Abstract
Study Objective:
Validate a novel method for sleep-wake staging in mice using noninvasive electric field (EF) sensors.
Methods:
Mice were implanted with electroencephalogram (EEG) and electromyogram (EMG) electrodes and housed individually. Noninvasive EF sensors were attached to the exterior of each chamber to record respiration and other movement simultaneously with EEG, EMG, and video. A sleep-wake scoring method based on EF sensor data was developed with reference to EEG/EMG and then validated by three expert scorers. Additionally, novice scorers without sleep-wake scoring experience were self-trained to score sleep using only the EF sensor data, and results were compared to those from expert scorers. Lastly, ability to capture three-state sleep-wake staging with EF sensors attached to traditional mouse home-cages was tested.
Results:
EF sensors quantified wake, rapid eye movement (REM) sleep, and non-REM sleep with high agreement (>93%) and comparable inter- and intra-scorer error as EEG/EMG. Novice scorers successfully learned sleep-wake scoring using only EF sensor data and scoring criteria, and achieved high agreement with expert scorers (>91%). When applied to traditional home-cages, EF sensors enabled classification of three-state (wake, NREM and REM) sleep-wake independent of EEG/EMG.
Conclusions:
EF sensors score three-state sleep-wake architecture with high agreement to conventional EEG/EMG sleep-wake scoring 1) without invasive surgery, 2) from outside the home-cage, and 3) and without requiring specialized training or equipment. EF sensors provide an alternative method to assess rodent sleep for animal models and research laboratories in which EEG/EMG is not possible or where noninvasive approaches are preferred.
Keywords: 3-State Sleep, Sleep-wake Scoring, Electric Field Sensor, Noninvasive, Rodent, REM sleep
Graphical Abstract
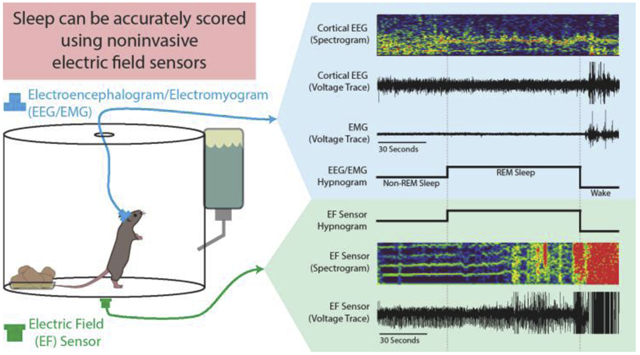
1. Introduction
Accurately characterizing sleep-wake is crucial to understanding its impact on health, cognition, and injury recovery.1-3 Sleep staging in humans relies on substantial non-invasive instrumentation (polysomnography) including electroencephalogram (EEG), electromyogram (EMG) of chin and limbs, electrooculogram (EOG) to detect rapid and rolling eye movements, along with chest wall or air flow movement, oximetry, and video.4-6 However, rodent studies typically rely on fewer signals, particularly invasive EEG and EMG electrodes, less commonly invasive EOG electrodes, and video analysis7-9. Invasive surgical implants can result in weight loss, extensive recovery, and commonly requires use of a tether cable.10-12 Moreover, surgical expertise, limited locations for electrode placement, and specialized equipment required to collect EEG and EMG data may restrict inclusion of sleep analysis in an experimental design. For these reasons, there is a need to develop noninvasive methods to assess sleep in preclinical rodent models11,13-17.
Most noninvasive rodent sleep assessment methods center around measures of gross body movement. Several video analysis techniques have been used to distinguish between 2-state conditions (wake or asleep), but are unable to reliably define three-state sleep-wake staging (henceforth staging) that further differentiates sleep into rapid-eye-movement (REM) and non-REM sleep.18-20 In individually housed animals, other remote methods using light reflection and Doppler techniques have been shown to distinguish REM from non-REM sleep in rats,21 but not in mice.22 Alternatively, force sensors placed inside modified rodent cages have used gross body movement to quantify sleep.10,23-26 These force sensors require animal contact, individual housing, and special analytical software to characterize sleep, but only one study showed classification of three-state sleep-wake in mice.10
Respiration allows three-state sleep staging through noninvasive movement-based measures, as respiration profiles can distinguish REM from non-REM sleep in humans,5,27 dogs,28,29 and in rodents.11,30 The more successful noninvasive approaches to quantify sleep incorporate respiration-related measures into their models10,11,21,23,25 and one study was able to reliably determine sleep-wake, including REM, in mice through respiration alone using whole body plethysmography.11
In this study, a new method to quantify three-state sleep staging was developed using electric field (EF) sensors. EF sensors continuously detect fluctuations in the local electric field caused by motion and have been shown to capture both gross body movement and respiration-related measures.31 EF sensors were mounted on the outside surface of cylindrical acrylic chambers housing mice instrumented with EEG and EMG electrodes. Simultaneously-collected data from EEG, EMG, and EF sensors were used to create rules for scoring sleep using EF sensors alone. A data set was recorded and then divided into separate EEG/EMG and EF recordings, scored by experts, then compared to determine whether EF sensors could reliably and accurately categorize sleep-wake. Additionally, novices were recruited to assess ability to accurately score sleep-wake using EF scoring alone. Overall, EF sensor data alone can reliably and easily distinguish three-state sleep-wake with comparable precision and accuracy to EEG/EMG and that novices can learn how to accurately score sleep from EF sensor data alone.
2. Materials and Methods
2.1. Animals
All work was conducted according to FASEB and Emory IACUC animal guidelines. Four adult mice (3-6 months old: wildtype female n=1, Vgatflox female n=2, male n=1, all backcrossed to C57BL/6J), participating as normal controls in an unpublished study, were used to validate the EF sensor technology.
2.2. Implanting EEG/EMG Electrodes
Hippocampal and contralateral fronto-parietal cortical EEG and EMG were recorded using custom-made headsets described in another publication.17 Briefly, mice were deeply anesthetized and placed on a stereotaxic frame. Meloxicam (5mg/kg, 1cc) was administered subcutaneously. The skull was prepared for aseptic surgery and lidocaine (2% in saline) was administered locally. The skull was exposed using a scalpel and surgical scissors, cleaned, and scored with the reverse side of a scalpel blade to provide a better substrate for dental cement adhesion. To accommodate EMG electrodes, the skin over the neck was blunt-dissected away from muscle. The mouse head was then leveled within the stereotaxic frame and holes were drilled stereotaxically to match the corresponding left frontal and right parietal cortical, left and right hippocampal, and posterior reference (over the midline cerebellum) locations of electrodes on the headplate. The headplate was then lowered into place and attached via screw electrodes (M0.8x3 stainless steel pan head), cyanoacrilic adhesive, and dental cement. Two EMG electrodes (0.005 inch diameter stainless steel wire with pads) were placed between the skin and underlying nuchal muscle. The wound was closed by drawing the skin over the adhesives and suturing (5-0 silk suture). The animals recovered for four days prior to tethering the EEG/EMG headplate to a preamplifier (Pinnacle Technology, Inc., 8406-SE31M, 100x gain), communicator (Pinnacle Technology, Inc., 8408), and A/D converter (Power1401-3A, Cambridge Electronic Design, UK). Recordings used for this study were collected more than one month after surgery.
2.3. Electric Field Sensors
Electric field (EF) sensors (Plessey Semiconductors, PS25251, 1 cm2, +/−5V) were adapted to interface with an A/D converter (Power1401-3A, Cambridge Electronic Design, UK). These EF sensors measure changes in the local electric field within their detection area caused by movement and translate it into a voltage trace (+/−5V, 2048Hz sampling rate). While EF sensors have a frequency response range within 0.1hz-10 kHz, a two-pole lowpass Chebychev filter with a 12 Hz cutoff frequency was added to exclude line noise while retaining frequency features required for sleep state differentiation. Recording features and validation of these EF sensors to measure respiration in rodents have been published previously.31 Here, when affixed outside the home-cage, the EF sensors detected large and small mouse motion with high temporal resolution at distances <6 inches from the sensor. Signal amplitude is distance-dependent. 31 Larger movements (e.g. rearing) generate larger electric field disruptions and corresponding voltage transients than smaller movements (e.g. resting respiration). While larger movements can consequently be detected further away, we found that the use of two spatially separated EF sensors reliably captured smaller movements with high signal to noise ratio in a chamber of 50 sq. inches.31 Moreover, a 16-sensor set-up capable of measuring 8 to 16 animals can be implemented for fewer than US $2000.
During Non-REM sleep, the predominant animal movement is caused by respiration via chest wall expansion and contraction. During REM sleep, respiration depth and frequency vary in addition to twitches of the distal digits of the limbs, tail, whiskers, and ears.32 In this study, these state-related movement changes were detected by EF sensors and validated against EEG and EMG.
2.4. EEG/EMG and EF Recording Set-up
To accommodate EEG/EMG recording, animals were housed singly in cylindrical acrylic chambers (150 oz. clear acrylic container, 8 inch diameter and 8 inches tall, Oggi Corporation, Anaheim, CA) with corncob bedding, nesting material, and free access to water and food. The lid contained a hole appropriate for the tether associated with the EEG/EMG recording equipment (Figure 1). Cameras (1 megapixel day/night dome USB security camera, ELP, 320, 30 frames per second, Shenzen, China) were placed above each cage for synchronized videography. Two EF sensors were placed on the exterior bottom of each cylindrical chamber, opposite each other and 2-3 inches from the chamber wall (Figure 1); only one was needed for characterization of respiratory changes during sleep. Housing room humidity was kept between 30-70%. Recordings were captured continuously for one week, in 12-hour segments, using Spike2 software and automated acquisition (Spike2 v8.09, CED, UK, 2048Hz sampling) with synchronization of the video, EEG, EMG, and EF signals. Although EF sensor data were collected here using CED hardware and Spike2 software, they can be recorded and analyzed using any other equipment that permits analog signal acquisition, such as Axon instruments Digidata/pCLAMP (Molecular Devices), National Instruments/LABVIEW, or MATLAB.
Figure 1. Cage Set-up for Electroencephalogram/Electromyogram (EEG/EMG) and Synchronized Electric Field (EF) Recordings.
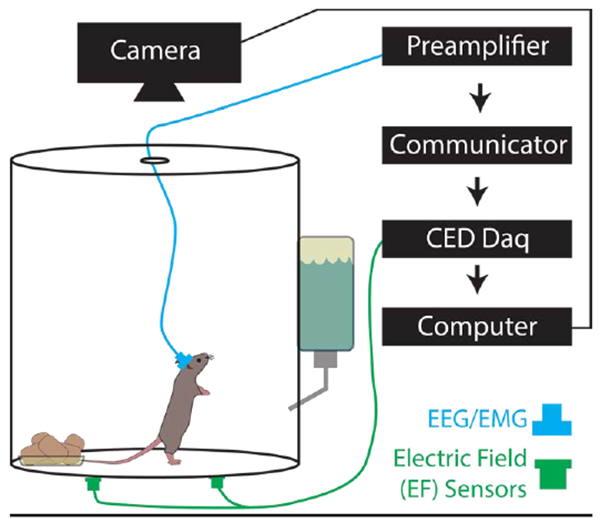
The animals were singly housed in cylindrical acrylic chambers (150 oz. 8 inch diameter, 8 inches tall). Head stages (blue) were surgically mounted prior to recordings and contain EEG and EMG electrodes connected to a preamplifier, communicator, then a Cambridge data acquisition box (CED Power 1401). EF sensors (green) were attached to the exterior of the cage bottom approximately 2-3 inches from the cage wall and connected directly to the Power 1401.
2.5. Scoring Sleep
Two 5.5-hour recording samples in four mice (totaling 8 recordings), randomly distributed within the 24-hour day period and representing a range of data quality (signal to noise ratio range 7.6-50.5 dB, average: 25.9±10.1 dB), were chosen to score sleep. Features and descriptions of each recording are summarized in Table 1. This design, with shorter than 24-hour duration, was intended to test whether EF sensor scoring could accurately discriminate three-state sleep scores when compared to conventional scoring.
Table 1. Description of Recordings used to Validate Electric Field (EF) Sensors.
Recordings were taken from 4 animals at random times during the circadian cycle specifically to assess whether EF sensors can accurately quantify three-state sleep staging. The purpose of this design is not to describe the sleep-wake behavior of these animals over a 24 hour period. Each recording consisted of 5.5 hours of data and was scored by three experts using electroencephalogram (EEG) and electromyogram (EMG) recordings to determine three-state sleep staging and the resulting percentage of each arousal state for each recording is given.
| Recording 1 |
Recording 2 |
Recording 3 |
Recording 4 |
Recording 5 |
Recording 6 |
Recording 7 |
Recording 8 |
|
|---|---|---|---|---|---|---|---|---|
| Animal | Animal 1 | Animal 1 | Animal 2 | Animal 2 | Animal 3 | Animal 3 | Animal 4 | Animal 4 |
| Gender | Female | Female | Female | Female | Female | Female | Male | Male |
| Strain | WT | WT | Vgatflox | Vgatflox | Vgatflox | Vgatflox | Vgatflox | Vgatflox |
| Time of Day (24-hour) | 16:00 | 8:00 | 11:00 | 13:00 | 22:00 | 4:00 | 8:00 | 2:00 |
| Total Number of 10-second Epochs | 1977 | 1977 | 1977 | 1977 | 1977 | 1977 | 1977 | 1977 |
| EEG/EMG Scored as wake (%) | 34.9 | 37.2 | 43.2 | 56.1 | 60.1 | 35.8 | 37.6 | 17.0 |
| EEG/EMG Scored as non-REM sleep (%) | 54.6 | 54.4 | 50.4 | 37.4 | 33.1 | 59.5 | 53.1 | 71.2 |
| EEG/EMG Scored as REM sleep (%) | 10.5 | 8.4 | 6.4 | 6.5 | 6.8 | 4.7 | 9.3 | 11.8 |
For each of the 8 recordings prepared for the development and validation of scoring, channels with EF signals and EEG/EMG were split to enable blinding to the other signal (Figure 2). In this way, each recording was scored purely on the basis of EEG/EMG or EF data without needing the video data. Recordings were then presented in random order and scored blindly; scorers knew whether the file contained EEG/EMG or EF data because different scoring rules were required for each method, but they did not know which EEG/EMG derivative recording went with its twin EF derivative recording. In addition, to assess intra-scorer reproducibility, three recordings each of EEG/EMG and EF taken from the same original recording were randomly chosen and blindly scored again within the data set.
Figure 2. Recording Set-Up for and Creating Definitions for Scoring.
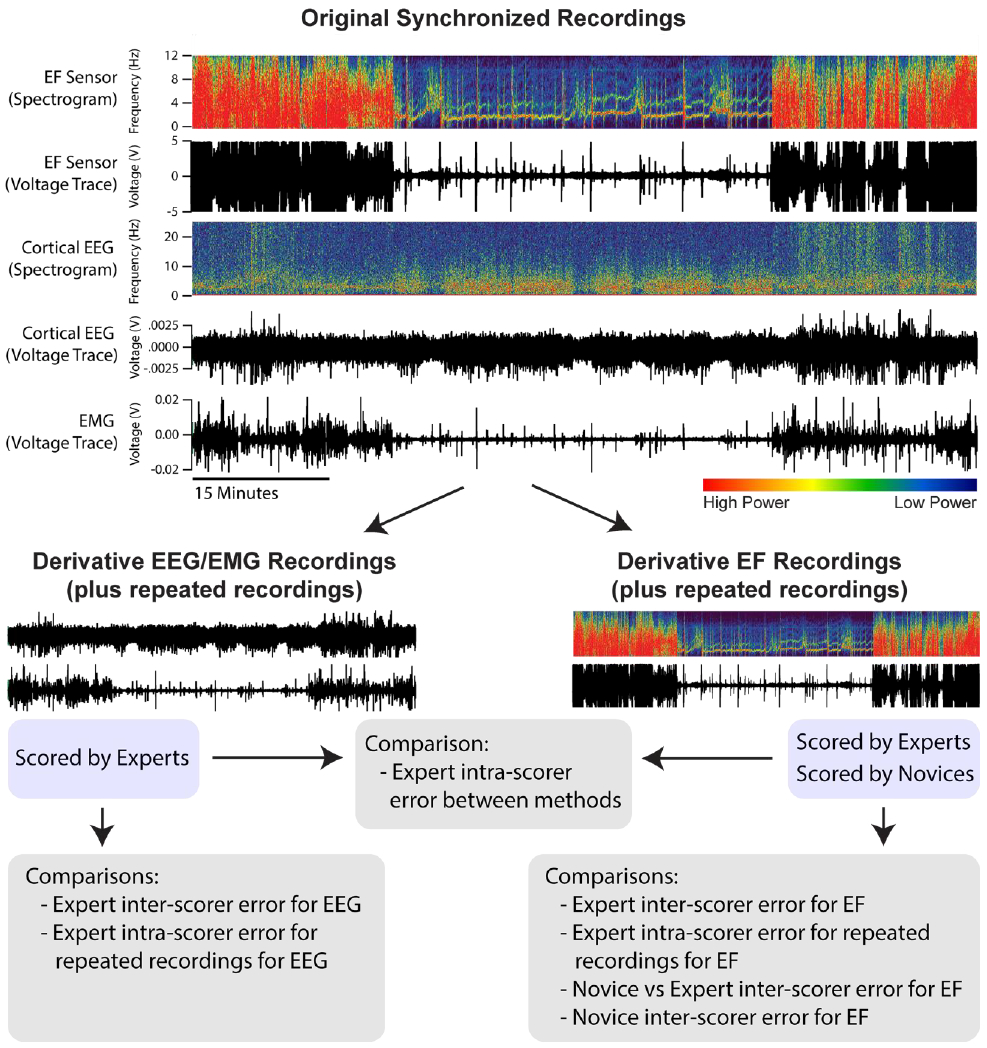
Each recording is created as synchronous voltage traces from electric field (EF), electroencephalogram (EEG), and electromyogram (EMG). As an example, one EF and EEG voltage trace are also represented as a spectrogram – a graphic in which the x-axis is time, the y-axis is frequency, and the color intensity denotes the power of respective frequencies present in the voltage trace. Each synchronized recording (8 total) is divided into two files that contain either the EEG/EMG or EF voltage traces for subsequent scoring and comparison.
2.6. Defining Criteria for Scoring Sleep using EF Sensors
All recordings were scored manually using Spike2 by three expert sleep scorers. For the EEG/EMG recordings, sleep-wake architecture (wake, REM sleep, or non-REM sleep) was manually scored in accordance with conventional Rechtschaffen and Kales33 criteria in 10-second epochs using only the cortical EEG electrode and EMG. Expert sleep scorers, with extensive experience scoring sleep-wake in mice, used 12 hours of EEG/EMG scored behavior to develop criteria for identification of sleep state by EF sensor recordings. EF sensors were found to reliably detect movement-related features that can discriminate between wake, non-REM, and REM states based on frequency domain analysis using Fast Fourier transform (FFT) as shown in Figure 3, with expanded settings described in Supplement 1, and detailed below. In this study, active and quiet wake were not distinguished from each other. A reference instruction document (Supplement 2) was generated to score sleep exclusively by EF sensor using the following criteria:
Figure 3. Wake, Non-REM Sleep, and REM Sleep Features are Unique for Electric Field (EF) Sensor Voltage Trace and Spectrogram.
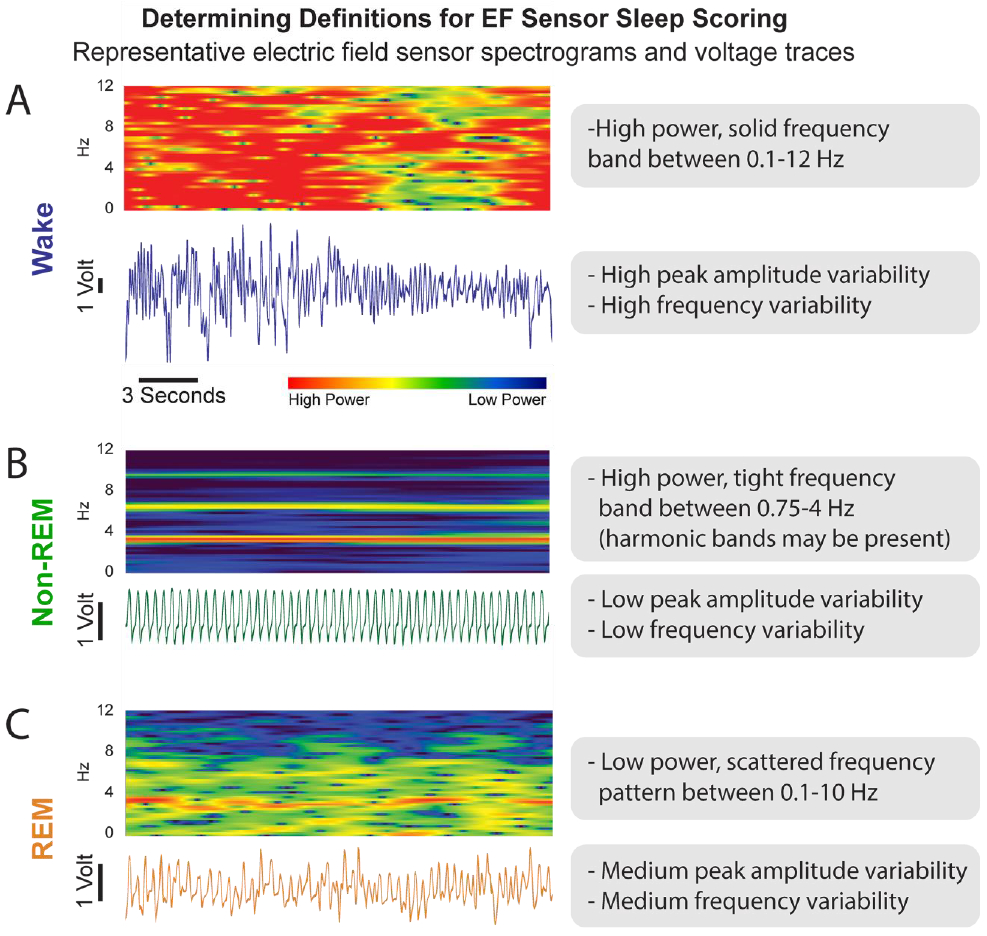
A) The EF sensor wake state data appears as both a spectrogram (top) and voltage trace (bottom in blue). Each voltage trace is the direct output from the EF sensors and represent animal movement. B) EF data for non-REM sleep (green) behavior. C) EF data for REM sleep (orange) behavior.
Each epoch is scored based on the majority (>50%) of the state present in that epoch.
REM sleep cannot transition directly from wake.
Non-REM cannot be scored as a single epoch
Single epochs of wake (brief arousals) are scored only if the wake-related movement represents both a) the majority of the epoch and b) endures for 10 seconds or more when looking at neighboring epochs.
These criteria and an expanded scoring protocol are detailed in another publication34.
During wake, the raw voltage output of the EF sensors (Figure 3A) is of high amplitude and erratic, reflecting the highly complex summations of movement for motor activity. Wake-state frequency domain patterns on FFT generally include multiple signals between 0.1-12 Hz and amplitude generally ranges 4-10 V (as measured between ±5V). These raw voltage traces were converted into Spike2 ‘sonograms’ which are fast Fourier transform (FFT) spectral plots (Hanning filter, 2-second window with 1 second offset overlapping bins) characterizing the frequency signals (y-axis, Hz) across time (x-axis, seconds) and their relative power (color intensity) that make up the raw voltage trace. During wake, the spectrogram shows frequency components with high power (i.e. red) that solidly span from near 0 Hz to 12 Hz.
During non-REM sleep, the raw voltage output of the EF sensors is consistently rhythmic and correlated with the repetitive motion of breathing during Non-REM sleep (Figure 3B). Non-REM-state frequency patterns generally includes a single signal between 2-4 Hz and amplitude generally ranges 0.1-2 V. A non-REM spectrogram, likewise, exhibits a high power (i.e. red), consistent frequency band associated with respiratory rate typically occurring between 2-4 Hz. Occasionally, lower power harmonic artifacts at whole number multiples of respiration frequency will appear in the spectrogram of non-REM sleep given the non-sinusoidal nature of the signal.
During REM sleep, the raw voltage output of the EF sensors increases in frequency variability relative to non-REM, but amplitude changes are minimal (Figure 3C). REM-state frequency patterns generally include multiple signals from 1-10 Hz and amplitude generally ranges 0.4-3 V. Spectrograms of REM sleep show these multiple frequency signals as fragmented spots of frequencies ranging from just below the corresponding non-REM respiratory rate (2-4 Hz) to 10 Hz. The frequency band associated with respiration often appears on the spectrogram during a REM event, but becomes less consistent in both frequency and intensity. The relative power of these cumulative signals during REM sleep is typically equivalent or slightly less than the corresponding non-REM power, but with much lower power than during wake. Overall, as shown in Figure 3, REM and non-REM states are easily discriminable.
2.7. Scoring Sleep using EF Sensors
Three expert sleep scorers used the developed criteria for scoring sleep using EF sensors. Additionally, two novice scorers used the same criteria to score the same EF recordings under two different conditions: Novice #1 scored EF recordings given only the EF sleep-wake scoring training document (Supplement 2) while Novice #2 was also given two example recordings of sleep-scored EF sensor data to aid assessment of scoring (Supplement 3).
2.8. Statistical Validation of EF Sensor Sleep Scores to EEG/EMG Sleep Scores
EEG/EMG and EF recordings were scored independently by the three expert sleep scorers. Comparisons were made on an epoch-by-epoch basis. Intra-scorer (e.g. Scorer #1 EF compared to Scorer #1 EEG/EMG) and inter-scorer (e.g. Scorer #1 EF compared to Scorer #2 EF) percentage agreement was calculated from the total number of epochs and represents consensus agreement – percent of epochs in which all three expert scorers agreed. 2-way paired t-tests with an alpha of 0.05 were performed on the percentage agreement measures.
To determine the reliability and reproducibility of EF sensor scoring and EEG/EMG scoring, intra-class correlation coefficients (ICCs) were calculated. ICCs describe how well data scored across graders or methods resemble each other accounting for grouping rather than only as paired observations. Where appropriate, single measure ICCs were reported for intra-scorer comparisons, average measures ICCs were reported for inter-scorer comparisons, and 2-way paired t-tests with an alpha of 0.05 were calculated to compare ICCs between scoring methods.
The two novice EF scores were compared to each of the three expert scores individually to produce three different percentage agreements and ICCs per novice. These percentage agreements and ICCs were averaged and compared using 2-way paired t-tests with an alpha of 0.05.
Percentage agreement and descriptive correlation statistics (ICCs, Pearson’s R, or Cohen’s kappa) are the most commonly reported variables to compare two sleep-wake scoring methods8,11,20,33,35-39. They are representative of how well broad sleep measures might perform (i.e. total time asleep), but not other measures that require more detail (e.g. sleep fragmentation). To assess how EF sleep-wake scoring matches EEG/EMG sleep-wake scoring with more resolution, the number of transitions between arousal states (into wake, into non-REM sleep, and into REM sleep) were calculated for each scorer and recording. Differences between state transition values for EF and EEG/EMG methods were assessed using 2-way paired t-tests with an alpha of 0.05. Unless otherwise stated, data are presented as mean ± standard deviation.
Sensitivity (false positivity rate) and specificity (false negative rate) were calculated for intra-scorer agreements (e.g. Scorer #1 EF compared to Scorer #1 EEG/EMG, where EEG/EMG is considered the ground truth) and novice agreements with expert scorers (where the expert score was considered the ground truth). Sensitivity and specificity are also reported broken down by arousal state (wake, non-REM sleep, and REM sleep).
2.9. Applying EF Sensors to Traditional Mouse Home-Cages to Assess Sleep
Once the EF sensor technology was validated against EEG/EMG in the cylindrical chambers, three typical rectangular vivarium mouse home-cages (32cmx18cmx14cm, Super Mouse™ microisolator) were shielded (VeilShield™, >40 dB signal reduction, 0.1 Ohms/sq resistivity, nickel-coated copper fabric-like mesh) instrumented with EF sensors to determine whether sleep could be measured in an environment without EEG/EMG methods. In these experiments, 6 female C57BL/6 mice (3 months old) were pair-housed in standard 12:12 hour light, food, water, and temperature home-cage conditions. During the 12-hour dark cycle recording period, the home-cages were temporarily divided into two electrically shielded compartments (16cmx18cmx14cm, 288 cm2 floor area) using a tension-fit custom insert composed of two clear acrylic sheets (18cmx14cmx0.25cm) glued together with grounded VeilShield™ between such that the mice were still able to see, smell, and hear their cage-mate. Recordings were performed during the dark cycle to minimize environmental disruptions. EF sensors were attached to the home-cage exterior allowing each animal to be recorded individually with negligible crosstalk between the chambers (Figure 4A). Once acclimated to the divided cage set-up, data were collected for 12 hours during the dark cycle using the Digidata/pClamp acquisition platform (Molecular Devices). Collected data was then imported into Spike2 (CED) where sleep was scored by one of the expert scorers. A single EF sensor was sufficient to accurately detect mouse sleep over an area of 50 sq. inches in a cylindrical cage and 45 sq. inches (288 cm2) of a traditional rectangular mouse home-cage. However, at least two sensors per animal are recommended to ensure redundant detection of mouse sleep and for the highest quality data, especially if larger cages or those of different shape are used.
Figure 4. Home-Cage Set-up with only Electric Field (EF) Sensors and Description of 12-Hour Dark Cycle Sleep-Wake Staging.
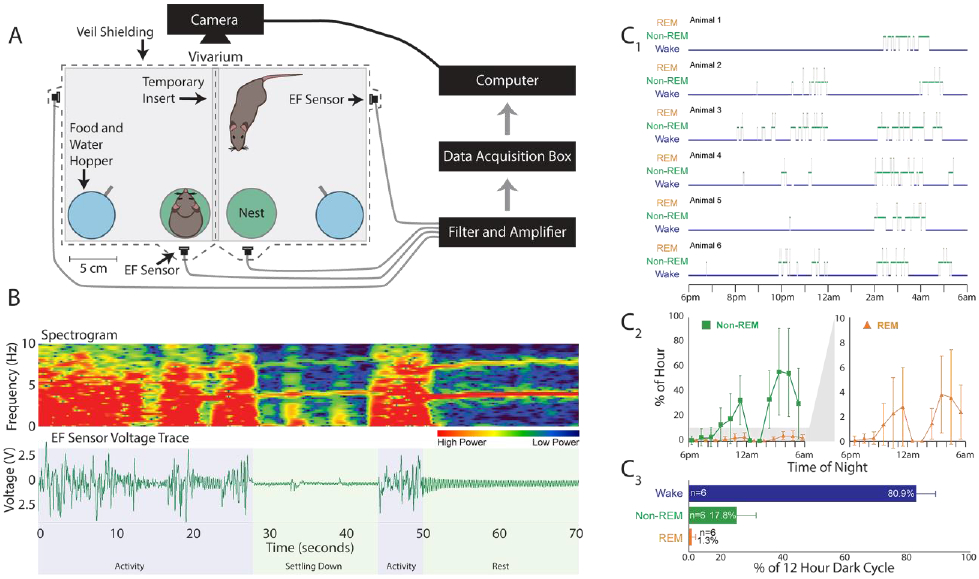
A) Home-cage set-up in which the animals are separated by a transparent shielded insert during testing that allows visual, olfactory, and thermal interactions between the animals. Each animal has free access to food and water and a 60 mm Petri dish to use as a nest. The electric field (EF) sensors are attached to the home-cage exterior and connected to a filter/amplifier box, an Axon instruments data acquisition box, then to the computer. B) Representative spectrogram and raw voltage trace from home-cages that are indistinguishable from the data collected on the electroencephalogram/electromyogram (EEG/EMG) validation cages. C1) three-state hypnogram describing the sleep results for 6 animals recorded overnight between 6pm and 6am. C2) The average percentage of sleep (non-rapid eye movement – non-REM – sleep plus REM sleep time) of all 6 animals per hour over the 12 hours. C3) The 12-hour average of all 6 animals for each arousal state. Data are presented as mean ± standard deviation.
3. Results
3.1. Sleep-wake scoring had high agreement within and between EEG/EMG and EF scoring methods
The three expert scorers were compared within each sleep-wake scoring method, EEG/EMG or EF, to determine method-related error. Within both EEG/EMG and EF scoring methods, consensus agreement of the expert scores were above 93%, comparable with prior reports,40 and ICCs were above 0.97 (Table2; Figure 5A). Furthermore, manual scoring times between EEG/EMG and EF methods were 10.9±2.7 minutes and 11.9±3.8 minutes, respectively, to score 5.5 hours of behavior.
Table 2. Summary of % Agreement and Intra-class Correlation Coefficients (ICCs).
for different comparisons in validating electric field (EF) sensors sleep performance against the electroencephalogram (EEG) and electromyogram (EMG) method. Data presented as mean ± standard deviation.
| % Agreement | Single Measure ICC |
Average Measures ICC |
|
|---|---|---|---|
| EEG/EMG Inter-scorer | 93.9±2.0 | - | 0.98±0.01 |
| EF Inter-scorer | 93.4±2.6 | - | 0.97±0.01 |
| EEG/EMG vs EF Intra-scorer | 94.1±0.5 | 0.90±0.01 | - |
| EEG Inter-scorer Repeated Recordings | 97.4±1.0 | 0.96±0.01 | - |
| EF Inter-scorer Repeated Recordings | 96.9±0.7 | 0.94±0.02 | - |
| EF Inter-scorer Novice #1 vs Experts | 87.6±1.5 | - | 0.89±0.03 |
| EF Inter-scorer Novice #2 vs Experts | 91.2±1.8 | - | 0.91±0.02 |
Figure 5. Agreement Between Electric Field (EF) and Electroencephalogram/Electromyogram (EEG/EMG) Sleep-Wake Scoring Methods.
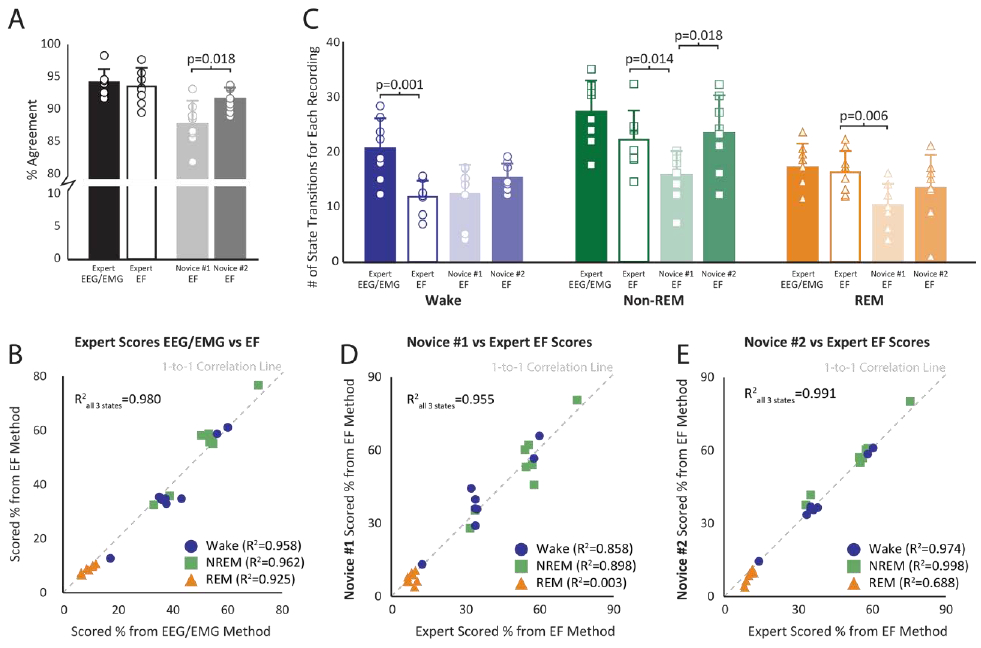
A) The black and white bars represent expert intra-scorer agreement for both electric field (EF) and electroencephalogram/electromyogram (EEG/EMG) methods. The light and dark gray bars represent novice agreements with expert scores for the EF sleep-wake scoring method. The bars represent the mean ± standard deviation. The circles represent the specific results from each of the 8 files. B) A correlation between the EEG/EMG and EF three-state sleep-wake scoring results for each of the 8 files. Percent time spent in wake (blue circle), non-rapid eye movement (non-REM) sleep (green square), and REM sleep (orange triangle) were calculated for each file and represent the average score from the three expert scorers. C) Bar (mean ± standard deviation) and raw data for each recording representing the number of calculated state transitions for each scoring method (EF vs EEG/EMG) and scorer skill (novice vs expert). D) Novice #1’s three-state scoring results for each file plotted against the average three expert scorers’ results for the EF sensor method. Novice #1 self-trained using only the Supplement 2 document. E) Novice #2’s three-state scoring results for each file plotted against the average three expert scorers’ results for the EF method. Novice #2 used both Supplement 2 and Supplement 3 to iteratively self-train and improve sleep skill prior to scoring data.
The combined three-state scores for EF and EEG/EMG methods were highly correlated (R2=0.98) including when assessed within individual states (Figure 5B; R2>0.92). The number of state transitions into non-REM and REM sleep were not different between the two scoring methods, though EF results identified fewer transitions into wake than EEG/EMG (p=0.001) (Figure 5C).
Intra-scorer agreement between EEG/EMG and EF methods averaged 94.1 ± 0.5% with a single measure ICC of 0.90 ± 0.01 (Table 2) and aggregate sensitivity and specificity above 90% (Table 3, Supplement 4). Of the three arousal states, REM sleep reported the lowest sensitivity and specificity for all comparisons, again as is typically noted given some ambiguity of the non-REM to REM sleep transition period.
Table 3. Summary of Sensitivity (false positive rate) and Specificity (false negative rate).
Summary of Sensitivity (false positive rate) and Specificity (false negative rate) when comparing the electric field (EF) sensor-based method against the electroencephalogram (EEG) and electromyogram (EMG) method (considered the ground truth) for scoring sleep. Novice scorer sensitivity and specificity, in which expert scorers are considered the ground truth, are calculated for the EF scoring method. Data are broken down by arousal state and summarized with an aggregate. P-values represent the comparison Novice #1 vs. Novice #2. Data represented as mean ± standard deviation.
| Sensitivity | Specificity | Sensitivity | Specificity | |||||
|---|---|---|---|---|---|---|---|---|
| EEG/EMG vs EF |
EEG/EMG vs EF |
EF Novice #1 vs Experts |
EF Novice #2 vs Experts |
p-value | EF Novice #1 vs Experts |
EF Novice #2 vs Experts |
p-value | |
| Wake | 93.8±3.2 | 97.3±1.1 | 96.1±2.5 | 96.4±1.5 | 92.8±6.1 | 97.9±0.8 | p=0.004 | |
| Non-REM | 94.3±1.4 | 92.3±2.9 | 87.2±7.1 | 94.6±2.3 | 88.9±9.4 | 91.4±3.9 | ||
| REM | 83.4±6.0 | 98.3±0.7 | 58.6±21.9 | 68.8±10.0 | p=0.008 | 97.1±1.6 | 97.8±1.5 | |
| Aggregate | 90.5±2.9 | 96.0±1.4 | 80.8±7.1 | 86.6±3.8 | p=0.025 | 92.9±2.7 | 95.7±1.5 | p=0.002 |
Reproducibility for both methods was assessed by blind inclusion of previously scored recordings. For both EEG/EMG and EF methods, average agreement of repeated recordings was above 96% with ICCs above 0.94 (Table 2), again in agreement with prior reports.40
3.2. There was high EF scoring agreement between Novice and Expert
To determine the level of instruction needed to achieve high scoring agreement with experts, novice scorers were given an instruction document (Novice #1) as well as two sleep scored training files (Novice #2) to classify three-state sleep scores with EF sensor data alone.
On average, Novice #1 produced agreements with the three expert scorers of 87.6±1.5% and an average ICC of 0.89±0.03 (Table 2). Overall sleep state identification for Novice #1 correlated well with the expert scorers (R2=0.96, p<0.001; Figure 5D). However, when broken down by state, wake and non-REM sleep scores correlated well (R2>0.86, p<0.001) but REM sleep scores did not (R2=0.004). Moreover, the number of state transitions into non-REM and REM sleep were lower (p=0.014 and p=0.006, respectively) compared to the expert scorers, but the number of wake transitions were not different.
Novice #2 produced higher percentage agreement with experts than Novice #1 (91.2±1.8%, p=0.018) but had similar ICCs (0.91±0.02; Table 2). Novice #2’s overall wake and non-REM sleep state identification correlated well with the expert scorers (R2>0.97, p<0.001; Figure 5E). Importantly, Novice #2 REM sleep state scores were well correlated with expert scores (R2=0.69 p<0.001) unlike Novice #1 (R2=0.004 p=0.89). Novice #2 also produced higher REM sleep sensitivity (p=0.008), wake specificity (p=0.004), and aggregate sensitivity and specificity (p=0.025 and p=0.002, respectively) to expert scorers than Novice #1 (Table 3, Supplement 4).
3.3. EF Sensors are able to Assess Sleep from Traditional Mouse Home-Cages Without EEG/EMG
EF sensors placed on traditional mouse home-cages were able to detect animal movement with high fidelity (Figure 4B). The voltage traces and spectrograms from these recordings appear indistinguishable from those collected during the validation experiment using the cylindrical acrylic chambers with EEG/EMG equipment. Moreover, three-state sleep-wake scoring was able to be performed from the EF data collected from the traditional home-cages (Figure 4C1). The animals exhibited a biphasic sleep pattern during the dark night cycle (Figure 4C2). Over the course of the 12-hour phase of the light-dark cycle from 6pm-6am, the animals spent 17.8±5.6% of the night asleep (Figure 4C3). As expected, both the average time spent sleeping and the biphasic distribution of sleep match previously reported behaviors for the C57BL/6 strain of mouse.38,41
4. Discussion
Knowing that respiration changes associate with sleep state, 5,11,27,30 this work demonstrated that EF sensors could capture these changes to quantify three-state sleep staging with comparable accuracy as the gold standard EEG/EMG method. The EF sensor-based sleep scores had comparable or better agreement (i.e. less error),10,11,20-22 sensitivity (i.e. false positive rate),10,20,21 and specificity (i.e. false negative rate)20,21,42 than other studies comparing respiration-related changes to EEG/EMG to assess three-state sleep staging in rodents.
The EF sensor recording approach offers several advantages over other recording technologies. First, the approach is non-invasive and can be conducted on many mice more easily and cheaply than with a tether or surgically implanted sensor system. Furthermore, it allows recordings to be undertaken in the home-cage of pair-housed animals. The cages in this study were temporarily divided for pair-housed animals with an electrically shielded barrier to isolate animal recordings. Dividing a home-cage to isolate individuals during recordings limit social contact and related sensory/behavioral interactions that may influence outcome. Future experimental designs that enable separate capture from individual animals without imposed social isolations may be possible with added shielding and selective animal identification. For example, with an EF sensor approach we have found it possible to record individual nest behavior in pair-housed mice by constructing two shielded and size-restricted nest cubicles with RFID tracking for individual animal identification. Other advantages of the EF sensor approach include that: 1) sensors can be placed outside the animal’s environment, 2) can be undertaken in both rats and mice with equal ease,31 3) there is no required specialized software for analyses, 4) components are inexpensive to purchase and easy to construct,31 and 5) remove the need to used cranial electrodes to capture sleep thus freeing them to record other EEG features. This approach is expected to be applicable to many disease models that impact various sensory, motor, and autonomic physio-behavioral states. In preliminary work in a limited mobility high T2 thoracic transection paraplegia spinal cord injury model of dysautonomia, we found that mice still generate micro movements while awake that enable the EF sensors to readily distinguish wake from sleep (Kloefkorn et al unpublished). Though mouse sleep can be measured using other noninvasive approaches, EF sensor recordings have the additional ability to simultaneously quantify other motor behaviors such as ventilation profiles in non-REM and REM sleep, grooming, locomotion, eating, and drinking using only a frequency-based features of a single voltage channel.31 Other noninvasive movement-based methods to measure rodent sleep have been reported using video,18-20,22,43 whole body plethysmography,7,11 infrared beams,19,44 pulse Doppler radar,21 piezoelectric films,10,24,45-47 and actimetry.48,49 Several of these approaches, namely video, infrared beams, and actimetry, cannot distinguish non-REM from REM sleep18,19,22,43-49 limiting their applications to when only sleep/wake discrimination is sufficient. Of the published studies capable of noninvasively measuring 3-state sleep architecture, the reported overall accuracies to EEG/EMG range from 84% to 91%,10,11,20,21,24. Specifically, piezoelectric approaches can record in unaltered home-cage environments and distinguish REM from non-REM sleep, having comparable accuracy (piezoelectric = 90±0.9%, EF = 94±0.5%), sensitivity (piezoelectric = 79±12%, EF = 90±3%), and specificity (piezoelectric = 92±1%, EF = 96±1%) to EF sensor performance. However, piezoelectric approaches require contact to determine animal movement and EF sensors do not, making EF sensors a more versatile option.10,24 Importantly, sleep scoring using the EF sensor data was easy to learn and novices were able to achieve scoring results comparable to expert sleep scorers. Moreover, the rules that form the basis for scoring based on EF voltage traces and spectrograms are also applicable to automated detection algorithms for high-throughput sleep analyses.
EF sensor sleep-wake scoring has inherent limitations common to most sleep-wake scoring methods. As with EEG/EMG scoring, the majority of error occurred at state transitions, brief arousals, and at the non-REM to REM transition.36,37 EF sensors detect only movement-related variables, and brief electrocortical arousal events that are visible on EEG can occur independent of movement. Consequently, the developed rules that rely on detection of movement-related variables required longer periods than EEG/EMG for the EF sensor method to be scored as wake. This led to missed brief arousals and likely contributed toward less accurate assessment of brief arousals, seen as reduced transitions into wake (Figure 5). Since brief arousals account for a small percentage of total assessed time, missing these brief events does not affect overall wake time but could negatively impact calculation of other sleep measures (e.g. sleep event duration or sleep fragmentation).
Similar to missed brief arousal detection, transitions into REM sleep were another common source of error for EF sensor sleep-wake scoring. The EF sensors, again, detect movement but REM sleep may show up on an EEG slightly prior to the start of summed body movements detected by the EF sensors. Often this discrepancy was a single 10-second epoch, but occasionally more. REM-related scoring error is also common in EEG/EMG sleep-wake scoring methods partially due to their low occurrence (typically 5-15% of total sleep time)36,37 and placement of EEG electrodes.50 However, accuracy in identifying REM sleep was not affected by the quality (i.e. signal to noise ratio) of the data. REM sleep has a unique frequency profile allowing scorers to distinguish it from wake. Even in the validation recording with the worst signal to noise ratio (7.6 dB) with EF sensors 4-6 inches away from mice, overall expert scorer epoch agreement was 96% with REM sleep epoch agreement more than 88%. That 90% of errors in REM sleep scoring were misidentifications as non-REM rather than Wake further argues against signal quality as a source of error.
While not the focus of this study, differentiating quiet wake from active wake can be useful when defining sleep quality51. Quiet wake represents a period where the animal is awake without exhibiting large motor movement and occurs ~1-3% of the 24-hour cycle in rodents30,51,52. Because of the lack of gross body movement during quiet wake, it may appear similar to non-REM sleep on EF sensor voltage traces and spectrograms and represents a potential source of scoring error. To explore this, EEG/EMG-scored quiet wake51 was quantified in four validation recordings (total 22 hours) reported in this study and compared to the 3-state sleep-wake EF sensor scores. Of the epochs defined as quiet wake by EEG/EMG (1.8±0.3% total time), EF sensors identified quiet wake as wake in 87.8±6.5% of epochs and as non-REM sleep in the remaining 12.2%. In sum, the current rules for scoring wake using EF sensors presented here are not able to identify quiet wake nor differentiate it from active wake. However, the resulting overall error accounts for only 0.3% of total epochs; this is well below typical inter-rater scoring discrepancies and thus is unlikely to negatively impact calculated sleep measures.
Overall, novice scorer error was better than reported values for other respiration-related, non-contact methods to assess rodent sleep.11 Accurately quantifying REM sleep events proved to be the poorest agreement with the expert scorers due largely to Novice #1 missing REM events entirely. Novice #2 was able to improve on Novice #1’s REM sleep scoring challenges putatively because Novice #2 also received samples of EF scored sleep (Supplement 3). These sleep-scored samples allowed Novice #2 to score recordings then compare the results to the expert scores provided. This process increased accuracy of sleep-wake scoring by correcting mis-classification. Novice #2’s overall agreement with experts was nearly similar to the inter-expert scoring variability, but, as with Novice #1, REM events were the source of greatest error. This suggests that accurate determination of REM sleep to be the most challenging aspect of sleep-wake scoring for the EF method (and arguably EEG/EMG methods). This is worth noting for other groups wishing to implement EF sensor sleep assessment into their work. Overall, with appropriate training resources provided, researchers with no sleep-wake scoring experience may be able to undertake sleep-wake scoring with comparable accuracy to experts using traditional EEG/EMG methodology.
EF sensors were also able to continuously acquire, and permit the accurate scoring of sleep-wake-related data in traditional mouse home-cages overnight, without the need for EEG/EMG equipment, and reproduced previously reported sleep patterns for same C57BL/6 strain of mice.38,41 Though only overnight data was presented in this study, the EF sensors have since proven to be easy to use, consistently and continuously measure animal movement over days and weeks, as well as detect behavioral differences between healthy and injured mice of multiple models and strains (unpublished observations). These strengths are tempered by environmental limitations that affect the EF sensors recording quality,31 and are important to emphasize here. First, response magnitude is highly distance-dependent indicating magnitude of voltage response may not itself identify a movement related event. In related fashion, placement of additional sensors at multiple locations may be required to accurately detect smaller movements including respiration. Second, EF sensors are dependent on the electrostatics of the environment which are reduced with humidity.
Overall, the EF sensors scored sleep accurately and novice scorers were able to reproduce expert sleep scores using only self-training. The EF sensors are adaptable, noninvasive, and able to detect a wealth of behavioral information continuously over long timelines. Though the EF sensors have limitations, they provide a more ethological method to assess sleep in the animal’s home-cage without need for surgical implants, including in cage environments unsuitable for EEG/EMG equipment.
Supplementary Material
Supplement 1. Spike2 Spectrogram Settings. Five-minute segments of electric field (EF) sensor recordings from the validation dataset are shown here with sleep scores (10-second epochs), spectrogram, voltage trace, and the Spike2 software spectrogram channel settings. The Spike2 settings consist of a “top dB”, a “range dB”, and a “block size”. Top dB sets the intensity of the colormap, range dB dictates the range of data to display, and the block size represents the number of data points used in the fast Fourier transform. W = wake (blue), N = non-REM sleep (green), and R = REM sleep (orange).
Highlights.
Electric field (EF) sensors detect mouse movement noninvasively from outside the home-cage
EF sensors distinguish 3-state sleep-wake architecture with high agreement to EEG/EMG
EF sensors provide an alternative method to assess 3-state sleep-wake in rodents without EEG/EMG
Statement of Significance:
Quantifying sleep in rodent models is crucial to understanding the impact of sleep status on disease, injury, and recovery. However, sleep assessment methods in rodents commonly require invasive electrodes and specialized equipment for recording. Noninvasive methods to identify sleep exist, but have limited ability to score sleep or require specialty housing and recording environments. Noninvasive electric field sensors attached to the animal home-cage exterior were used to detect respiration and other movements and shown to accurately and reliably quantify three-state rodent sleep. These sensors provide an easy and inexpensive method to quantify sleep in the home-cage environment.
Acknowledgments
This work was supported by grants from the Craig H. Nielsen Foundation and the NIH (NPP: K08NS105929 and HK: 5k12-Gm000680). We would also like to express our gratitude to reviewer #1 for the exceptional effort made to improve the quality of the manuscript.
Abbreviations
- EEG
electroencephalogram
- EMG
electromyogram
- EF
sensors – electric field sensors
- REM
sleep – rapid eye movement sleep
- Non-REM
sleep – non-rapid eye movement sleep
Footnotes
Disclosure Statement
Financial Disclosure: HK, WG, and SH are co-inventors of US patent application 16/095,906, filed 10/23/2018, that includes use of EF sensor methodology for non-contact physio-behavioral monitoring of movements including respiration. NPP is a member of the scientific advisory board for Dixi Medical USA (unrelated to this work).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Ramar K, Olson EJ. Management of Common Sleep Disorders. Am Fam Physician. 2013;88(4):231–238. [PubMed] [Google Scholar]
- [2].Irwin MR. Why Sleep is Important for Health: a Psychoneuroimmunology Perspective. Annu Rev Psychol. 2016;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jensen MP, Hirsh AT, Molton IR, Bamer AM. Sleep Problems in Individuals With Spinal Cord Injury: Frequency and Age Effects. Rehabil Psychol. 2010;54(3):323–331. doi: 10.1037/a0016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sadeh A Sleep assessment methods. Monogr ofthe Soc Res Child Dev. 2015;80:33–48. [DOI] [PubMed] [Google Scholar]
- [5].Douglas NJ, White DP, Pickett CK, Weil J V, Zwillich C. Respiration during sleep in normal man. Thorax. 1982;37:840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carrubba S, Kim PY, McCarty DE, Chesson AL, Frilot C, Marino AA. Continuous EEG-based dynamic markers for sleep depth and phasic events. J Neurosci Methods. 2012;208(1):1–9. doi: 10.1016/j.jneumeth.2012.04.018 [DOI] [PubMed] [Google Scholar]
- [7].Hernandez AB, Kirkness JP, Smith PL, et al. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol. 2012;112(4):671–680. doi: 10.1152/japplphysiol.00818.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fulda S, Romanowski CPN, Becker A, Wetter TC, Kimura M, Fenzel T. Rapid eye movements during sleep in mice: High trait-like stability qualifies rapid eye movement density for characterization of phenotypic variation in sleep patterns of rodents. BMC Neurosci. 2011;12:1–13. doi: 10.1186/1471-2202-12-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McKenna JT, Cordeira JW, Christie MA, et al. Assessing sleepiness in the rat: A multiple sleep latencies test compared to polysomnographic measures of sleepiness. J Sleep Res. 2008;17(4):365–375. doi: 10.1111/j.1365-2869.2008.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yaghouby F, Donohue K, O’Hara B, Sunderam S. Noninvasive Dissection of Mouse Sleep Using Piezoelectric Motion Sensor. J Neurosci Methods. 2017;259:90–100. doi: 10.1016/j.jneumeth.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bastianini S, Alvente S, Berteotti C, et al. Accurate discrimination of the wake-sleep states of mice using non-invasive whole-body plethysmography. Sci Rep. 2017;7(December 2016):41698. doi: 10.1038/srep41698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tang X, Sanford LD. Telemetric Recording of Sleep and Home Cage Activity in Mice. Sleep. 2002;25(6):677–685. [PubMed] [Google Scholar]
- [13].Toth LA. The influence of the cage environment on rodent physiology and behavior: Implications for reproducibility of pre-clinical rodent research. Exp Neurol. 2015;270:72–77. doi: 10.1016/j.expneurol.2015.04.010 [DOI] [PubMed] [Google Scholar]
- [14].Febinger HY, George A, Priestley J, Toth LA, Opp MR. Effects of housing condition and cage change on characteristics of sleep in mice. J Am Assoc Lab Anim Sci. 2014;53(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- [15].Choi JH, Koch KP, Poppendieck W, Lee M, Shin HS. High resolution electroencephalography in freely moving mice. J Neurophysiol. 2010;104(3):1825–1834. doi: 10.1152/jn.00188.2010 [DOI] [PubMed] [Google Scholar]
- [16].Rensing N, Moy B, Friedman JL, Galindo R, Wong M. Longitudinal analysis of developmental changes in electroencephalography patterns and sleep-wake states of the neonatal mouse. PLoS One. 2018;13(11):1–17. doi: 10.1371/journal.pone.0207031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu KJ, Aiani LM, Pedersen NP. Reconfigurable 3D-Printed Headplates for Reproducible and Rapid Implantation of EEG , EMG and Depth Electrodes in Mice. J Neurosci Methods. 2020;333. doi: 10.1016/j.jneumeth.2019.108566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fisher SP. Rapid Assessment of Sleep-Wake Behavior in Mice. J Biol Rhythms. 2012;27:48–58. doi: 10.1177/0748730411431550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pack AI, Galante RJ, Maislin G, et al. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–238. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- [20].McShane BB, Galante RJ, Biber M, Jensen ST, Wyner AJ, Pack AI. Assessing REM Sleep in Mice Using Video Data. Sleep. 2012;35(3):433–442. doi: 10.5665/sleep.1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zeng T, Mott C, Mollicone D, Sanford L. Automated determination of wakefulness and sleep in rats based on non-invasively acquited measures of movement and respiratory activity. J Neurosci Methods. 2012;204(2):276–287. doi: 10.1016/j.jneumeth.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brown LA, Hasan S, Foster RG, Peirson SN. COMPASS: Continuous Open Mouse Phenotyping of Activity and Sleep Status. Wellcome Open Res. 2017;1(May):2. doi: 10.12688/wellcomeopenres.9892.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Donohue KD, Medonza DC, Crane ER, Hara BFO. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed Eng Online. 2008;7(14):1–14. doi: 10.1186/1475-925X-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mang GM, Nicod J, Emmenegger Y, Donohue KD, Hara BFO, Franken P. Evaluation of a Piezoelectric System as an Alternative to Electroencephalogram / Electromyogram Recordings in Mouse Sleep Studies. Sleep. 2014;37(8):1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Flores AE, Flores JE, Deshpande H, et al. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans Biomed Eng. 2007;54(2):225–233. doi: 10.1109/TBME.2006.886938 [DOI] [PubMed] [Google Scholar]
- [26].Xie XS, Zhang J, Zou B, et al. Rodent Behavioral Assessment in the Home Cage Using the SmartCageTM System In: Chen J, Xu X-M, Xu ZC, Zhang JH, eds. Animal Models of Acute Neurological Injuries II: Injury and Mechanistic Assessments, Volume 1 Totowa, NJ: Humana Press; 2012:205–222. doi: 10.1007/978-1-61779-576-3_13 [DOI] [Google Scholar]
- [27].Kirjavainen T, Cooper D, Polo O, Sullivan C. Respiratory and body movements as indicators of sleep stage and wakefulness in infants and young children. J Sleep Res. 1996;5:186–194. [DOI] [PubMed] [Google Scholar]
- [28].Phillipson EA. Regulation of breathing during sleep. Am Rev Respir Dis Suppl. 1977;115:217–244. [DOI] [PubMed] [Google Scholar]
- [29].Murphy DJ, Renninger JP, Schramek D. Respiratory inductive plethysmography as a method for measuring ventilatory parameters in conscious, non-restrained dogs. J Pharmacol Toxicol Methods. 2010;62(1):47–53. doi: 10.1016/j.vascn.2010.04.006 [DOI] [PubMed] [Google Scholar]
- [30].Friedman L, Haines A, Klann K, et al. Ventilatory behavior during sleep among A/J and C57BL/6J mouse strains. J Appl Physiol. 2004;97:1787–1795. doi: 10.1152/japplphysiol.01394.2003. [DOI] [PubMed] [Google Scholar]
- [31].Noble DJ, MacDowell CJ, McKinnon ML, Neblett TI, Goolsby WN, Hochman S. Use of electric field sensors for recording respiration, heart rate, and stereotyped motor behaviors in the rodent home cage. J Neurosci Methods. 2017;277:88–100. doi: 10.1016/j.jneumeth.2016.12.007 [DOI] [PubMed] [Google Scholar]
- [32].Tiriac A, Uitermerkt B, Fanning A, Sokoloff G, Blumber M. Rapid Whisker Movements in Sleeping Newborn Rats. Curr Biol. 2012;22(21):2075–2080. doi: 10.1080/10810730902873927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moser D, Anderer P, Gruber G, et al. Sleep Classification According to AASM and Rechtschaffen & Kales: Effects on Sleep Scoring Parameters. Sleep. 2009;32(2).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2635577/pdf/aasm.32.2.139.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kloefkorn H, Aiani LM, Hochman S, Pedersen NP. Scoring Sleep Using Respiration and Movement-Based Features. Co-Submitted to MethodsX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hallgren KA. Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutor Quant Methods Psychol. 2012;8(1):23–34. doi: 10.1080/11035896009449194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Younes M, Raneri J, Hanly P. Staging Sleep in Polysomnograms : Analysis of Inter-Scorer Variability. J Clin Sleep Med. 2016; 12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Younes M, Hanly PJ. Minimizing Interrater Variability in Staging Sleep by Use of Computer-Derived Features. J Clin Sleep Med. 2016;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Veasey SC, Valladares O, Fenik P, et al. An Automated System for Recording and Analysis of Sleep in Mice. Sleep. 2000;23(8): 1–16. [PubMed] [Google Scholar]
- [39].Keenan BT, Galante RJ, Lian J, et al. High-throughput sleep phenotyping produces robust and heritable traits in Diversity Outbred mice and their founder strains. Sleep. 2020;(February): 1–17. doi: 10.1093/sleep/zsz278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Costa-Miserachs D, Portell-Corte I, Torras-Garcia M, Morgado-Bernal I. Automated sleep staging in rat with a standard spreadsheet. J Neurosci Methods. 2003;130:93–101. doi: 10.1016/S0165-0270(03)00229-2 [DOI] [PubMed] [Google Scholar]
- [41].Franken P, Malafosse A, Tafti M. Genetic Determinants of Sleep Regulation in Inbred Mice. Sleep. 1999;22(2): 155–169. [PubMed] [Google Scholar]
- [42].Yaghouby F, Schildt CJ, Donohue KD, O’Hara BF, Sunderam S. Validation of a closed-loop sensory stimulation technique for selective sleep restriction in mice. 2014 36th Annu Int Conf IEEE Eng Med Biol Soc EMBC 2014 2014:3771–3774. doi: 10.1109/EMBC.2014.6944444 [DOI] [PubMed] [Google Scholar]
- [43].Singh S, Bermudez-Contreras E, Nazari M, Sutherland RJ, Mohajerani MH. Low-cost solution for rodent home-cage behaviour monitoring. PLoS One. 2019;14(8):1–18. doi: 10.1371/journal.pone.0220751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Saré RM, Lemons A, Torossian A, Beebe Smith C. Noninvasive, high-throughput determination of sleep duration in Rodents. J Vis Exp. 2018;2018(134): 1–6. doi: 10.3791/57420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Donohue KD, Medonza DC, Crane ER, O’Hara BF. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed Eng Online. 2008;14:1–14. doi: 10.1186/1475-925X-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Flores AE, Flores JE, Deshpande H, et al. Pattern Recognition of Sleep in Rodents Using Piezoelectric Signals Generated by Gross Body Movements. IEEE Trans Biomed Eng. 2007;(March). doi: 10.1109/TBME.2006.886938 [DOI] [PubMed] [Google Scholar]
- [47].Sato M, Sagawa Y, Hirai N, et al. Noninvasive detection of sleep-wake changes and cataplexy-like behaviors in orexin/ataxin-3 transgenic narcoleptic mice across the disease onset. Exp Neurol. 2014;261:744–751. doi: 10.1016/j.expneurol.2014.08.004.Noninvasive [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Khroyan T V, Zhang J, Yang L, et al. Rodent motor and neuropsychological behavior measured in home cages using the integrated modular platform SmartCage ™. Clin Exp Pharmacol Physiol. 2012;39(7):614–622. doi: 10.1111/j.1440-1681.2012.05719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Suzuki M, Nakayama M, Ando KB, et al. Sleep disturbance and hyperactivity detected by actigraphy in rats with allergic rhinitis or attention-deficit hyperactivity disorder. Tohoku J Exp Med. 2018;246(2):65–71. doi: 10.1620/tjem.246.65 [DOI] [PubMed] [Google Scholar]
- [50].Sunderam S, Chernyy N, Peixoto N, et al. Improved sleep-wake and behavior discrimination using MEMS accelerometers. J Neurosci Methods. 2007;163(2):373–383. doi: 10.1016/j.jneumeth.2007.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mavanji V, Teske JA, Billington CJ, Kotz CM. Elevated sleep quality and orexin receptor mRNA in obesity-resistant rats. Int J Obes. 2010;34(11):1576–1588. doi: 10.1038/ijo.2010.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Münch A, Dibué M, Hescheler J, Schneider T. Cav2.3 E-/R-type voltage-gated calcium channels modulate sleep in mice. Somnologie. 2013;17(3):185–192. doi: 10.1007/s11818-013-0628-7 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Spike2 Spectrogram Settings. Five-minute segments of electric field (EF) sensor recordings from the validation dataset are shown here with sleep scores (10-second epochs), spectrogram, voltage trace, and the Spike2 software spectrogram channel settings. The Spike2 settings consist of a “top dB”, a “range dB”, and a “block size”. Top dB sets the intensity of the colormap, range dB dictates the range of data to display, and the block size represents the number of data points used in the fast Fourier transform. W = wake (blue), N = non-REM sleep (green), and R = REM sleep (orange).


