 | - | - | - | 86.8 | 13.2 | |
| >16 | 29 | 43.3 | - | - | - | 86.2 | 13.8 | 1.1 (0.3-4.3) |
| Cumulative BED3 (Gy) | ||||||||
| <200 | 31 | 57.4 | 96.8 | 3.2 | 83.9 | 16.1 | ||
| ≥200 | 23 | 42.6 | 65.2 | 34.8 | 16.0 (1.8-140.0) | 82.6 | 17.4 | 1.3 (0.3-5.7) |
Abbreviations: BED = biologically equivalent dose; CI = confidence interval; EBRT = external beam radiation therapy; HR = hazard ratio; NSCLC = non–small cell lung cancer.
Efficacy: local control and pain relief
The median follow-up was 81.6 months (range, 60.3-133.5 months). Pain relief was achieved after 89% of treatments. The crude local failure rate was 44.4% (n = 24). Actuarial 1-year, 5-year, and 10-year rates of local control were 82.7%, 57.7%, and 54.3%, respectively (Fig. 1). The median time to local recurrence was 21.0 months (range, 2.0-137.4 months). Ten local recurrences occurred outside the radiation field, 13 occurred in-field, and 1 occurred both within and outside the field. Age, prior surgery, primary tumor type, level treated, SRS dose, and treatment volume were not associated with local control. Salvage therapy consisted of repeat SRS in 16 cases, surgery in 4, surgery followed by repeat SRS in 2, systemic therapy in 1, and none in 1 case.
Fig. 1.
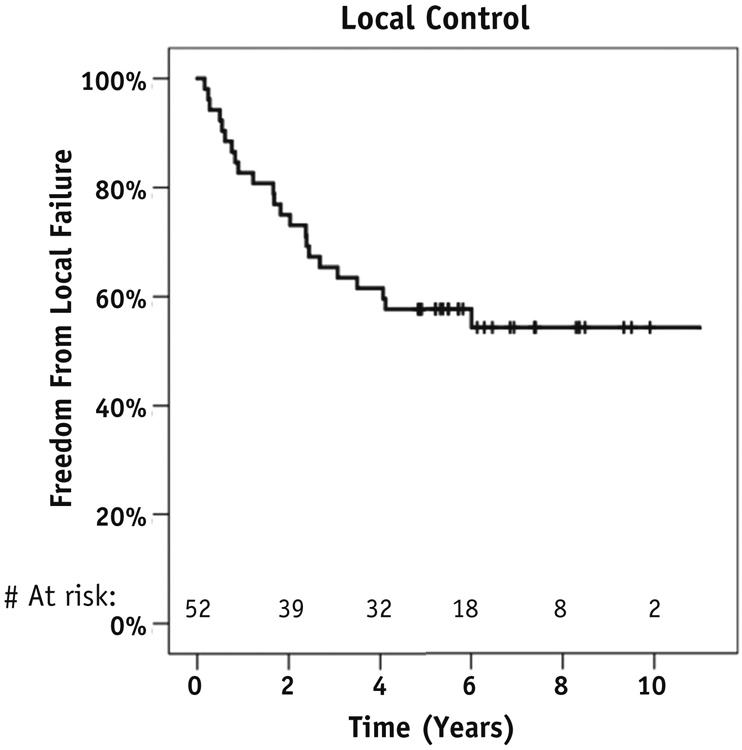
Kaplan-Meier analysis of local control.
Late toxicities
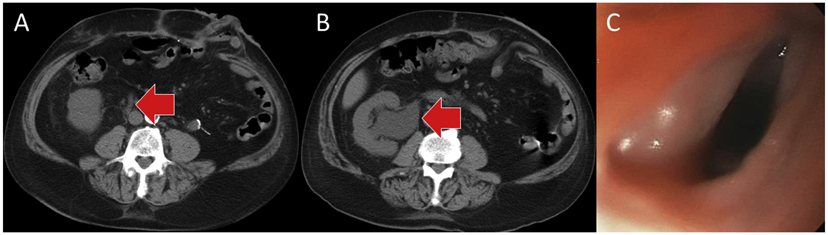
Nine patients (20.9% of patients, 16.7% of treated sites) developed grade ≥2 late toxicities at a median time of 12.8 months after SRS (range, 4.2-59.0 months) (Table 2). This included 5 patients who developed grade 3 late toxicity consisting of painful sensory neuropathy (n = 3), esophageal stricture (n = 1), and radiation-induced ureteral stricture requiring chronic stent placement (n = 1) (Fig. 2). The patient with the ureteral stricture also developed a grade 4 nonhealing wound secondary to radiation-related ischemia, requiring hyperbaric oxygen therapy.
Table 2.
Late toxicities
| Toxicity | Grade 2 |
Grade 3 |
Grade 4 |
|||
|---|---|---|---|---|---|---|
| n | %* | n | %* | n | %* | |
| Neuropathy | 4 | 7.4 | 3 | 5.6 | 0 | 0.0 |
| Esophageal stricture | 0 | 0.0 | 1 | 1.9 | 0 | 0.0 |
| Skin | 0 | 0.0 | 0 | 0.0 | 1 | 1.9 |
| Ureteral stricture | 0 | 0.0 | 1 | 1.9 | 0 | 0.0 |
Percentage is out of a total of 54 treated spine sites.
Fig. 2.
(A) Grade 3 radiation-induced right ureteral stricture at 5 years after stereotactic radiosurgery, causing hydronephrosis as shown in (B), requiring a chronic ureteral stent. (C) Grade 3 radiation-induced esophageal stricture at 53 months after stereotactic radiosurgery.
All 3 patients who developed grade 3 sensory neuropathy had been treated with external beam radiation therapy to the sacrum, followed by 3 to 5 courses of SRS to the L5–sacrum region. Management of grade 3 neuropathy cases is described in Table E1 (available online at www.redjournal.org). Management of patients with grade 2 painful neuropathy consisted of medications for neuropathic pain, physical therapy, or narcotics, as needed. Table E2 (available online at www.redjournal.org) shows the number and intervals of re-treatments by presence or absence of grade ≥2 late neuropathy.
Actuarial 5- and 10-year rates of grade ≥2 and grade ≥3 toxicity were 17%/17% and 9%/9%, respectively. On univariate analysis, cumulative BED3 of all prior treatments > 200 Gy was associated with any grade ≥2 late toxicity (P = .012), and there was a trend to higher rates of grade ≥2 late toxicity with treatment of sacral lesions (P = .055) (Figs. 3A and 3B). On multivariate analysis, only cumulative BED3 > 200 Gy remained significant (P = .036, hazard ratio 12.05, 95% confidence interval [CI] 1.2-123.0). Age, sex, and primary tumor site were not associated with late toxicity. Although there was no significant interaction between BED3 > 200 Gy and treated spine level according to χ2 testing (P = .21), a larger proportion of sacral lesions (75.0%) received BED3 > 200 Gy, compared with 33.3% of cervical lesions, 41.7% of thoracic lesions, and 30.8% of lumbar lesions. When we repeated the analysis using EQD22Gy, we found that EQD22Gy > 130 Gy is an equivalent predictor to the cut point of BED3 > 200 Gy.
Fig. 3.
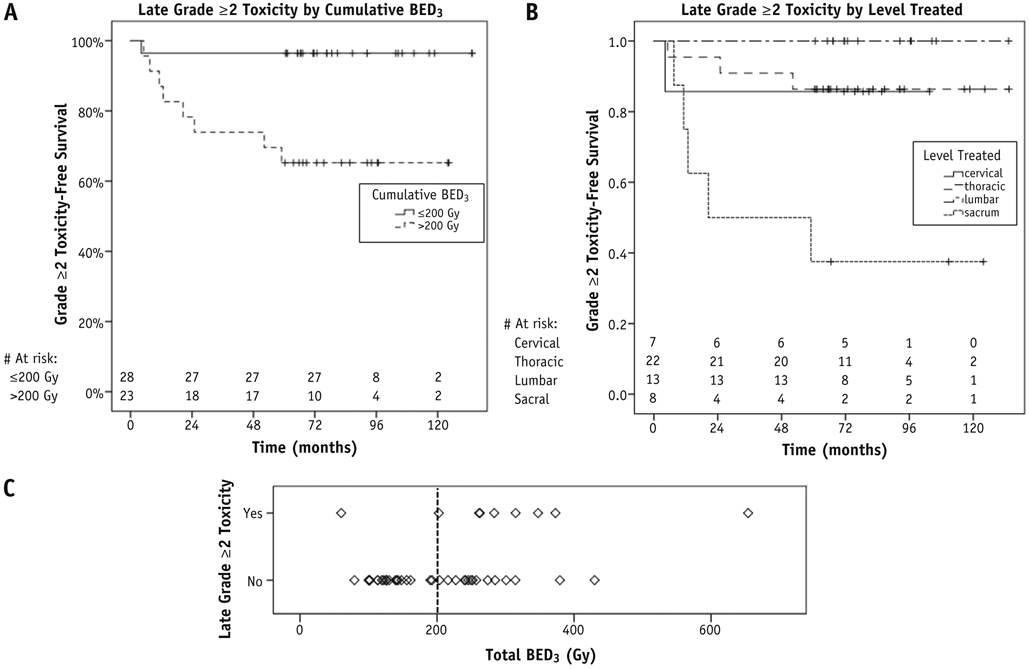
(A, B) Grade ≥2 late toxicity by cumulative biologically effective dose (BED3) and by spine level treated, respectively. (C) Cumulative biologically effective dose cut-off point of 200 Gy, above which the vast majority of late toxicities occurred.
A receiver operating characteristic (ROC) curve was generated to better assess the most reliable BED3 threshold and its accuracy in predicting for grade ≥2 late toxicity (Fig. E1, available online at www.redjournal.org). The area under the curve value was 0.86 (95% CI 0.7-1.0), suggesting that the BED3 is a good predictor for toxicity. In reviewing the coordinates of the curve, which are displayed in Table E3 (available online at www.redjournal.org), the optimal cut-off point seems to be at a BED3 threshold of 259 Gy, where the sensitivity is 89% and the specificity is 89%. This threshold is higher than the threshold of 200 Gy identified on multivariate analysis. Eight of 9 patients who experienced grade ≥2 late toxicities all received a BED3 of >260 Gy to a single treatment site. No late toxicity was seen below a cumulative BED3 of 260 Gy (Fig. 3C), with the exception of 1 patient who received a single SRS treatment to the C2 level to a BED3 of 60 Gy, with no prior external beam radiation therapy, and subsequently developed bilateral hand and finger numbness 3 months after SRS, with repeat spine MRI negative for tumor progression. Multiple cut-off points were tested during logistic regression, and in fact both >260 Gy and >200 Gy were identified as significant predictors on univariate analysis; however, >200 Gy was ultimately chosen for inclusion in multivariate analysis because it was the more conservative value.
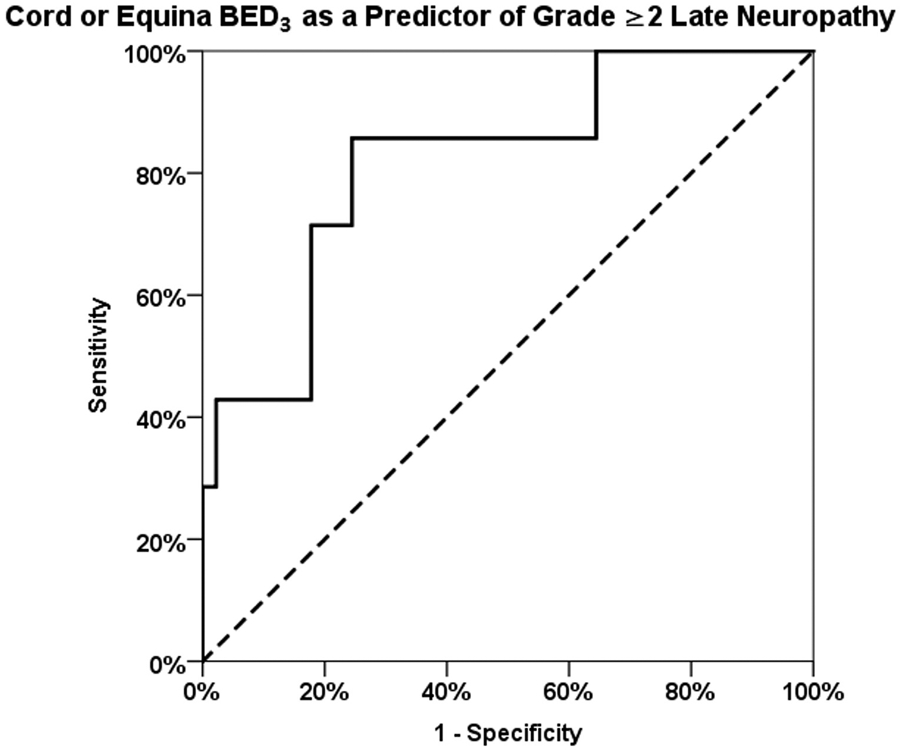
Maximum point BED3 > 110 Gy delivered to the spinal cord or cauda equina was associated with grade ≥2 late neuropathy (P = .017). Although multiple cut-off points were tested, 110 Gy was the most conservative. A ROC analysis showed an area under the curve value of 0.82 (95% CI 0.7-1.0) (Fig. E2 and Table E4, available online at www.redjournal.org). The ROC analysis suggests an optimal cut-off at a BED3 threshold of 121 Gy, where the sensitivity is 86% and the specificity is 76%. When we repeated the analysis using EQD22Gy, we found that a maximum point EQD22Gy > 70 Gy is an equivalent predictor to maximum point BED3 > 110 Gy.
Vertebral compression fractures
Sixteen lesions (22.9%) were managed with spinal surgery before SRS, including 9 kyphoplasties for pre-existing pathologic fracture and 5 decompressive surgeries. The decision for surgical stabilization, either using percutaneous cement augmentation or instrumented fixation, was based on the degree of mechanical instability as determined by the Spinal Instability Neoplastic Score (13).
Of 54 sites, 9 VCFs (16.7% of treated sites) occurred at a median time of 10.2 months (range, 3.2-57.2 months). The 5- and 10-year rates of VCF according to whether surgery was performed before first course of SRS are described in Table E5 (available online at www.redjournal.org) and Figure 4. Seven vertebral compression fractures (77.8%) occurred de novo, and 2 (22.2%) were progression of a pre-existing fracture. Eight fractures (88.9%) were symptomatic with pain, and 5 (55.6%) required surgical stabilization with kyphoplasty (n = 4) or posterior spinal fusion (n = 1). Among the patients who were symptomatic but did not undergo surgical treatment of their fracture, 1 patient was offered a kyphoplasty but refused; 1 patient was managed nonsurgically because she also developed grade 3 neuropathic pain, eventually managed with a morphine pump, and it was not thought that a kyphoplasty would alleviate her symptoms; and 1 patient had had prior kyphoplasty, although subsequent imaging showed loss of 50% of anterior vertebral height, and was offered conservative management. Age, sex, primary tumor type, sclerotic versus lytic lesion type, presence of pre-existing compression fracture, SRS dose, cumulative BED3 of all prior treatments, and gross tumor volume did not predict for vertebral compression fracture.
Fig. 4.
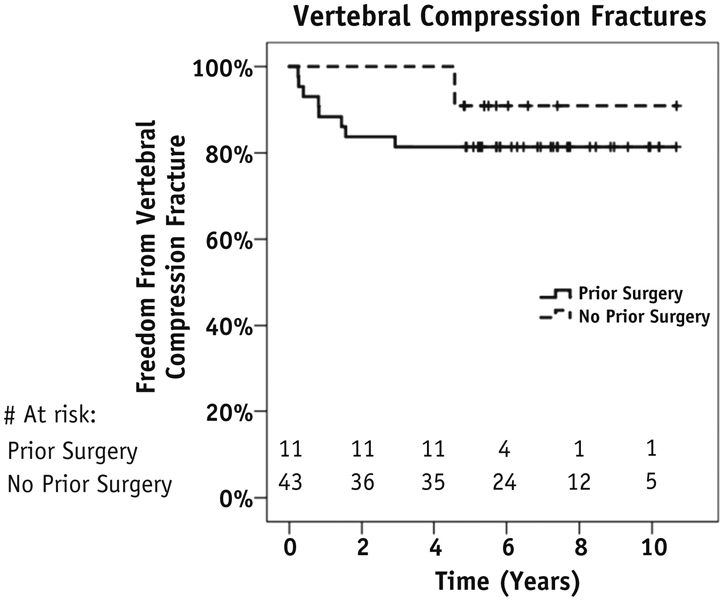
Kaplan-Meier analysis of long-term risk of vertebral compression fractures.
Discussion
Re-irradiation of spinal metastases with traditional external beam radiation therapy techniques is limited by dose tolerance limits of normal organs, especially the spinal cord. Although numerous series have demonstrated the safety and efficacy of SRS, follow-up in most series is limited to 1 to 2 years (1-6). In the era of improving systemic therapy, the effect of late toxicities on long-term survivors becomes increasingly important. Our series is the first to report long-term follow-up up to 10 years in this population, with a median follow-up of 81 months per lesion. We found that SRS for the primary treatment or reirradiation of spine metastases is associated with 5-year rates of grade ≥2 and grade ≥3 late toxicity of 17% and 9%, respectively, with no further increase in toxicity up to 10 years. Similarly, we found the 5-year and 10-year rates of vertebral compression fracture were both 17%, indicating a plateau in incidence after 5 years.
The majority of late toxicities observed in this study consisted of nervous system injury. Preclinical studies on spinal cord tolerance in animals suggest that the pathogenesis of radiation-induced spinal cord injury includes vascular endothelial damage as the primary event leading to white matter necrosis and resultant radiation myelopathy (14). Animal studies on the repair kinetics of radiation injury to the spinal cord support a time-dependent model of spinal cord recovery (15-18). Using a rat model, the half-time of repair for the spinal cord has been estimated at 1.5 hours (18), whereas others have used primate models to develop a long-term reirradiation tolerance model wherein the estimated long-term recovery at 1, 2, and 3 years was 34 Gy, 28 Gy, and 45 Gy, respectively (16).
In the setting of reirradiation with conventional external beam radiation therapy, the risk of radiation myelopathy has been minimized with maintaining a cumulative BED2 < 120 Gy, although other factors affecting the safety of reirradiation include the reirradiation interval and volume of spinal cord irradiated (12, 19). A BED2 of 120 Gy is equivalent to 60 Gy in 2-Gy fractions, which is roughly half the cumulative EQD22Gy limit of 130 Gy that we identified in our study. Our results demonstrate the utility of SRS in allowing safe dose escalation in the setting of reirradiation, where not possible with external beam radiation therapy.
We identified spine or cauda equina maximum point EQD22Gy > 70 Gy as a predictor for grade ≥2 late neuropathy. Despite variations in the methods for delineation of the spinal cord, this finding corroborates a small, earlier study by Sahgal et al (12), who similarly recommended limiting the maximum dose to the thecal sac for reirradiation SRS to an EQD22Gy of 25 Gy, for a cumulative EQD22Gy of 70 Gy. Others have not identified a dose–response relationship for radiation-induced toxicity. Thibault et al (20) reported on 56 spinal levels in 40 patients irradiated with a second course of SRS to the same level, to a median dose of 30 Gy in 4 fractions (20). With a median follow-up of 6.8 months, no radiation-induced VCF or myelopathy was observed, likely a result of the limited follow-up time. The dose–response relationship identified herein, with cumulative EQD22Gy > 130 Gy or BED3 > 200 Gy predicting for grade ≥2 toxicity, may help guide the application of reirradiation with SRS, because these patients often have limited additional treatment options and are otherwise left to endure the sequelae of uncontrolled spine metastases.
We identified a trend to higher rates of grade ≥2 late toxicity with treatment of sacral lesions (P = .055) on univariate analysis, although this factor was nonsignificant on multivariate analysis. We hypothesize an interplay between dose and level treated, whereby higher doses are delivered to sacral lesions owing to the perceived higher dose tolerance of the cauda equina compared with the spinal cord. However, our data suggest that the cauda equina may not have as high a tolerance as previously thought. Others have noted that single-fraction spine SRS for spine metastases is associated with a <3% risk of peripheral nervous system injury at 3 years, with the most common sites of injury being lumbosacral and brachial plexus (21).
The Memorial Sloan-Kettering Cancer Center recently published a series on 31 patients with 36 treated spinal lesions who survived 5 years after treatment with single-fraction SRS to 24 Gy (22). In contrast to our results, no dose–response relationship was identified, and there were no high-grade late toxicities. Late toxicities were reported in 8 patients (22%) who developed grade 2 neurologic morbidity consisting of paresthesia or neuropathic pain, 8 (22%) with skin toxicity, 3 (8%) with myalgia or myositis, and 2 patients (6%) with gastrointestinal discomfort. The lack of late high-grade toxicity may be explained by the fact that 35 of the lesions (97%) were treated with spinal SRS as the first course of irradiation, whereas 56% of treatment sites in our study had received prior external beam radiation therapy, with some patients later receiving repeat spinal SRS at the same site for local failure.
In the Memorial Sloan-Kettering study, VCF occurred at 13 treated levels (36%), of which 5 (14%) were symptomatic and required percutaneous cement augmentation or surgical management (22). Our VCF rate was lower at 17% overall, which may be due to our institutional preference for aggressive pre-SRS surgical stabilization in patients with a potentially unstable pre-existing pathologic fracture. In our series, 23% of lesions were stabilized with spinal surgery before SRS, an approach that has been shown to be an effective management strategy for patients presenting with pathologic fractures secondary to spine metastasis (23). Our VCF rate was within the range of 6% to 20% reported in most other series (24-28). Although others have reported various predictive factors for VCF, such as percentage of vertebral body tumor replacement, presence of lytic disease, and pre-existing VCF (27-29), our study did not identify any predictive factors, likely owing to our smaller sample size consisting of only those with long-term follow-up. The plateau of VCF beyond 5 years supports the idea that VCF remains predominantly an acute or subacute phenomena.
A recent multi-institutional analysis was published on 215 patients with 247 spinal target volumes treated with salvage reirradiation SRS to the spine, at a median interval of 13.5 months after prior conventional external beam radiation therapy (30). The median dose of initial external beam radiation therapy was 30 Gy in 10 fractions, and the median SRS dose used was 18 Gy in 1 fraction. With a limited median follow-up of 8.1 months, no cases of radiation myelopathy or radiculopathy were identified, and the VCF rate was 4.5%. Of the 11 VCFs identified, 5 were de novo, and 6 were fracture progression.
Our study is limited by the inherent biases of its retrospective design, including a lack of standardization of volume definition over time, treatment delivery, follow-up, and imaging. In addition, radiobiological data suggest that direct tumor cell death cannot account entirely for the efficacy of SRS. Preclinical evidence suggests that irradiation of tumors with high dose (>10 Gy) per fraction damages tumor vasculature and thus destroys the tumor microenvironment, leading to indirect, ischemic tumor cell death (31). Therefore, our calculation of BED and EQD2 based on the linear-quadratic model, which only accounts for direct cell death caused by DNA strand breaks, may not be an accurate representation of the true dose delivered (32). Nevertheless, our data still indicate an association between cumulative dose delivered and risk of late effects.
Another limitation of this study stems from the inability to differentiate radiation-induced neuropathy from other, confounding causes of neuropathy. The majority of patients experiencing late toxicity in this series had painful neuropathy as a component of their symptoms. However, some patients’ symptoms may have been multifactorial owing to the complex interplay between potentially radiation-induced neuropathy or myelopathy, neurotoxic chemotherapy, and subsequent salvage surgeries leading to increased risk of nerve injury. Nevertheless, to avoid underreporting of toxicity, such patients were conservatively graded to have radiation-related toxicity for the purposes of this analysis. Thus, late toxicity may have been overestimated in our study.
Conclusion
Extreme hypofractionation with SRS for the primary treatment and reirradiation of spinal metastases achieves excellent pain relief and durable local control and is associated with a moderate risk of late toxicity with 10-year follow-up. Risk of late toxicity is a function of total dose and is significantly higher with cumulative BED3 > 200 Gy (or EQD22Gy > 130 Gy) and spinal cord or cauda equina point BED3 > 110 Gy (or EQD22Gy > 70 Gy). Patients remain at moderate risk of VCF up to 5 years after treatment, with a plateau in incidence thereafter up to 10 years.
Extended Data
Fig. E1.
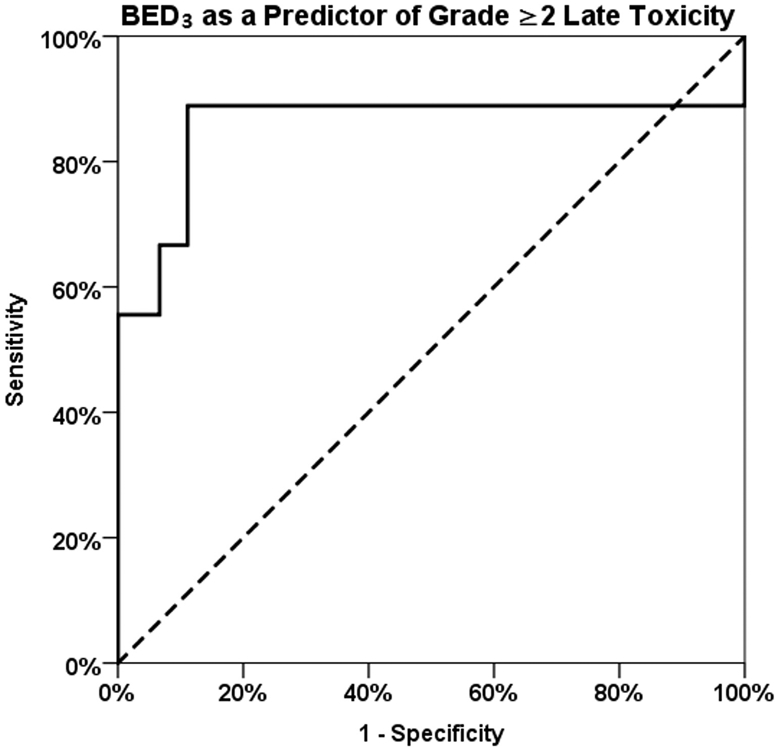
Fig. E2.
Supplementary Material
Footnotes
Conflict of interest: J.A.V. receives speaking honoraria from BrainLAB.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Guckenberger M, Mantel F, Gerszten PC, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: A multi-institutional analysis. Radiat Oncol 2014;9:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahgal A, Ames C, Chou D, et al. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys 2009;74:723–731. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys 2012;82:e803–e809. [DOI] [PubMed] [Google Scholar]
- 4.Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: A phase 1-2 trial. Lancet Oncol 2012;13:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 2007;7:151–160. [DOI] [PubMed] [Google Scholar]
- 6.Puvanesarajah V, Lo SL, Aygun N, et al. Prognostic factors associated with pain palliation after spine stereotactic body radiation therapy. J Neurosurg Spine 2015;23:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2012;83:e597–e605. [DOI] [PubMed] [Google Scholar]
- 8.Redmond KJ, Robertson S, Lo SS, et al. Consensus contouring guidelines for postoperative stereotactic body radiation therapy for metastatic solid tumor malignancies to the spine. Int J Radiat Oncol Biol Phys 2017;97:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thibault I, Change EL, Sheehan J, et al. Response assessment after stereotactic body radiotherapy for spinal metastasis: A report from the SPIne Response Assessment in Neuro-Oncology (SPINO) group. Lancet Oncol 2015;16:e595–e603. [DOI] [PubMed] [Google Scholar]
- 10.Schultheiss TE. The radiation dose-response of the human spinal cord. Int J Radiat Oncol Biol Phys 2008;71:1455–1459. [DOI] [PubMed] [Google Scholar]
- 11.Nieder C, Grosu AL, Andratschke NH, et al. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys 2006;66:1446–1449. [DOI] [PubMed] [Google Scholar]
- 12.Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:107–116. [DOI] [PubMed] [Google Scholar]
- 13.Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: An analysis of reliability and validity from the spine oncology study group. J Clin Oncol 2011;29:3072–3077. [DOI] [PubMed] [Google Scholar]
- 14.Coderre JA, Morris GM, Micca PL, et al. Late effects of radiation on the central nervous system: Role of vascular endothelial damage and glial stem cell survival. Radiat Res 2006;166:495–503. [DOI] [PubMed] [Google Scholar]
- 15.Ang KK, van der Kogel AJ, van der Schueren E. The effect of small radiation doses on the rat spinal cord: The concept of partial tolerance. Int J Radiat Oncol Biol Phys 1983;9:1487–1491. [DOI] [PubMed] [Google Scholar]
- 16.Ang KK, Jiang GL, Feng Y, et al. Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys 2001;50:1013–1020. [DOI] [PubMed] [Google Scholar]
- 17.Wong CS, Hao Y. Long-term recovery kinetics of radiation damage in rat spinal cord. Int J Radiat Oncol Biol Phys 1997;37:171–179. [DOI] [PubMed] [Google Scholar]
- 18.Ruifrok AC, Kleiboer BJ, van der Kogel AJ. Repair kinetics of radiation damage in the developing rat cervical spinal cord. Int J Radiat Biol 1993;63:501–508. [DOI] [PubMed] [Google Scholar]
- 19.Rades D, Rudat V, Veninga T, et al. Prognostic factors for functional outcome and survival after reirradiation for in-field recurrences of metastatic spinal cord compression. Cancer 2008;113:1090–1096. [DOI] [PubMed] [Google Scholar]
- 20.Thibault I, Campbell M, Tseng CL, et al. Salvage stereotactic body radiotherapy (SBRT) following in-field failure of initial SBRT for spinal metastases. Int J Radiat Oncol Biol Phys 2015;93:353–360. [DOI] [PubMed] [Google Scholar]
- 21.Stubbenfield MD, Ibanez K, Riedel ER, et al. Peripheral nervous system injury after high-dose single-fraction image-guided stereotactic radiosurgery for spine tumors. Neurosurg Focus 2017;42:E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moussazadeh N, Lis E, Katsoulakis E, et al. Five-year outcomes of high-dose single-fraction spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2015;93:361–367. [DOI] [PubMed] [Google Scholar]
- 23.Gerszten PC, Germanwala A, Burton SA, et al. Combination kyphoplasty and spinal radiosurgery: A new treatment paradigm for pathological fractures. J Neurosurg Spine 2005;3:296–301. [DOI] [PubMed] [Google Scholar]
- 24.Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: A multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol 2013;31:3426–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jawad MS, Fahim DK, Gerszten PC, et al. Vertebral compression fractures after stereotactic body radiation therapy: A large, multi-institutional, multinational evaluation. J Neurosurg Spine 2016;24:928–936. [DOI] [PubMed] [Google Scholar]
- 26.Boyce-Fappiano D, Elibe E, Schultz L, et al. Analysis of the factors contributing to vertebral compression fractures after spine stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2017;97:236–245. [DOI] [PubMed] [Google Scholar]
- 27.Cunha MV, Al-Omair A, Atenafu EG, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): Analysis of predictive factors. Int J Radiat Oncol Biol Phys 2012;84:e343–e349. [DOI] [PubMed] [Google Scholar]
- 28.Boehling NS, Grosshans DR, Allen PK, et al. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J Neurosurg Spine 2012;16:379–386. [DOI] [PubMed] [Google Scholar]
- 29.Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol 2009;27:5075–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashmi A, Guckenberger M, Kersh R, et al. Re-irradiation stereotactic body radiotherapy for spinal metastases: A multi-institutional outcome analysis. J Neurosurg Spine 2016;25:646–653. [DOI] [PubMed] [Google Scholar]
- 31.Park HJ, Griffin RJ, Hui S, et al. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012;177:311–327. [DOI] [PubMed] [Google Scholar]
- 32.Kim M, Kim W, Park IH, et al. Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery. Radiat Oncol J 2015;33:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.