Abstract
Culture of cells as three-dimensional (3D) aggregates can enhance in vitro tests for basic biological research as well as for therapeutics development. Such 3D culture models, however, are often more complicated, cumbersome, and expensive than two-dimensional (2D) cultures. This paper describes a 384-well format hanging drop culture plate that makes spheroid formation, culture, and subsequent drug testing on the obtained 3D cellular constructs as straightforward to perform and adapt to existing high-throughput screening (HTS) instruments as conventional 2D cultures. Using this platform, we show that drugs with different modes of action produce distinct responses in the physiological 3D cell spheroids compared to conventional 2D cell monolayers. Specifically, the anticancer drug 5-fluorouracil (5-FU) has higher anti-proliferative effects on 2D cultures whereas the hypoxia activated drug commonly referred to as tirapazamine (TPZ) are more effective against 3D cultures. The multiplexed 3D hanging drop culture and testing plate provides an efficient way to obtain biological insights that are often lost in 2D platforms.
Introduction
Three-dimensional (3D) cell culture is motivated by the need to work with cellular models that better mimic physiological tissues. Cellular functions and responses that are present in tissues are often lost in conventional ‘dish’-based two-dimensional (2D) cell cultures limiting predictive capability of drug assays and skewing cell biological research results.1 Consequently, many researches have been devoted to develop in vivo like 3D cell culture techniques. Spheroid formation is one of the most well characterized models for 3D culture and screening due to its simplicity, reproducibility, and similarity to physiological tissues compared to other methods involving extracellular matrix (ECM) scaffolds and hydrogel systems.2,3 Spheroids are self-assembled spherical clusters of cell colonies cultured in environments where cell-cell interactions dominate over cell-substrate interactions, and they naturally mimic avascular tumors with inherent metabolic (oxygen) and proliferative (nutrient) gradients.2,3 Therefore, spheroids serve as excellent physiologic tumor models known to provide more reliable and meaningful therapeutic readouts compared to 2D tests.3 Spheroids allow cellular self-organization of appropriate 3D ECM assembly with complex cell-matrix and cell-cell interactions that mimic functional properties of the corresponding tissue in vivo.2 Most importantly, spheroids can be monitored easily for practical daily observations. As a result, spheroid cultures have been valued as a physiologically relevant alternative to 2D cultures for decades.4–6
Although these advantages of spheroids have been widely recognized, it has been difficult to scale up spheroid culture in a high-throughput manner for screening and testing. Typical spheroid formation methods include hanging drops on the underside of culture plate lids, culture of cells on non-adherent surfaces, spinner flask cultures, and rotary cell culture systems.6 These traditional spheroid formation and culture systems, however, are often tedious, produce variable size spheroids, low-throughput, and hard to handle. Recently, various microfluidic (spheroids on a chip) devices have also been developed7–14 to increase spheroid formation efficiency, offer better control of spheroid sizes, as well as simplify handling procedures. Many of these techniques, however, still suffer from problems such as long-term culture and device compatibility with drugs. Most importantly, these techniques are often not compatible with existing liquid handling robots for performing high-throughput screening (HTS). In this paper, we describe a 384-well format spheroid culture plate based on the scientifically proven but traditionally tedious hanging drop method. The developed hanging drop array platform allows for efficient formation of uniformly-sized spheroids, their long-term culture, and drug testing using existing HTS instruments (e.g. liquid handling robots and plate readers) (Fig. 1d). Utilizing this platform, we show that drugs with different modes of action produce distinct responses in the physiological 3D cell spheroids compared to conventional 2D cell monolayers.
Fig. 1.
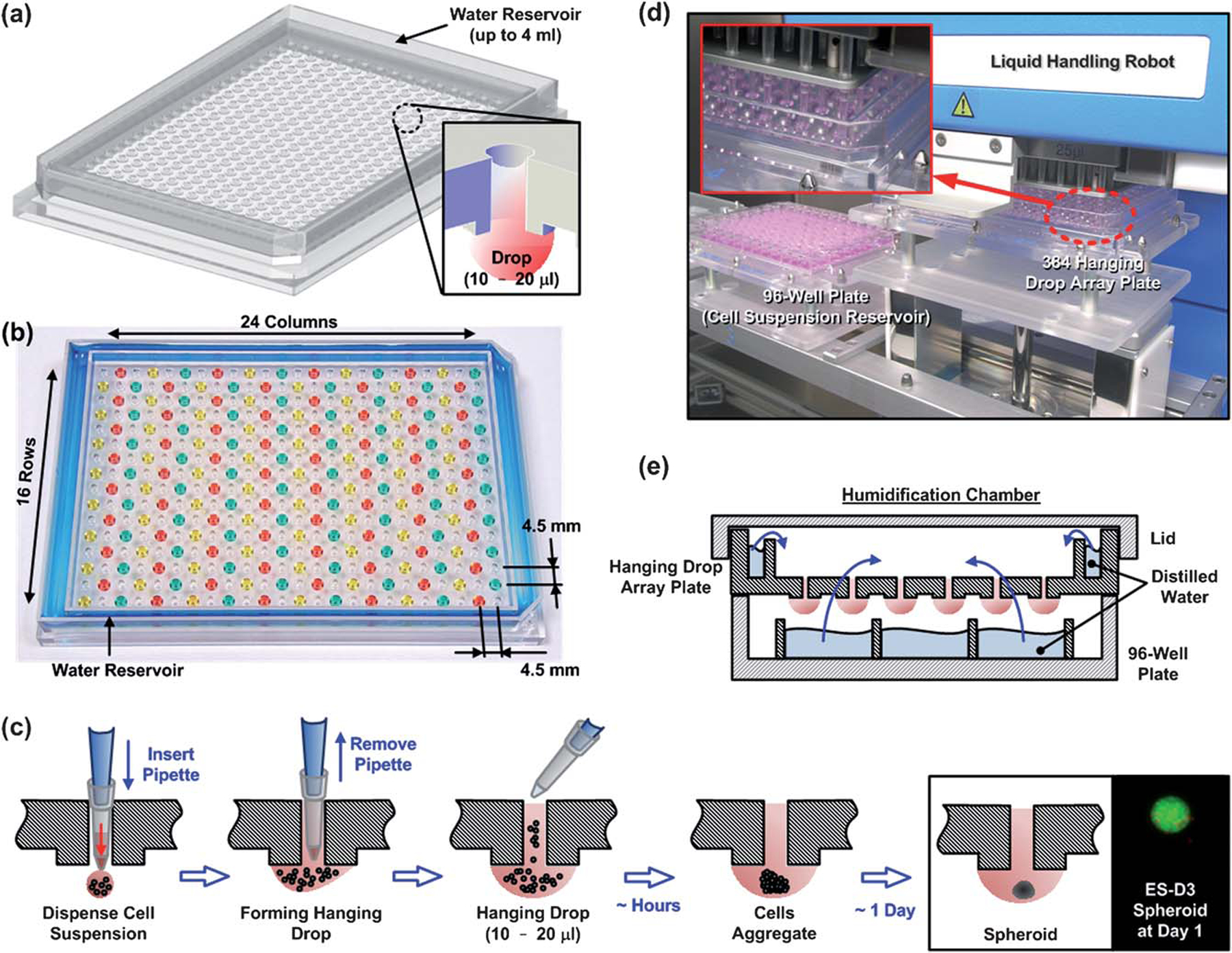
(a) Illustration of the designed 384 hanging drop spheroid culture array plate, and its cross-sectional view. (b) Photo and key dimensions of the array plate. (c) Cartoon of the hanging drop formation process in the array plate. The pipette tip is first inserted through the access hole to the bottom surface of the plate, and cell suspension is subsequently dispensed. Cell suspension is quickly attracted to the hydrophilic plate surface and a hanging drop is quickly formed and confined within the plateau. Within hours, individual cells start to aggregate and eventually form into a single spheroid around 1 day. (d) Photo of the 384 hanging drop array plate operated with liquid handling robot capable of simultaneously pipetting 96 cell culture sites. (e) Cartoon of the final humidification chamber used to culture 3D spheroids in the hanging drop array plate. The 384 hanging drop array plate is sandwiched between a 96-well plate filled with distilled water and a standard-sized plate lid. Distilled water from the bottom 96-well plate and the peripheral water reservoir prevent serious evaporation of the small volume hanging drops.
Experimental
Plate design, fabrication, and hanging drop formation
The hanging drop array plate is made of polystyrene, and fabricated by injection molding. To overcome the drawback in liquid handling and substrate inversion of the conventional hanging drop method, each cell culture site has an access hole (diameter = 1.6 mm) through the substrate with a plateau on the bottom surface (diameter = 3mm, height = 0.5 mm) (Fig. 1a). These cell culture sites are arranged in the standard 384-well plate format (16 rows, 24 columns, and 4.5 mm apart in both directions as shown in Fig. 1b). To alleviate the commonly encountered evaporation problem with the small volume hanging drops (tens of μl), a water reservoir is constructed around the periphery of the culture sites (Fig. 1a, b, and e).
Prior to usage, a hydrophilic coating (0.1%, Pluronic F108, BASF Co., Ludwigshafen, Germany) is applied onto the entire plate surface. The plate is subsequently UV sterilized before cell seeding. To form hanging drops, cell suspension solution is pipetted from the top side through the access holes with the end of each pipette tip inserted into the access hole to guide the sample liquid to the bottom surface (Fig. 1c). The liquid or cell samples can also be removed from the drop through the access holes using pipettes or slot pins (V&P Scientific, Inc., San Diego, CA). The size of the hanging drop is confined by the diameter of the plateau on the bottom surface.
General cell culture
To investigate the stability of long-term hanging drop spheroid culture using the designed array plate, osmolality measurements were performed while culturing three types of cells: African green monkey kidney fibroblast cell (COS7), murine embryonic stem (mES) cell (ES-D3), and human epithelial carcinoma cell that stably express mesothelin (A431.H9).15 Prior to performing hanging drop culture using the plate, ES-D3 cells were cultured in dishes coated with 0.1% w/v porcine gel (Sigma-Aldrich Co.) and maintained in medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco 11960, Invitrogen Co., Carlsbad, CA) with 15% v/v fetal bovine serum (FBS) (Gibco 10082, Invitrogen Co.), 4 mM L-glutamin (Invitrogen Co.), 0.1mM 2-mercapto-ethanol (Sigma-Aldrich Co.), 0.02% v/v sodium pyruvate (Sigma-Aldrich Co.), 100 U ml-1 penicillin (Invitrogen Co.), 100 U ml-1 streptomycin (Invitrogen Co.), and 1000 U ml-1 ESGRO (Invitrgoen Co.) which contains leukemia inhibitory factor (LIF). COS7 and A431.H9 cells were cultured in DMEM (Gibco 11965, Invitrogen Co.) with 10% v/v FBS (Gibco 10082, Invitrogen Co.), and 1% v/v antibiotic-antimicotic (Gibco 15240, Invitrogen Co.). All the cells were cultured in a humidified incubator (37 °C in an atomosphere of 5% CO2). Cell suspensions for the hanging drop experiments were made by dissociating cells with 0.25% trypsin-EDTA (Gibco 25200, Invitrogen Co.), centrifugation of dissociated cells at 1000 rpm for 1 min at room temperature, and re-suspended in growth media. Cell density was estimated using a hemocytometer.
Hanging drop spheroid culture, culture media exchange, and osmolality measurement
On the spheroid culture plate, a 15 μl cell suspension was dispensed into the access hole at each cell culture site to form a hanging drop (Fig. 1c). In order to prevent evaporation, 4 μl of distilled water was added into the peripheral water reservoir. In addition, the plate was sandwiched by a well-plate lid and a 96-well plate filled with distilled water, and wrapped using Parafilm (Fig. 1e). The growth media was exchanged every other day by taking 5 μl solution from a drop, and adding 7 μl fresh growth media into a drop. For the osmolality measurement, 10 μl sample solution was pipetted out from a drop and transferred to a vapor pressure osmometer (Vapro Model 5520, Wescor Inc., Logan, UT) for analysis.
Anticancer drug sensitivity testing
For demonstration of anticancer drug sensitivity testing, A431.H9 spheroids at three different sizes (300, 1500, and 7500-cell spheroids) were tested under the effect of two types of drug—tirapazamine (TPZ) (Toronto Research Chemicals Inc.) and 5-fluorouracil (5-FU) (Sigma-Aldrich Co.). According to the procedure mentioned above, A431.H9 spheroids at the specified cell numbers were formed, and their growth media were exchanged every other day. TPZ and 5-FU stock solutions of four times the final testing concentrations (0, 0.1, 1, 10, 100, 1000, 5000 μM) were initially prepared in Dulbecco’s phosphate buffered saline (D-PBS) (Gibco 14190, Invitrogen Co.). On day 2 of A431.H9 spheroid culture, 5 μl of the appropriate concentration of TPZ (or 5-FU) stock solutions were subsequently added to each of the 15 μl A431.H9 cell hanging drop droplets to generate 20 μl hanging drops of cells with drugs. Cellular viability was monitored at 24, 48, 72, and 96 h of drug incubation using alamarBlue (DAL1025, Invitrogen Co.). Following manufacturer’s protocol, 2 μl (one-tenth of each hanging drop sample volume) of alamarBlue was added to each A431.H9 hanging drop spheroid sample and incubated for 2 h. Following incubation, each A431.H9 hanging drop spheroid sample plate was read using a plate reader (FLx800 Fluorescence Microplate Reader, BioTek Instruments Inc., Winooski, VT) at 525 nm excitation and 590 nm emission to obtain fluorescence intensity readouts. As the fluorescence intensity of alamarBlue is directly proportional to cell number (Fig. S1c), the average percent cell viability for each drug concentration could be calculated by normalizing to the 0 μM untreated spheroid control. The viability results achieved by the alamarBlue assay were further compared to the viability results obtained by fluorescence microscopy imaging using live/dead stain (LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells, L3224, Invitrogen Co.). The detailed comparison is shown in supplementary information and Fig. S1. Anticancer drug sensitivity experiments under 2D control conditions were performed in standard tissue culture treated 96-well plates (Corning Costar 3596, Corning Inc., Lowell, MA), with everything else being the same as the 3D spheroid experiments.
Results and discussion
Formation of hanging drops for spheroid culture
A schematic of the 384 hanging drop array plate is shown in Fig. 1a and an actual picture of the plate containing 192 hanging drops arranged in an alternating fashion is shown in Fig. 1b. The hanging drop spheroid culture sites are arranged in the standardized 384-well plate format with 16 rows and 24 columns separated by 4.5 mm apart in both directions. A water reservoir designed in the outer ring of the plate further holds up to 4 μl of water to alleviate evaporation problem (Fig. 1a, b, and e). The enlarged cartoon in Fig. 1a further shows the access hole on the top surface of the plate with a liquid droplet hanging and confined by the diameter of the plateau on the bottom surface. As a result, the geometry of the hanging drop can be kept consistent during the culturing process without spreading out, which leads to more robust and stable culturing conditions not possible on conventional flat hanging drop substrates. Fig. 1c illustrates the droplet and spheroid formation process in the 384 hanging drop array plate. After a cell suspension droplet is successfully formed, cells slowly aggregate in the bottom center of the droplet and eventually form into spheroid. The access holes allow direct manipulation of the droplets from the top, thus greatly simplifying the initial droplet formation and subsequent media exchange procedures by eliminating the tedious hanging drop culture dish inversion required in the conventional hanging drop method. Fig. 1d is a snapshot of the hanging drop formation process in the 384 hanging drop array plate by a commercially available liquid handler (CyBi-Well, CyBio Inc.).
Long-term culture of spheroids in hanging drops
In order to culture spheroids over long periods of time, the osmolality of the cell culture media in the hanging drops must be kept stable. Due to the small volume nature of the hanging drops, evaporation is inherently rapid and can cause large osmolality shifts in the culture media. In order to prevent this during spheroid culture, the 384 hanging drop array plate was sandwiched by a well-plate lid and a 96-well plate filled with distilled water, and the whole setup subsequently wrapped in parafilm (Fig. 1e). The water-filled 96-well plate directly on the bottom of the hanging drops provides significant humidification to the hanging drops. In addition, the water reservoir (Fig. 1a, b, and e) in the periphery of the plate further prevents serious evaporation from the hanging drops near the edges of the plate where droplets are more prone to evaporation. To investigate the long-term stability of the hanging drop spheroid cultures, osmolality measurements were performed. Fig. 2a shows a plot of the average osmolality of the COS7, mES, and A431.H9 cell culture media versus time over 7 to 12 days. With exchange of approximately 30% of the culture media every other day, the osmolality of the media was kept in the optimal culture range of 300 to 360 mmol/kg.16–18 Fig. 2b shows the live/dead images of the COS7 and mES cell spheroids, indicating that most cells (>90%) were still alive after 12 days of culture. Fig. 2c shows that A431.H9 spheroids of various initial sizes are still proliferating over a 7-day culture period. The ease of media exchange and stability of the drop geometry enabled by the inverted plateau structures of the custom 384 drop plate allow for convenient long-term spheroid culture in ways not possible with the conventional hanging drop culture method.
Fig. 2.
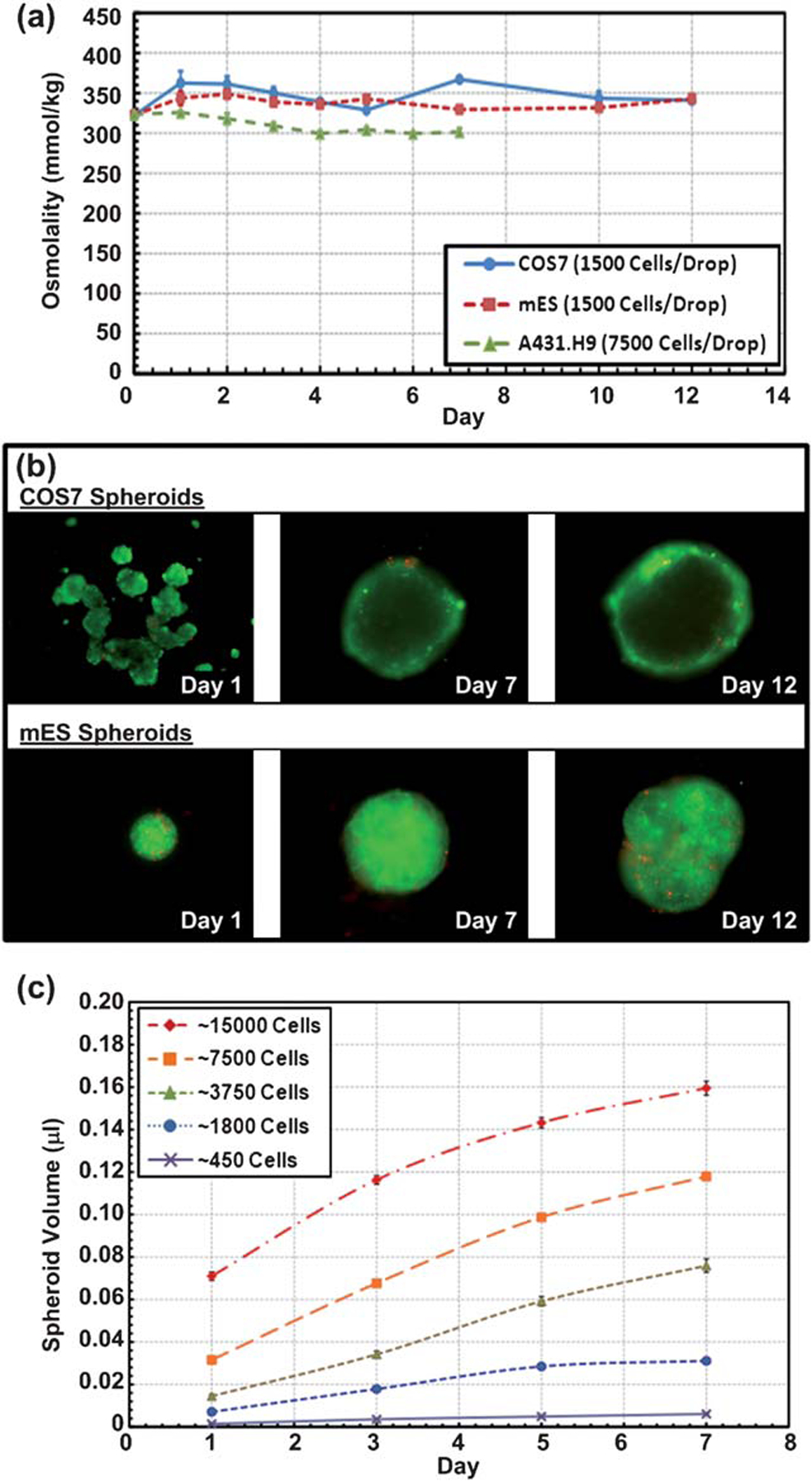
(a) Osmolality of COS7, mES, and A431.H9 cell spheroids with various cell populations over a 7- and 12-day culture. Data are expressed as the mean s.e.m. (b) Fluorescence images of live/dead stained COS7 and mES cell spheroids over a 12-day culture. (c) Volume of A431.H9 spheroids over a 7-day culture for various initial cell numbers per spheroid. n = 14 for each initial cell number condition. Data are expressed as the mean ± s.e.m.
Anticancer drug sensitivity testing
To analyze cell-based assay capability, an anticancer drug sensitivity test was performed using 2 drugs with distinctly different activity profiles: a conventional anticancer drug 5-fluorouracil (5-FU, Sigma-Aldrich Co., St. Louis, MO) that inhibits cellular proliferation,19 and a hypoxia-triggered cytotoxin tirapazamine (TPZ, Toronto Research Chemicals Inc., Ontario, Canada) that causes DNA damage,20 on A431.H9 cells under both 2D and 3D spheroid culture conditions. Fig. 3a shows cell viability at 10 μM 5-FU 96 h after drug treatment for 7500-cell A431.H9 spheroids and 2D culture condition. At the same 5-FU concentration, there is only 5% viability relative to untreated control for 2D cultures, but still 75% viability relative to control for 3D spheroids. This clearly shows that A431.H9 cells are more resistant to 5-FU in 3D than 2D cultures. Fig. 4a and b further show that the IC50 of A431.H9 cells cultured in 2D condition is about 0.1 μM, while the IC50 of the A431.H9 3D spheroids is more resistant with an IC50 of 1 to 100 μM. Due to the 3D integrity of spheroids, it is more difficult for 5-FU to diffuse and penetrate into the center cell mass. Furthermore, 5-FU specifically targets proliferating cells, and thus would not kill the quiescent cells in the spheroids. Whereas in 2D monolayer cultures, cells proliferate at a faster rate and thus 5-FU inhibits cellular growth more effectively.
Fig. 3.
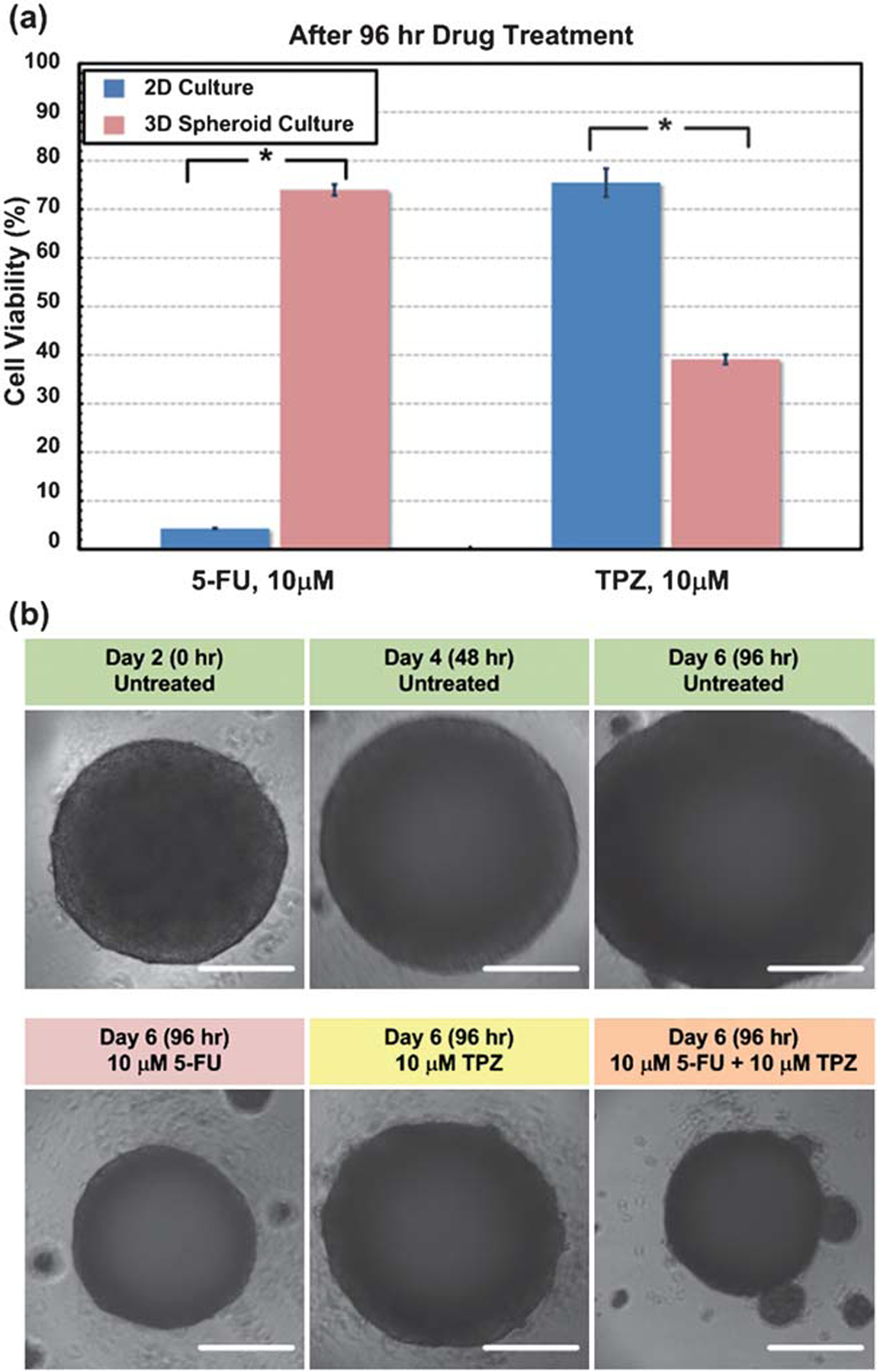
(a) Bar graph of the cell viability at 10 μM 5-FU, and 10 μM TPZ 96 h after drug treatment for 2D A431.H9 monolayer culture and 7500-cell A431.H9 3D spheroid culture conditions. For both drugs, the viability of A431.H9 cells was statistically different between 2D monolayer and 3D spheroid culture conditions. Statistical significance is determined by two-tailed Student’s t-Test (*, P < 0.01) P = 1.75 × 10−16 for 5-FU, P = 1.22 × 10−6 for TPZ. n = 8 for 2D culture condition and Data are expressed as the n = 14 for 3D spheroid culture condition. Mean × s.e.m. (b) Time-lapse images of control untreated 7500-cell A431.H9 spheroid, and spheroids treated with 10 μM 5-FU, 10 μM TPZ, and 10 μM 5-FU + 10 μM TPZ 96 h after treatment. Scale bar is 200 μm.
Fig. 4.
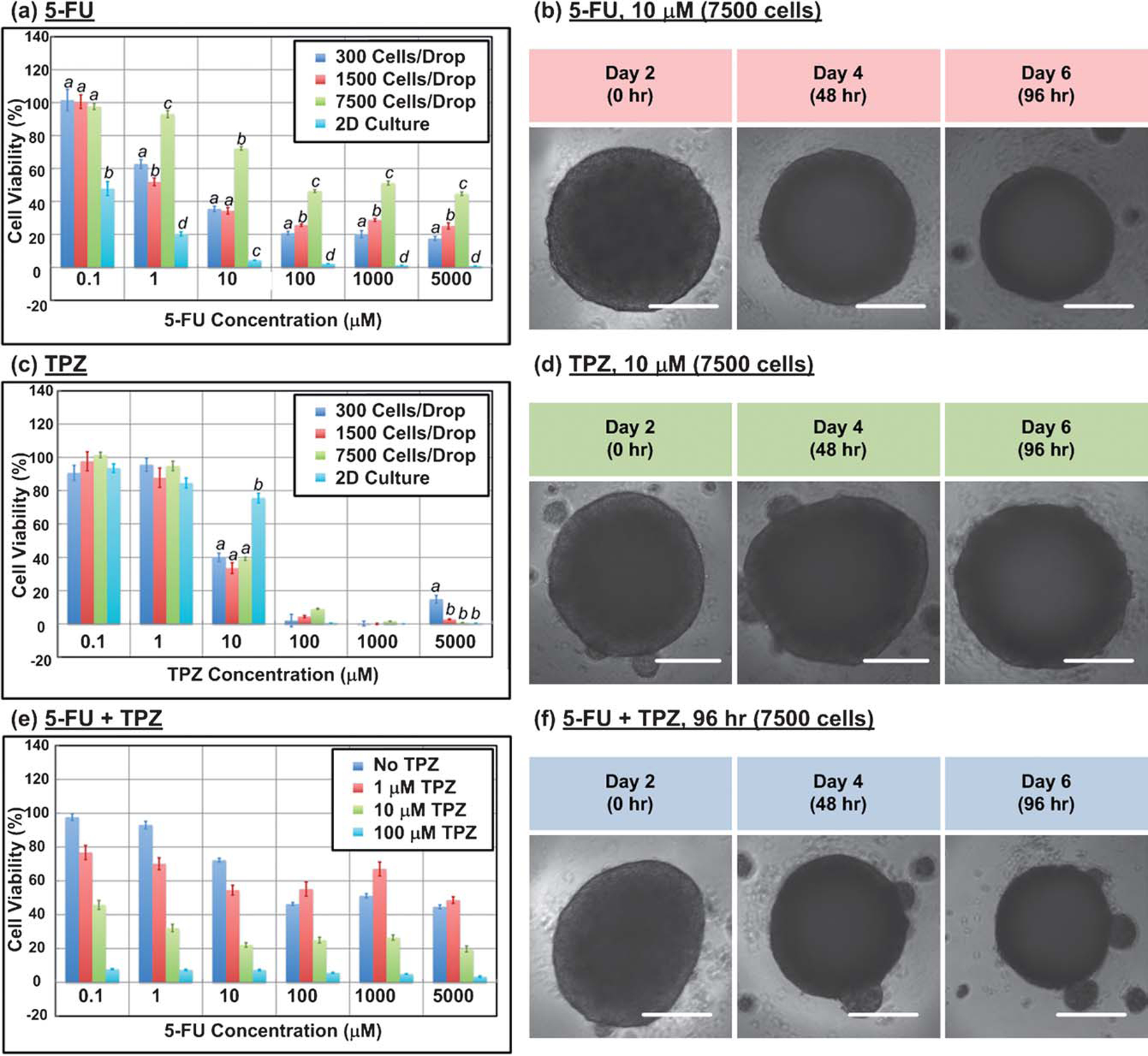
(a) Bar graph of the cell viability at various 5-FU concentrations 96 h after drug treatment for 300, 1500, and 7500-cell A431.H9 spheroids and 2D culture condition. Different letters between culture conditions (spheroid size or 2D) within a 5-FU concentration represent a significant difference between the spheroid sizes or 2D (a, b, c, d = p < 0.01). (b) Time-lapse images of 7500-cell A431.H9 spheroids treated with 10 μM 5-FU. (c) Bar graph of the cell viability at various TPZ concentrations 96 h after drug treatment for 300, 1500, and 7500-cell A431.H9 spheroids and 2D culture condition. Different letters between culture conditions (spheroid size or 2D) within a TPZ concentration represent a significant difference between the spheroid sizes or 2D (a, b = p < 0.01). (d) Time-lapse images of 7500-cell A431.H9 spheroids treated with 10 μM TPZ. (e) Bar graph of the cell viability at various 5-FU concentrations 96 h after drug treatment for 7500-cell A431.H9 spheroids with 0, 1, 10, and 100 μM TPZ. (f) Time-lapse images of 7500-cell A431.H9 spheroids treated with 10 μM 5-FU + 10 μM TPZ. Statistical analysis was performed by ANOVA followed by Holm-Sidak tests. Spheroid size or 2D groups that are statistically significantly different are designtated with different letters (a, b, c, d). n = 8 for 2D culture condition and n 14 for 3D spheroid culture condition. Data are expressed as the mean ± s.e.m. Scale bar is 200 μm.
In contrast, TPZ is a hypoxia-activated cytotoxin. Fig. 3a shows that at 10 μM TPZ 96 h after drug treatment, there is still 75% viability relative to control for 2D cultures, but only 40% viability for 7500-cell A431.H9 spheroids. The IC50 of A431.H9 cells cultured in 2D is about 50 μM, while the IC50 of the A431.H9 3D spheroids for all 3 sizes is about 8 μM (Fig. 4c and d). Here, A431.H9 cells are more resistant to TPZ when cultured under 2D rather than 3D conditions. This is likely because TPZ is activated more in spheroids where active oxygen consumption by cells and limits in diffusive oxygen transport creates a hypoxic core similar to actual solid tumors.21 Such distinct cellular responses from the same cells to the same drugs tested under 2 different culture conditions highlights the importance of using 3D models in drug screening and testing. Statistical analysis ANOVA followed by pairwise comparisons between the culture conditions (spheroid sizes or 2D) using Holm-Sidak tests were performed for each 5-FU and TPZ concentration groups. The statistically significantly different groups are shown in Fig. 4a and c.
Finally, we performed combination drug treatment (5-FU and TPZ) on the 7500-cell A431.H9 spheroids. The combined treatment has an additive trend. The viability is 75% and 40% for spheroids treated with 10 μM of 5-FU and 10 μM of TPZ, respectively (Fig. 3a). But the viability decreased to only 20% when the spheroids were under combined treatment of 10 μM 5-FU and 10 μM TPZ (Fig. 4e). The additive effect is reasonable since 5-FU is an anti-proliferation drug that targets proliferating cells in the peripheral layers of spheroids and TPZ is a hypoxic drug that kills cells in the hypoxic core of spheroids.
Conclusions
We describe the design and fabrication of a high-throughput and versatile 384 hanging drop array plate for cellular spheroid formation, culture, and drug testing. The platform greatly simplifies the proven but traditionally inconvenient hanging drop culturing method in a format that is compatible with existing liquid handling robots. Anticancer drug sensitivity testing on A431.H9 cells show that cytotoxicity can be drastically different in the physiological 3D spheroids formed in the 384 hanging drop array plates compared to 2D monolayer cultures in conventional multiwell plates. Although this study focused on response of cancer spheroids, the user-friendly high-throughput 3D culture system is applicable to multiple cell types. We believe the platform will be valuable in a wide range of studies where 3D spheroid cultures and high-throughput multiplexing is needed.
Supplementary Material
Acknowledgements
We thank Dr Keisuke Suzuki for helpful discussion. This material is based upon work supported by the Coulter Foundation, the College of Engineering Translational Research Fund, and gifts from Jacque Passino. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Electronic supplementary information (ESI) available: Viability Validation assay and Fig. S1.
Notes and references
- 1.Pampaloni F, Reynaud EG and Stelzer EHK, Nat. Rev. Mol. Cell Biol, 2007, 8, 839–845. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich J, Seidel C, Ebner R and Kunz-Schughart LA, Nat. Protoc, 2009, 4, 309–324. [DOI] [PubMed] [Google Scholar]
- 3.Kunz-Schughart LA, Freyer JP, Hofstaedter F and Ebner R, J. Biomol. Screening, 2004, 9, 273–285. [DOI] [PubMed] [Google Scholar]
- 4.Kubbies AIM, Biomol J. Screening, 2006, 11, 922–932. [DOI] [PubMed] [Google Scholar]
- 5.Del Duca D, Werbowetski T and Del Maestro RF, J. Neuro-Oncol, 2004, 67, 295–303. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich J, Ebner R and Kunz-Schughart LA, Int. J. Radiat. Biol, 2007, 83, 849–871. [DOI] [PubMed] [Google Scholar]
- 7.Torisawa Y, Chueh BH, Huh D, Ramamurthy P, Roth TM, Barald KF and Takayama S, Lab Chip, 2007, 7, 770–776. [DOI] [PubMed] [Google Scholar]
- 8.Sakai Y and Nakazawa K, Acta Biomater, 2007, 3, 1033–1040. [DOI] [PubMed] [Google Scholar]
- 9.Ungrin MD, Joshi C, Nica A, Bauwens C and Zandstra PW, PLoS One, 2008, 3, e1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee WG, Ortmann D, Hancock MJ, Bae H and Khademhosseini A, Tissue Eng., Part C, 2010, 16, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torisawa Y, Takagi A, Nahimoto Y, Yasukawa T, Shiku H and Matsue T, Biomaterials, 2007, 28, 559–566. [DOI] [PubMed] [Google Scholar]
- 12.Wu LY, DiCarlo D and Lee LP, Biomed. Microdevices, 2008, 10, 197–202. [DOI] [PubMed] [Google Scholar]
- 13.Toh YC, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, van Noort D, Hutmacher DW and Yu H, Lab Chip, 2007, 7, 302–309. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, Blumling J, Wang CF, Kohane DS and Langer R, Biomaterials, 2006, 27, 5259–5267. [DOI] [PubMed] [Google Scholar]
- 15.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T and Pastan I, Clin. Cancer Res, 2005, 11, 3814–3820. [DOI] [PubMed] [Google Scholar]
- 16.Ozturk SS and Palsson O, Biotechnol. Bioeng, 1991, 37, 989–993. [DOI] [PubMed] [Google Scholar]
- 17.Zhou W, Chen CC, Buckland B and Aunins J, Biotechnol. Bioeng, 1997, 55, 783–792. [DOI] [PubMed] [Google Scholar]
- 18.Takagi M, Hayashi H and Yoshida H, Cytotechnology, 2000, 32, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valeriote F and Santelli G, Pharmacol. Ther, 1984, 24, 107–132. [DOI] [PubMed] [Google Scholar]
- 20.Peters KB, Wang H, Brown JM and Iliakis G, Cancer Res, 2001, 61, 5425–5431. [PubMed] [Google Scholar]
- 21.Sutherland RM, Science, 1988, 240, 177–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


