Abstract
Objective:
Angiotensin II (Ang II) exerts its effect through two G-protein coupled receptors: Angiotensin II type 1 receptors (AT1) and type 2 receptors (AT2). Both these receptor subtypes are poorly understood in asthma. In this study, we investigated effects of AT1 receptor antagonist losartan, novel AT2 receptor agonist novokinin and AT2 receptor antagonist PD123319 in a mouse model of asthma.
Methods:
Mice were divided into control (CON) and allergen sensitized (SEN) groups. SEN was sensitized with ovalbumin (OVA) on day 1 and 6 (30μg; i.p.), followed by 5% OVA aerosol challenge (days 11–13). Treatments included a) losartan (SEN+LOS; 20 mg/kg i.p., day 14), b) Novokinin (SEN+NOV; 0.3 mg/kg i.p., day 14) c) PD123319 (SEN+PD; 5 mg/kg i.p., day 14). Experiments for airway responsiveness, bronchoalveolar lavage and tracheal ring reactivity using isolated organ bath were performed.
Results:
Airway responsiveness to methacholine (48 mg/ml) was significantly higher in SEN (563.71±40% vs 294.3±123.84 in CON).). This response was potentiated in SEN+PD group (757±30%; p<0.05 compared to SEN). SEN+LOS (247.61±86.85%) and SEN+NOV (352±11%) had significantly lower response compared to SEN. SEN+LOS (26.22±0.29%) and SEN+NOV (46.20±0.76%) treatment significantly (p<0.001) attenuated total cell count and eosinophils compared to SEN group (69.38±1.5%),while SEN+PD (73.04±0.69%) had highest number of eosinophils. Tracheal response to methacholine was significantly higher in SEN group compared to controls, and this response was significantly lowered with the losartan and novokinin treatments.
Conclusion:
These data suggest that AT1 and AT2 receptors have opposite effects in modulating airway hyperresponsiveness and inflammation in asthma.
Keywords: Angiotensin II, asthma, Angiotensin II receptors, airway hyperresponsiveness, inflammation
INTRODUCTION
Asthma is a chronic lung disease characterized by airway hypersensitivity, inflammation and difficulty in breathing. The renin-angiotensin system (RAS) plays a key role in the regulation of homeostasis and fluid balance [1]. Angiotensin II (Ang II), an effector octa-peptide of RAS, is a potent vasoconstrictor of vascular smooth muscle and causes increased blood pressure, inflammation and vascular remodeling [2–4]. Ang II exerts its effect by binding to two GPCRs: angiotensin type 1 (AT1) and type 2 (AT2) receptors [5]. Several reports indicate the involvement of AT1 receptors in inflammation. Studies have shown that a number of inflammatory molecules, such as interleukin-1 beta (IL-1β), are induced via AT1 receptors [6]. Ang II stimulation of the AT1 receptor-subtype is known to increase the production of prostaglandins and TLR-4 expression, which initiate the inflammatory response and oxidative injury, as well as exerts its major cardiovascular effects like vasoconstriction [7–9].
Ang II is produced locally in lungs [10, 11]. It is also known that Ang II can potentiate airway responsiveness in asthma, which contributes to the fact that it plays a role in bronchoconstriction [12]. AT2 receptors are known to counterbalance some of the AT1 mediated-effects, though the mechanisms involved are not fully known yet. AT2 receptor activation inhibits AT1 receptor-induced vasoconstriction by inducing vasodilation in kidneys [13]. These effects are observed due to production of cGMP and nitric oxide (NO), suggesting vasodilatory role for AT2 receptors in that tissue [14].
However, the effects of AT1 and AT2 receptors are not completely understood asthma, even though it appears that Ang II is pro-inflammatory and has effects in airway responsiveness in asthmatic patients. This represents a gap in knowledge and limitation, given that AT1 antagonists are used therapeutically for conditions like hypertension. The role of AT1 antagonists, AT2 agonists and antagonists in asthma are unknown. Thus, in this study, we investigated the effects of a novel AT2 receptor agonist (novokinin), AT2 antagonist (PD123319) as well as AT1 receptor antagonist (losartan) on airway responsiveness and inflammation using an asthmatic mouse model. The aim of our study was to elucidate the roles of AT1 and AT2 receptors in asthma and if either or both receptors were involved in modulating inflammation and airway reactivity.
MATERIALS AND METHODS
Animals
C57BL/6J mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME) and then maintained at the Long Island University animal facility. Equal number of males and females (6–12 weeks old) were used for studies. Free access to food and water was given to all animals. The animal facility was maintained at 23° C ±5° C, 50–60% humidity and 12-hour day/light cycle. The Institutional Animal Care and Use Committee of Long Island University approved this study.
Animal Sensitization
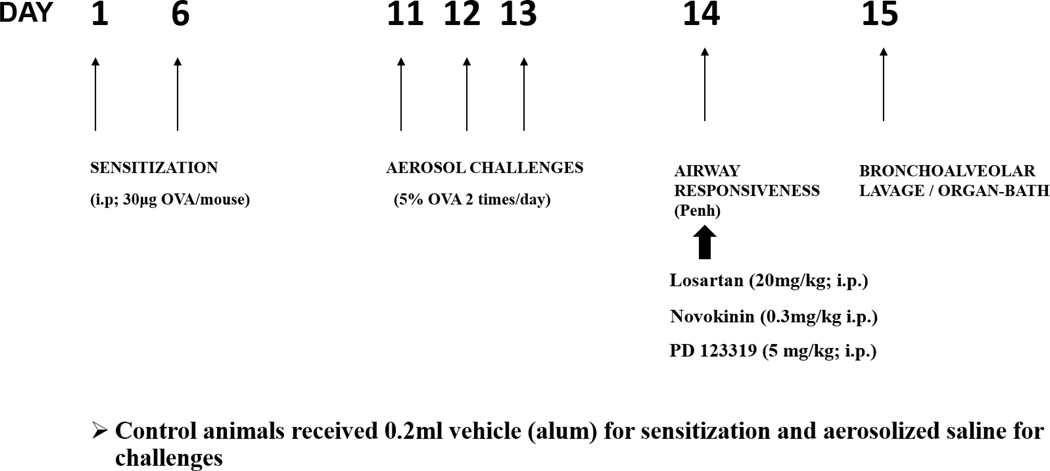
The animal sensitization protocol (Fig 1) for our experiments used ovalbumin (OVA) as the allergen and has been previously established [15]. Briefly, animals were sensitized by i.p. injection (30 μg/mouse) on days 1 and 6 with OVA (Sigma-Aldrich, St.Louis MO) suspended in Imject alum (Thermo Fisher Scientific). Control groups received same volume of Imject alum. On days 11, 12 and 13, the allergen sensitized mice were exposed to challenges of aerosolized 5% OVA in 0.9% saline (vehicle) while the controls were exposed to vehicle only for 20 minutes, twice a day with a minimum interval of at least 6 hours using an ultrasonic nebulizer (De Vilbiss Healthcare, Somerset, PA). Flow rate of aerosol was 2mL/min.
Fig 1:
Mice were divided into control (CON) and allergen sensitized (SEN) groups. Mice were sensitized with ovalbumin (OVA) on day 1 and 6 (30μg; i.p.), followed by 5% OVA aerosol challenge (days 11–13) to obtain the SEN group. Treatments included a) losartan (SEN+LOS; 20 mg/kg i.p., day 14), b) Novokinin (SEN+NOV0.3 mg/kgi.p., day 14) c) PD123319 (SEN+PD; 5 mg/kg i.p., day 14). Experiments for airway responsiveness, protein expression, bronchoalveolar lavage and tracheal ring reactivity using isolated organ bath were performed.
Study Groups:
Animals were divided into 6 groups. The following study groups were used (Table 1): Non-asthmatic mice: Control (CON), Control treated with AT1 antagonist losartan (CON+LOS), Control treated with AT2 agonist novokinin (CON+NOV), Control treated with AT2 antagonist PD 123319 (CON+PD). Asthmatic mice: Allergen sensitized-challenged (SEN), Asthmatic mice receiving losartan (SEN+LOS), Asthmatic mice receiving novokinin (SEN+NOV), Asthmatic mice receiving PD 123319 (SEN+PD).
Table 1:
Study Groups
| NON-ASTHMA GROUPS | ASTHMA GROUPS |
|---|---|
|
Vehicle treated mice CONTROL (CON) |
Allergen-sensitized and challenged SENSITIZED (SEN) |
|
Vehicle treated+ losartan CON+LOS |
Allergen-sensitized and challenged+ losartan SEN+LOS |
|
Vehicle treated+ novokinin CON+NOV |
Allergen-sensitized and challenged+ novokinin SEN+NOV |
|
Vehicle treated+ PD123319 CON+PD |
Allergen-sensitized and challenged+PD123319 SEN+PD |
Treatments:
CON+LOS and SEN+LOS groups received bolus dose of losartan (20 mg/kg; i.p.) on day 14. CON+NOV and SEN+NOV groups received novokinin (0.3 mg/kg; i.p.) on day 14. CON+PD and SEN+PD groups received PD on day 14 (5mg/kg; i.p.). The doses selected were based on literature [16–20] and preliminary studies from our lab.
Assessment of airway responsiveness to methacholine
Airway responsiveness was measured using unrestrained whole-body plethysmography (Buxco electronics, Troy, NY) by comparing a factor called enhanced pause (Penh) on day 14 of protocol. Penh is an index in which the change in airflow pattern can be measured while the animal breathes inside the chamber and is fully conscious. Mice were placed inside plexiglass chambers that were calibrated to an airflow of 1.5–2.5 ml/sec. The baseline was recorded for 15 minutes until constant airflow. Response to 0.9% saline (vehicle) and increasing doses of methacholine (MCh; 1.5mg/mL-48 mg/mL) were measured to construct a dose-response curve. Each higher dose of MCh was introduced only after baseline was achieved from the previous dose.
Bronchoalveolar lavage (BAL) and differential cell counts
BAL was performed on day 15 of protocol using a previously described protocol [21]. Mice were euthanized using pentobarbital sodium (65mg/kg). The lungs were lavaged with 3 volumes (1ml each time) of cold saline. Collected BAL was then kept on ice for total cell count and differential cell count. BAL was centrifuged at 1800 rpm at 4° C for 8 minutes. Supernatant was carefully collected and cryopreserved in liquid nitrogen and transferred to 80° C for further experiments. The cell pellet was resuspended with normal saline and mixed thoroughly to make a cell suspension. 10 ¼l of this suspension was mixed with of 0.4% trypan blue and a hemocytometer was used to count the viable cells. The remaining cell-pellet suspension was used for the differential cell count. The cell suspension was centrifuged at 800 rpm for 5min onto poly lysine coated glass slides. Slides were left to dry overnight at room temperature and then stained using Shandon Kwik-Diff set (Thermo Fisher Scientific, USA). Differential count of 300 cells was done per slide and then data was calculated as a percentage of total cell count. ELISA analysis was performed on the BAL supernatant using kits for Th2 cytokines IL-5 (Invitrogen, USA) and IL-13 (Millipore Sigma, USA). The plates were prepared as per the manufacturers’ instructions and absorbance was measured in plate reader at 450nm.
Preparation of mouse tracheal rings and isometric force measurement
Mice were euthanized using pentobarbital sodium (65 mg/kg; i.p.) on day 15. These experiments were performed using separate groups from those used for airway responsiveness and BAL studies. Thoracotomy was performed, and trachea was removed carefully and cleaned of connective tissue and fat. Trachea then was cut transversally in two 3–4mm rings. Cleaning of trachea was carried out in oxygenated ice-cold buffer. The rings were mounted vertically between two stainless steel wire hooks and then suspended in 5-mL organ baths (EZ-Bath, GlobalTown Microtech, FL), containing Krebs-Henseleit buffer. The Krebs-Henseleit buffer (pH 7.4), containing 118 nm NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 11mM glucose, and 2.5 mM CaCl2 was maintained at 37° C with continuous bubbling of 95% O2 and 5% CO2. Rings were equilibrated with a resting force of 1g tension for at least 90 minutes and buffer solution was changed every 15 minutes. This protocol was previously studied by Talukder MA and Mustafa SJ [22, 23]. KCl (60 mM) was added to organ bath to check viability of tracheal rings. After administration of KCl several times to check reproducible contraction, rings were allowed to return to baseline for at least 20 minutes. A cumulative concentration-response curve with MCh (10−11 – 10−5 M) was constructed after precontraction of rings by KCl, with an interval of at least 10 minutes between concentrations of MCh. Half of the chambers were incubated with Ang II (0.1 μM) for 30 minutes prior to KCl precontraction. Tracheal contraction was measured as percentage increase or decrease with respect to KCl precontraction. Isometric tension was measured continuously with fixed range precision force transducer (i-FOT, BRAM-4B, GlobalTown Microtech) connected to MP150, BIOPAC digital acquisition system. Data was analyzed using Acknowledge 4.4 software (BIOPAC system).
Western blot analysis
Whole lungs from control (CON) and sensitized (SEN) mice were isolated, and each sample was homogenized with 130 μl RIPA buffer (Cell Signaling Technology) on wet ice. The samples were vortexed and centrifuged for 5 min at 12,000 rpm at 4°C. Lysates were sonicated and stored at −80°C. Protein was measured using Bio-Rad assay based on the Bradford dye procedure with bovine serum albumin (BSA) as a standard. The protein mixture was divided into aliquots and stored at −80°C. At the time of analysis, samples were thawed and 30 μg of total protein per lane were loaded on a slab gel. Proteins were separated by SDS-PAGE using 10% acrylamide gels (1-mm thick). After electrophoresis, the proteins on the gel were transferred to nitrocellulose membrane (Hybond-ECL) by electroelution. Protein transfer was confirmed by employing prestained molecular weight markers (Thermo Fisher). After blocking was completed with 5% nonfat dry milk, the nitrocellulose membranes were incubated with primary antibodies for the AT1 (Cell Signaling, Danvers, MA) and AT2 1 (Santa Cruz Biotechnology, Santa Cruz, CA). The AT1 antibody detects at 45 kDa, while AT2 antibody detects at 50Kda. Both antibodies were used at dilutions of 1:1000. β-Actin antibody (Cell Signaling) was used as an internal control to normalize the target protein expression in each lane. The secondary antibody was a horseradish peroxidase-conjugated anti-rabbit or anti-goat IgG. The membranes were developed using enhanced chemiluminescence (GE Healthcare) and imaged by Amersham Imager 600 (GE Healthcare). The data are presented as the ratio of target protein expression to β-actin. ImageJ software was used to quantify the data.
Drugs and Chemicals
Losartan and methacholine (used for vivo studies) were dissolved in 0.9% saline. KCl, PD123319 (Tocris), Angiotensin II and novokinin (Abcam) were dissolved in water. Unless stated otherwise, all chemicals were of the highest grade available and were purchased from Sigma Chemicals (St. Louis, MO).
Statistical analysis
The data are expressed as mean ±SEM. Comparison between different groups was performed using non- parametric one-way ANOVA followed by Tukey’s multiple comparison test. Unpaired t-test was used to assess the comparison between two groups. A p value less than 0.05 (p<0.05) was considered as significant. All statistical analyses were performed using GraphPad Prism software (GraphPad Software, San Diego, CA).
RESULTS
1) Airway Responsiveness to methacholine
The airway responsiveness to increasing doses of methacholine (MCh) was measured using whole body plethysmography as described above, 24 hours post-treatment in all groups: Control (CON; non-allergic), Control treated with AT1 antagonist losartan (CON+LOS), Control treated with AT2 agonist novokinin (CON+NOV), Control treated with AT2 antagonist PD 123319 (CON+PD), allergen sensitized-challenged (SEN; asthmatic mice with no treatments), asthmatic mice receiving losartan (SEN+LOS), asthmatic mice receiving novokinin (SEN+NOV), and asthmatic mice receiving PD 123319 (SEN+PD). The baseline responses were measured first in all groups, followed by responses to the vehicle (0.9% saline). The Penh values for vehicle in all groups showed no significant difference. Inhalation of MCh induced dose-dependent increases in responses in all mice. The results of the different treatments are explained below:
a) Effect of AT1 antagonist (losartan) on airway responsiveness
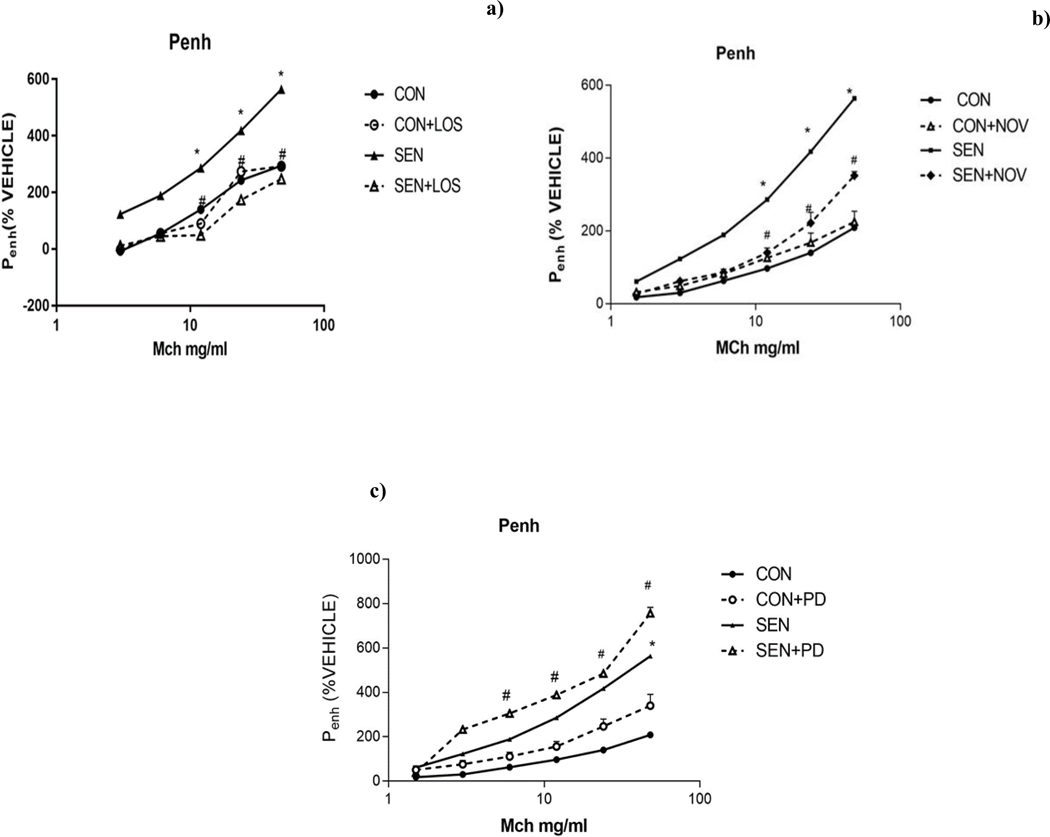
SEN group (non-treated asthmatic mice) had significantly higher airway responsiveness to MCh (48mg/ml) compared to non-asthmatic controls (563.71±40% in SEN vs 294.3±123.84 in CON, p<0.05). Treatment with losartan significantly (p<0.05) attenuated the MCh response (247.61±86.85% in SEN+LOS) compared to non-treated asthmatic mice (563.71±40% in SEN), suggesting that blocking of AT1 receptors by losartan reduced the airway responsiveness in asthmatic mice (Fig 2a).
Figure 2:
Airway responsiveness a) Effect of AT1 antagonist losartan b) Effect of AT2 agonist novokinin c) Effect of AT2 antagonist PD123319 on methacholine-induced airway hypersensitivity. Losartan (SEN+LOS) and novokinin (SEN+NOV) reduced airway responsiveness in asthmatic mice (SEN), while PD 123319 (SEN+PD) increased responsiveness. Values are expressed as mean±SEM, *p<0.05 compared to CON; # p<0.05 compared to SEN, n=4–12 mice.
b) Effect of AT2 agonist novokinin on airway responsiveness
Treatment with novokinin in sensitized mice (SEN+NOV) attenuated methacholine response (48 mg/ml) compared to non-treated SEN group (563.71±40% in SEN vs. 352±11% in SEN+NOV, p<0.001). These data suggest that activation of AT2 receptors with novokinin lowered the MCh-induced airway responsiveness in asthmatic mice (Fig 2b).
c) Effect of AT2 antagonist PD 123319 on airway responsiveness
Treatment with PD 123319 in sensitized mice (SEN+PD) resulted in significantly higher MCh response compared to non-treated SEN group (757±30% in SEN+PD 12239 vs. 563.71±40% in SEN; p<0.05) (Fig 2c). This suggested that blocking AT2 receptors resulted in increased airway responsiveness in asthmatic mice.
These data indicate that blocking AT1 receptors with losartan lessens airway responsiveness, suggesting a role for AT1 receptors in bronchial constriction in asthma. By using AT2 agonist novokinin, the airway responsiveness was also lowered, which suggests effects that are opposite to those of AT1 activation. The role of AT2 receptors was confirmed by use of the AT2 antagonist PD 123319 that increased airway responsiveness to MCh in asthmatic mice.
2) Differential cell studies of bronchoalveolar lavage
The total and differential cell count studies were done 24 hours post-aerosolized methacholine challenge. Total cell counts and cell counts for eosinophils, macrophages and neutrophils were obtained for all study groups (Fig 3 a–d).
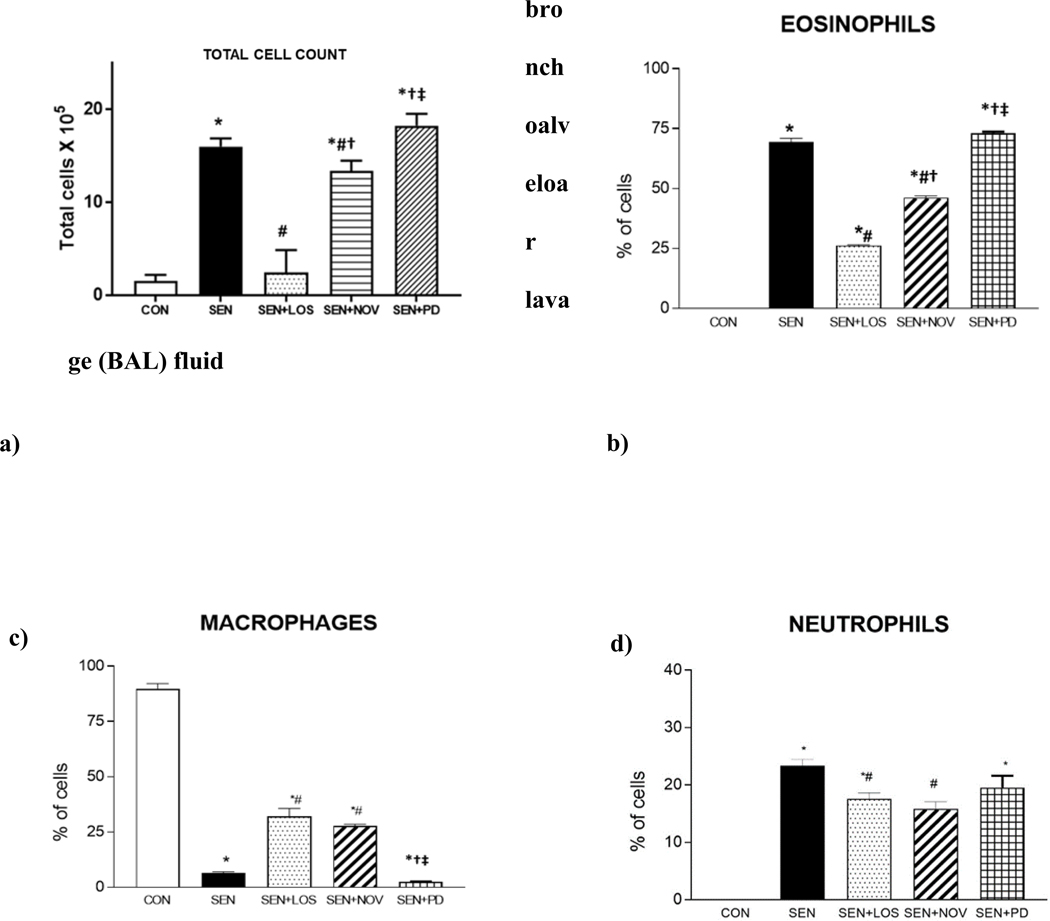
Fig 3:
a) Total cell count in BAL from study groups control (CON), allergen sensitized (SEN), allergen sensitized+ AT1 antagonist losartan (SEN+LOS), allergen sensitized+AT2 agonist novokinin (SEN+NOV) and allergen sensitized+AT2 antagonist PD 123319 (SEN+PD) groups. Losartan and novokinin reduced post-allergen inhalation mediated increase in total lung cell infiltrate, while PD 123319 had no effect. Values are described as mean±SEM, *p<0.05 compared to CON; #p<0.05 compared to SEN; †p<0.05 compared to SEN+LOS; ‡p<0.05 compared to SEN+NOV; n=4–12 Fig 3 b) Eosinophils c) Macrophages d) Neutrophils from BAL in control (CON), allergen sensitized (SEN), allergen sensitized+ AT1 antagonist losartan (SEN+LOS), allergen sensitized+AT2 agonist novokinin (SEN+NOV) and allergen sensitized+AT2 antagonist PD 123319 (SEN+PD) groups. Eosinophilia and neutrophilia were significantly reduced in allergic mice treated with losartan (SEN+LOS) and novokinin (SEN+NOV) compared to non-treated SEN. PD 123319 had no effect in allergic mice. With respect to macrophages, both losartan and novokinin lowered the attenuation of macrophages in treated allergic mice (SEN+LOS, SEN+NOV), while PD had no effect. Values are described as mean±SEM, *p<0.05 compared to CON; #p<0.05 compared to SEN; †p<0.05 compared to SEN+LOS; ‡p<0.05 compared to SEN+NOV; n=4–12
a) Total cell count of BALF (Fig 3a)
Total cell count analysis showed significantly increased number of cells in BALF from non-treated sensitized groups (SEN) compared to CON group (16×105 ±6.03 in SEN vs 1.5×105 ±0.7 in CON, p<0.05; Fig 3a). Treatment with losartan (p<0.05) and novokinin both lowered the total cell counts in BAL fluid (2.43×105 ±2.43 in SEN+LOS), (13.37×105 ± 1.11 in SEN+NOV) in the respective treated asthmatic groups. PD123319 treatment in asthmatic mice increased the total number of cells compared to SEN group but this was not significant (18.25×155 ± 1.29, SEN+PD; ns). In addition, losartan-treated asthmatic mice (SEN+LOS) had significantly lower total cell count compared than novokinin-treated asthmatic mice (SEN+NOV). This suggests that blocking AT1 receptors with losartan had a more potent effect on lowering the cell infiltrate than activating AT2 receptors with novokinin in the asthmatic mice.
b) Comparison of the effects of losartan, novokinin and PD 123319 on eosinophils (Fig 3b)
SEN mice (non-treated asthmatic group) had significantly higher number of eosinophils compared to control group (0±0% in CON vs. 69.38±1.5 % in SEN; p<0.0001). Losartan (26.22±0.29% in SEN+LOS; p<0.0001) and novokinin (46.20±0.76 % in SEN+NOV; p<0.0001) both significantly reduced number of eosinophils in asthmatic mice compared to non-treated SEN groups (Fig 3b). Treatment with PD123319 (73.04±0.69% in SEN+PD; p=0.051) increased number of eosinophils compared to SEN group. We also observed that SEN+LOS had significantly lower eosinophils compared to SEN+NOV; again this was suggestive of losartan (blocking AT1 receptors) having a more potent effect on lowering the eosinophilia than novokinin (activation of AT2 receptors).
c) Comparison of the effects of losartan, novokinin and PD 123319 on macrophages (Fig 3c)
In all control groups, macrophages were the primary cells with no significant difference between these groups. In SEN group, macrophages were reduced significantly, suggesting the presence of other inflammatory cells contributing to the cellular infiltrate (6.51±0.569% in SEN vs. 89.74±2.35% in CON; p<0.001). In SEN+LOS (32±3.7%) and SEN+NOV (27.83±0.731%), the decrease in macrophages was significantly attenuated compared to SEN (p<0.001). SEN+PD group lowest number of macrophages (2.4±0.16 %) that was significantly different from CON, SEN+LOS and SEN+NOV groups.
d) Comparison of the effects of losartan, novokinin and PD 123319 on neutrophils (Fig 3d)
Neutrophils were significantly increased in SEN group compared to CON (0.0±0% in CON vs 23.39±1.08% of cells in SEN; p<0.0001). Treatment with losartan (17.56±1.06% of cells in SEN+LOS) and novokinin significantly reduced the neutrophil count (15.83±1.20% of cells in SEN+NOV) in asthmatic mice compared to SEN (p<0.001). There was also significantly lower neutrophils in SEN+LOS compared to SEN+NOV, suggesting that losartan had a greater effect to reduce the neutrophils. PD compound had very little effect on neutrophils compared to SEN. The differential cell counts for CON+LOS, CON+NOV, CON+PD are shown in Table 2
Table 2:
Differential cell count in BALF of control groups treated
| GROUP | EOSINOPHILS | MACROPHAGES | NEUTROPHILS |
|---|---|---|---|
| CON+LOS | 0.5±0.3 | 81±2% | 2±0.8% |
| CON+NOV | 0±0% | 93.37±0.38% | 0±0% |
| CON+PD | 0±0% | 85.6±0.26% | 0±0% |
These data suggest that both losartan and novokinin lowered the inflammation in asthmatic mice, although the amount of response was different for each agent.
3) ELISA analysis of BALF supernatant for IL-5 and IL-13
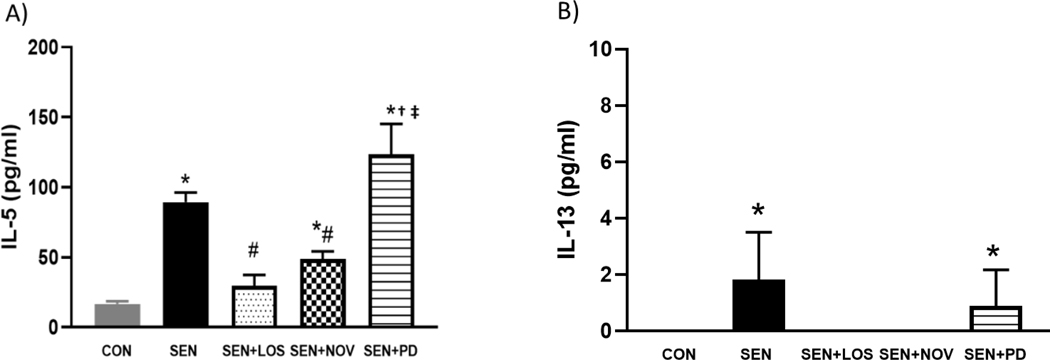
The supernatant of the BALF was analyzed for IL-5 (Fig 4A) and 1L-13 (Fig 4B). IL-5 was significantly elevated in SEN (89.48±6.84 pg/ml compared to 16.76±1.82CON; p<0.05). Losartan and novokinin significantly reduced IL-5 in treated asthmatic mice (29.75±7.71 pg/ml in SEN+LOS and 48.86±5.31 in SEN+NOV; p<0.05) compared to the non-treated SEN mice. SEN+PD group had the highest level of IL-5 (123.57±39 pg/), although this was not significant compared to SEN. IL-13 was only detected in SEN (1.83±1.67 pg/ml) and SEN+PD (0.88±1.29pg/ml) groups. These data suggest that losartan and novokinin reduced the inflammatory cytokines in the allergic mice.
Fig 4.
(A): IL-5 (B) IL-13 in BALF in control (CON), allergen sensitized (SEN), allergen sensitized+ AT1 antagonist losartan (SEN+LOS), allergen sensitized+AT2 agonist novokinin (SEN+NOV) and allergen sensitized+AT2 antagonist PD 123319 (SEN+PD) groups. IL-5 and IL-13 were significantly elevated in allergic (SEN) mice as compared to control. Losartan and novokinin significantly reduced IL-5 in allergic mice compared to SEN, while IL-13 was not detected in these groups (SEN+LOS, SEN+NOV). Groups treated with PD123319 showed no changes in IL-5 and IL-3 compared to SEN. Values are expressed as ±SEM, *p<0.05 compared to CON; #p<0.05 compared to SEN; †p<0.05 compared to SEN+LOS; ‡p<0.05 compared to SEN+NOV; n=3 mice per group.
4) Isometric tracheal reactivity
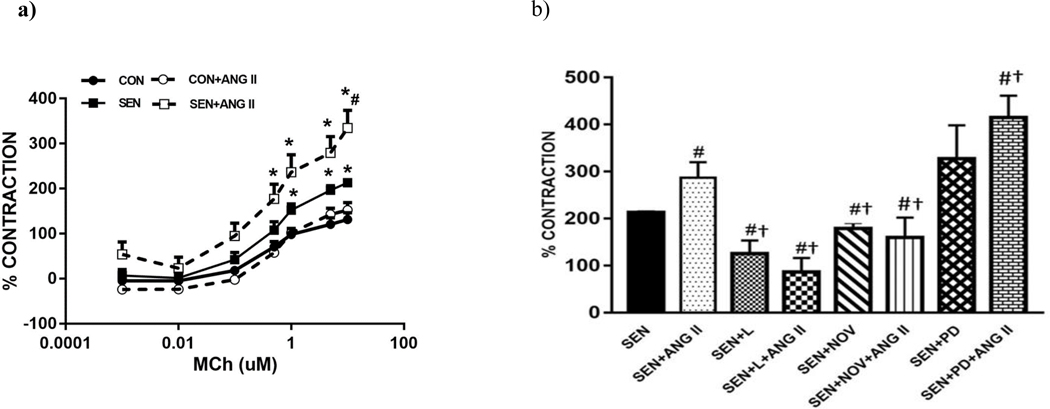
Methacholine (MCh) concentration-response (Fig 5a) curve was obtained for tracheal rings (10−11M – 10−5M) from CON and SEN groups. Some of the tracheal rings were incubated (ex vivo) with Ang II (0.1 μM; CON+ANG II and SEN+ANG II) in organ bath for 30 minutes prior to performing the MCh concentration-response. SEN group had significantly higher contraction to MCh compared to CON group (213±2.08 in SEN vs 131.31±15.12 in CON; p<0.05). Incubation with Ang II significantly increased contraction in SEN+ANG II (334.79±38.97) compared to SEN, indicating that Ang II potentiated the MCh-induced tracheal contraction response. MCh concentration response was also obtained in trachea from all sensitized+treatment groups (Fig 5b) and response was compared at the highest concentration of MCh (10−5M). Losartan-treated asthmatic mice (SEN+LOS; 125.68±27.68%) had significantly (p<0.05) lower contraction to MCh alone compared to the non-treated SEN group. Incubation of SEN+LOS group tracheal rings with Ang II had a further lowering of the contraction response (86.68±29.63 in SEN+LOS+ANG II; p<0.05 compared to SEN), although this was not significant from SEN+LOS. These data suggest that the potentiating effect of Ang II on the MCh response was inhibited by blocking of the AT1 receptors. It is also probable that binding of Ang II to unopposed AT2 receptors lowered the contraction further, as Ang II can activate both AT1 and AT2 receptors. Treatment with novokinin also reduced (p=0.051) methacholine-induced contraction in tracheal rings compared to SEN group (178±12.21 in SEN+NOV; Fig 5b) while no difference was observed in SEN+NOV group incubated with Ang II compared to SEN+NOV. SEN+ PD had increased contraction compared to SEN group and Ang II incubation in this group had the highest contraction in tracheal, rings suggesting that blocking of AT2 receptors can lead to more reactivity of airway smooth muscle in asthma (327±71.06 in SEN+PD, 414.65±46.87 in SEN+PD+ANGII). Complete concentration responses for the drug treatments are included in Supplementary Data: Appendix A.
Fig 5:
Methacholine-induced contraction in isolated tracheal rings a) Effect of Angiotensin II on methacholine response. SEN group had significantly higher contraction in trachea compared to CON. ANG II incubation i(SEN+ANG II) potentiated contraction compared to SEN group. b) Comparison of the effects of losartan, novokinin and PD 123319 on methacholine (10¼M) response in tracheal rings. SEN+LOS treated group had lower contraction compared to SEN group. ANG II incubation in same group has a further reduction in contraction. SEN+NOV had similar response as losartan. SEN+PD group had highest contraction indicating blocking of AT2 receptors potentiated the contraction response in isolated trachea. Values are indicated as mean±SEM, *p<0.05 compared to CON, #p<0.05 compared to SEN and †p<0.05 compared to SEN+ANG II; n=4–8
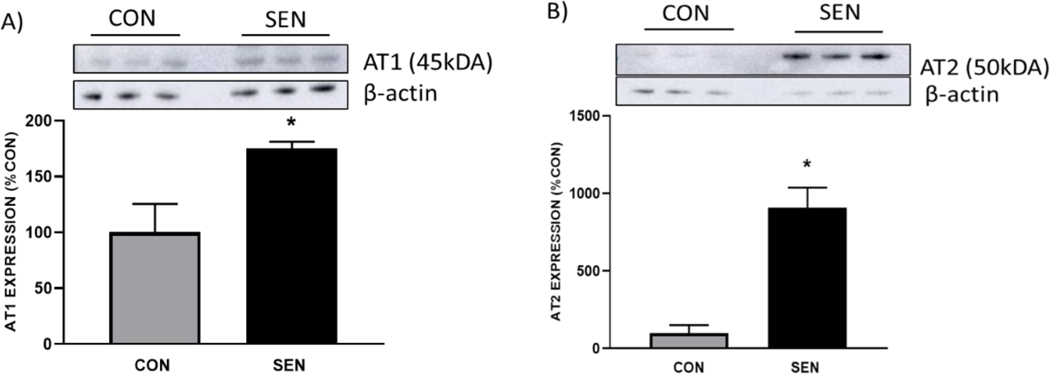
5) Lung expression of AT1 and AT2 receptors in control and sensitized mice
The protein expressions of AT1 and AT2 receptors were determined in whole lungs from control (CON) and asthmatic mice (SEN) to assess if any asthma induced any changes in the levels of these receptors (Fig. 6). Both AT1 and AT2 receptor expressions were significantly elevated in SEN group as compared to CON. For AT1, we observed expression in SEN was almost 2 fold higher than CON (175.29± 5.84% in SEN vs. 100 ± 25.47% in CON, Fig. 6A, p<0.05) and for AT2, the fold difference was almost 9-fold higher (905.71 ± 130.63% in SEN vs. 100 ± 50.13% in CON, Fig. 6B, p < 0.05). This data suggests that AT1, which may increase lung inflammation, is also expressed at higher levels in asthma. AT2, which modulates AT1 function, shows a corresponding upregulation in asthma.
Fig 6:
Protein expression of a) AT1 and b) AT2 receptors in lungs from control and asthmatic mice. Expression of both AT1 and AT2 was significantly elevated in asthmatic (SEN) mice as compared to nonasthmatic controls (CON). Values are indicated as mean±SEM, *p<0.05 compared to CON; n=3 mice
DISCUSSION
The primary goal of this study was to elucidate the roles of Ang ll AT1 and AT2 receptors in inflammation and airway reactivity using a mouse model of asthma. Our aim was to determine the pharmacological effects of losartan (AT1 antagonist), novokinin (AT2 agonist) and PD123319 (AT2 antagonist) on airway hyperreactivity, inflammation and airway smooth muscle contractility in trachea. The major finding of this study was that using AT1 receptor antagonist losartan reduced inflammation, airway hyperreactivity and methacholine-induced contraction in trachea, suggesting that Angiotensin II acting via AT1 receptors potentiates the symptoms of asthma. In addition, using the AT2 receptor agonist novokinin reduced both inflammation and airway reactivity, suggesting a potential role for AT2 receptor-activation in reduction of asthma symptoms. We also noted that losartan appeared to have greater effects in lowering the inflammation in the asthmatic mice compared to novokinin. We found that the expression of AT1 and AT2 receptors was upregulated in asthmatic lungs, with both receptors being significantly elevated compared to non-asthmatic controls.
Asthma is a common chronic lung disease characterized by inflammation and airway hypersensitivity, affecting almost 260 million people around the world. Treatments such as leukotrienes inhibitors and corticosteroids have been used to manage acute episodes of asthma and control the inflammation. However, occurrence of this disease has not declined [24]. A more thorough knowledge of mechanisms and identification of potential therapeutic targets is needed in order to reduce the incidence of asthma.
Angiotensin II (Ang II) is main effector octapeptide in renin angiotensin system (RAS; [5]. Apart from regulating blood pressure, increased concentration of Ang II also leads to elevation of pro-inflammatory cytokines and ultimately leading to infiltration of inflammatory cells in tissues [25]. Ang II works via two G- protein coupled receptors: AT1 and AT2[5]. These receptors mediate different biological effects. Elevated level of Ang II leads to increased hypertension and other cardiovascular diseases via AT1 receptor signaling. Activation of AT1 receptors is also involved in increased systemic inflammation, oxidative stress and vascular inflammation[26]. With respect to the airways, AngII causes contraction in vitro as evidenced by its effect on isolated human bronchial rings and also potentiates bronchoconstriction in asthmatics in vivo [12]. On other hand, AT2 receptors are known to counter balance effects of AT1 receptor. Binding of Ang II to AT2 receptors increases vasodilation and cardiac protective effects in cardiovascular disease [27]. AT2 activation via Ang II also exhibits anti-inflammatory properties in kidney [28]. Successful cloning of Ang II AT2 receptors and understanding their pathophysiological effects have made these receptors as an emerging target for various conditions, mainly those due to the deleterious effects of Ang II AT1 receptors [29]. However, roles of Ang II AT1 and AT2 receptors in asthma are not fully understood. Due to the availability of selective receptor antagonists and agonists for the Ang II AT1 and AT2 receptors, it is possible to study the pharmacological effects of these receptors. Losartan blocks AT1 receptors, whereas PD123319 effectively blocks AT2 receptor [30]. Novokinin binds to AT2 receptors and is a selective AT2 agonist [31]. This a novel agent, and our study is the first to use this peptide to study AT2-mediated effects in asthma.
We first assessed airway responsiveness by using whole body plethysmography. While the use of whole body plethysmography is controversial, it has been used previously by several investigators alone [32, 33] or in conjunction with invasive techniques [34, 35], and continues to be used to assess airway responsiveness [36–39]. In this study, we measured airway responsiveness as enhanced pause (Penh) using increasing doses of methacholine to determine the effects of losartan, novokinin and PD123319 in order to establish the roles of AT1 and AT2 receptors in asthma. Significant increases in Penh values were observed in asthmatic mice compared to non-asthmatic controls, suggesting increases in airway responsiveness in the allergen-sensitized mice. Treatment of losartan in vivo in asthmatic mice significantly reduced airway hyperreactivity compared to allergen sensitized group suggesting that losartan maybe beneficial to lower airway responsiveness. This also indicates that activation of AT1 receptors in asthma may lead to higher bronchoconstriction. Groups treated with novokinin also displayed similar results as losartan indicating a beneficial role for AT2 agonist novokinin in asthma. PD123319, which blocks AT2 receptors, had maximum Penh values, suggesting that blocking of AT2 receptors leads to exacerbation of airway responsiveness. These data suggest that AT2 receptors may be potential targets for reduction of bronchoconstriction in asthma patients and is a novel finding.
We also measured protein expression of AT1 and AT2 receptors in the lungs from control and asthmatic mice by Western blot to assess if differential expression of these receptors was observed in asthma. We found significant upregulation of both AT1 and AT2 receptors in lungs from the asthmatic mice as compared to control animals. In the control mice, clearly observable expression of AT1 receptors was noted, while AT2 was detected at extremely low levels. However, the AT2 receptor expression increased tremendously in asthmatic mice. It is known that AT2 receptors may modulate function of the AT1 receptors [27], and this could explain the high expression of AT2 receptors in asthma.
Increased level of inflammation is observed in asthmatic patients and mouse models of asthma [40]. In this study, total cell count in the BAL fluid from the SEN group was significantly higher than controls, indicating the presence of cell infiltration in the lungs of the allergen-sensitized mice. The total cell counts in asthmatic mice were lowered with both losartan and novokinin respectively, suggesting that blocking of AT1 receptors and activating AT2 receptors reduced the lung cellular infiltrate. We also observed that losartan had a greater effect in lowering the cell count than novokinin. This finding was suggestive of perhaps a more dominant role for AT1 receptors in mediating inflammation in asthma. Many studies indicate the involvement of AT1 receptor activation in inflammatory response and that blocking AT1 receptors lowers inflammation [41–44]. We assessed the lung inflammation in our study groups by differential analysis of the BAL cell counts to identify which cells were present in the BAL. Recruitment of eosinophils in the BAL describes presence of inflammation and exacerbates airway hyperreactivity [45, 46] and previous studies indicate increased number of eosinophils in bronchial lavage of asthmatic patients [47]. We found that asthmatic mice (SEN) and asthmatic mice treated with AT2 antagonist PD 123319 had the highest number of eosinophils. This also correlated with the airway responsiveness data. Further, we found that in vivo treatment with losartan reduced the eosinophilia in asthmatic mice. Treatment with novokinin also significantly reduced number of infiltrated cells in BAL, indicating an anti-inflammatory role for AT2 receptors in asthma. Again, we noted that the effect of losartan on reducing the eosinophils was greater than that of novokinin. Thus, blocking of AT1 receptors with losartan or activation of AT2 receptors reduced eosinophilia but the reduction was greater with AT1 blockade.
Other than eosinophils, studies have also shown that neutrophils are higher in asthmatics than in healthy individuals [48]. We found that sensitized mice had significantly greater number of neutrophils compared to controls. Groups treated with losartan had significantly reduced neutrophils, which again suggests an anti-inflammatory role for losartan. Likewise, we found that novokinin also reduced the number of neutrophils in asthmatic mice. The predominant cell type in the BAL fluid from healthy individuals are non-activated macrophages [49]. In the present study, macrophages were the highest in control groups indicating the lack of active inflammatory processes and cellular infiltration. We also observed significantly lower macrophages in asthmatic mice. Use of losartan and novokinin attenuated this decrease in macrophages in treated asthmatic mice. Blocking AT2 receptors with PD123319 had no effect on reducing eosinophils, neutrophils and the attenuation of decreased macrophages in asthmatic mice. There were also increased levels of IL-5 and IL-13 in the allergic mice that were lowered with either losartan or novokinin. PD 123319 on the other hand, either increased the cytokine levels or had no effect on lowering them in asthmatic mice. Thus, our data shows that losartan and novokinin both have effects on lowering lung inflammation and that effects of losartan appear to be greater in comparison to novokinin. These data thus confirm that AT2 receptors may have an anti-inflammatory role compared to AT1 receptors, which are pro-inflammatory in asthma.
Studies have shown that Ang ll is a bronchoconstrictor in asthmatics and potentiates response to methacholine in vitro in bronchial rings from asthmatics [13]. To assess the effects of Ang ll in isolated airway rings, we also measured tracheal contractility to methacholine ex vivo and evaluated the effect of Ang II on methacholine-induced contraction. Our data showed that trachea from allergen-sensitized mice had the highest contraction response to methacholine. Ang II incubation prior to the methacholine concentration response potentiated the contraction. Allergen-sensitized mice treated with either losartan or novokinin had lower contraction response to methacholine and the Ang II potentiation of contraction was abolished. These data further confirm the role of Ang II in mediating increased airway contractility in asthma via the activation of AT1 receptors. As for the AT2 receptors, their activation leads to a decrease in tracheal contractility. We also found that blocking AT2 receptors with PD 123319 (SEN+PD group) increases contractility in sensitized mice. These data again suggest that the AT2 receptors counterbalance the effects of AT1 activation. Further studies are required to understand the mechanisms involved in the contractility.
CONCLUSION
In summary, our data shows that increases in airway inflammation and airway responsiveness in allergic mice were reduced with the ATI antagonist losartan. The use of AT2 agonist also lowered lung inflammation and airway reactivity. However, it appears blocking AT1 receptors has a greater effect on reducing both inflammation and airway hyperreactivity in asthma compared to activation of AT2 receptors. The lung expression of AT1 and AT2 receptors is significantly upregulated in asthma. These novel findings support modulatory roles for both AT1 and AT2 receptors in asthma and these receptors are potential new targets for developing therapies to manage this airway disease. Further studies are required to elucidate signaling mechanisms and identify molecular targets in the signal transduction pathway downstream of these receptors.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by LIU startup funds (DSP) and National Institutes of Health grant HL027339 (SJM).
Funding Source: Long Island University start-up funds (DSP), HL027339 (SJM)
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
The authors declare that they have no conflict of interest in this work.
REFERENCES
- 1.Reid IA, Morris BJ, and Ganong WF, The renin-angiotensin system. Annu Rev Physiol, 1978. 40: p. 377–410. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension, 2001. 37(4): p. 1047–52. [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ and Re R, Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation, 1994. 89(1): p. 493–8. [DOI] [PubMed] [Google Scholar]
- 4.Touyz RM and Schiffrin EL, Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev, 2000. 52(4): p. 639–72. [PubMed] [Google Scholar]
- 5.Inagami T, The renin-angiotensin system. Essays Biochem, 1994. 28: p. 147–64 [PubMed] [Google Scholar]
- 6.Hein L, et al. , Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol, 1997. 11(9): p. 1266–77. [DOI] [PubMed] [Google Scholar]
- 7.Catt KJ, et al. , The role of angiotensin II receptors in vascular regulation. J Cardiovasc Pharmacol, 1984. 6 Suppl 4: p. S575–86. [DOI] [PubMed] [Google Scholar]
- 8.Ji Y, et al. , Angiotensin II induces inflammatory response partly via toll-like receptor 4dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem, 2009. 23(4–6): p. 265–76. [DOI] [PubMed] [Google Scholar]
- 9.Lv J, et al. , Candesartan attenuates Angiotensin II-induced mesangial cell apoptosis via TLR4/MyD88 pathway. Biochem Biophys Res Commun, 2009. 380(1): p. 81–6. [DOI] [PubMed] [Google Scholar]
- 10.Marshall RP, et al. , Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol, 2004. 286(1): p. L156–64. [DOI] [PubMed] [Google Scholar]
- 11.Phillips MI, et al. , Dynamic changes in hypothalamic angiotensin II levels and release in association with progesterone-induced luteinizing hormone surge. Endocrinology, 1993. 132(4): p. 1637–42. [DOI] [PubMed] [Google Scholar]
- 12.Millar EA, Nally JE, and Thomson NC, Angiotensin II potentiates methacholine-induced bronchoconstriction in human airway both in vitro and in vivo. Eur Respir J, 1995. 8(11): p. 1838–41. [DOI] [PubMed] [Google Scholar]
- 13.Endo Y, et al. , Function of angiotensin II type 2 receptor in the postglomerular efferent arteriole. Kidney Int Suppl, 1997. 63: p. S205–7. [PubMed] [Google Scholar]
- 14.Harada S, et al. , Contrasting effects of angiotensin type 1 and 2 receptors on nitric oxide release under pressure. Hypertens Res, 2002. 25(5): p. 779–86. [DOI] [PubMed] [Google Scholar]
- 15.Ponnoth DS, et al. , Involvement of A1 adenosine receptors in altered vascular responses and inflammation in an allergic mouse model of asthma. Am J Physiol Heart Circ Physiol, 2010. 299(1): p. H81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz MC, et al. , Chronic blockade of the AT2 receptor with PD123319 impairs insulin signaling in C57BL/6 mice. Peptides, 2017. 88: p. 37–45. [DOI] [PubMed] [Google Scholar]
- 17.Bivalacqua TJ, et al. , Effects of candesartan and PD123319 on responses to angiotensin II in the anesthetized mouse. J Am Soc Nephrol, 1999. 10 Suppl 11: p. S98–100. [PubMed] [Google Scholar]
- 18.Wagenaar GT, et al. , Angiotensin II type 2 receptor ligand PD123319 attenuates hyperoxia-induced lung and heart injury at a low dose in newborn rats. Am J Physiol Lung Cell Mol Physiol, 2014. 307(3): p. L261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki M, et al. , Losartan ameliorates progression of glomerular structural changes in diabetic KKAy mice. Life Sci, 2004. 75(7): p. 869–80. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, et al. , A potent hypotensive peptide, novokinin, induces relaxation by AT2- and IP-receptor-dependent mechanism in the mesenteric artery from SHRs. Biosci Biotechnol Biochem, 2008. 72(1): p. 257–9. [DOI] [PubMed] [Google Scholar]
- 21.Oldenburg PJ and Mustafa SJ, Involvement of mast cells in adenosine-mediated bronchoconstriction and inflammation in an allergic mouse model. J Pharmacol Exp Ther, 2005. 313(1): p. 319–24. [DOI] [PubMed] [Google Scholar]
- 22.Teng B, et al. , Isolation and characterization of coronary endothelial and smooth muscle cells from A1 adenosine receptor-knockout mice. Am J Physiol Heart Circ Physiol, 2006. 290(4): p. H1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talukder MA, Morrison RR, and Mustafa SJ, Comparison of the vascular effects of adenosine in isolated mouse heart and aorta. Am J Physiol Heart Circ Physiol, 2002. 282(1): p. H49–57. [DOI] [PubMed] [Google Scholar]
- 24.Braman SS, The global burden of asthma. Chest, 2006. 130(1 Suppl): p. 4S–12S. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, et al. , Inflammation and angiotensin II. Int J Biochem Cell Biol, 2003. 35(6): p. 881–900. [DOI] [PubMed] [Google Scholar]
- 26.Fyhrquist F and Saijonmaa O, Renin-angiotensin system revisited. J Intern Med, 2008. 264(3): p. 224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulman IH and Raij L, The angiotensin II type 2 receptor: what is its clinical significance? Curr Hypertens Rep, 2008. 10(3): p. 188–93. [DOI] [PubMed] [Google Scholar]
- 28.Esteban V, et al. , Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J Am Soc Nephrol, 2004. 15(6): p. 1514–29. [DOI] [PubMed] [Google Scholar]
- 29.Chang Y and Wei W, Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol, 2015. 179(2): p. 137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmermans PB, et al. , Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev, 1993. 45(2): p. 205–51. [PubMed] [Google Scholar]
- 31.Yoshikawa M, Ohinata K, and Yamada Y, The pharmacological effects of novokinin; a designed peptide agonist of the angiotensin AT2 receptor. Curr Pharm Des, 2013. 19(17): p. 3009–12. [DOI] [PubMed] [Google Scholar]
- 32.Drazen JM, Finn PW, and De Sanctis GT, Mouse models of airway responsiveness: physiological basis of observed outcomes and analysis of selected examples using these outcome indicators. Annu Rev Physiol, 1999. 61: p. 593–625. [DOI] [PubMed] [Google Scholar]
- 33.Fan M and Mustafa SJ, Adenosine-mediated bronchoconstriction and lung inflammation in an allergic mouse model. Pulm Pharmacol Ther, 2002. 15(2): p. 147–55. [DOI] [PubMed] [Google Scholar]
- 34.Bosnjak B, et al. , Tiotropium bromide inhibits relapsing allergic asthma in BALB/c mice. Pulm Pharmacol Ther, 2014. 27(1): p. 44–51. [DOI] [PubMed] [Google Scholar]
- 35.Rajavelu P, et al. , Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest, 2015. 125(5): p. 2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng BH, et al. , Yan-Hou-Qing formula attenuates allergic airway inflammation via upregulation of Treg and suppressing Th2 responses in Ovalbumin-induced asthmatic mice. J Ethnopharmacol, 2019. 231: p. 275–282. [DOI] [PubMed] [Google Scholar]
- 37.Low A, et al. , Lung Function, Inflammation, and Endothelin-1 in Congenital Heart Disease-Associated Pulmonary Arterial Hypertension. J Am Heart Assoc, 2018. 7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Hashim AZ, Mathews S, and Al-Shamlan F, Central adenosine A1 receptors inhibit cough via suppression of excitatory glutamatergic and tachykininergic neurotransmission. Br J Pharmacol, 2018. 175(15): p. 3162–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JC, et al. , Multispecies probiotics combination prevents ovalbumin-induced airway hyperreactivity in mice. Allergol Immunopathol (Madr), 2018. 46(4): p. 354–360. [DOI] [PubMed] [Google Scholar]
- 40.Jousilahti P, et al. , The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol, 2002. 89(4): p. 381–5. [DOI] [PubMed] [Google Scholar]
- 41.Duff JL, et al. , Angiotensin II signal transduction and the mitogen-activated protein kinase pathway. Cardiovasc Res, 1995. 30(4): p. 511–7. [PubMed] [Google Scholar]
- 42.Klahr S and Morrissey J, Angiotensin II and gene expression in the kidney. Am J Kidney Dis, 1998. 31(1): p. 171–6. [DOI] [PubMed] [Google Scholar]
- 43.Bernstein KE, et al. , New insights into the cellular signaling of seven transmembrane receptors: the role of tyrosine phosphorylation. Lab Invest, 1998. 78(1): p. 3–7. [PubMed] [Google Scholar]
- 44.Marrero MB, et al. , Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature, 1995. 375(6528): p. 247–50. [DOI] [PubMed] [Google Scholar]
- 45.Kay AB, The role of eosinophils in the pathogenesis of asthma. Trends Mol Med, 2005. 11(4): p. 148–52. [DOI] [PubMed] [Google Scholar]
- 46.Trivedi SG and Lloyd CM, Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci, 2007. 64(10): p. 1269–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bousquet J, et al. , Eosinophilic inflammation in asthma. N Engl J Med, 1990. 323(15): p. 1033–9. [DOI] [PubMed] [Google Scholar]
- 48.Wenzel SE, et al. , Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med, 1997. 156(3 Pt 1): p. 737–43. [DOI] [PubMed] [Google Scholar]
- 49.Eschenbacher WL and Gravelyn TR, A technique for isolated airway segment lavage. Chest, 1987. 92(1): p. 105–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.