Abstract
Herein, a novel method for carbon-11 labeling of acyl sulfonamides by a one-step insertive [11C]CO carbonylative cross-coupling reaction between aryl halides and sulfonamides is presented. Various model compounds as well as drug molecules LY573636 (tasisulam) and ABT-199 were obtained in excellent yields. This method provides a valuable and widely applicable contribution to the continuously expanding radiochemical toolbox for PET research.
Positron emission tomography (PET), an imaging technique that allows for quantitative visualization of biological processes at a molecular level in vivo at high spatial and temporal resolution, is widely used in clinical diagnosis, biomedical research and drug development.1–3 PET depends on the production of radiopharmaceuticals, which are generally biologically active molecules (e.g., ligands, inhibitors, substrates) labelled with PET isotopes such as carbon-11 (t1/2 = 20.3 minutes) and fluorine-18 (t1/2 = 109.8 minutes).4 Carbon-11 is an especially attractive radioisotope for radiolabelling small molecules, since carbon is widely present in drug-like compounds. The shorter half-life, compared to fluorine-18, also offers a lower patient radiation burden which then allows the possibility for multiple PET studies to occur within the same day for a single subject. On the contrary, the short half-life of carbon-11 also presents a challenge for the radiochemist to complete the radiochemistry and use the radiolabelled compounds within a shorter timeframe.5,6 Continuous improvement and expansion of the radiochemical toolbox are essential to empower the radiochemist to make more compounds available for radiolabelling.
A common and versatile radioactive synthon in carbon-11 chemistry is [11C]CO, typically obtained in high yields via online heterogeneous catalytic reduction of cyclotron-produced [11C]CO2 over heated zinc or molybdenum, although other methods are available as well.7–10 [11C]CO has been historically used in insertive transition metal-mediated cross-coupling reactions for the synthesis of carbon-11 labelled amides, esters, carboxylic acids, aldehydes, ketones, carbamates and ureas, among others.11,12 More recently, various techniques that allow carbon-11 carbonylations to be executed at atmospheric pressure have emerged.13–15 The resulting decreased demand for highly specialized and expensive high-pressure synthesis equipment has made [11C]CO chemistry accessible to more PET centres worldwide.16,17 However, to the best of our knowledge, [11C]CO carbonylation chemistry has not been applied for the synthesis of acylsulfonamides. As a well-known carboxylic acid isostere,18 acylsulfonamides present a common motif in organic and medicinal chemistry and have been applied in development of inhibitors of hepatitis C virus NS3/4A protease inhibitors, inhibitors of the sodium channel blocker NaV1.7 used in pain management, angiotensin II inhibitors, antiproliferative agent LY573636, hereafter referred to as tasisulam, anti-apoptotic Bcl-2 proteins inhibitors amongst others.19–21 To the best of our knowledge, only one example of a carbon-11 labelled acyl sulfonamide exists (Fig. 1).22 This carbon-11 labelled angiotensin II inhibitor was synthesized in a three step procedure including preparation and purification of carbon-11 benzoyl chloride and subsequent reaction with a sulfonamide precursor molecule.22 Although impressive by itself and successful for this specific compound, such a challenging radiosynthetic strategy results in unreliable production runs and thus hampers efforts towards clinical translation. In fact, analogues of the compound have been developed since that allow for more straightforward one-step methylation procedure using [11C]CH3I.23 The synthesis of isotopically non-enriched sulfonamides using Mo(CO)6 as a solid carbon source in combination with microwave heating has previously been reported.24
Fig. 1.

Synthesis of carbon-11 labelled sulfonamides via (A) multistep synthesis via a Grignard reagent to obtain the carbon-11 carboxylic acid, conversion to acyl chloride and subsequent condensation reaction towards acyl sulfonamide22 and (B) direct synthesis of carbon-11 acyl sulfonamides via insertive transition metal mediated carbonylation reaction (this work). The position of the carbon-11 label is depicted with *.
The aim of the current study was to develop an efficient, reliable and broadly applicable method for the radiosynthesis of carbon-11 labelled sulfonamides to provide a valuable contribution to the radiochemical toolbox. Keeping the limitations of carbon-11 chemistry in mind, the envisioned method is focusing on a short reaction time (5 min), low reagent concentrations compatible with semi-preparative HPLC purification of crude reaction mixtures, and broad applicability by using conventional radiochemistry equipment towards efficient synthesis of carbon-11 labelled acylsulfonamides.
Initially, a small series of acyl sulfonamides was prepared according to a previously described literature procedure, i.e., a microwave assisted palladium catalyzed carbonylation reaction between aryl halides and sulfonamides with Mo(CO)6 as source of carbon monoxide (Fig. 2).24 All compounds were obtained in good yields, with the exception of 4-nitro-N-tosylbenzamide 9 (no product formed). Compound 9, however, was eventually obtained in acceptable yield by reacting nitrobenzoyl chloride with toluene-sulfonamide. The obtained acylsulfonamides were characterized by 1H, 13C-NMR, and mass spectrometry and used as HPLC reference standards for subsequent radiochemical reactions.
Fig. 2.

Overview of model acyl sulfonamides prepared in this work (see ESI† for experimental conditions).
For efficiently conducting the radiochemical reactions described herein, a recently reported [11C]CO dispensing system was used25 which resulted in a 10-fold increase in efficiency compared to traditional methods. Radiochemical reaction conditions were derived from conditions used for synthesis of unlabelled acylsulfonamide,24 with modifications suitable for carbon-11 chemistry. In contrast to the 15 minute reaction time for unlabelled chemistry reactions, a five minute reaction time was chosen to minimize product loss due to the rapid decay of carbon-11 (t1/2 20.3 minutes). Reactant and reagent amounts were drastically reduced (for sulfonamides and aryl halides 20 μmol vs. 0.40 mmol), to allow for rapid one-step purification of products by means of semi-preparative HPLC. In addition, THF was selected as a solvent, as this is the generally preferred solvent in carbon-11 carbonylation reactions.13,14 Finally, microwave heating was substituted for conventional heating to increase the applicability to PET centres where microwave systems are not available within the radiochemical facility.
Initially, the feasibility of [11C]CO carbonylation chemistry towards synthesizing acylsulfonamides was explored by preparing [11C]1. Using Pd(OAc)2 as the catalyst (entry 1, Table 1), a quantitative [11C]CO trapping efficiency was observed, corresponding well with a previous report using xantphos as the supporting ligand.14 Importantly, the radiochemical purity after reaction was 80%, demonstrating for the first time the synthesis of acylsulfonamides by [11C]CO carbonylation chemistry. A rapid screen using several commonly used catalysts showed that Pd(PPh3)2Cl2 was the optimal catalyst for this particular reaction (entry 3), resulting in [11C]1 in 97% radiochemical yield. Use of Herrmann’s palladacycle22 resulted in the lowest trapping efficiencies (71%), whereas Pd2dba3 gave the lowest radiochemical purity (56%).
Table 1.
Screening of various palladium sources and solvents for the formation of [11C]1
 | |||||
|---|---|---|---|---|---|
| Entry | Pd-Source | Solvent | TE (%) | RCP (%) | RCY (%) |
| 1 | Pd(OAc)2 | THF | 99.9 ± 0 | 80.4 ± 3 | 80.4 ± 3 |
| 2 | Pd2dba3 | THF | 99.5 ± 1 | 56.3 ± 2 | 56.0 ± 1 |
| 3 | PdCl2(PPh3)2 | THF | 99.9 ± 0 | 97.0 ± 3 | 97.0 ± 3 |
| 4 | Palladacycle | THF | 71.2 ± 2 | 64.7 ± 11 | 45.9 ± 7 |
| 5 | Pd(PPh3)4 | THF | 99.9 ± 0 | 93.0 ± 2 | 92.9 ± 2 |
| 6 | [(Cinnamyl)PdCl]2 | THF | 99.3 ± 1 | 96.9 ± 0 | 96.2 ± 1 |
| 7 | PdCl2(PPh3)2 | Xylene | 98.0 ± 3 | 94.0 ± 1 | 92.1 ± 1 |
| 8 | PdCl2(PPh3)2 | Toluene | 99.9 ± 0 | 94.7 ± 2 | 94.6 ± 2 |
| 9 | PdCl2(PPh3)2 | Chlorobenzene | 91.5 ± 4 | 79.8 ± 2 | 71.8 ± 5 |
| 10 | PdCl2(PPh3)2 | DCM | 75.1 ± 16 | 77.0 ± 11 | 57.6 ± 13 |
| 11 | PdCl2(PPh3)2 | Dioxane | 64.1 ± 23 | 86.0 ± 7 | 56.9 ± 23 |
| 12 | PdCl2(PPh3)2 | EtOAc | 95.9 ± 3 | 99.0 ± 0 | 94.9 ± 3 |
| 13 | PdCl2(PPh3)2 | DMF | 84.0 ± 8 | 68.2 ± 2 | 57.3 ± 6 |
| 14 | PdCl2(PPh3)2 | DMSO | 85.6 ± 1 | 74.7 ± 7 | 64.1 ± 6 |
| 15 | PdCl2(PPh3)2 | MeCN | 96.5 ± 3 | 92.7 ± 1 | 89.5 ± 4 |
TE: trapping efficiency; RCP: radiochemical purity; RCY: radiochemical yield. Results are expressed as average standard deviation (n = 3). The position of the carbon-11 label is depicted with *.
Continuing with Pd(PPh3)2Cl2 as the optimized catalyst, various solvents ranging from non-polar solvents such as xylene and dichloromethane to polar solvents like acetonitrile, DMSO and DMF were screened (entries 7–15) and generally resulted in high radiochemical yields of [11C]1 between 57–95%. Surprisingly, despite the moderate solubility of the reagents in toluene and xylene, these solvents provided both high trapping efficiencies as well as high radiochemical purities. The polar solvent DMSO and DMF resulted in low yields (57%), whereas MeCN, also a polar solvent, resulted in considerably higher yield (90%). Overall, no correlation between trapping efficiency, radiochemical purity or radiochemical yield and solvent polarity was found. The high radiochemical purity using chlorobenzene as solvent (80%) demonstrated the negligible reactivity of aryl chlorides vs. aryl iodides. The highest radiochemical yield in this solvent screen for this particular reaction were found using THF (97.0 ± 2.8, entry 3).
Next, a series of both aryl halides and sulfonamides were subjected to the optimized reaction conditions (entry 3 in Table 1). First, the aryl halide was varied (Table 2, entries 1–10). All compounds were prepared in high radiochemical yields (62–99%). Both electron donating as well as electron withdrawing groups were tolerated as substituents on the aryl halide. By using both iodo- and bromobenzene (entries 1 and 2) towards [11C]2, a higher reactivity of iodobenzene was demonstrated. The high yielding reaction using 1-bromo-4-iodobenzene (entry 6) confirmed a preference for oxidative addition at iodo-position over the bromo-position, corresponding to the more reactive iodo-functionality. The same effect for aryl bromides over aryl chlorides was demonstrated using 1-bromo-4-chlorobenzene (entry 5). Interestingly, nitro-N-tosylbenzamide was synthesized in good yield (62%), despite the fact that this compound was previously not obtained in this study as a reference standard using non-radioactive carbonylation chemistry.24 Various sulfonamides with either electron donating or electron withdrawing groups showed good reactivity (entries 13–17). Besides the generally high yields, radioHPLC chromatograms of the crude reactions mixtures showed minimal side product formation. Additionally, excess of unreacted precursors and reagents eluted either earlier (sulfonamides) or later (aryl halides) than the desired radiolabelled products. Together, this will facilitate future product purification by decreasing the risk of collecting (radio)chemical contaminations in the final product formulation. Following the optimal reaction conditions, [11C]1 was synthesized and isolated in 66.8 ± 5.3% decay corrected yield (n = 3, calculated from the amount of [11C]CO produced, decay-corrected to end-of-synthesis, non-decay corrected: 33.8 ± 2.7%) in an overall synthesis time of 30.6 ± 2 minutes from end of bombardment (n = 3, see ESI† for HPLC chromatograms of crude reaction mixture and purified [11C]1). Starting with 0.50 GBq of [11C]CO, a total of 0.17 ± 0.013 GBq [11C]1 was obtained with high chemical and radiochemical purities with a molar activity of 20.6 ± 7 GBq μmol−1 at the end-of-synthesis, corresponding to general molar activities obtained in our lab from such short and low-powered irradiations.
Table 2.
Synthesis of various model acyl sulfonamides using [11C]CO carbonylation chemistry
 | |||||
|---|---|---|---|---|---|
| Entry | Product | Aryl halide | TE (%) | RCP (%) | RCY (%) |
| 1 | [11C]2 | Iodobenzene | 98.6 ± 1 | 97.6 ± 3 | 96.2 ± 3 |
| 2 | [11C]2 | Bromobenzene | 70.8 ± 11 | 89.5 ± 4 | 63.4 ± 11 |
| 3 | [11C]3 | 1-Iodo-4-methoxybenzene | 99.9 ± 0 | 98.3 ± 1 | 98.2 ± 1 |
| 4 | [11C]4 | 1-Iodonaphthalene | 82.1 ± 4 | 99.8 ± 0 | 81.3 ± 4 |
| 5 | [11C]5 | 1-Bromo-4-chlorobenzene | 85.1 ± 7 | 95.1 ± 0 | 81.0 ± 6 |
| 6 | [11C]6 | 1-Bromo-4-iodobenzene | 90.1 ± 3 | 99.9 ± 0 | 90.0 ± 3 |
| 7 | [11C]7 | 4-Iodobenzonitrile | 80.7 ± 14 | 98.3 ± 1 | 79.4 ± 14 |
| 8 | [11C]8 | 1-Iodo-4-(trifluoromethyl)benzene | 83.6 ± 6 | 98.5 ± 1 | 82.3 ± 7 |
| 9 | [11C]9 | 4-Iodonitrobenzene | 72.2 ± 1 | 86.5 ± 1 | 62.4 ± 1 |
| 10 | [11C]10 | Ethyl 4-iodobenzoate | 90.0 ± 5 | 93.7 ± 3 | 84.2 ± 4 |
| 11 | [11C]11 | tert-Butyl(4-iodophenyl)carbamate | 97.1 ± 2 | 81.3 ± 1 | 79.0 ± 1 |
| 12 | [11C]l2 | 3-Iodothiophene | 99.6 ± 0 | 99.0 ± 0 | 98.6 ± 0 |
| 13 | [11C]l3 | 4-Iodo-toluene | 98.2 ± 1 | 93.1 ± 1 | 91.5 ± 2 |
| 14 | [11C]14 | 4-Iodo-toluene | 97.7 ± 2 | 75.8 ± 6 | 74.1 ± 8 |
| 15 | [11C]15 | 4-Iodo-toluene | 98.6 ± 1 | 92.7 ± 4 | 91.4 ± 4 |
| 16 | [11C]16 | Bromobenzene | 72.7 ± 8 | 73.6 ± 21 | 57.0 ± 17 |
| 17 | [11C]16 | Iodobenzene | 96.5 ± 3 | 99.5 ± 1 | 96.0 ± 2 |
The position of the carbon-11 label is depicted with *.
To further explore this radiochemistry and expand this methodology to biologically relevant and more complex structures, tasisulam and ABT-199 were selected for radiolabelling (Fig. 3).19,20 Tasisulam is an anticancer compound that has a non-conventional dual mode of action, both inhibiting angiogenesis and mitosis as well as inducing apoptosis via cytochrome c release and caspase activation,19 and has been under investigation in multiple clinical trials to treat various cancers. ABT-199 (also Venetoclax, marketed as Venclexta in the USA and Venclyxto in the EU) is a Bcl-2 inhibitor (IC50: 10 pM) developed by AbbVie that was recently approved by the FDA as a second line treatment of chronic lymphocytic lymphoma or small lymphocytic lymphoma. For both compounds, the only potential position for carbon-11 radiolabelling is the acylsulfonamide carbonyl functionality. Without any further reaction optimization, [11C]tasisulam was obtained in good radiochemical yield (31.1 ± 2.6%, n = 3), despite the multiple aryl halide functionalities present in the starting materials.
Fig. 3.
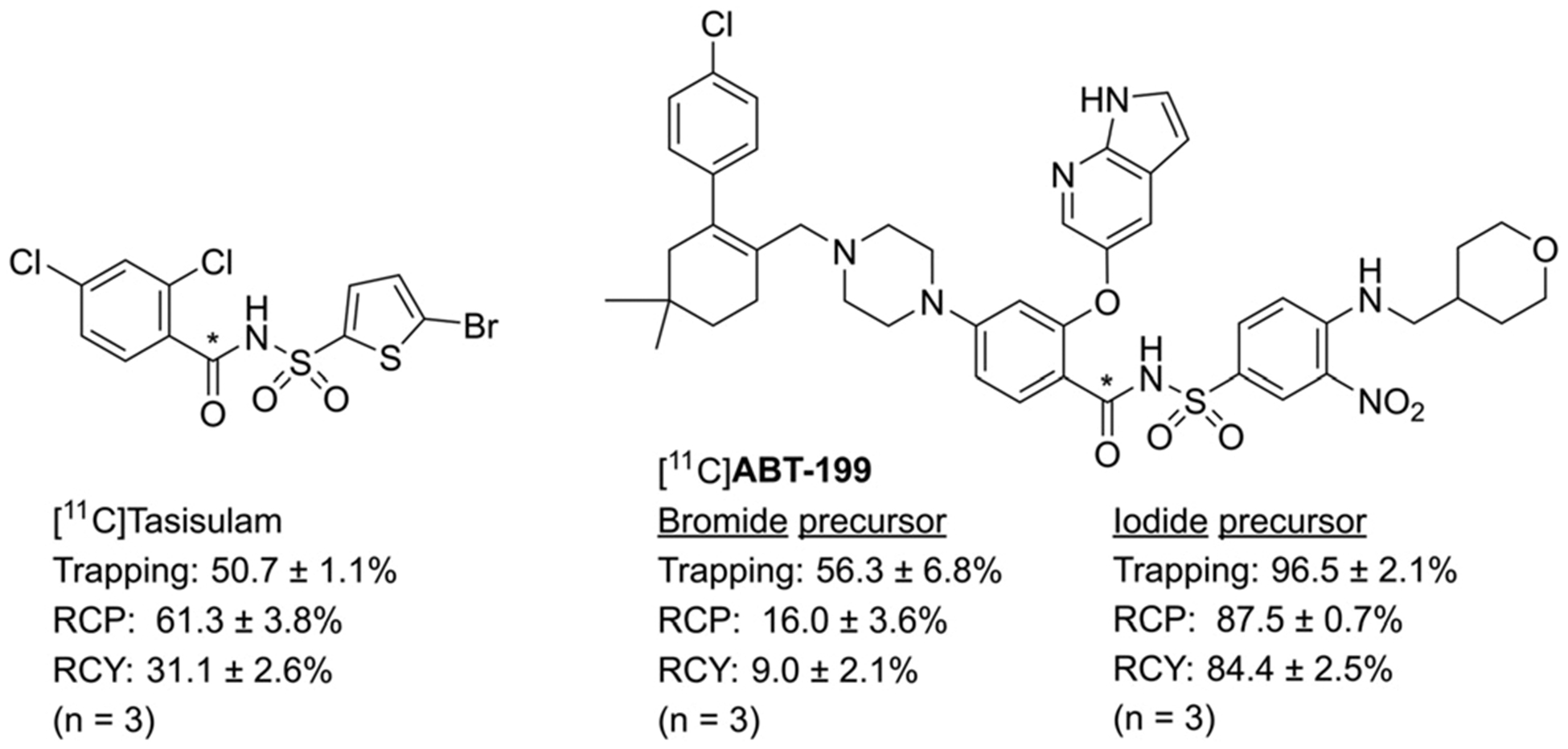
Radiosynthesis and yields of drug molecules [11C]tasisulam and [11C]ABT-199. The position of the carbon-11 label is depicted with *.
The majority of the activity was likely lost as unreacted [11C]CO as the trapping efficiency was only 50.7 ± 1.1%. Towards carbon-11 synthesis of ABT-199, aryl bromide and iodide precursors were prepared based on literature procedures and subjected to [11C]CO carbonylation (see ESI† for experimental conditions). As a result, [11C]ABT-199 was obtained in a moderate yield when using aryl bromide precursor 24 (9.0 ± 2.1%) (n = 3). However, employing aryl iodide precursor 27 the yields were drastically increased (84 ± 3%).
Overall, an efficient, reliable and broadly applicable method for the radiosynthesis of carbon-11 labelled acylsulfonamides by means of palladium mediated [11C]CO carbonylation was developed. The wide substrate scope and broad applicability have been exemplified by the successful synthesis of both [11C]tasisulam and [11C]ABT-199. Despite the fact that no further reaction optimization towards these two specific drug molecules were performed, current radiochemical yields are sufficient for evaluating these compounds in appropriate animal models for studying drug pharmacokinetics towards understanding tumour biology and, potentially future clinical applications. Preclinical studies are currently underway and will be reported in due time.
Supplementary Material
Acknowledgments
This work was achieved with financial support from the NIH (R21 CA205564) and the Ben & Catherine Ivy Foundation.
Footnotes
Electronic supplementary information (ESI) available: Synthesis and character-isation of (labelled) compounds and experimental details. See DOI: 10.1039/c8cc09661a
Conflicts of interest
The authors have no conflict of interest to declare.
Notes and references
- 1.Phelps ME, Proc. Natl. Acad. Sci. U. S. A, 2000, 97, 9226–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones T and Rabiner EA, J. Cereb. Blood Flow Metab, 2012, 32, 1426–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergström M, Grahnén A and Långström B, Eur. J. Clin. Pharmacol, 2003, 59, 357–366. [DOI] [PubMed] [Google Scholar]
- 4.Miller PW, Long NJ, Vilar R and Gee AD, Angew. Chem., Int. Ed. Engl, 2008, 47, 8998–9033. [DOI] [PubMed] [Google Scholar]
- 5.Antoni G, Labelled Compd J. Radiopharm, 2015, 58, 65–72. [DOI] [PubMed] [Google Scholar]
- 6.Tu Z and Mach R, Curr. Top. Med. Chem, 2010, 10, 1060–1095. [DOI] [PubMed] [Google Scholar]
- 7.Welch MJ and Ter-Pogossian MM, Radiat. Res, 1968, 36, 580–587. [PubMed] [Google Scholar]
- 8.Zeisler SK, Nader M, Theobald A and Oberdorfer F, Appl. Radiat. Isot, 1997, 48, 1091–1095. [Google Scholar]
- 9.Taddei C, Bongarzone S, Dheere A and Gee A, [11C]CO2 to [11C]CO conversion mediated by [11C]silanes: a novel route for [11C]carbonylation reactions, Chem. Commun, 2015, 51, 11795–11797. [DOI] [PubMed] [Google Scholar]
- 10.Anders AD, Bongarzone S, Fortt R, Gee AD and Long NJ, Electrochemical [11C]CO2 to [11C]CO conversion for PET imaging, Chem. Commun, 2017, 20, 2982–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Långström B, Itsenko O and Rahman O, J. Labelled Compd. Radiopharm, 2007, 50, 794–810. [Google Scholar]
- 12.Kealey S, Gee A and Miller PW, J. Labelled Compd. Radiopharm, 2014, 57, 195–201. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson J, van den Hoek J and Windhorst AD, J. Labelled Compd. Radiopharm, 2012, 55, 223–228. [Google Scholar]
- 14.Dahl K, Schou M, Amini N and Halldin C, Eur. J. Org. Chem, 2013, 1228–1231. [Google Scholar]
- 15.Kealey S, Miller PW, Long NJ, Plisson C, Martarello L and Gee AD, Chem. Commun, 2009, 3696–3698. [DOI] [PubMed] [Google Scholar]
- 16.Verbeek J, Eriksson J, Syvänen S, Labots M, De Lange ECM, Voskuyl RA, Mooijer MPJ, Rongen M, Lammertsma AA and Windhorst AD, EJNMMI Res., 2012, 2, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahl K, Itsenko O, Rahman O, Ulin J, Sjöberg CO, Sandblom P, Larsson LA, Schou M and Halldin C, J. Labelled Compd. Radiopharm, 2015, 58, 220–225. [DOI] [PubMed] [Google Scholar]
- 18.Ballatore C, Huryn DM and Smith AB, ChemMedChem, 2013, 8, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier T, Uhlik M, Chintharlapalli S, Dowless M, van Horn R, Stewart J, Blosser W, Cook J, Young D, Ye X, Evans G, Credille K, Ballard D, Huber L, Capen A, Chedid M, Ilaria R, Smith MC and Stancato L, Mol. Cancer Ther, 2011, 10, 2168–2178. [DOI] [PubMed] [Google Scholar]
- 20.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH and Elmore SW, Nat. Med, 2013, 19, 202–210. [DOI] [PubMed] [Google Scholar]
- 21.Ammazzalorso A, De Filippis B, Giampietro L and Amoroso R, Chem. Biol. Drug Des, 2017, 90, 1094–1105. [DOI] [PubMed] [Google Scholar]
- 22.Mathews WB, Burns HD, Dannals RF, Ravert HT and Naylor EM, J. Labelled Compd. Radiopharm, 1995, 36, 729–737. [Google Scholar]
- 23.Hamill TG, Burns HD, Dannals RF, Mathews WB and Naylor EM, Appl. Radiat. Isot, 1996, 47, 211–218. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Rönn R, Gossas T and Larhed M, J. Org. Chem, 2005, 70, 3094–3098. [DOI] [PubMed] [Google Scholar]
- 25.van der Wildt B, Shen B and Chin FT, J. Labelled Compd. Radiopharm, 2018, 61, 1110–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


