Abstract
Dendritic cells (DCs) play a key role in the initiation of an immune response and are known as “professional” antigen-presenting cells because of their ability to activate naïve T cells. A widely used method to generate DCs in vitro is to culture bone marrow cells, or blood monocytes in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4. Here we show that a small population of NK cells residing in the bone marrow of RAG−/−, but not RAG−/− γc chain−/− mice, remain in the DC culture and is the source of IFN-γ produced after stimulation with Lipopolysaccharide (LPS). These cells, which may represent early promoters of LPS-induced responses, have to be taken into account when interpreting experiments using bone marrow-derived DCs.
Introduction
Dendritic cells (DCs) are bone marrow-derived myeloid cells considered “professional” antigen-presenting cells (1) with the unique ability to induce primary immune responses by activating naïve T cells (2, 3). They are used extensively both in fundamental research on the immune system, and in clinical protocols (4). Because of the difficultly in isolating DCs from tissues, much of the current knowledge about them has been gained using DC generated in vitro from both humans and mice (3, 5, 6). The most common method to generate mouse DCs is to differentiate bone marrow (BM) cells into bone marrow-derived DCs (BMDCs) by culturing them with granulocyte-macrophage colony-stimulating factor (GM-CSF), an important cytokine for in vitro DC development (7, 8). Most investigators obtain BM cells from normal mice, which are known to harbor B and memory T cells (9–11). BMDCs that are differentiated under these conditions are exposed to T cells or their product(s) during the in vitro cultures. In previous studies, we found that the presence of such T cells in these cultures has functional consequences, as they can influence IL-12 production by BMDCs when stimulated with LPS (12). Thus, to eliminate the possibility of T cell contribution in DC cultures, we generally use BM cells from RAG−/− mice to generate DCs (12–14).
BMDC populations have previously been shown to include macrophages, in addition to dendritic cells (7, 8, 15, 16). Therefore, the effector molecules that are produced by such cultures after stimulation could be attributed to either one of these two cell populations. For example, the inflammasome activation and IL-1β secretion that occur after stimulating BMDCs with LPS was previously attributed to DCs. However, a recent study has been shown that IL-1β secretion occurs in the macrophages found in DC cultures (17). In the process of exploring the origin of IFN-γ produced during TH1 type responses, we found a difference between LPS-stimulated BMDC cultures established from normal mice versus those deficient in the common gamma chain of the IL-2 receptor (IL-2Rγc). Because mice deficient in IL-2Rγc lack natural killer (NK) cells, we asked if the difference might be due to a population of NK cells in the BMDC cultures. Using flow cytometry and a single-cell ELISPOT assay, we show here that a small population of NK1.1+NKp46+ cells residing in the BM of RAG−/− but not double mutant RAG−/− γC−/− mice is a source of IFN-γ when LPS is added to DCs obtained from GM-CSF and IL-4 cultures. Remarkably, these NK cells survive the in vitro differentiation conditions used to generate DCs and retain their ability to produce IFN-γ. Based on these observations, the functional contribution of NK cells should be taken into account when cultured BMDCs are used.
Materials and Methods
Mice
All studies were carried out and approved in accordance with the Institutional Animal Care and Use Committee (IACUC) of the NIH, an ALAAC approved facility. Adult 8–14-week-old male and female C57BL/6J and C57BL/6-IFN-γ−/− (Jackson Laboratory), C57BL/6-Rag2−/− γC−/− double knockout, B10.A Rag2−/− and C57BL/6J RAG1−/− mice were generated at the NIAID/Taconic Farms, Inc. These mice can be purchased from the NIAID/Taconic Farms, Inc. exchange.
Media and Reagents
Bacterial LPS was from Escherichia (E.) coli Serotype 0127: B8. Bovine Serum Albumin (BSA) 30% solution was from Sigma. Recombinant mouse GM-CSF and IL-4 were purchased from Peprotech Inc. Culture medium used throughout was Iscove’s Modified Dulbecco’s Medium (IMDM: GibcoBRL), which consisted with 10% heat-inactivated Fetal Bovine Serum (FBS: tested to be free of endotoxin, mycoplasma, virus and bacteriophage GibcoBRL), plus 2 mM L-glutamine, 55 μM 2β-Mercaptoethanol (GibcoBRL), penicillin, streptomycin and gentamicin (Biosource). The following antibodies were purchased from Biolegend: anti-mouse NK1.1-biotin (PK136), anti-NKp46-biotin (29A1.4), anti-CD49b-biotin (DX5), anti-IFN-γ BV421 (XMG1.2), anti-CD11c BV605 (N418), anti-NKp46 PerCp-Cy5.5 (29A1.4), anti-NK1.1 APC (PK136), anti-CD19 AF700 (6D5), and anti-CD16/32 (LEAF clone 93). Zombie Aqua was used to exclude dead cells.
Generation of bone marrow derived DCs (BMDC)
Generation of bone marrow derived DCs was done as described previously, (12). Briefly, bone marrow cells were flushed out of the femurs and tibias of 8–12-week-old mice into complete medium and pipetted vigorously to make a single cell suspension, then passed through a cell strainer (70-μm Nylon mesh; BD Falcon ™). Erythrocytes were lysed using ACK lysis buffer (BioSource) and cells washed twice with complete medium. At this point, BM cells were either cultured immediately or cryopreserved for later use. BM cells were cultured at 1×106 cells/well in a 24-well plate in a final volume of 2 ml with complete medium supplemented with 6 ng/ml (30 U/ml) GM-CSF and 3 ng/ml (30 U/ml) IL-4. Starting at the 3rd day of culture, half of the medium was replaced each day with fresh pre-warmed medium supplemented with GM-CSF and IL-4. Loosely adherent cells were harvested on day 6, washed twice with cold complete medium, and used as BMDCs.
Cytokine measurements
The concentration of IFN-γ in the cell-free medium culture supernatant (CSN) was determined by Aushon Biosystems (Billerica, MA) using the SearchLight Multiplex cytokine array, via a service contract.
IFN-γ ELISPOT
Mouse IFN-γ ELISpotPLUS kit was from MABTECH (3321–4APT-10, Cincinnati, OH), and the manufacturer’s guidelines were used for all experiments. Briefly, BM cells or BMDCs were incubated overnight at 1×105 cells/well (or as indicated) in the presence or absence of 1 μg/ml LPS at 37°C 18–20 h in a final volume of 200 μl/well. The plates were washed 4 times with PBS with using a squirt bottle. The spots were enumerated by ZellNet Consulting Inc (FortLee, NJ).
Magnetic Depletion of NK cells
BM cells from RAG−/− mice were washed twice with cold PBS plus 0.5% BSA (PBS/BSA) and resuspended at 1×107 per 1 ml of PBS/BSA. The Fc receptors were blocked with 5 μg/ml of anti-CD16/32 for 20 min on ice. 125 μl of a cocktail of biotinylated anti-NK antibodies (NK1.1, NKp46 and DX5) was then added to the cells and kept on ice for 30 min with occasional mixing. Cells were washed with 14 ml PBS/BSA and resuspended in 1 ml PBS/BSA. Magnetic separation was accomplished using 9×107 per ml of Dynal beads in a final volume of 2 ml in the cold at 4°C room on a rotor mixer for 60 min before magnetic separation. After magnetic removal of bound cells, unbound cells were washed twice with complete medium and cultured as described for BMDCs.
Flow Cytometry Analysis and Intracellular Staining for IFN-γ
Fresh BM cells and BMDCs were stimulated with 200 ng/ml LPS for a total of 4 h at 37°C. After 1 h, Brefeldin A (BD Biosciences) was added at 5 μg/ml, and then the cells were incubated an additional 3 h. Fc receptors were then blocked by incubation with anti-mouse CD16/32 (eBioscience) for 20 min on ice. Cells were labeled with Zombie Aqua Fixable Viability dye for exclusion of dead cells (Biolegend). Following surface staining for NKp46, NK1.1, CD19, and Gr-1, cells were washed, treated with FoxP3/Transcription Factor Fixation/permeabilization Buffer (eBioscience) for 30 min on ice, and then washed twice with BD Perm/wash buffer. Cells were stained for intracellular IFN-γ using XMG1.2 antibody for 30 min on ice. Flow cytometry data were collected on an LSR-II, and the data were analyzed using Flow Jo v9 (FLowJo, LLC).
Statistical analysis
Statistical analyses Student’s t-test and analysis of variance (ANOVA) were performed using Prism.
Results
Having found in the past that antigen-activated T cells can stimulate BMDCs to produce IL-12 leading to the production of IFN-γ (12, 14), we were surprised when a comparison of normal (WT) and RAG−/− mice showed that LPS-stimulated BMDCs from RAG−/− mice still harbored cells that produce IFN-γ. To determine the number of cells in a BMDC culture that produce IFN-γ in response to LPS, we used a sensitive ELISPOT assay that can detect single IFN-γ-producing cells (Figure 1A). The frequency of these IFN-γ-producing cells varied from culture to culture, but on average, it was about 1 out of 2500 BMDCs (Figure 1B). In contrast, IFN-γ-producing cells were completely absent from BMDCs generated from BM of RAG−/− γC−/−, and from IFN-γ−/− mice (Figure 1C). The magnitude of IFN-γ production at the single cell level was very heterogeneous among the cells secreting IFN-γ in the BMDC cultures (Figure 1D). A few cells made a very high amount of IFN-γ, some cells made intermediate amounts, and the majority of cells produced a small amount.
Figure 1. BMDCs obtained from RAG−/− mice harbor cells that produce IFN-γ.
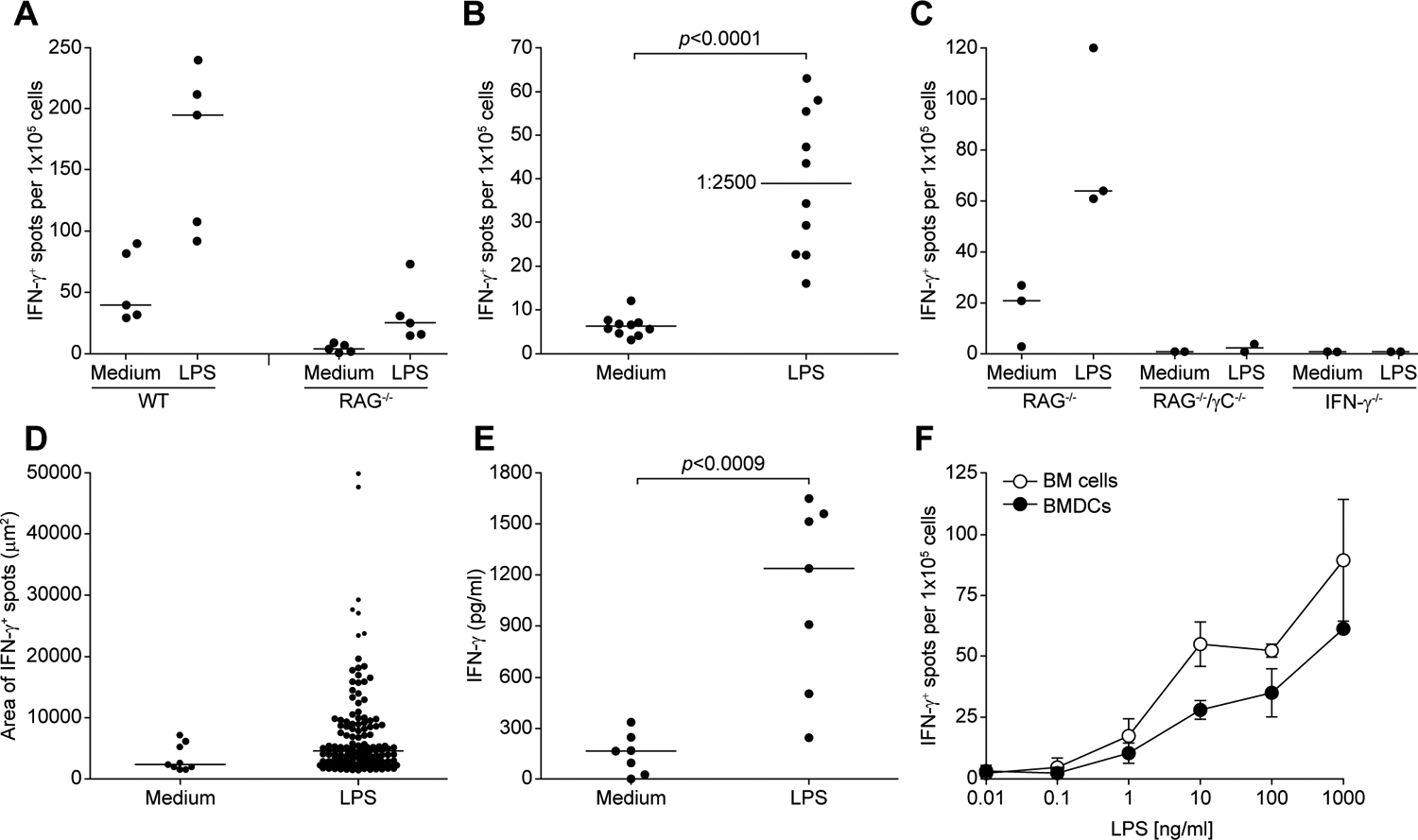
(A) BMDCs were added to a 96-well plate ELISPOT at 1×105 cells/well and incubated in the presence of absence of 1 μg/ml LPS for 18–20 h at 37°C, and IFN-γ−secreting cells were enumerated as described in materials and methods. The data represent five independent experiments. (B) BMDCs from RAG−/− mice were treated as in (A). Each dot represents an independent experiment. The median is shown as a bar and corresponds to frequency of one IFN-γ-secreting cell per 2500 cells in the BMDC culture. Statistical significance was assessed by Student’s t test. (C) BMDCs generated from RAG−/−, RAG−/−/γC−/−, and IFN-γ−/− mice were analyzed as in (A). Each dot represents an independent experiment calculated from triplicate wells. (D) The area of each IFN-γ spot in the ELISPOT from a well seeded with 1×105 BMDCs was measured. (E) BM cells from RAG−/− mice were cultured at 1×106 cells/well in a 24-well plate with GM-CSF and IL-4 for 6 days, and were stimulated with 200 ng/ml LPS for 18–20 h. Culture supernatant was tested for the presence of IFN-γ. Each dot represents an independent experiment. Statistical significance was assessed by Student’s t test. (F) Freshly isolated BM cells and BMDCs from RAG−/− mice were compared after stimulation with different concentrations of LPS. Data represent mean ± SD of triplicate wells from one representative experiment.
Thus, a non-T-cell capable of secreting IFN-γ is present in cultures routinely used to generate DCs (Figure 1E). To determine if these IFN-γ producers are generated in the cultures (perhaps from bone marrow stem cells) or whether they pre-exist in the bone marrow, we compared the number of LPS-stimulated IFN-γ-producers in fresh BM cells and BMDC cultures from RAG−/− mice. A similar number of cells were found in BM cells and BMDC cultures, which had similar response to various doses of LPS (Figure 1F). It is possible that these IFN-γ-producing cells in the BM can survive culture conditions used to generate DCs.
The nature of the IFN-γ producing cells in the BMDCs, and the contribution of NK cells as a source of IFN-γ production were examined by multicolor flow cytometry and intracellular staining for IFN-γ. We isolated BM cells from RAG−/− mice and compared freshly isolated BM cells with BMDCs generated by culture with GM-CSF and IL-4. We gated on NK1.1+NKp46+ cells and measured intracellular IFN-γ in cells that had been stimulated with LPS or medium alone. Approximately 10% of ex-vivo BM NK cells (Figure 2A top panel) and 30% of NK cells in the BMDC cultures (Figure 2A bottom panel) were positive for IFN-γ. Consistent with the ELISPOT data (Figure 1D), the mean fluorescence intensity of intracellular IFN-γ was heterogeneous among IFN-γ+ cells in both BM cells and BMDCs (Figures 2A top panel and bottom panel). The proportion of NKp46+NK1.1+ cells that produce IFN-γ in several experiments is shown in Figure 2B. Indeed, IFN-γ producing cells were detected only in NK1.1+NKp46+ but not in NK1.1−NKp46− cell population (Supplemental Fig. 1). There are different methods both in mouse and human to generated DCs in vitro. Some investigators use GM-CSF alone (7) and others use GM-CSF plus IL-4 (6). We have adapted a method to use GM-CSF plus IL-4 to generate BMDCs because we had found that the addition of IL-4 to these cultures reduces the number of macrophages, which this finding has been corroborate in recent report (16). Therefore, to test the role of IL-4 in the generation or survival of NK cells in BMDCs, we used two different strains of RAG−/− mice (B6 and B10.A) using a low and a higher dose of GM-CSF in the absence of IL-4. We found that IFN-γ producing cells still present in BMDC cultures generated in the presence of GM-CSF alone. Although, the frequency of IFN-γ producing cells in GM-CSF plus IL-4 cultures was 3–4-fold higher when stimulated with LPS when compared with BM cells cultured in GM-CSF alone, in addition, it appeared that IL-4 can stimulate IFN-γ production from NK cells in absence of LPS (Supplemental Fig. 2). These data suggest that IL-4 is not required for the generation or maintenance of NK cells in BMDC cultures.
Figure 2. IFN-γ production by NK cells in RAG−/− BMDCs.

(A) Fresh BM cells (top panels) and 6-day-old BMDCs (lower panels) from RAG1−/− mice were stimulated with 200 ng/ml LPS for 4 h with 5μg/ml Brefeldin A included for the last 3 h. Dot plots depict intracellular IFN-γ expression in live 7AAD−CD19−Gr-1−NK1.1+NKp46+ cells. IFN-γ expression was not detected in 7AAD− NK1.1−NKp46−CD19−Gr-1− gated cells within the same samples (Supplemental Fig. 1). (B) The proportion of NKp46+NK1.1+ cells that produce IFN-γ was determined in 3 independent experiments. Statistical significance was assessed by one-way ANOVA. (C) Comparison of the number of IFN-γ forming spots in wells seeded with 1×105 BMDCs that were obtained from RAG−/− BM cells (No depletion) or RAG−/− BM cells that had been depleted of NK cells (NK-depletion) before culture in GM-CSF and IL-4. At day 6, BMDCs were stimulated with 200 ng/ml LPS for 18–20 h at 37°C. These data are expressed as the mean ± SD composed of three independent experiments. Statistical significance was assessed by one-way ANOVA with Sidak-adjusted comparisons.
To confirm that NK cells are a source of IFN-γ in the BMDC cultures, and to test whether IFN-γ−producing-NK cells were generated in the BMDC cultures or may have persisted as NK cells already present in BM, NK cells were depleted from RAG−/− BM cells using antibodies to NK cell markers and magnetic-depletion. NK-depleted BM cells cultured in GM-CSF and IL-4 for six days to generate BMDCs had very small numbers of IFN-γ+ cells in response to LPS, which were comparable to the numbers of IFN-γ+ cells observed in the absence of LPS stimulation of both NK-depleted and undepleted BMDCs (Figure 2C).
Discussion
Some of the knowledge of DC immunobiology has been derived from studies using in vitro differentiation of mouse bone marrow cells with GM-CSF in the presence or absence of IL-4. Such BMDC cultures are, however, heterogeneous. In addition to DCs, they include other myeloid cells such as granulocytes and macrophages (7, 16). Our present work has uncovered a previously unappreciated presence of NK cells in BMDC cultures. Given that such cultures, which have been a useful tool to generate large number of DCs from BM cells do not represent physiological conditions, we have not investigated how NK cells persist under such conditions. Instead, in view of the IFN-γ produced by NK cells after LPS addition to BMDCs, we recommend that BM cells be depleted of NK cells prior to culture in GM-CSF and IL-4 in order to obtain BMDCs devoid of potentially confounding IFN-γ producing cells.
These NK1.1+ IFN-γ producing cells were observed in BMDCs generated from bone marrow cells of RAG−/− mice, but not mice that lack both RAG and the γc chain that is essential for survival of NK cells through stimulation by IL-15 or IL-2. The original study of RAG−/− mice demonstrated that the spleen of these mice lack T and B cells, but had increased numbers of NK cells compared with WT mice (18). Furthermore, in addition to their increased numbers, NK cells in RAG−/− mice have enhanced cytotoxic activities on a per cell basis (19). Doubly deficient RAG−/−/γC−/− lack not only T and B cells, but also NK cells (20), and have normal DC development (21). Therefore, IFN-γ producing cells in RAG−/− BMDC cultures were likely NK cells since only the NK1.1+NKp46+ but not NK1.1−NKp46− cells shown to make IFN-γ. A simple depletion of NK cells from BM cells using standard antibodies to NK cell markers, prior to culture in GM-CSF and IL-4, eliminated the presence of cells that produced IFN-γ in response to LPS. This depletion is advisable considering that IFN-γ production by NK cells in standard BMDC cultures could influence the response of DCs and other myeloid cells.
Supplementary Material
Acknowledgments
We thank Jeff Skinner for helping with statistical analysis, Joanna Ireland, Elina Stregevsky for help with flow cytometry and Alan Hoofring and Ethan Tyler for data illustrations.
Footnotes
Conflict of interest
The authors have no financial conflict of interest.
The Intramural Research Program at the NIH, NIAID supported this work.
References
- 1.Lassila O, Vainio O, and Matzinger P. 1988. Can B cells turn on virgin T cells? Nature 334: 253–255. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Turley SJ, and Steinman RM. 1998. Antigen processing for amateurs and professionals. Trends Cell Biol 8: 231–237. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, and Palucka K. 2000. Immunobiology of dendritic cells. Annu Rev Immunol 18: 767–811. [DOI] [PubMed] [Google Scholar]
- 4.Palucka K, Banchereau J, and Mellman I. 2010. Designing vaccines based on biology of human dendritic cell subsets. Immunity 33: 464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM 1991. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 9: 271–296. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, and Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179: 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, and Steinman RM. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176: 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caux C, Dezutter-Dambuyant C, Schmitt D, and Banchereau J. 1992. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature 360: 258–261. [DOI] [PubMed] [Google Scholar]
- 9.Di Rosa F, and Santoni A. 2002. Bone marrow CD8 T cells are in a different activation state than those in lymphoid periphery. Eur J Immunol 32: 1873–1880. [DOI] [PubMed] [Google Scholar]
- 10.Becker TC, Coley SM, Wherry EJ, and Ahmed R. 2005. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol 174: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 11.Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, Silberstein LE, and von Andrian UH. 2005. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity 22: 259–270. [DOI] [PubMed] [Google Scholar]
- 12.Abdi K, Singh N, and Matzinger P. 2006. T-cell control of IL-12p75 production. Scand J Immunol 64: 83–92. [DOI] [PubMed] [Google Scholar]
- 13.Abdi K, Singh NJ, and Matzinger P. 2012. Lipopolysaccharide-activated dendritic cells: “exhausted” or alert and waiting? J Immunol 188: 5981–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdi K, Laky K, Padhan K, Petrovas C, Skinner J, Kabat J, Dorward DW, Brzostowski J, Long EO, Trinchieri G, and Varma R. 2018. Cutting Edge: Quantitative Determination of CD40L Threshold for IL-12 and IL-23 Production from Dendritic Cells. J Immunol 201: 2879–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santiago-Schwarz F, Belilos E, Diamond B, and Carsons SE. 1992. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukoc Biol 52: 274–281. [PubMed] [Google Scholar]
- 16.Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, and Reis e Sousa C. 2015. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity 42: 1197–1211. [DOI] [PubMed] [Google Scholar]
- 17.Erlich Z, Shlomovitz I, Edry-Botzer L, Cohen H, Frank D, Wang H, Lew AM, Lawlor KE, Zhan Y, Vince JE, and Gerlic M. 2019. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat Immunol 20: 397–406. [DOI] [PubMed] [Google Scholar]
- 18.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, and et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68: 855–867. [DOI] [PubMed] [Google Scholar]
- 19.Karo JM, Schatz DG, and Sun JC. 2014. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell 159: 94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colucci F, Soudais C, Rosmaraki E, Vanes L, Tybulewicz VL, and Di Santo JP. 1999. Dissecting NK cell development using a novel alymphoid mouse model: investigating the role of the c-abl proto-oncogene in murine NK cell differentiation. J Immunol 162: 2761–2765. [PubMed] [Google Scholar]
- 21.Ohteki T, Suzue K, Maki C, Ota T, and Koyasu S. 2001. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol 2: 1138–1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


