Abstract
Objectives:
Public health policies are often enacted without adequate consideration of the existing market structure or their impacts on that market structure. This paper provides context for the potential impact of regulations on nicotine vaping products (NVP) use by providing a structural analysis of competition in the US NVP market before FDA regulation.
Methods:
A literature review was conducted with the aim of providing a framework for analysis that: 1) defines the market; 2) evaluates market concentration; 3) identifies entry barriers; and 4) examines firm conduct.
Results:
The NVP market includes retail, internet sellers and vape shops. Although conventional retail became more concentrated after the major cigarette companies entered the NVP market, the vape shop and internet sectors remain substantially less concentrated, producing an overall low market concentration, with few entry barriers and competitive behavior.
Conclusions:
The largely unregulated US NVP market has been highly competitive, with a high degree of innovation. However, new FDA deeming regulations as applied to NVPs could make it difficult for smaller companies to remain in the market and could discourage new companies and new product innovations from entering the market.
Keywords: e-cigarette, vaping, industry, market competition
INTRODUCTION
The use of nicotine vaping products (NVPs), including e-cigarettes, has grown rapidly in the US.1,2 While there has been debate about whether NVPs should be considered tobacco products,3 they are defined and regulated as such under the Tobacco Control Act and are part of a newly shifting nicotine-delivery landscape. NVPs have been shown to efficiently deliver nicotine4,5 and provide sensorimotor experiences and “throat-hit” similar to cigarettes.6 By 2015, at least 40% of US adult smokers had tried NVPs and 10% were current users.7,8
NVPs were initially manufactured and marketed by companies not in the cigarette business (“independents”), such as NJOY.9 The independents had strong incentives to market their NVPs as a substitute for cigarettes.10 Starting in 2012, however, the major cigarette companies entered the NVP market; Lorillard acquired Blu and Japan Tobacco acquired Logic, while RJ Reynolds and Phillip Morris developed their own e-cigarettes.2,11 With high cigarette profits and established customer brand loyalties,12 the cigarette companies, unlike independents, have financial incentives to protect their cigarette sales from being replaced by NVPs.13 They have emphasized improved technology and avoiding smoke-free air laws in their marketing of NVPs,14 while de-emphasizing possible benefits of NVPs for smoking cessation.15 Accordingly, some observers are concerned that cigarette companies’ involvement in the NVP market may reduce their potential as harm-reducing smoking substitutes.16,17
The FDA’s deeming rule made NVPs subject to the US Tobacco Control Act and FDA’s related tobacco control regulations as of August 2016.18 FDA’s deeming rule prevents new or significantly changed NVPs from entering the US market without formal permission from the FDA, and existing NVPs must submit a pre-market application by August 2022 to remain on the market. The deeming rule also prohibits marketing of NVPs with reduced-risk claims without permission from the FDA, banned NVP sales to those under age 18, and prohibited distribution of free samples. These requirements may affect product innovation, availability, marketing and pricing in the NVP market, as well as alter the role of cigarette companies relative to independents. Because most NVP purchasers are adult current or former smokers,7,8 the changes prompted by FDA’s regulation of NVPs are likely to have an impact on cigarette smoking.10,19
To provide insight into how FDA’s regulation of NVPs could change the NVP market and its impact on cigarette sales and smoking, this paper offers an economic analysis of competition in the US NVP market. With FDA regulations still in transition, we focus on the largely unregulated market prior to deeming to focus on the role of competition in that market. No prior peer-reviewed study has provided such analysis.
METHODS
We adopt a competitive analysis framework based on the Horizontal Merger Guidelines used by the US Federal Trade Commission and Department of Justice,20 which includes: 1) define the relevant market; 2) evaluate market concentration; 3) identify entry barriers; and 4) examine firm conduct.
We apply information available in published sources, such as the Surgeon General’s Report,2 and industry analyses, including by stock market analysts. Since formal studies have not been conducted of the NVP market, we do not attempt to conduct a structured review, but consider studies that provide information on different aspects of the market. Since the information is limited, we suggest the information needed to better gauge market impact.
THE RELEVANT MARKET
From an economic perspective, the relevant market is defined in order to determine the relevant arena for competition and the potential for market power. Market power is gauged by the ability to raise price despite related declines in sales.20 The potential loss in sales depends on whether customers are willing and able to switch to other products.
The Relevant Product
NVPs can be distinguished by at least three physical product dimensions;type, flavors, and other constituents.5,21,22
The three broad classes of NVP products are disposables, closed reusable systems, and open reusable systems. All systems require liquid and a core device that vaporizes the liquid. Disposables include the self-contained device and the liquids as closed systems. They are discarded after the liquid is used, and often resemble cigarettes (“cigalikes”). Closed, reusable systems follow a ‘razor and blade’ model, where customers purchase a core device and then purchase refill portions (“cartridges”) that are generally tied to that manufacturer’s device. Thereby, firms can sell the device at a lower price and profit from refills. These systems tend to have limited options (eg, users cannot adjust heating temperature). Many of the reusable systems were designed to look like cigarettes, but recent devices such as the Juul use USB-shaped, closed pod systems. Open systems have a tank or reservoir that the user can fill with liquids of their choice. Although up-front costs of a closed system are typically lower, long-run costs may favor open system NVPs because of lower costs for refill liquids.2 These devices often allow options for customization, e.g., to adjust the heat, amount of nicotine used, and mix flavors. At present, cigarette companies only sell closed reusable systems (eg, Vuse, MarkTen), while independents sell closed and open systems.15
Flavors are an important product feature for many vapers,23,24 and may impact use. Open system devices allow for greatest variety, since they are not confined to specific flavors provided by the device manufacturer and the cost of producing greater flavor variety is less.
The amount of other constituents, such as nicotine or certain toxicants, received by users varies with the device, the flavors25,26 and the way that the device is used.27,28 NVPs share the common characteristic of delivering nicotine into the respiratory system, without any combustion of tobacco, and thereby provide reduced risk. Although the long-term health risks of vaping have yet to be characterized, biomarker data indicate that NVPs are less harmful than smoking cigarettes.29–31 Not surprisingly, vapers generally perceive NVPs to be less harmful than cigarettes, while non-vapers express more concern about the toxicity of using NVPs.5,32,33
Distinct preferences have been found for the different types of vaping devices,6,34,35 especially by smokers who are considering quitting or have recently quit smoking.36 Those trying to quit smoking often prefer open systems (“they don’t look like cigarettes”).37 However, while regular NVP consumers may prefer a particular vaping device, they often switch between closed and open systems,4 suggesting direct competition between products. Another indication of direct competition is the high levels of responsiveness to price found for disposable and non-disposable NVPs individually and the high cross-price responsiveness between products.38,39 Consumers do not appear to have sorted themselves into different groups that each buy only certain types of NVPs;37 implying that specific submarkets have not clearly emerged.
Thus, the current market can be defined as all NVPs, including disposables, closed reusables, and open systems. Although NVPs often compete against cigarettes, the difference in risks between inhaling nicotine without combustion and inhaling combusted tobacco suggests that NVPs can be viewed as lower-risk products. In addition, the variety of product styles and flavors adds to the appeal of NVPs as distinct from cigarettes, which are relatively uniform in design with limited flavor options. While we focus on the NVP market as separate from cigarettes, NVPs have become increasingly substitutable for cigarette consumers. In addition, the relevant market could expand if new types of non-combustible nicotine-delivery products, such as heat-not burn (HNB) products, are able to secure FDA permission to be marketed.
Firms Involved in the Production and Sale of NVPs
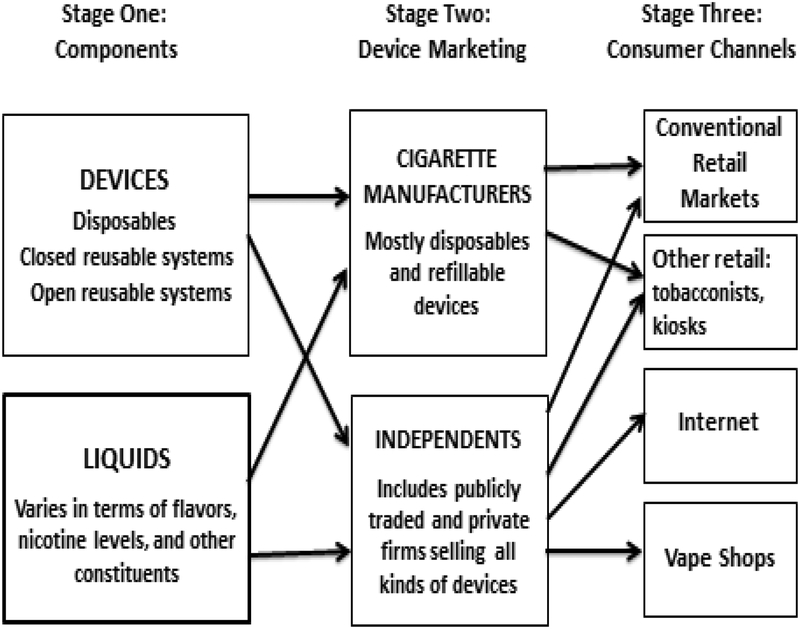
The industry is composed of firms involved at different stages in the production and sale of NVPs (eg, different levels of vertical integration).40 NVPs are produced and marketed in three stages: 1) production of vaping devices and liquids; 2) marketing of products; and 3) distribution and sale to consumers via the internet, vape shops and other retail (see Figure 1).
Figure 1.
Structure of the Vaporized Nicotine Product Industry
Vaping devices are often produced in China,2 but some companies, such as BAT and Altria, manufacture their product in the US. Liquid refills for open systems are usually sold in bottles, and refills for closed systems typically take the form of cartridges. Some liquids are produced in China, but are increasingly from other countries, including the US.2 For open systems, mixtures of nicotine and flavoring may be prepared by vape shops or the individual consumer.
Unlike the cigarette market, in which manufacturers primarily sell through wholesalers to bricks-and-mortar retailers, NVPs were first sold exclusively by internet retailers as early as 2006. Purchase channels expanded to shopping mall kiosks and more conventional retail outlets between 2009 and 2012, with firms selling mostly disposable and rechargeable cigalikes through conventional retail.2,41 By 2013, some retailers began selling tank-style devices;41 but vape shops played an increasing role in the market.2,42,43 Vape shops have provided a variety of brands and flavors (although mostly brands sold by independents), and serve as a point where consumers can discuss products, try samples, and get individualized product information.2,17,44–46
Table 1 provides an overview of the NVP purchase channels,47 distinguishing e-cigarettes (disposables and closed reusables) from vaporizers (tanks and mods). In 2017, sales were estimated at $1.4 billion in conventional retail (convenience store, food, drug and grocery), $1.4 billion in online and other retail (kiosks and tobacconists), and $1.8 billion in vape shops. Both e-cigarettes and vaporizers are sold through retail and online, while cigarette companies currently sell NVPs mostly in conventional retail market.
Table 1.
Estimated Sales of the US Vapor Market (In millions)
| Purchase Channel Categories | 2014 | 2015 | Market Shares 2015*** | 2016 | 2017 | Market Shares 2017*** | 2018 expected | Market Shares 2017*** |
|---|---|---|---|---|---|---|---|---|
| E-cigarettes (Disposables and Closed Systems) | 1,000 | 1,400 | 42.4% | 1,600 | 1,400 | 31.8% | 3,800 | 57.6% |
| Mass Market Retail* | 600 | 600 | 18.2% | 700 | 700 | 15.9% | 2,600 | 39.4% |
| Online | 200 | 400 | 12.1% | 500 | 400 | 9.1% | 800 | 12.1% |
| Other Retail** | 200 | 400 | 12.1% | 400 | 300 | 6.8% | 400 | 6.1% |
| Vapors/Tanks/Mods & Personal Vaporizers (Open System) | 1,500 | 1,900 | 57.6% | 2,500 | 3,000 | 68.2% | 2,800 | 42.4% |
| Mass Market Retail* | 300 | 300 | 9.1% | 500 | 500 | 11.4% | 400 | 6.1% |
| Online and Other Retail | 300 | 400 | 12.1% | 600 | 700 | 15.9% | 600 | 9.1% |
| Vape Shops | 900 | 1,200 | 36.4% | 1,400 | 1,800 | 40.9% | 1,800 | 27.3% |
| Total | 2,500 | 3,300 | 100.0% | 4,100 | 4,400 | 100.0% | 6,600 | 100% |
Also called conventional retail, includes convenience, food, and drug stores
includes tobacconists and kiosks
calculated as a percent of the total
Source: Wells Fargo Securities Equity Research51
While consumers using each of these purchase channels may have different smoking and socio-demographic characteristics, consumers switch across the channels. Consumers often learn about NVPs through the internet.48,49 Many users, especially regular users,50 continue to buy over the internet, although some use vape shops and other retail outlets. In addition, products of the same manufacturer may be sold over each of the three channels. Almost half of online vendors also sold in retail settings, and 60% of online vendors offered wholesale opportunities for their products.51 Vape shops may also be associated with an internet business.52
Although liquids are part of the NVP market, entry barriers are more likely to occur in producing vaping devices because their production is more complicated and specialized than for liquids. As described below, much of the advertising and marketing is by device sellers. Accordingly, our analysis of market competition focuses on competition among device sellers. However, purchases by consumers of devices may occur directly via the internet or mail order sales, or indirectly through vape shops, conventional or other retail. With the competition between channels, the relevant consumer market is vaping device sales in all purchase channels.
INDUSTRY CONCENTRATION
The potential influence of individual firms in a market is indicated by their respective market shares (ie, their sales relative to total industry sales). However, the ability of a single firm or small group of firms to exert monopoly power depends on overall market concentration. Concentration is often measured as the summed market shares of the largest four firms or the sum of the squared market shares of all firms in the industry.20
Industry Concentration Among Vaping device Sellers
Focusing on closed systems, the 2016 Surgeon General’s Report on e-cigarettes2 lists three primary groups of vaping device sellers in the US market: 1) four cigarette companies that also sell NVPs, 2) seven independent public companies, and 3) 22 independent privately-owned NVP companies, including NJOY, Ballantyne Brands, CB Distributors, International Vapor Group, and VMR. Although this tally may have missed some smaller companies, at least 33 (4+7+22) firms sold vaping devices as of early 2015, despite a spate of acquisitions.2
Conventional Retail Sales
Conventional, mass market retail (including food, drug and convenience stores) e-cigarette sales, were initially dominated by independent firms (21st Century, NJOY, Mistic and Logic).2 This segment of the market was highly concentrated in 2010, but less so by mid-2012.2 The major cigarette companies then began entering the market, in some cases by acquiring existing firms (eg, blu by Lorillard). In late 2013, Reynolds brought VUSE onto the conventional retail market with aggressive advertising and price discounts.2 By the end of 2014, VUSE became the market leader in conventional retail, with its share reaching 36% in late 2015.47 Altria began marketing MarkTen in 2014, with its conventional retail share reaching 16% by the end of 2015.47
Total conventional retail dollar sales of e-cigarettes (not including open systems), doubled each year from 2011 through 2014, and then fell between 2015 and 2017.1 By the end of 2014, the top five NVP brands accounted for more than 87% of conventional retail,2 with NVPs sold by the major cigarette companies (Blu, Vuse and MarkTen) accounting for 63% of the market, and the major NVPs sold by independent firms accounting for 24% (7% NJOY and 17% Logic). The cigarette companies’ combined share in conventional retail reached 72% in 2015 with JTI’s acquisition of Logic.51
While the large cigarette companies controlled 72% of conventional retail in 2015, that sector accounted for only 27% [(900)/3300] of the total NVP market, where 900 is total mass market and 3300 is total sales (see Table 1), and the major cigarette companies’ NVP sales at vape shops or via the Internet were relatively small. Accordingly, their share of the total NVP market was less than 20% (72% x 27%). In addition, while shares of individual independent firms were all less than 5% of conventional retail in 2015, they remained viable with a combined 25% market share in conventional retail.
Due to the availability of Nielsen data, sales figures are often presented only for conventional retail, but disposable and closed systems sales from conventional retail represent only 15.9% and open systems from conventional retail represented 11.4% of the whole NVP market in 2017. While cigarette companies had a significant share of conventional retail, the level of concentration for conventional retail alone would not signal the potential for monopoly behavior even if that market were independent of other channels.
Vape Shops
Vape shops mostly sell products of independents rather than cigarette companies,17,52 reflecting an anti-cigarette orientation of many vape shops.53,54 Estimates of the number of vape shops in the US have varied due to growth in this sector, and the lack of an accepted definition2 and uniform registration system.55 The 2016 Surgeon General’s Report2 offers a low-end estimate of 3,500 vape shops (2013),43 with intermediate estimates of 6,000–15,000 (2015),56,57 and a high estimate of 35,000 vape shops (2014).17 The American Vaping Association estimated 10,000 to 15,000 vape shops in 2014.58 However, like conventional retail, NVP consumers are likely to frequent vape shops within a reasonable distance of their home or workplace. While there may be less availability of shops in their local area, especially more rural areas,55 vape shops add to the potential purchase channels for many, if not most consumers.
Internet
In those areas with few vape shops (eg, rural areas), the internet may be a more convenient source for NVPs. Internet-using consumers in the US have ready access to both domestic and international firms. While data is scarce and difficult to obtain, a recent study59 found nearly 400 internet NVP vendors with 3-fold growth between 2013 and 2014. Most sold disposables or closed systems, but many were transitioning to tanks and mods. Studies50,60 have found substantial variety in pricing and marketing practices of online sellers.
Summary
With conventional retail only a portion of overall NVP sales and minimal sales outside of that sector, the share of the NVP market held by cigarette companies has been limited. Shares of other vaping device manufacturers are mostly unknown, but 29 other mostly closed system firms were identified as of 2015.2 Many open system companies had yet to be identified. Thus, overall NVP market concentration by 2017 was certainly low. Besides the relatively large number of firms, new firms may enter the market and grow rapidly.
Some information on NVP sales is publicly available for publicly traded vaping device companies, although often not distinguished by product lines. Less information is available for privately owned manufacturers. The shares of these firms in the market will depend on their sales in all sectors, including the internet and vape shops. While Nielsen data on firm-level NVP sales is available for conventional cigarette selling retail outlets, the market shares of device sellers in other purchase channels, such as online and vape shops, is lacking. Although US shares of particular brands may be gleaned from surveys,61 even rough measures of US NVP market concentration by device sellers are generally not available, but market concentration appears to be low by antitrust standards at least through 2016.
ENTRY BARRIERS
Firms cannot exercise and maintain monopoly power unless barriers to market entry make it difficult for new firms to enter the market and effectively compete by offering better products or lower prices.21 Profitable entry depends on the ability to reach an efficient scale of operation, have the necessary technological and marketing knowledge, gain reputation, obtain essential inputs, and meet regulatory constraints.21
In the manufacturing of vaping devices, technological entry barriers from scale economies or proprietary knowledge appear to be minimal. While firms faced some early legal patent rights challenges, innovative designs have been developed and proprietary knowledge apparently has not been a hindrance. In addition, those marketing devices in the US have been able to buy devices designed and manufactured by Chinese firms, eg, via an original design manufacturer arrangement.2 Except where subject to an exclusivity arrangement, potential vaping device marketers have been able to purchase slightly modified products from the same Chinese firms for rebranding in the US. Indeed, Chinese device manufacturers have incentives to enter into such arrangements to encourage increased competition, and thereby prevent market concentration that could produce monopoly price mark-ups that ultimately reduce the demand for their products.62 Alternatively, US firms have been able to design their own NVPs and contract with an outside firm for manufacture exclusively on their behalf.63
Economies of scale in marketing also do not appear to be a major source of entry barriers. Much marketing occurs over the internet,2,21,43,45,49 especially through social media, and through word of mouth, where economies of scale do not readily apply. More costly forms of advertising such as through mass broadcast media, are more likely to be subject to economies of scale, but appear to be less important in marketing NVPs than cigarettes.2,42,44,48 While retail NVP marketing expenditures grew rapidly between 2010 and 2014,65 advertising expenditures as a percent of sales even among closed system NVPs remains much lower than for cigarettes.66 Nevertheless, cigarette companies may still have some advantages in marketing their NVPs, because of larger financial resources, lists of potential customers (eg, smokers) and prior marketing experience.2
While advertising appears to have had minimal effect on NVP market entry, slotting fees, often found in conventional retail stores, have been found to be an important entry deterrent in the cigarette market.67 Through slotting fees, cigarette companies pay for scarce shelf space for their products, which have been combined with retail-based promotions, price discounts, and sales incentives, to control how their products are sold.68,69 Thereby, cigarette firms have used slotting fees to limit the shelf space and target price promotion to impede competitors’ entry or growth. Cigarette companies could potentially apply this practice to retail sales of NVPs as well. However, the appearance of open systems in conventional retail and the recent growth of Juul indicates that this potential barrier can be overcome by an innovative product. Furthermore, limiting “shelf space” is not relevant to internet sales and does not appear relevant in vape shops.
Vape shops are also relatively easy to launch, with a plethora of websites providing guidance.71,72 The cost has been estimated at $25,000 for a small shop and $50,000 for a large shop, which includes the cost to design and build the space, rent, licensing and inventory (starter kits, mods, parts, e-liquids).72 In addition, although single-location vape shops are in the majority, 46% of locations in a recent survey were found to be part of a multi-store chain.56 In 2015, one firm franchised 14 shops,73 with individual stores paying a $25,000 franchise fee and $50,000 for initial stocking, while being provided training and help to set up the store. While a particular franchisor can gain significant market share in a local market, franchisors from other markets may enter if prices are raised or brand availability is restricted. Establishing online NVP distribution can also be accomplished with relatively small financial investment and limited know-how,21 thereby providing another potential outlet for vaping device firms.
In summary, market entry barriers, at least before the deeming rule, had been limited, as indicated by the large number of firms and continual new entrants selling vaping devices in the major purchase channels. The large cigarette companies may have had some advantage in marketing their products in conventional retail, due primarily to slotting allowances, but such advantages appear to be minimal and are unimportant in the internet or vape shop sectors. In addition, many vape shop owners have focused on helping smokers to quit, and customers view non-cigarette firm ownership important to their decision to use vape shops,43,46,56,74–75 thereby creating a competitive disadvantage to cigarette firms in that channel. However, while our focus is the pre-deeming period, regulatory restrictions applied to NVPs by the FDA deeming rule may have already created significant entry barriers.
FIRM CONDUCT
From an economic perspective,20 conduct in the relevant product market is generally evaluated in terms of pricing, firm market share stability, innovation, and marketing practices.
Prices of retail NVPs fell sharply in 2009–2010 and then more gradually through 2014.2,76 Like other dynamic consumer durable markets, prices for new products are often higher than for existing products, but typically decline over time as other new products enter the market. Since cigarette companies have especially high profit margins for cigarettes, in the range of 40% to 50%,10,77 they might be expected to raise NVP prices to try to secure comparable profit margins and avoid smoking customers switching to NVPs (“cannibalization”). However, with cigarette companies having a small share of the overall NVP market and consumers responsive to price,38,39 any attempt by cigarette companies to raise their NVP prices is likely to greatly reduce their own NVP sales without substantially reducing overall NVP sales. In addition, profit margins from cigarette firms’ sales of NVPs are estimated to be substantially lower than for cigarettes and even negative in some years.10
In addition to falling prices, competition can be gauged by the lack of coordinated pricing behavior.20 Substantial variation has been found for internet prices,60,78 with heavy advertising of price discounts79,80 including tweets on price promotions.81 Vape shops also commonly engage in price promotions44 and retailers display considerable price variation.76 Market analysts cited significant (coupon) price drops driving sales of cigarette companies.47 Besides price, other marketing practices vary, as evidenced by the variation in warning labels by different firms.82
The instability of market shares and proliferation of products also reveals intense competition. Table 2 shows the market shares of the firms selling e-cigarettes in conventional retail sales.83 Between 2010 and 2014, the market leader changed from Gamucci to NJOY, to 21st Century Smoke, to Mistic, back to NJOY, to blu, and finally to VUSE.2 By 2017, Juul had become the market leader. The overall market has also seen a proliferation of new products, with major shifts from cigalikes to vaporizers. In addition, there has been considerable variation in the type of products offered, with substantial improvements in the ability to satisfy nicotine cravings.4,36 For example, Juul63,84 is said to deliver nicotine more efficiently than other closed systems. Industry analysts see improved product technology and consumer satisfaction as major drivers of NVP market growth.85
Table 2.
Market Shares of the Three Leading Firms in Conventional Retail, by Year
| Company/Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|
| Ballantyne | 17% | |||||||
| CB Distributors | 23% | 24% | ||||||
| NJOY | 30% | 22% | 22% | |||||
| Imperial | 17% | 42% | 33% | 22% | 28% | |||
| Japan Tobacco | 12% | 16% | 14% | |||||
| BAT (Reynolds) | 15% | 33% | 38% | 30% | 21% | |||
| Altria | 14% | 15% | 12% | |||||
| Juul | 25% | 55% |
Cowen Reports83
Although recent deeming regulations may have discouraged innovation, competition is intense and the market is still in the growth stage. Competition intensity may, however, lessen as the market becomes more established. Like cigarettes,11 brand name may become increasingly important for vaping devices.10 Much will depend on the FDA and other regulatory constraints as they affect innovation and the number of firms that survive in the market.
DISCUSSION
The NVP market over the past decade has seen rapid evolution in products, prices, distribution channels, and, despite mergers and acquisitions, has not become concentrated. At least prior to the recent deeming rule, the market has not been subject to major entry barriers. While independent vaping device sellers may have had less access to conventional retail due to slotting allowances, they have still been able to offer their products in these locations and even take sales from the cigarette companies. Further, conventional retail accounts for less than 30% of total NVP sales, and faces stiff competition from the internet and vape shops, also subject to relatively low entry barriers.
The dynamic competition in the market for vaping devices differs markedly from the highly concentrated market for traditional cigarettes.86 Historically, cigarette market concentration has driven up prices, consistent with tobacco control aims, but probably also discouraged innovation in alternative nicotine delivery products.86 In particular, restrictions on media advertising likely hindered new firm entry due to the limited ability to effectively promote new and especially innovative products, while retail point-of-sale advertising and accompanying slotting allowances restricted competitors from access to shelf space.
The lack of competition in the overall tobacco market appears to have changed with the emergence of NVPs. Much of the growth in the NVP market is spurred by information provided over the internet and through vape shops. Unlike the marketing of NVPs by cigarette companies, which is often geared toward dual use with cigarettes, information, especially from vape shops, is often geared toward moving cigarette smokers exclusively to NVPs.2 Moreover, one of the hallmarks of the NVP market has been that products are continually improving in their ability to serve as substitutes for cigarettes.4,87,88
We have focused primarily on the NVP market as it developed prior to the FDA’s deeming regulations in 2016. While regulatory hurdles to market entry had been minimal, the future impact of the NVP market on cigarette use will likely depend heavily on how FDA regulates NVPs. The newly applicable TCA provisions prohibit NVPs from being marketed with reduced-risk or reduced-exposure claims without first obtaining formal permission from the FDA. In addition, all new or significantly changed NVPs must obtain a permissive order from the FDA before entering the US market, which can be difficult and costly and lead to substantial delays. Such major hurdles for new firms and products may thereby discourage NVP market growth and innovation. Industry analysts have predicted that the deeming rule would create entry barriers and favor the large cigarette companies, “because of their larger financial resources and regulatory experience.”89,90
Since the original deeming regulations, the FDA has extended the deadlines for new and existing products and sought guidance on how to make the procedures less burdensome. While it is difficult to assess the effect of deeming to date, it bears mentioning that Pax Labs, an independent, entered the retail market with Juul in June 2015 and replaced Vuse for first position by the end of 2017, with sales reaching a 73% share in dollar sales and a 55% in unit sales by September 2018.47 While it is difficult to gauge their market share of the overall NVP market without knowing their share of internet and vape shop sales, Juul’s share from conventional retail in 2018 would have been about 25% (0.55%*3000/6600), where 3000 is total mass market and 6600 is total sales (see Table 1). By that time, sales of the four cigarette companies’ NVPs plummeted to 30% of conventional retail dollar sales and 44% of unit sales -- or roughly 12% of total NVP dollar sales from all sales channels, thus suggesting that there continues to be strong market competition.47
It appears that the FDA has not been strict in enforcing product application requirements. However, the TCA barriers to relative-risk claims by NVPs and its obstacles to technological improvements to NVPs and to market entry by new types of NVP products (eg, HNB products) could be having a major impact on the U.S. markets for cigarettes and NVPs. With the absence of relative-risk claims, technological improvements, and new types of products, any effect is not visible and therefore not as striking as concrete, visible impacts would be. Moreover, while some new/changed products have been able to enter the market and some peripheral products have been able to make relative-risk claims without the FDA stopping them, the tobacco companies appear to be abiding by the law. For example, without the existing constraints, IQOS may have already been marketed in the USA. In addition, companies might be marketing their e-cigs (and HNBs) with careful relative-risk claims, and major-company and smaller-company e-cigarette technological changes may be more quickly appearing.
While regulations are likely to create hurdles for small firms and new entrants, the FDA could act in ways to encourage vaping. In particular, product standards that make NVPs more reliable and less toxic could enable the NVP market to evolve and more successfully replace smoking, especially if the risks of NVPs relative to cigarettes are communicated to smokers. In addition, stricter regulations on cigarettes, especially reducing the nicotine content in cigarettes, could also encourage instead of smoking.91
FDA regulation will also influence competition from other potential nicotine delivery products besides NVPs. In particular, Philip Morris International has submitted a new product application to the FDA for iQOS, a HNB tobacco product, which has already gained substantial market share in Japan,92 and other tobacco companies are developing their own versions.90 Like NVPs, HNBs are inhaled and have similar sensorimotor experiences and “throat-hit” to cigarettes.92 However, the HNB technology is proprietary and protected by patent, and thus more likely than NVPs to be a profitable business line for the major cigarette firms holding those patents. If FDA allows HNB products on the U.S. market, the major cigarette companies may aggressively market HNB products as a way to fend off competition from independent NVP manufacturers.93 At the same time, their ability to control the alternative nicotine delivery market is constrained by competition from NVPs.
The future of the NVP industry and the cigarette industry will also depend on other federal, state, and local tobacco control policies, such as the extent to which state and local governments extend smoke-free laws to cover vaping, and whether any governments start to provide smokers with information about the relative harms from NVPs compared to smoking or actively encourage switching.2 In addition, with smoking and vaping both sensitive to product prices,38,39 future levels of NVP taxes relative to cigarette taxes will directly impact both initiation into smoking and vaping and whether smokers switch to vaping. Indeed, keeping NVP taxes low and raising cigarette taxes may increase incentives for cigarette firms to increase cigarette prices in order to reap higher profits before losing substantial customers to NVPs (ie, “making hay while the sun shines”), especially if they can encourage their customers to move to their own brands of NVPs or HNB products.
In conclusion, the framework and analysis provided here should directly assist the FDA in the development of tobacco control regulations and policies. Regulatory policies can more effectively promote public health by taking account the existing market structure, the impacts of regulatory actions on that structure, and the related public health consequences from changes in market structure. To improve the ability of policymakers to accomplish these aims, it will be important for regulatory bodies to carefully monitor the evolving NVP market structure and its impact on the public health.
IMPLICATIONS FOR TOBACCO REGULATION:
To provide insight into how FDA’s regulation of NVPs could change the US NVP and cigarette markets, this paper offers a structured analysis of competition in the US NVP market. With FDA regulations still in transition, we focus on the largely unregulated market prior to the FDA deeming rule to help understand the role of competition in that market. The pre-deeming market is found to exhibit strong competitive tendencies, as exhibited by low levels of market concentration, low entry barriers into the market, and competitive pricing. To date, the cigarette industry appears to have minimal NVP market share. Competition has spurred the innovation of new products, which appear to be increasingly better substitutes for cigarettes. While competition has been intense in the pre-deeming market, the availability of products, innovation of products and use patterns of these products vis-a vis cigarettes will depend on future regulation by the FDA and industry responses. It will be important for regulatory bodies to carefully monitor the evolving NVP market structure and its impact on the public health.
Acknowledgements:
Funding was received by the authors from the National Cancer Institute under grant P01-CA200512.
Footnotes
Human Subjects: This study is exempt from review, as this study used secondary data sources.
Conflict of Interests: FJC and KMC have served as an expert witness in litigation against the cigarette industry. MLG received a research grant from Pfizer and served as an advisory board member to Johnson & Johnson.
Contributor Information
David T. Levy, Cancer Prevention and Control, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC..
Eric N. Lindblom, Tobacco Control and Food & Drug Law, O’Neill Institute for National & Global Health Law, Georgetown University Law Center, Washington, DC..
David T. Sweanor, Adjunct Professor, Faculty of Law, University of Ottawa, Canada..
Frank Chaloupka, Health Policy Center, Institute for Health Research and Policy, University of Illinois at Chicago, Chicago, Illinois..
Richard J. O’Connor, Department of Health Behavior, Roswell Park Cancer Institute, Buffalo, NY..
Ce Shang, Department of Pediatrics and Oklahoma Tobacco Research Center, Stephenson Cancer Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK..
Thomas Palley, School of Business, Indiana University..
Geoffrey T. Fong, Department of Psychology, University of Waterloo, Waterloo, Ontario, Canada,.
K. Michael Cummings, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC..
Maciej L. Goniewicz, Department of Health Behavior, Division of Cancer Prevention and Population Studies, Roswell Park Cancer Institute, Buffalo, NY..
Ron Borland, Nigel Gray Distinguished Fellow in Cancer Prevention, Cancer Council Victoria, Melbourne, Victoria, Australia..
References
- 1.Marynak KL, Gammon DG, King BA, et al. National and state trends in sales of cigarettes and e-cigarettes, U.S., 2011–2015. Am J Prev Med. 2017;53(1):96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2016. [Google Scholar]
- 3.Siegel M What the FDA Gets Wrong About E-Cigarettes (online). Available at: https://www.bloomberg.com/view/articles/2017-03-16/what-the-fda-gets-wrong-about-e-cigarettes. Accessed December 12, 2018.
- 4.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4(4133):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med. 2017;52(2):e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawkins L, Turner J, Roberts A, Soar K. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013;108(6):1115–1125. [DOI] [PubMed] [Google Scholar]
- 7.Kasza KA, Bansal-Travers M, O’Connor RJ, et al. Cigarette smokers’ use of unconventional tobacco products and associations with quitting activity: findings from the ITC-4 U.S. cohort. Nicotine Tob Res. 2014;16(6):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy DT, Yuan Z, Luo Y, Abrams D. The relationship e-cigarette use to cigarette quit attempts and cessation: insights from a large, nationally representative U.S. survey. Nicotime Tob Res. 2018;20(8):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose SW, Barker DC, D’Angelo H, et al. The availability of electronic cigarettes in U.S. retail outlets, 2012: results of two national studies. Tob Control. 2014;23 Suppl 3:iii10–iii16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branston JR, Sweanor D. Big tobacco, e-cigarettes, and a road to the smoking endgame. Int J Drug Policy. 2016;29:14–18. [DOI] [PubMed] [Google Scholar]
- 11.Vapor--Revolutionizing the Tobacco Industry. Wells Fargo Securities Equity Research; 2014. [Google Scholar]
- 12.Dawes J Cigarette brand loyalty and purchase patterns: an examination using US consumer panel data. Journal of Business Research. 2014;67:1933–1943. [Google Scholar]
- 13.Abrams DB. Potential and pitfalls of e-cigarettes--reply. JAMA. 2014;311(18):1922–1923. [DOI] [PubMed] [Google Scholar]
- 14.Haardorfer R, Cahn Z, Lewis M, et al. The advertising strategies of early e-cigarette brand leaders in the United States. Tob Regul Sci. 2017;3(2):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidenberg AB, Jo CL, Ribisl KM. Differences in the design and sale of e-cigarettes by cigarette manufacturers and non-cigarette manufacturers in the USA. Tob Control. 2016;25(e1):e3–e5. [DOI] [PubMed] [Google Scholar]
- 16.Tobacco Free CA. Big tobacco advocates for e-cigarettes (online). Available at: http://stillblowingsmoke.org/#bigtobacco. Accessed Nov 30 2017.
- 17.Kamerow D the battle between big tobacco and vape shops. BMJ. 2014;349:g5810. [Google Scholar]
- 18.U.S. Department of Health and Human Services Food and Drug Administration, Extension of Certain Tobacco Product Compliance Deadlines Related to the Final Deeming Rule: Guidance for Industry (online). Available at: https://www.fda.gov/downloads/TobaccoProducts/Labeling/RulesRegulationsGuidance/UCM557716.pdf. Accessed November 2, 2017.
- 19.Caponnetto P, Saitta D, Sweanor D, Polosa R. What to consider when regulating electronic cigarettes: pros, cons and unintended consequences. Int J Drug Policy. 2015;26(6):554–559. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Justice and the Federal Trade Commission. Horizontal Merger Guidelines. Washington DC: U.S. Department of Justice and the Federal Trade Commission; 2010. [Google Scholar]
- 21.Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23 Suppl 3:iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23 Suppl 2:ii4–ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiffman S, Sembower MA, Pillitteri JL, Gerlach KK, Gitchell JG. The impact of flavor descriptors on nonsmoking teens’ and adult smokers’ interest in electronic cigarettes. Nicotine Tob Res. 2015;17(10):1255–1262. [DOI] [PubMed] [Google Scholar]
- 24.Villanti AC, Johnson AL, Ambrose BK, et al. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH study (2013–2014). Am J Prev Med. 2017;53(2):139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klager S, Vallarino J, MacNaughton P, Christiani DC, Lu Q, Allen JG. Flavoring chemicals and aldehydes in e-cigarette emissions. Environ Sci Technol. 2017;51(18):10806–10813. [DOI] [PubMed] [Google Scholar]
- 26.Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob Control. 2016;25(Suppl 2):ii81–ii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eissenberg T, Shihadeh A. Nicotine flux: a potentially important tool for regulating electronic cigarettes. Nicotine Tob Res. 2015;17(2):165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield:measurements and model predictions. Nicotine Tob Res. 2017;17(2):150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;(17)6:704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royal College of Physicians. Nicotine without smoke: tobacco harm reduction. London: Royal Chapter of Physicians; 2016. [Google Scholar]
- 31.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper M, Case KR, Loukas A, Creamer MR, Perry CL. E-cigarette dual users, exclusive users and perceptions of tobacco products. Am J Health Behav. 2016;40(1):108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Guo Y, Liu K, Liu Z, Wang X. E-Cigarette awareness, use, and harm perception among adults: a meta-analysis of observational studies. PLoS One. 2016;11(11):e0165938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–28. [DOI] [PubMed] [Google Scholar]
- 35.Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int J Clin Pract. 2011;65(10):1037–1042. [DOI] [PubMed] [Google Scholar]
- 36.Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 2017;26(e1):e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nonnemaker J, Kim AE, Lee YO, MacMonegle A. Quantifying how smokers value attributes of electronic cigarettes. Tob Control. 2016;25(e1):e37–43. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Tauras J, Chaloupka FJ. The impact of price and tobacco control policies on the demand for electronic nicotine delivery systems. Tob Control. 2014;23 Suppl 3:iii41–iii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Y, Zhen C, Dench D, Nonnemaker JM. U.S. Demand for tobacco products in a system framework. Health Econ. 2016;26(8):1067–1086. [DOI] [PubMed] [Google Scholar]
- 40.Levy D The transactions cost approach to vertical integration: an empirical examination. Review of Economics and Statistics. 1985;August 1985:438–45. [Google Scholar]
- 41.Giovenco DP, Hammond D, Corey CG, Ambrose BK, Delnevo CD. E-Cigarette market trends in traditional U.S. retail channels, 2012–2013. Nicotine Tob Res. 2015;17(10):1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose SW, Barker DC, D’Angelo H, et al. The availability of electronic cigarettes in US retail outlets, 2012: results of two national studies. Tob Control. 2014;23 Suppl 3:iii10–iii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YO, Kim AE. ‘Vape shops’ and ‘e-cigarette lounges’ open across the USA to promote ENDS. Tob Control. 2015;24:410–412. [DOI] [PubMed] [Google Scholar]
- 44.Cheney M, Gowin M, Wann TF. Marketing practices of vapor store owners. Am J Public Health. 2015;105(6):e16–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheney MK, Gowin M, Wann TF. vapor store owner beliefs and messages to customers. Nicotine Tob Res. 2016;18(5):694–699. [DOI] [PubMed] [Google Scholar]
- 46.Sussman S, Garcia R, Cruz TB, Baezconde-Garbanati L, Pentz MA, Unger JB. Consumers’ perceptions of vape shops in Southern California: an analysis of online Yelp reviews. Tob Induc Dis. 2014;12(22):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen: Tobacco ‘All Channel’ Data - 11/3- Cig Vol Decelerate Strongly, Wells Fargo Securities Equity Research; 2018. [Google Scholar]
- 48.Emery SL, Vera L, Huang J, Szczypka G. Wanna know about vaping? Patterns of message exposure, seeking and sharing information about e-cigarettes across media platforms. Tob Control. 2014;23 Suppl 3:iii17–iii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang J, Kornfield R, Emery SL. 100 million views of electronic cigarette youtube videos and counting: quantification, content evaluation, and engagement levels of videos. J Med Internet Res. 2016;18(3):e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunlop S, Dessaix A, Currow D. How are tobacco smokers using e-cigarettes? patterns of use, reasons for use and places of purchase in New South Wales. Med J Aust. 2016;204(9):355. [DOI] [PubMed] [Google Scholar]
- 51.Mackey TK, Miner A, Cuomo RE. Exploring the e-cigarette e-commerce marketplace: Identifying Internet e-cigarette marketing characteristics and regulatory gaps. Drug Alcohol Depend. 2015;156:97–103. [DOI] [PubMed] [Google Scholar]
- 52.The Evolution of Vape Shops. Roebling Research. (online). Available at: http://roeblingresearch.com/blog/2015/6/23/the-evolution-of-vape-shops. Accessed December 12, 2018.
- 53.Mickle T Japan Tobacco to Acquire U.S. electronic cigarette company logic technology development. Wall Street Journal. 2015;April 30, 2015. [Google Scholar]
- 54.Nielsen: Tobacco ‘All Channel’ Data Through 9/8 - Cig Vol Decelerates, Wells Fargo Securities Equity Research; 2018. [Google Scholar]
- 55.Sussman S, Baezconde-Garbanati L, Garcia R, et al. Commentary: forces that drive the vape shop industry and implications for the health professions. Eval Health Prof. 2016;39(3):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allem JP, Unger JB, Garcia R, Baezconde-Garbanati L, Sussman S. tobacco attitudes and behaviors of vape shop retailers in los angeles. Am J Health Behav. 2015;39(6):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CounterTobacco. Licensing E-Cigarette Retailers and Vape Shops. (online). Available at: http://countertobacco.org/resources-tools/stories-from-the-field/licensing-e-cigarette-retailers-and-vape-shops/. Accessed Novermber 30 2017.
- 58.Bour N How many vape shops are there in the USA? (online). Available at: <https://vapenews.com/november-2014/how-many-vape-shops-are-there-in-the-u-s-a/. Accessed Novermber 30 2017.
- 59.Williams RS, Derrick J, Liebman AK, LaFleur K. Content analysis of e-cigarette products, promotions, prices and claims on Internet tobacco vendor websites, 2013–2014. Tob Control. 2018;27(e1):e34–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuomo RE, Miner A, Mackey TK. Pricing and sales tax collection policies for e-cigarette starter kits and disposable products sold online. Drug Alcohol Rev. 2016:;35(1):110–114. [DOI] [PubMed] [Google Scholar]
- 61.Gravely S, Fong GT, Cummings KM, et al. Awareness, trial, and current use of electronic cigarettes in 10 countries: findings from the ITC Project. Int J Environ Res Public Health. 2014;11(11):11691–11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levy D, Reiffen D. vertical integration in a spatial setting: implications of the successive monopoly distortion. Economic Letters. 1989;1:77–81. [Google Scholar]
- 63.Juul website. Manufacturing Quality. 2017. (online). Available at: https://support.juulvapor.com/home/learn/faqs/manufacturing-quality (accessed November 30 2017).
- 64.Kim AE, Hopper T, Simpson S, et al. Using twitter data to gain insights into e-cigarette marketing and locations of use: An Infoveillance Study. J Med Internet Res. 2015;17(11):e251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kornfield R, Huang J, Vera L, Emery SL. Rapidly increasing promotional expenditures for e-cigarettes. Tob Control. 2015;24(2):110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24(4):341–347. [DOI] [PubMed] [Google Scholar]
- 67.Lariviere MA, Padmanabhan, V. Slotting Allowances and new product introductions. Marketing Science. 1997;16(2):112–128. [Google Scholar]
- 68.Levy DT, Lindblom EN, Fleischer NL, et al. Public health effects of restricting retail tobacco product displays and ads. Tob Regul Sci. 2015;1(1):61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feighery EC, Ribisl KM, Clark PI, Haladjian HH. How tobacco companies ensure prime placement of their advertising and products in stores: interviews with retailers about tobacco company incentive programmes. Tob Control. 2003;12(2):184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shopkeep. Starting a vape shop? here are the 4 things you will need. (web). Available at: https://www.shopkeep.com/blog/starting-a-vape-shop#step-12017. Accessed August 7 2017. [Google Scholar]
- 71.VapeMentors. How to start a vape shop. (online). Available at: http://vapementors.com/how-to-start-a-vape-store/. Accessed August 7 2017.
- 72.Mincer J In rise of U.S. vape shops, owners eye new marijuana market. (online). Available at: http://www.reuters.com/article/usa-ecigarettes-shops-idUSL1N1072E620150729. Accessed August 7 2017.
- 73.Burbank AD, Thrul J, Ling PM. A pilot study of retail ‘vape shops’ in the San Francisco Bay area. Tob Prev Cessat. 2016;2(Suppl):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hart JL, Walker KL, Sears CG, et al. Vape shop employees: public health advocates? Tob Prev Cessat. 2016;2(suppl):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sussman S, Allem JP, Garcia J, et al. Who walks into vape shops in Southern California?: a naturalistic observation of customers. Tob Induc Dis. 2016;14:18–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loomis BR, Rogers T, King BA, et al. National and state-specific sales and prices for electronic cigarettes-U.S., 2012–2013. Am J Prev Med. 2016;50(1):18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tobacco Talk: Independent vapor mfr. survey: more bullish than retailers-suggesting vapor growth remains robust, Wells Fargo Securities Equity Research; 2015. [Google Scholar]
- 78.Grana RA, Ling PM. “Smoking revolution”: a content analysis of electronic cigarette retail websites. Am J Prev Med. 2014;46(4):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang J, Kornfield R, Szczypka G, Emery SL. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23 Suppl 3:iii26–iii30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2014;24(4):341–347. [DOI] [PubMed] [Google Scholar]
- 81.Jo CL, Kornfield R, Kim Y, Emery S, Ribisl KM. Price-related promotions for tobacco products on Twitter. Tob Control. 2016;25(4):476–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shang C, Huang J, Chaloupka FJ, Emery SL. The impact of flavour, device type and warning messages on youth preferences for electronic nicotine delivery systems: evidence from an online discrete choice experiment. Tob Control. 2018;27(e2):e152–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cowen’s Cigarette Global Guidebook. Cowen Research, 2018. [Google Scholar]
- 84.Huddleston T Pax Labs takes on big tobacco for e-cig dominance, announces $46.7 million funding round. Fortune. June 2015. [Google Scholar]
- 85.Leinster Jonathan, Tobacco-changing the business model, Berenberg, 2018. [Google Scholar]
- 86.Levy D, Chaloupka F, Lindblom E, Sweanor DT, O’Connor RJ, Shang C, Borland B, The US cigarette industry: an economic and marketing perspective. Tob Reg Sci. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations between e-cigarette type, frequency of use, and quitting smoking: findings from a longitudinal online panel survey in Great Britain. Nicotine Tob Res. 2015;17(1):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dawkins L, Kimber C, Puwanesarasa Y, Soar K. First- versus second-generation electronic cigarettes: predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction. 2015;10(4):69–77. [DOI] [PubMed] [Google Scholar]
- 89.Final deeming e-cigs regs released--quick take, Wells Fargo Securities Equity Research; 2016. [Google Scholar]
- 90.Good for the goose, less for the gander, Wells Fargo Securities Equity Research; 2016. [Google Scholar]
- 91.Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med 2017;377(12):1111–4. [DOI] [PubMed] [Google Scholar]
- 92.iQOS in pictures_a visual overview of the global opportunity through 2025. Well Fargo Equity Research; 2017. [Google Scholar]
- 93.The new world of tobacco – JUUL starting to disrupt U.S. cigarette industry. Citi Research, 2018. [Google Scholar]