Abstract
Objective:
Compare rates of hypoxemia during transpyloric and gastric feedings in very preterm infants with severe bronchopulmonary dysplasia.
Design:
N-of-1 multiple crossover trial with individual patient and pooled data analyses.
Setting:
Level IV intensive care nursery.
Patients:
Infants receiving positive airway pressure between 36-55 weeks postmenstrual age were enrolled 12/2014-7/2016.
Intervention:
N-of-1 trial consisting of 2 blocks, each with a 4-day gastric and 4-day transpyloric feeding period assigned in random order.
Main Outcome Measures:
The primary outcome was the frequency of daily intermittent hypoxemic events (SpO2 ≤80% lasting 10-180s). Secondary outcomes included the daily proportion of time with an SpO2 ≤80% and mean daily fraction of inspired oxygen.
Results:
Of 15 infants, 13 completed the trial and 2 stopped early for transient worsening in respiratory status during gastric feedings. In the intention-to-treat analyses, transpyloric feedings resulted in increased rates of intermittent hypoxemia in 5 infants, greater time per day in hypoxemia in 3 infants, and more supplemental oxygen use in 3 infants. One infant received more supplemental oxygen during gastric feedings. The remaining study outcomes were similar between the feeding routes in all other infants. Pooling all data, transpyloric feedings resulted in a higher frequency of intermittent hypoxemic events (median 7.5/day [IQR 1-23.5] vs. 3/day [1-11]; adjusted incidence rate ratio 1.8, 95% CI 1.3-2.5) and a greater proportion of daily hypoxemia time (median 0.8% [IQR 0.1-2.3] vs. 0.4% [0.07-1.8]; adjusted mean difference 1.6, 95% CI 1.1-2.5).
Conclusions:
Transpyloric compared to gastric feedings modestly increased rates of hypoxemia among study participants.
Introduction
Bronchopulmonary dysplasia (BPD) predisposes very preterm infants to severe, enduring cognitive and physical impairments.1–4 Gastroesophageal reflux (GER) and pulmonary aspiration may contribute to these adverse sequelae by inducing lung inflammation, bronchospasm, and recurrent hypoxemia.5–13 Transpyloric feedings reduce aerodigestive sequelae of GER in older children and adults.14,15 However, the safety and efficacy of this feeding practice in infants with BPD is uncertain. The limited data from randomized trials conducted in preterm newborns show that early use of transpyloric compared to gastric feedings does not prevent aspiration pneumonia and may increase mortality risk.16 In contrast, recent observational studies found that transpyloric feedings were associated with less frequent apnea and bradycardia in preterm infants and a lower risk of death or BPD.17–19 These data also suggest that any potential benefit with transpyloric feedings may vary between infants.18
Because of the heterogeneity of GER in preterm infants20,21 and potential intra-patient variability in the response to transpyloric feedings,18 individualized feeding route selection in infants with BPD may be optimal. N-of-1, or single patient trials, are a methodologically robust means to select a treatment strategy in individual patients when the preferred therapy may vary between subjects.22–24 Data from N-of-1 trials can also be pooled to estimate overall treatment effects. We report a series of N-of-1 trials comparing the safety and efficacy of gastric and transpyloric feedings in infants with severe BPD at the individual and group levels.
Methods
Study Infants
Infants born <32 weeks’ gestation with severe BPD25 who were receiving positive airway pressure and full enteral nutrition at 360/7 to 556/7 weeks postmenstrual age were eligible. Infants with clinical signs attributed to gastroesophageal reflux (GER) by their medical team (e.g. frequent regurgitation, suspected aspiration) were targeted for enrollment. No infants underwent diagnostic evaluation for GER, as this is not part of our routine practice. Infants with recent, significant changes to their medical care (e.g. change in mode of ventilation, new medication), a gastric fundoplication, or severe congenital malformation were excluded. Informed consent was obtained from a parent or guardian of each infant. The Institutional Review Board at the Children’s Hospital of Philadelphia approved this study. The trial was registered at clinicaltrials.gov ( NCT 02142621).
N-of-1 Trial Design
Each N-of-1 trial lasted 16 days and consisted of two blocks. Each block consisted of a 4-day gastric and 4-day transpyloric feeding period assigned in random order using a computer-generated sequence. A “washout period” was not utilized between feeding periods owing to the need to provide enteral nutrition throughout the study.
Intervention
All feedings were administered using 6f CORFLO nasogastric/nasointestinal feeding tubes (Halyard Health, Alpharetta, GA) placed by nursing staff. Gastric tube position was confirmed by aspirate pH testing (acceptable pH≤5) or abdominal radiograph if the pH result was inconclusive. Transpyloric tubes were placed in the second portion of the duodenum and confirmed by radiograph. Feeding route allocation could not be blinded as clinicians needed to monitor the feeding tube location. Gastric feedings were run continuously for the first 24 hours of each assigned period. Thereafter, clinicians were allowed to condense feedings. Transpyloric feedings were run continuously throughout the study. All other care decisions were at the discretion of the medical team. However, clinicians were asked to avoid making non-urgent changes in medical management during the trial. Following each trial, the study team discussed the results with the medical team and provided a summary report (online supplementary Figure 1). The treating physician selected the post-study feeding route.
Outcomes
The primary outcome was the daily number of intermittent hypoxemic events defined as a peripheral oxygen saturation (SpO2) ≤80% lasting between 10 seconds and 3 minutes.6,7 The secondary outcomes were the proportion of time per day with an SpO2 ≤80%, the daily number of prolonged hypoxemic events (SpO2 ≤80% lasting ≥60 seconds)13, and the daily time-averaged fraction of inspired oxygen (FiO2). All SpO2 data were recorded using a Masimo Rad-8 pulse oximeter (Masimo, Irvine, CA) set to 2-second averaging and sampling rates.26
Occurrence of the following pre-specified adverse events were recorded: (1) absolute increase in FiO2 >20% above baseline for ≥8 hours, (2) need for non-elective endotracheal intubation; (3) aspiration pneumonia defined as an observed regurgitation event with a new infiltrate on chest radiograph, increased respiratory support needs, and antibiotic therapy; (4) bag-valve ventilation for an acute hypoxemic event; (5) water loss stools with associated electrolyte abnormalities; (6) necrotizing enterocolitis or intestinal perforation; (7) and death. An independent data safety monitor reviewed the study data at half enrollment and adjudicated all adverse events.
Oximeter Data and Statistical Analysis
SpO2 data were downloaded from the study oximeters every 72 hours. Prior to any statistical analyses, oximeter values with a 0 pulse rate or a “Low Signal IQ” exception message were removed. The primary analyses utilized an intention-to-treat approach. Pre-specified as-treated analyses were also performed. Mixed effects negative binomial regression models were used to assess the relationship between the feeding route and rates of intermittent and prolonged hypoxemic events in the individual and pooled data. The daily proportion of time spent hypoxemic and the mean daily time-weighted FiO2 were log transformed and assessed using mixed effects linear regression models. A dichotomous variable distinguishing the first and second treatment blocks was included in all models to adjust for temporal variation in disease severity. The data for one infant with zero hypoxemia time observed on 2 days were replaced with the lowest percentage of hypoxemia time in the data set (0.003%) to generate analyzable log transformed results for those days. To account for within subject correlation of repeated outcome measures, a random effect for each patient with an unstructured covariance matrix and robust variance estimates was used in the mixed-effects models for the pooled analyses.
We assessed infant-level heterogeneity of treatment effects for the primary study outcome in two ways. Firstly, we tested the statistical significance of a treatment-by-participant interaction term added to the pooled-data regression model. Secondly, we tested the equivalence of the infant-level treatment effect estimates using Cochran’s chi-square and quantified the proportion of variation due to infant-level heterogeneity with the I2 statistic in a fixed effect inverse variance model.27,28 All statistical testing was conducted using Stata/SE, version 15.1 (StataCorp, College Station, TX).
Sample Size Determination
The sample size calculation for the pooled analysis utilized the following parameters: 2 paired crossovers, a within subject outcome correlation of 0.7, and an intra-subject variance in the frequency of intermittent hypoxemic events of 84.7,29 Based on these assumptions, 15 infants provides 90% power to detect a 13% relative reduction in the daily number of intermittent hypoxemic events between the two feeding routes (see additional description of the sample size calculation in the online supplement).
Results
Study Participants
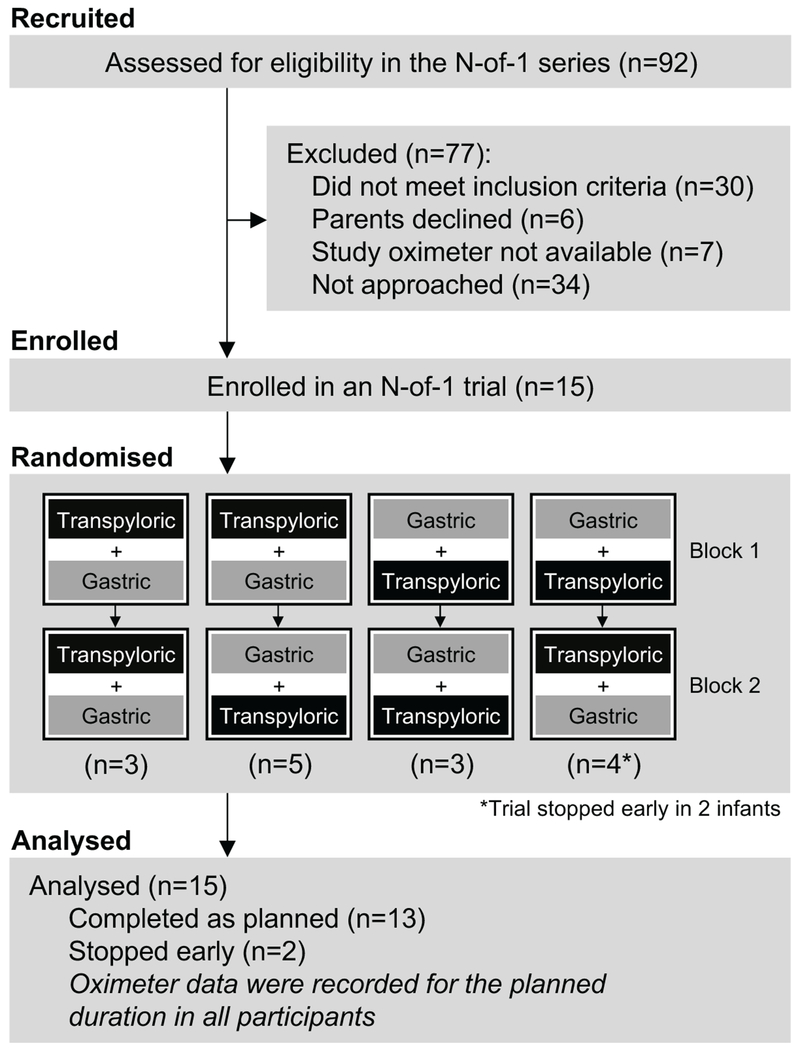
A total of 15 infants were enrolled between December 2014 and July 2016 (Figure 1). The majority were male, receiving non-invasive positive airway pressure, and clinically prescribed transpyloric feedings at enrollment (Table 1). Five infants (27%) received a proton pump inhibitor or histamine-2 antagonist during their N-of-1 trial. Pulse oximetry data were available for 77% of study period after excluding low perfusion readings and times when the oximeter was not attached to the infant. The proportion of analyzable data was similar between the feeding routes.
Figure 1.
Study flow diagram
Table 1.
Characteristics of the 15 study participants
| Gestational age, wk - median (IQR) | 26.0 (24.6-27.9) |
| Birth weight, g – median (IQR) | 750 (640-930) |
| Male, n (%) | 12 (80%) |
| Outborn, n (%) | 14 (93) |
| Postmenstrual age at enrollment, wk – median (IQR) | 47.3 (39.3-53.1) |
| Weight at enrollment, g – median (IQR) | 4400 (2923-5260) |
| Feeding route immediately prior to enrollment, (%) | |
| Gastric | 5 (33) |
| Transpyloric | 10 (67) |
| Respiratory support at enrollment, (%) | |
| High flow nasal cannula | 6 (40) |
| Nasal CPAP | 6 (40) |
| Mechanical ventilation | 3 (20) |
| Gastric acid suppression medication use at enrollment, n (%) | 5 (27) |
Individual Patient Analyses
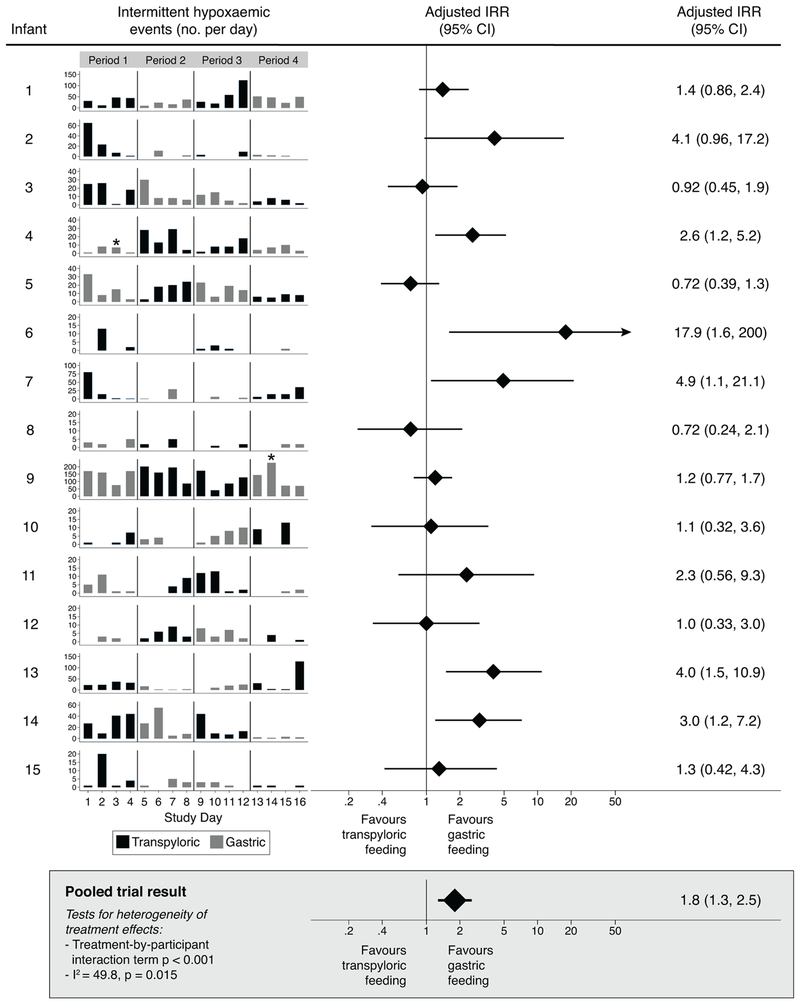
Five infants (20%) (no. 4, 6, 7, 13, and 14) experienced a significantly higher frequency of intermittent hypoxemic events per day while receiving transpyloric compared to gastric feedings (Figure 2). Rates of intermittent hypoxemia were similar between the two feeding routes in the remaining 10 infants. There was evidence of infant-level heterogeneity of treatment effects for this outcome (treatment-by-participant interaction term p<0.001; I2=49.8, p=0.015). Transpyloric feedings were also associated with a higher frequency of prolonged hypoxemic events in two infants (no. 4, 13) and a greater proportion of time per day with an SpO2 ≤80% in 3 infants (no. 2, 6, 13) (online supplementary Table 1). Three infants (no. 10, 12, 15) received more supplemental oxygen during transpyloric feedings; one (no. 5) received more during gastric feedings.
Figure 2. Primary outcome results for the individual patient and pooled analyses (shaded box).
Incidence rate ratios (IRR) calculated using mixed effects negative binomial regression. A dichotomous variable distinguishing treatment blocks 1 and 2 was included in each model to account for temporal variation in disease severity over time. IRR values indicate the average relative increase (IRR >1) or decrease (IRR<1) in the daily number of intermittent hypoxemic events with transpyloric compared to gastric feedings. Stars (*) show the approximate timepoints these infants were removed from the intervention portion of the trial and transpyloric feedings were administered thereafter. All study participants received gastric feedings run continuously when this feeding route was assigned except for infants no 12 and 14 who received bolus gastric feedings after the first 24 hours of each gastric feeding period. CI, confidence interval.
Adverse Events and As-Treated Analyses
No pre-defined adverse events were observed. However, the trial intervention was stopped early in two infants (no. 4 and 9) for clinical worsening during gastric feedings. Infant no. 4 received transpyloric feedings prior to enrollment. His oxygen requirement acutely worsened after approximately 48 hours of initial gastric feedings. This change was attributed to a clinically suspected post-emesis aspiration event that did not meet the pre-defined stopping rule for aspiration pneumonia. Gastric feedings were subsequently discontinued and transpyloric feedings resumed. The intention-to-treat analysis for this subject showed a higher frequency of intermittent hypoxemic events during transpyloric feedings (Figure 2). The as-treated analysis showed no difference between the feeding routes (IRR 3.0, 95% CI 0.81-11.4). Infant no. 9 was removed from the trial during the second gastric feeding period following an acute rise in FiO2. The independent data safety monitor determined this clinical change most likely resulted from a concomitant decrease in the ventilator mean airway pressure. After a brief period without enteral nutrition, the medical team chose to administer transpyloric feedings. Rates of intermittent hypoxemia in this infant were similar between the two feeding routes in the intention-to-treat and as-treated analyses.
Post-Trial Feeding Route and Discharge Status
Four of the 5 infants whose intention-to-treat analyses showed greater intermittent hypoxemia during transpyloric feedings were prescribed gastric feeding by their medical team following the N-of-1 trial (Table 2). This was a change from clinically prescribed, pre-trial transpyloric feedings in 2 of the 4 infants. Of the 10 infants with equivocal trial results for the primary outcome, 6 were prescribed post-trial gastric feedings (5 of whom received pre-trial transpyloric feedings) and 4 were prescribed transpyloric feedings (2 of whom received pre-trial transpyloric feedings). Rates of gastric fundoplication or jejunal tube feedings at discharge were similar among the infants whose N-of-1 trials favored gastric feedings and those with equivocal results (60% vs. 50%; p=1.0).
Table 2 –
Individual patient feeding route pre and post N-of-1 trial
| Infant | N-of-1 trial primary outcome result | Pre-trial Feeding route | Post-trial Feeding route | Feeding route at NICU discharge | Respiratory support at NICU discharge |
|---|---|---|---|---|---|
| 1 | Equivocal | Transpyloric | Gastric | Nasogastric tube | Room air |
| 2 | Equivocal | Transpyloric | Gastric | Oral | Room air |
| 3 | Equivocal | Transpyloric | Gastric | GT/Fundoplication | Nasal cannula |
| 4 | Gastric Bettera | Transpyloric | Transpyloric | Jejunal tube | Tracheostomy |
| 5 | Equivocal | Gastric | Gastric | Nasogastric tube | Nasal cannula |
| 6 | Gastric Better | Gastric | Gastric | GT/Fundoplication | Tracheostomy |
| 7 | Gastric Better | Transpyloric | Gastric | Gastrostomy tube | Nasal cannula |
| 8 | Equivocal | Transpyloric | Gastric | GT/Fundoplication | Nasal cannula |
| 9 | Equivocal | Transpyloric | Transpyloric | GT/Fundoplication | Tracheostomy |
| 10 | Equivocal | Transpyloric | Transpyloric | Care Withdrawn | Care Withdrawnb |
| 11 | Equivocal | Gastric | Transpyloric | GT/Fundoplication | Nasal cannula |
| 12 | Equivocal | Gastric | Transpyloric | Jejunal tube | Nasal cannula |
| 13 | Gastric Better | Transpyloric | Gastric | GT/Fundoplication | Nasal cannula |
| 14 | Gastric Better | Gastric | Gastric | Oral | Room air |
| 15 | Equivocal | Transpyloric | Gastric | Oral | Nasal cannula |
As-treated analysis showed equivocal results
Medical care was withdrawn long after the N-of-1 trial in this infant owing to significant unremitting cardiopulmonary disease
Abbreviations: GT, gastrostomy tube; NICU, neonatal intensive care unit
Pooled Data Analyses
In the pooled analyses, transpyloric compared to gastric feedings significantly increased the frequency of intermittent hypoxemic events and the proportion of time per day with an SpO2 ≤80% (Figure 2, Table 3). Stated as time, the differences in SpO2 levels equated to an average of 3 additional minutes (95% CI 0.3 to 8 minutes) of hypoxemia per day during transpyloric feedings. The feeding route was not associated with differences in FiO2 (Table 3). Results of the pooled as-treated analyses were similar to the intention-to-treat analyses (data not shown).
Table 3.
Pooled study results
| Outcome | Gastric feedings Median (IQR) | Transpyloric feedings Median (IQR) | IRR for transpyloric feedings (95% CI)a |
|---|---|---|---|
| Intermittent hypoxemic events (no. per day) | 3 (1-11) | 7.5 (1-23.5) | 1.8 (1.3, 2.5) |
| Prolonged hypoxemic events (no. per day) | 0 (0-1) | 0 (0-2.5) | 1.9 (1.1, 3.2) |
| Mean change with transpyloric feedings (95% CI)b | |||
| Proportion of time with SpO2 ≤ 80% per day (%) | 0.4 (0.07-1.8) | 0.8 (0.1-2.3) | 1.6 (1.1, 2.5) |
| Mean time-weighted daily FiO2 (%) | 29.2 (23.7-34.5) | 29.0 (24.0-35.9) | 1.0 (1.0, 1.0) |
Mixed effects negative binomial regression models were used to assess the relationship between the feeding route and rates of intermittent and prolonged hypoxemic events. IRR values indicate the average relative increase in the daily number of intermittent or prolonged hypoxemic events with transpyloric compared to gastric feedings.
The daily proportion of time spent hypoxemic and the mean daily time-weighted FiO2 were log transformed and assessed using mixed effects linear regression models. Values shown for these outcomes are back-transformed (exponentiated) model parameter estimates. They indicate the average relative increase (for values >1) in the proportion of time in hypoxemia per day and the time-weighted daily FiO2 exposure during transpyloric compared to gastric feedings.
A random effect from each individual patient and an unstructured covariance matrix with robust variance estimates were used in all models. A dichotomous variable distinguishing the first and second treatment blocks was included in the models to account for temporal variation in disease severity over time. Use of these model parameters account for the differences in the ratios of the median values for the study outcomes and the estimates of treatment effects shown in the table.
Abbreviations: CI, confidence interval; FiO2, fraction of inspired oxygen; IQR, interquartile range; IRR, incidence rate ratio; SpO2 peripheral capillary oxygen saturation level
Discussion
We conducted a series N-of-1 trials to compare the safety and efficacy of transpyloric to gastric feedings in individual very preterm infants with severe BPD. Transpyloric feedings resulted in a modest, but statistically significant increase in the rates intermittent hypoxemia in one third of study participants. No infants experienced more hypoxemia during gastric feedings. When pooling the data for the entire study cohort, transpyloric feedings resulted in greater hypoxemia and no difference in the prescribed FiO2.
We compared transpyloric and gastric feedings using N-of-1 trials rather than a parallel-arm group trial to enable real-time, individualized therapy selection. N-of-1 trials are a preferred option to personalize treatment choices when the following conditions are met: a relatively stable chronic illness, a therapy with heterogeneous treatment effects, availability of a clinically relevant short-term endpoint, a treatment effect that is expected to persist with continued use of the therapy, and a lack of adequate evidence from standard group trials establishing a preferred treatment.22 Transpyloric feeding in severe BPD meets these requirements. Severe BPD is a chronic illness marked by prolonged periods with “stable” physiology. GER with pulmonary aspiration likely exacerbates respiratory disease in some but not all infants with severe BPD.30 The utility of transpyloric feedings may vary among infants.18 Hypoxemia is easily measured in real-time and observational data suggest that transpyloric feeding may reduce episodic apnea and bradycardia within 72 hours in preterm infants.17 Pathologic GER persists throughout the first year of life in some infants with severe BPD, suggesting control of GER will provide stable treatment effects over time.31 Finally, there are no proven safe and effective treatments for GER in preterm infants.32
Our study findings raise concern that transpyloric feedings may carry potential for harm in some infants with severe BPD. The etiology of the apparent adverse effects is unclear. Data from chronically ill young children suggest that transpyloric feeding does not eliminate GER.33 Moreover, gastric feeding is an important buffer of gastric pH in infants and may act to reduce the acidity and irritability of esophageal refluxate.34 It is possible that GER occurring during transpyloric compared to gastric feedings has a higher concentration of gastric acid and injurious enzymes. Transpyloric feeding is also not physiologic. Rapid entry of fortified, high osmolarity nutrition into the jejunum can induce bloating and dumping. We did not observe significant diarrhea or electrolyte abnormalities during transpyloric feedings, but it is possible that more subtle effects on intestinal function could impact patient comfort and clinical stability. Although the existing neonatal trial data primarily include younger infants, these studies showed increased gastro-intestinal disturbances necessitating temporary cessation of enteral nutrition among infants randomized to receive transpyloric feedings.16 Variability in any of these multiple physiologic processes could explain why some but not all study participants experienced more hypoxemia during transpyloric compared to gastric feedings.
We acknowledge several study limitations. Firstly, the trials were conducted in a small and select cohort of infants. Although our findings suggest clinicians should carefully consider the potential risks of transpyloric feedings in infants with severe BPD, larger studies are needed to fully characterize the safety and efficacy of this feeding method in preterm infants. Secondly, the study endpoints focused on oxygen saturation stability and not the frequency or severity of GER or pulmonary aspiration. Continuous esophageal pH-multiple impedance monitoring may help evaluate the utility of transpyloric feeding. However, this diagnostic tool is not commonly used in our unit as it does not differentiate benign GER from esophageal reflux that may contribute to pulmonary aspiration and exacerbation of BPD. Thirdly, we did not include a “washout period” between feedings routes as it was necessary to maintain enteral feedings throughout the study. Whether the effects of one feeding route carried over to the other is unknown. To monitor the feeding tube location, investigators and clinicians were also not masked to the assigned feeding route. Lastly, 4-day trial periods may not provide sufficient time to fully characterize the risks and benefits of transpyloric feedings. The study duration in N-of-1 trials must be sufficiently long to provide meaningful information about a therapy but short enough to allow adequate post-trial time to positively influence patient care. The 16-day trial used in this study was chosen to balance these considerations. Future studies should explore the effects of transpyloric feedings administered over longer periods and in infants with objectively diagnosed GER.
In conclusion, this series of N-of-1 trials found that transpyloric feedings, on average, led to greater oxygen saturation instability among infants with severe BPD. Subsequent studies should investigate the physiologic effects of transpyloric feedings in preterm infants and further evaluate the safety and efficacy of this feeding strategy.
Supplementary Material
What is known about this topic?
Gastroesophageal reflux and pulmonary aspiration may contribute to hypoxemia in some very preterm infants with severe bronchopulmonary dysplasia (BPD).
Transpyloric feeding reduces the risk of aspiration pneumonia among adults receiving invasive mechanical ventilation; the utility in infants with severe BPD is uncertain and may vary between infants.
N-of-1, or single-patient, randomized crossover trials are a methodologically robust means to estimate treatment effects within individual patients and to personalize care.
What this study adds?
Transpyloric feeding increased rates of hypoxemia among some very preterm infants with severe BPD; no infants demonstrated less hypoxemia with transpyloric feedings.
N-of-1 trials are feasible to conduct in the intensive care nursery and may help individualize care in infants with severe BPD.
Acknowledgements:
The authors are grateful to the infants and their families who participated in this study and thank John Flibotte, M.D. for severing as the independent data safety monitor during this study.
Funding Source: Children’s Hospital of Philadelphia Center for Pediatric Clinical Effectiveness. EAJ was supported by a grant from the National Health, Lung, and Blood Institute (K23HL136843).
Footnotes
Trial Registration: ClinicalTrials.gov, number NCT 02142621.
Financial Disclosure: The authors have no relevant financial relationships to disclose.
Conflict of Interest: The authors have no relevant conflicts of interest to disclose.
References
- 1.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the national institutes of health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt B, Asztalos EV, Roberts RS, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months. JAMA 2003;289:1124–1129. [DOI] [PubMed] [Google Scholar]
- 3.Walsh MC, Morris BH, Wrage LA, et al. Extremely low birthweight neonates with protracted ventilation: Mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146:798–804. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Dysart K, Kendrick D, et al. Prolonged respiratory support of any type impacts outcomes of extremely low birth weight infants. Pediatr Pulmonol. 2018;53:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.See CC, Newman LJ, Berezin S, et al. Gastroesophageal reflux induced hypoxemia in infants with apparent life threatening events(s). Arch Pediatr Adolesc Med 1989;143:951. [PubMed] [Google Scholar]
- 6.Di Fiore JM, Kaffashi F, Loparo K, et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr Res. 2012;72:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Fiore JM, Bloom JN, Orge F, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimaguila MA, Di Fiore JM, Martin RJ, et al. Characteristics of hypoxemic episodes in very low birth weight infants on ventilatory support. J Pediatr. 1997;130:577–583. [DOI] [PubMed] [Google Scholar]
- 9.Warner B, Stuart L, Papes R, et al. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–L117. [DOI] [PubMed] [Google Scholar]
- 10.Farrokhi F, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux. Oral Dis. 2007;13:349–359. [DOI] [PubMed] [Google Scholar]
- 11.Jadcherla S Pathophysiology of aerodigestive pulmonary disorders in the neonate. Clin Perinatol. 2012;39:639–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radford PJ, Stillwell PC, Blue B, et al. Aspiration complicating bronchopulmonary dysplasia. Chest. 1995;107:185–188. [DOI] [PubMed] [Google Scholar]
- 13.Poets CF, Roberts RS, Schmidt B, et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. 2015;314:595–603. [DOI] [PubMed] [Google Scholar]
- 14.Jiyong J, Tiancha H, Huiqin W, et al. Effect of gastric versus post-pyloric feeding on the incidence of pneumonia in critically ill patients: Observations from traditional and bayesian random-effects meta-analysis. Clin Nutr. 2013;32:8–15. [DOI] [PubMed] [Google Scholar]
- 15.Lyons K, Brilli N, Wieman R, et al. Continuation of transpyloric feeding during weaning of mechanical ventilation and tracheal extubation in children: A randomized controlled trial. J Parenter Enteral Nutr. 2002;26:209–213. [DOI] [PubMed] [Google Scholar]
- 16.Watson J, McGuire W. Transpyloric versus gastric tube feeding for preterm infants. Cochrane Database Syst Rev. 2013;2:CD003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malcolm WF, Smith PB, Mears S, et al. Transpyloric tube feeding in very low birthweight infants with suspected gastroesophageal reflux: Impact on apnea and bradycardia. J Perinatol. 2009;29:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misra S, Macwan K, Albert V. Transpyloric feeding in gastroesophageal-reflux–associated apnea in premature infants. Acta Paediatrica. 2007;96:1426–1429. [DOI] [PubMed] [Google Scholar]
- 19.Wallenstein M, Brooks C, Kline T, et al. Early transpyloric vs gastric feeding in preterm infants: A retrospective cohort study. J Perinatol. 2019;39:837–841. [DOI] [PubMed] [Google Scholar]
- 20.Jadcherla S, Slaughter J, Stenger M, et al. Practice variance, prevalence, and economic burden of premature infants diagnosed with GERD. Hosp Pediatr. 2013;3:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalaf MN, Porat R, Brodsky NL, et al. Clinical correlations in infants in the neonatal intensive care unit with varying severity of gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2001;32:45–49. [DOI] [PubMed] [Google Scholar]
- 22.Duan N, Kravitz RL, Schmid CH. Single-patient (n-of-1) trials: A pragmatic clinical decision methodology for patient-centered comparative effectiveness research. J Clin Epidemiol. 2013;66:S21–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt G, Zhang Y, Jaeschke R, et al. N-of-1 Randomized Clinical Trials In: Guyatt G, Rennie D, Meade MO, et al. eds. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice, 3rd ed. New York, NY: McGraw-Hill; 2015. [Google Scholar]
- 24.Guyatt GH, Heyting A, Jaeschke R, et al. N of 1 randomized trials for investigating new drugs. Control Clin Trials. 1990;11:88–100. [DOI] [PubMed] [Google Scholar]
- 25.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed SJM, Rich W, Finer NN. The effect of averaging time on oximetry values in the premature infant. Pediatrics. 2010;125:e115–e121. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 29.Martin RJ, Wang K, Köroğlu Ö, et al. Intermittent hypoxic episodes in preterm infants: Do they matter? Neonatology. 2011;100(3):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farhath S, He Z, Nakhla T, et al. Pepsin, a marker of gastric contents, is increased in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatrics. 2008;121:e253–e259. [DOI] [PubMed] [Google Scholar]
- 31.Singer L, Martin RJ, Hawkins SW, et al. Oxygen desaturation complicates feeding in infants with bronchopulmonary dysplasia after discharge. Pediatrics. 1992;90:380–384. [PMC free article] [PubMed] [Google Scholar]
- 32.Eichenwald EC, AAP Committee on Fetus and Newborn. Diagnosis and management of gastroesophageal reflux in preterm infants. Pediatrics. 2018;142e20181061. [DOI] [PubMed] [Google Scholar]
- 33.Rosen R, Hart K, Warlaumont M. Incidence of gastroesophageal reflux during transpyloric feeds. J Pediatr Gastroenterol Nutr. 2011;52:532–535. [DOI] [PubMed] [Google Scholar]
- 34.Omari T, Davidson G. Multipoint measurement of intragastric pH in healthy preterm infants. Arch Dis Childhood Fetal Neonatal Ed. 2003;88:F517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.