Abstract
Rationale
The corticotropin-releasing hormone (CRH) system is a key mediator of stress-induced responses in alcohol seeking behavior. Recent research has identified the central nucleus of the amygdala (CeA), a brain region involved in the regulation of fear and stress-induced responses that is especially rich in CRH-positive neurons, as a key player in mediating excessive alcohol seeking. However, detailed characterization of the specific influences that local neuronal populations exert in mediating alcohol responses is hampered by current limitations in pharmacological and immunohistochemical tools for targeting CRH receptor subtype 1 (CRHR1).
Objective
In this study, we investigated the effect of cell- and region-specific overexpression of CRHR1 in the CeA using a novel transgenic tool.
Methods
Co-expression of CRHR1 in calcium-calmodulin-dependent kinase II (αCaMKII)-neurons of the amygdala was demonstrated by double immunohistochemistry using a Crhr1-GFP reporter mouse line. A Cre-inducible Crhr1 expressing-adeno-associated virus (AAV) was site-specifically injected into the CeA of αCaMKII-CreERT2 transgenic rats to analyze the role of CRHR1 in αCaMKII-neurons on alcohol self-administration and reinstatement behavior.
Results
48% of CRHR1-containing cells showed co-expression of αCaMKII in the CeA. AAV mediated gene transfer in αCaMKII-neurons induced a 24-fold increase of Crhr1 mRNA in the CeA which had no effect on locomotor activity, alcohol self-administration or cue-induced reinstatement. However, rats overexpressing Crhr1 in the CeA increased responding in the stress-induced reinstatement task with yohimbine serving as a pharmacological stressor.
Conclusion
We demonstrate that CRHR1 overexpression in CeA-αCaMKII neurons is sufficient to mediate increased vulnerability to stress-triggered relapse into alcohol seeking.
Keywords: Alcohol, transgenic rats, AAV mediated gene transfer, Corticotropin-releasing hormone receptor subtype 1, calcium-calmodulin-dependent kinase II neurons, amygdala, stress-induced reinstatement
Introduction
One of the key brain regions that mediate stress and stress-induced relapse to alcohol-seeking is the amygdala, particularly the central nucleus (CeA) (Simms et al. 2012). Through projections to other brain regions, e.g. the bed nucleus of the stria terminalis (BNST), the hippocampus, and the hypothalamus (Pitkanen et al. 2000), the CeA relays information concerning stress and anxiety-like behavior. The CeA is also involved in the acquisition and expression of conditioned fear responses, and is a key nucleus of the emotional reward circuitry (Davis and Shi 2000; LeDoux 2000; Pare et al. 2004; Shinnick-Gallagher et al. 2003). Within the amygdala, both glutamatergic (excitatory) and GABAergic (inhibitory) connections mediate signaling and output from this region. The basolateral amygdala (BLA), which receives most of the sensory input to the amygdala, sends glutamatergic projections to the lateral part of the CeA (CeL) (Dong et al. 2001; Ehrlich et al. 2009; McDonald 1982). Within the CeA, the majority of neurons are GABAergic (Cassell et al. 1986; Ehrlich et al. 2009; McDonald 1982; Swanson and Petrovich 1998), as are projections from intercalated cell masses (ITCs), which surround the BLA and act as an interface between the BLA and CeA (Ehrlich et al. 2009; Millhouse 1986).
Alpha calcium/calmodulin-dependent protein kinase II (αCaMKII) plays a crucial role in the modulation of synaptic function via calcium-dependent activation (Yamauchi 2005). It is generally considered as a marker of excitatory neurons (Benson et al. 1992), although recent studies suggest the involvement of αCaMKII in inhibitory transmission within the amygdala (Huang et al. 2014). αCaMKII has been found in GABAergic cells (Beckerman et al. 2013; Huang et al. 2014; Meins et al. 2010) in the amygdala including the CeA (Wang et al. 2013), and a recent proteomics study has shown that moderate alcohol drinking in mice induces adaptation in αCaMKII-AMPA receptor pathway in the amygdala that regulates the positive reinforcing effect of alcohol (Salling et al. 2016).
For the modulation of CeA transmission and functional activity, the corticotropin releasing hormone (CRH) system plays an important role. CRH and CRH receptor subtype 1 (CRHR1) (Bale and Vale 2004; Hauger et al. 2006), are present in glutamatergic and GABAergic neurons in many brain regions (Bonfiglio et al. 2011; Lemos et al. 2012; Refojo et al. 2011). Activation of CRHR1 is central in the initiation of behavioral responses to stress (Coste et al. 2006; Hauger et al. 2006; Janssen and Kozicz 2013). There are several studies suggesting that CRH and CRHR1 play a key role in stress-induced alcohol seeking behavior (Bjork et al. 2010; Hansson et al. 2006; Le et al. 2002; Marinelli et al. 2007; Meinhardt and Sommer 2015; Noori et al. 2014; Spanagel et al. 2014) and the effects of alcohol on stress-related behavior (Lee et al. 2004; Lee et al. 2001; Ogilvie et al. 1998; Pastor et al. 2008). Crhr1 mRNA abundance is increased in the amygdala of alcohol dependent rats (Sommer et al. 2008). This translates into increased protein and function, as CRHR1-containing neurons in the CeA show an enhanced activity in response to alcohol (Herman et al. 2013). It has also been suggested in several studies that the CRH system mediates the effects of acute and chronic alcohol exposure via modulation of glutamatergic and GABAergic signaling (Nie et al. 2004; Nie et al. 2009; Roberto et al. 2010; Silberman et al. 2015; Silberman and Winder 2015). Together, these studies demonstrate that amygdala CRHR1 plays a key role in the expression of relapse-like behavior, particularly the stress-induced reinstatement of responding for alcohol. A remaining question is whether increased CRHR1 expression is sufficient to elicit relapse-like behavior.
To address this issue, we assessed the role of CRHR1 in the CeA in cue- and stress-induced reinstatement of alcohol responding. We employed a novel approach for time-, region-, and cell type specific CRHR1 overexpression using alpha calcium/calmodulin-dependent protein kinase II (αCaMKII) Cre ERT2-transgenic rats (Schonig et al. 2012) and adeno-associated virus (AAV) gene transfer to investigate the role of CRHR1 in CeA neurons during reinstatement behavior.
Material and Methods
Experimental animals.
For behavioral experiments αCaMKII-CreERT2 rats were used. In our hands rats are a better model system to study behaviors related to alcohol addiction (Vengeliene et al. 2014). Due to the lack of a specific antibody directed to CRHR1, the Crhr1-GFP transgenic reporter mouse (Bernardi et al. 2017; Justice et al. 2008) was used for immunohistochemical assays. Male Crhr1-GFP mice with a mixed BALB/cJ x C57BL/6J genetic background (Justice et al. 2008) were kept single housed with water and food available ad libitum, under a 12 h light/dark cycle (light off at 6:00 pm). αCaMKII-CreERT2 rats (Sprague-Dawley genetic background, (Schonig et al. 2012)) weighing 190 – 290 g at the beginning of the experiment were bred at the Central Institute of Mental Health (Mannheim, Germany). They were housed in standard cages of four rats per cage with ad libitum access to food and water, under a 12 h light/dark cycle (lights off at 5:00 am). All behavioral testing was performed during the dark phase. Experiments were carried out in accordance with the ethical guidelines for the care and use of laboratory animals, and were approved by the local animal care committee (Regierungspräsidium Karlsruhe, Germany, AZ 35-9185.81/G-183/09, AZ 35-9185.81/G-221/15).
Double Immunohistochemistry.
Crhr1-GFP mice were deeply anesthetized and perfused with 0,9% NaCl solution with 10000 IE/1 heparin followed by fixation with phosphate solution containing 4% paraformaldehyde and 14% saturated picric acid. Brains were isolated, post-fixed for 1-2h, dehydrated in 1xPBS solution with 10% sucrose for 3 d and quickly frozen at −80°C. For fluorescent double-labeling 16 μm coronal sections (Bregma −0.7 mm to −1.7 mm, (Paxinos 2004)) were cut with a cryostat (Leica, Germany), mounted on gelatin-coated slides and stored at −20°C. Sections were rehydrated, dipped in 0.01 M PBS buffer, and incubated with a mixture of anti-GFP (rabbit, 1:500 dilution, ThermoFisher Scientific, USA) and anti-αCaMKII (mouse, 1:500 dilution, Pierce Biotechnology, USA) primary antibodies at 4°C overnight (Pfarr et al. 2015). Brain sections were rinsed in 0.01 M PBS/0.3%Triton solution containing the secondary antibody Alexa Fluor 488-labeled donkey anti-rabbit (1:1000 dilution, ThermoFisher Scientific, USA) and Alexa Fluor 594-labeled donkey anti-mouse (1:1000 dilution, ThermoFisher Scientific, USA) and incubated for 1–2 h at room temperature. Sections were rinsed briefly, mounted and coverslipped. Slides were analyzed using a Leica TCS SP2 imaging system mounted on a DM IRE2 microscope (Leica Microsystems, Germany) using x20 and x63 oil planchromat lenses, and recorded with argon ion laser (458-514 nm) and green neon laser (543 nm). Images were acquired with a Z-stack of 0.49 μm from 3 Crhr1-GFP mouse brains. For quantification, the number of cells at 2 different levels of the CeA (between AP: −1.22 and −1.46 mm according to (Paxinos and Watson 1998)) was counted in each hemisphere. Both GFP-stained cells and GFP-αCaMKII co-localized cells were quantified using the cell counter analysis macro of Image J. The percentage of co-localization was calculated for each recording and the mean ± SEM was calculated.
DNA constructs and AAV vector generation.
A rat cDNA encoding Crhr1 was cloned using BamHI/EcoRI from pCRII into an AAV plasmid containing the cytomegalovirus immediate early enhancer/chicken β-actin (CAG) promoter, the woodchuck hepatitis virus posttranscriptional regulatory element and the bovine growth hormone polyadenylation sequence flanked by AAV2 inverted terminal repeats. A transcriptional terminator element, flanked by loxP sites preceding the cDNA (pAAV-Stop-Crhr1). was employed to enable cell type-selective transgene expression following Cre recombinase-mediated recombination (Guggenhuber et al. 2010). The same expression cassette, carrying the humanized renilla green fluorescent protein (hrGFP) reporter (Guggenhuber et al. 2010), was used as a control. In addition a plasmid encoding Cre recombinase fused to a nuclear localization signal (pAAV-Cre) was used for in vitro validation of pAAV-Stop-Crhr1.
Transfection.
Co-transfection of pAAV-Stop-Crhr1 and pAAV-Cre in human embryonic kidney cells (HEK) gown on coverslips was performed by standard calcium phosphate precipitation. Forty-eight hours later, cells were fixed and subjected to immunocytochemistry by permeabilization in 0.1 %Triton-X100 in 1% normal goat serum followed by incubation in primary anti-CRHR1 antibody (1:500; Santa Cruz Biotechnology, USA, sc-1757) overnight. Cells were washed and then incubated for 1h with the appropriate Alexa488-conjugated goat IgG (1:1000; Invitrogen, USA). Before the third wash in PBS, cells were counterstained with the nuclear dye 4′,6-diamidino-2-phenylindole (DAPI) for 5 min. Coverslips were then mounted and fluorescence was visualized using a Zeiss Z1 AxioExaminer NLO710 confocal microscope (Carl Zeiss Microimaging, Germany). Production of pseudotyped rAAV1/2 mosaic vectors and determination of genomic titers was performed as described (Klugmann et al. 2005).
Intra-amygdala virus administration.
Rats were injected with either the Crhr1 overexpression virus or the control virus. They were anaesthetized with isoflurane and immobilized in a Kopf stereotaxic frame. A WPI microinjection pump with a 33 gauge blunt needle was used to deliver 500 nl of the virus (5 x 108 vector genomes) bilaterally into the central amygdala (CeA; AP:−2.3 mm, ML: ± 4.2 mm, DV: −7.8 mm according to (Paxinos and Watson 1998)) at a flow rate of 100 nl/min. The needle was left in place for 5 min after the end of the injection to avoid backflow.
Induction of Crhr1 overexpression by tamoxifen injection.
5 d after virus injection, tamoxifen was injected ip for 5 consecutive days, with one injection on day 1, 3 and 5 and two injections on day 2 and 4. After the injections were completed, the rats were allowed to recover for 6 weeks, during which the overexpression of CRHR1 take effect.
Open field locomotor test.
Locomotor activity was measured 6 weeks after virus injection in an open field arena (51 cm x 51 cm x 50 cm) made of dark PVC, at 10.5 lux for 15 min. A center zone (24.9 cm χ 22.2 cm) was defined in the middle of each box. For the test, the animal was placed in the center of the box and the experimenter then left the room while a camera above recorded the animal’s movements. The observation program Viewer2 (Biobserve GmbH, Bonn, Germany) was used for tracking and analysis of locomotor activity (Distance traveled [cm], center time [s] and velocity [cm/s]).
Drugs.
Alcohol (10% v/v) was prepared using 96-97% ethyl alcohol (Sigma-Aldrich, Germany) and tap water. Tamoxifen (Sigma-Aldrich, Germany) was dissolved in medium-chain triglycerides (Stadtklinik Frankenthal, Frankenthal, Germany) for intraperitoneal (ip) injection at a concentration of 20 mg/ml and administered at a dose of 40 mg/kg. Yohimbine hydrochloride (Sigma-Aldrich, Germany) was dissolved in distilled water for ip injection and administered at a dose of 1.25 mg/kg and a volume of 1 ml/kg.
Cue- and stress-induced reinstatement of alcohol-seeking behavior.
The cue- and stress-induced reinstatement procedure was performed as recently described by (Hansson et al. 2006; Hansson et al. 2017; Hirth et al. 2016; Meinhardt et al. 2013; Pfarr et al. 2015; Pfarr et al. 2018; Uhrig et al. 2017).
Operant self-administration.
The alcohol-seeking experiment was performed during the dark phase in operant conditioning chambers (MED Associates) enclosed in ventilated sound-attenuating cubicles. Each chamber was equipped with a retractable response lever on each side panel of the chamber. Pressing the left lever activated a syringe pump, which delivered 30 μl of fluid into a liquid receptacle next to the lever. A light stimulus (house light) was mounted above the response lever. The light was only illuminated in response to left lever presses. An IBM-compatible computer controlled the delivery of fluids, presentation of stimuli, and data recording.
Saccharin-fading procedure and alcohol self-administration training.
Rats were trained to differentiate between active and inactive levers using a saccharin-fading procedure (Suppl. Figure 1), as Sprague-Dawley rats do not learn this behavior well if trained only with alcohol reward. A saccharin-fading procedure was employed according to Tolliver et al. (1988). During the first six training sessions, left lever presses were rewarded with the delivery of 30 μl of 0.2% saccharin on a fixed-ratio 1 (FR1) schedule, while right lever presses were recorded, but did not have any programmed consequences. The rats were water-deprived 16 hours before each of the first three training sessions, after which they had access to water ad libitum. Each of six 0.2% saccharin sessions were followed by 1 d of 0.2% saccharin + 5% (v/v) alcohol, 1 d of 5% alcohol, 1 d of 0.2% saccharin + 8% alcohol, 1 d of 8% alcohol, 1 d of 0.2% saccharin + 10% alcohol. In the final two sessions only 10% alcohol was delivered. Once rats were trained to press the active lever to receive a 10 % alcohol fluid reward, responses to the left lever also activated the house light (1 light flash per second for 3 s), which also indicated a 3 s “time-out” period, during which additional lever presses of the active lever were recorded but did not lead to further delivery of 10% alcohol. In addition to the conditioned light stimulus (discrete cue), orange odor was presented during the entire session as a contextual cue, by adding 6 drops of orange extract to the bedding of the operant chambers. The bedding was changed and the trays cleaned at the end of each training session (Ciccocioppo et al. 2003; Ciccocioppo et al. 2002). After 10 self-administration sessions, a stable baseline was reached. A criterion for a stable baseline is met when the mean number of the reward lever presses in each of the last three sessions not varies by more than 20%.
Extinction of alcohol self-administration behavior.
During extinction training, responses on the left lever did not result in delivery of alcohol or activation of the light flashing, and the environmental stimulus was omitted. The extinction criterion was set to <10 % of baseline active lever presses, calculated from the final 3 retraining sessions after virus injection (Fig. 2A).
Figure 2. Stress-induced alcohol seeking behavior in αCaMKIICreERT2 rat injected with Crhr1-overexpression virus.
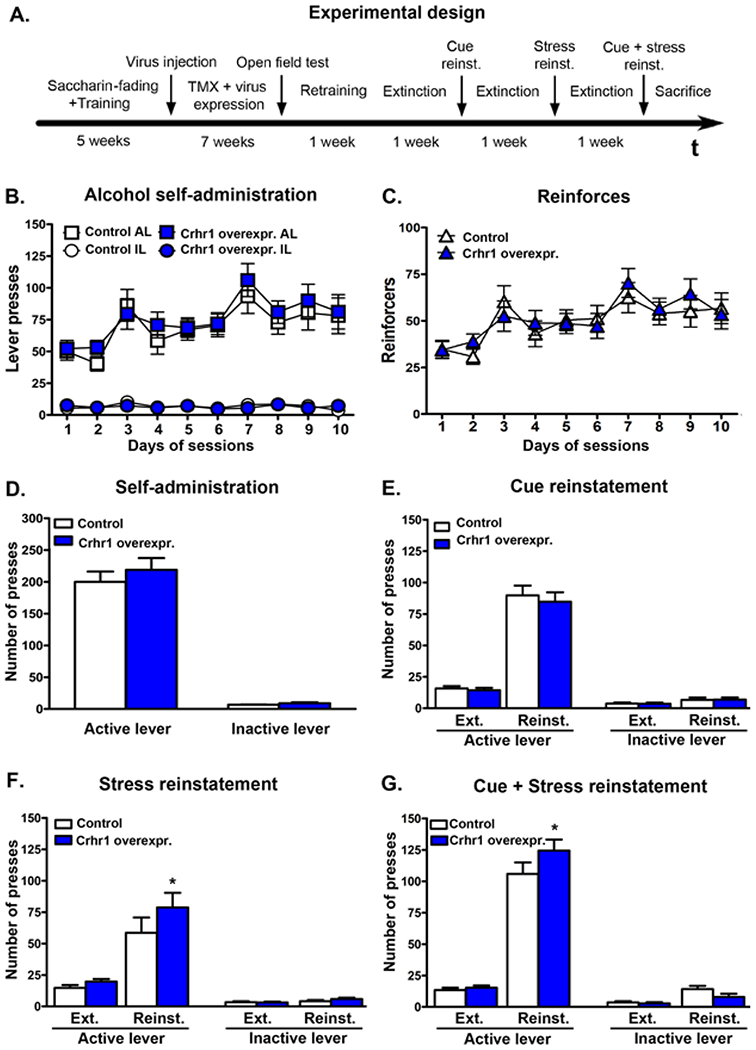
(A.) Experimental design and time line of the operant behavior. (B.) Active and inactive lever presses and (C.) number of reinforcers during training sessions with conditioned and environmental cue within the prospective treatment groups. (D.) Baselines of the last three training sessions after intra-amygdala injection of Crhr1 overexpression or control virus. (E.) Cue-induced, (F.) yohimbine-induced and (G.) combined cue- and yohimbine-induced reinstatement of alcohol-seeking behavior. Bar graphs illustrate number of lever presses of control AAV rats (white bars) and rats with Crhr1 overexpression in the CeA (dark-blue bars). Stress was induced by yohimbine (1.25 mg/kg, ip). Statistical analysis was performed by repeated measures ANOVA, followed by Newman-Keuls post-hoc test if appropriate; n=12-13; p values: *p<0.05 Crhr1 overexpression vs control AAV rats. TMX, tamoxifen; AL, active lever; IL, inactive lever; Ext, extinction; Reinst., reinstatement; Yoh., yohimbine; ind, induced.
Cue-induced Reinstatement.
The conditions during cue-induced reinstatement were identical to training sessions, except that the pressing of the left lever did not result in alcohol delivery. The conditioned discrete and contextual cues were presented.
Yohimbine-induced Reinstatement
Yohimbine was injected ip 30 min before the start of the test session to serve as a stressor. The conditions in the operant conditioning chamber were identical to the extinction phase.
Cue- and Yohimbine-induced Reinstatement
Rats were injected with yohimbine (ip) 30 min before the start of the test sessions. During cue-induced reinstatement trials, the conditioned discrete and contextual cues were presented, but active lever pressing did not result in the delivery of alcohol (Le et al. 2005).
In situ hybridization.
At the end of behavioral testing, rats were decapitated, brains quickly removed then snap-frozen in isopentane at −40°C. Brains were stored at −80°C before the preparation of serial 12 μm coronal brain slices at Bregma −1.8 mm to – 2.8 mm according to The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson 1998). Sections were mounted on SuperFrost glass slides and stored at −80°C until further use. Antisense and sense RNA probe generation (Table 1) and in situ hybridization was performed as previously described (Bernardi et al. 2014; Hansson et al. 2006; Sommer et al. 2008; Uhrig et al. 2017). In situ hybridization of viral WPRE, αCaMKII and Crhr1 were used to verify the injection sites and the successful Crhr1 mRNA overexpression. Animals in which the injection did not lead to Crhr1 mRNA overexpression in the CeA were excluded from the analysis.
Table 1.
Primers used for RNA probe generation
| mRNA | DNA | FW-Primer | RV-Primer |
|---|---|---|---|
| CreERT2 | Plasmid (Feil et al. 1997) | 5′-GGGCTGCCA CGACCAAG-3′ |
5′-GCTACACCAG AGACGGAAATC-3′ |
| αCaMKII | NM_009792.3 | 5′-AGGAAGTCTCTC GCTGGTTG-3′ |
5′-AACTGAACGC TGGAACTGGAC-3 |
| WPRE | Plasmid #021_pAM/CBA-CRHR1-WPRE-bGH | 5′-TGGTTGCTGTCTCTT TATGAGGAGTTGTGGC CCGTTGTCAGGCAACG TGGCGTGGTGTGCACT GTGTTTGCTGACGCAA CCCCCACTGGTTGG-3′ |
5′-GGCATTGCCACC TGTCAGCTCCTTTCCGG GACTTTCGCTTTCCCCC TCCCTATTGCCACGGC GGAACTCATCG-3′ |
| Crhr1 | NM_030999 | 5′-CGCTGTGAGA ACCTGTCCCTG-3′ |
5′-TAGGATGAA AGCCGAGATG-3′ |
Statistical analysis.
Data are expressed as mean ± SEM. For statistical evaluation, the Statistica software (StatSoft, Germany) was used. In situ hybridization and open field data were analyzed by one-way ANOVA (control vs. overexpression virus). A repeated measures ANOVA (with the factor treatment, i.e. control vs. overexpression virus, and with session, i.e. self-administration sessions or extinction and reinstatement, as repeated measures, and considering active and inactive levers) was used. In cases where the ANOVA revealed significant differences, Newman-Keuls post-hoc test was used for self-administration sessions and reinstatement tests.
Results
CRHR1 and αCaMKII are co-localized in the CeA.
Because it has been difficult to obtain specific CRHR1 immunostaining, we used BAC transgenic mice expressing GFP under the control of the Crhr1 genomic locus (Justice et al. 2008). Using these reporter mice, we were able to determine if and to what extent CRHR1 is expressed in αCaMKII-expressing CeA cells. Double fluorescence immunostainings were performed on coronal amygdala brain sections according to Pfarr et al. (2015). αCaMKII-ir neurons were distributed throughout the amygdala with the highest expression levels in the BLA and the CeA. CRHR1-GFP positive neurons were found mainly along the ITC clusters and in the CeL (Figures 1A–B). Quantification revealed that 48% of αCaMKII-ir- positive cells within the CeA co-express CRHR1-GFP (Figure 1C).
Figure 1. Co-located CRHR1 and αCaMKII in the CeA.
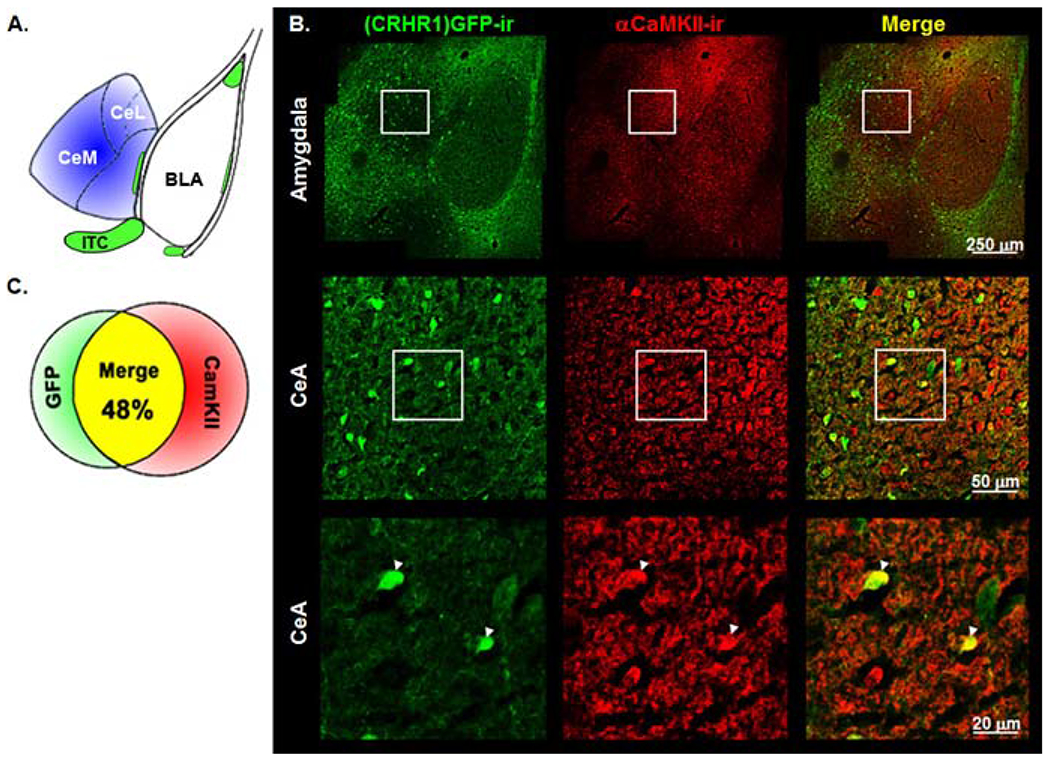
(A.) Schematic illustration of the amygdala brain region of the mouse (Paxinos 2004). (B.) Immunofluorescence staining in the amygdala for (CRHR1)GFP-ir (green), αCaMKII-ir (red) and with merged channels (yellow) in Crhr1-GFP reporter mouse line. White arrow heads indicate (CRHR1)GFP-ir and αCaMKII-ir co-localization. (C.) Quantification of co-located (CRHR1)GFP-ir- and αCaMKII-ir -positive cells in the CeA. BLA, basolateral amygdala; CeA, central amygdala; CeM, medial division of the CeA; CeL, lateral division of the CeA; ITC, intercalated cell cluster. For more details, see Material and Methods.
Behavioral effects of Crhr1 overexpression in the CeA
Crhr1 overexpression in the CeA does not alter alcohol self-administration.
The timeline of the behavioral experiments is shown in Figure 2A. Self-administration training, extinction and reinstatement were analyzed using repeated measures ANOVA followed by Newman-Keuls post-hoc analysis using virus treatment as a between group factor and time points (baseline before virus injection, baseline after injection, extinction, reinstatement) as well as active vs. inactive lever presses as within group factors. αCaMKII-CreERT2 rats were trained to self-administer 10% alcohol and assigned to prospective treatment groups (baseline: 84.1 ± 10.1 vs. 77.0 ± 11.7 active lever presses, designated Crhr1 overexpression and control group, respectively). No differences in active or inactive level presses were found between the assigned groups. After virus injection and tamoxifen treatment, rats had a six-week recovery period after which they were tested in the open field to check for effects of the vector on basal locomotor behavior. No differences between Crhr1 overexpression and control vector injected rats were found in the open field test (velocity: controls: 4.6±0.5 cm/s, Crhr1 overexpression: 5.2±0.5 cm/s; distance traveled: controls: 4143.7±405.3 cm, Crhr1 overexpression: 4717.9±460.8 cm; activity: controls: 21.2±1.8 %, Crhr1 overexpression: 24.2±2.2 %; center time: controls: 47.6±6.9 s, Crhr1 overexpression: 44.9±8.3 s). Next, rats underwent 10 self-administration sessions. The baseline of both groups increased significantly compared to lever presses before surgery (controls: 200.0±16.3 active lever presses, F[1,22]=37.78, p=0.000003; Crhr1 overexpression: 218.9±18.7 active lever presses, F[1,24]=40.45, p=0.000001), but there was no difference in ethanol self-administration between active and control virus injected rats (Figures 2B–D).
Yohimbine- but not cue-induced reinstatement is increased by intra-amygdala Crhr1 overexpression.
After extinction, rats were tested successively for cue-induced, yohimbine-induced, and combined cue- and stress-induced reinstatement of alcohol seeking. Both groups showed a significant reinstatement in all tests (repeated measures analysis with extinction and reinstatement as within group effect: Cue-induced reinstatement: F[1,23]=200.1, p<0.001; yohimbine-induced reinstatement: F[1,23]=39.2, p<0.001; Cue- and yohimbine-induced reinstatement: F[1,23]=287.3, p<0.001). Furthermore, overall effect on lever presses showed that animals clearly distinguished between the active and inactive levers (repeated measures analysis lever effect: Cue-induced reinstatement: F[1,23]=264.9, p<0.001; yohimbine-induced reinstatement: F[1,23]=84.1, p<0.001; Cue- and yohimbine-induced reinstatement: F[1,23]=292.3, p<0.001). Crhr1 overexpression in the CeA had no effect on cue-induced reinstatement (Figure 2E). However, post-hoc analysis revealed that animals with Crhr1 overexpression showed increased responding at the active lever during yohimbine-induced reinstatement compared to control rats (p=0.025), as well as in the combined cue- and yohimbine-induced reinstatement (p=0.011, Figures 2F–G).
Confirmation of AAV mediated Cre-dependent Crhr1 overexpression In vitro validation of Cre-dependent Crhr1 overexpression.
To demonstrate the functionality of the Cre-dependent Crhr1 expression construct, human embryonic kidney (HEK293) cells were transfected with the Cre-inducible Crhr1 AAV-vector plasmid. As expected, immunostaining for the receptor could only be detected after co-transfection of the Crhr1 construct with a Cre-expression plasmid (Figure 3A).
Figure 3. In vitro and in vivo validation of AAV-Crhr1 overexpression.
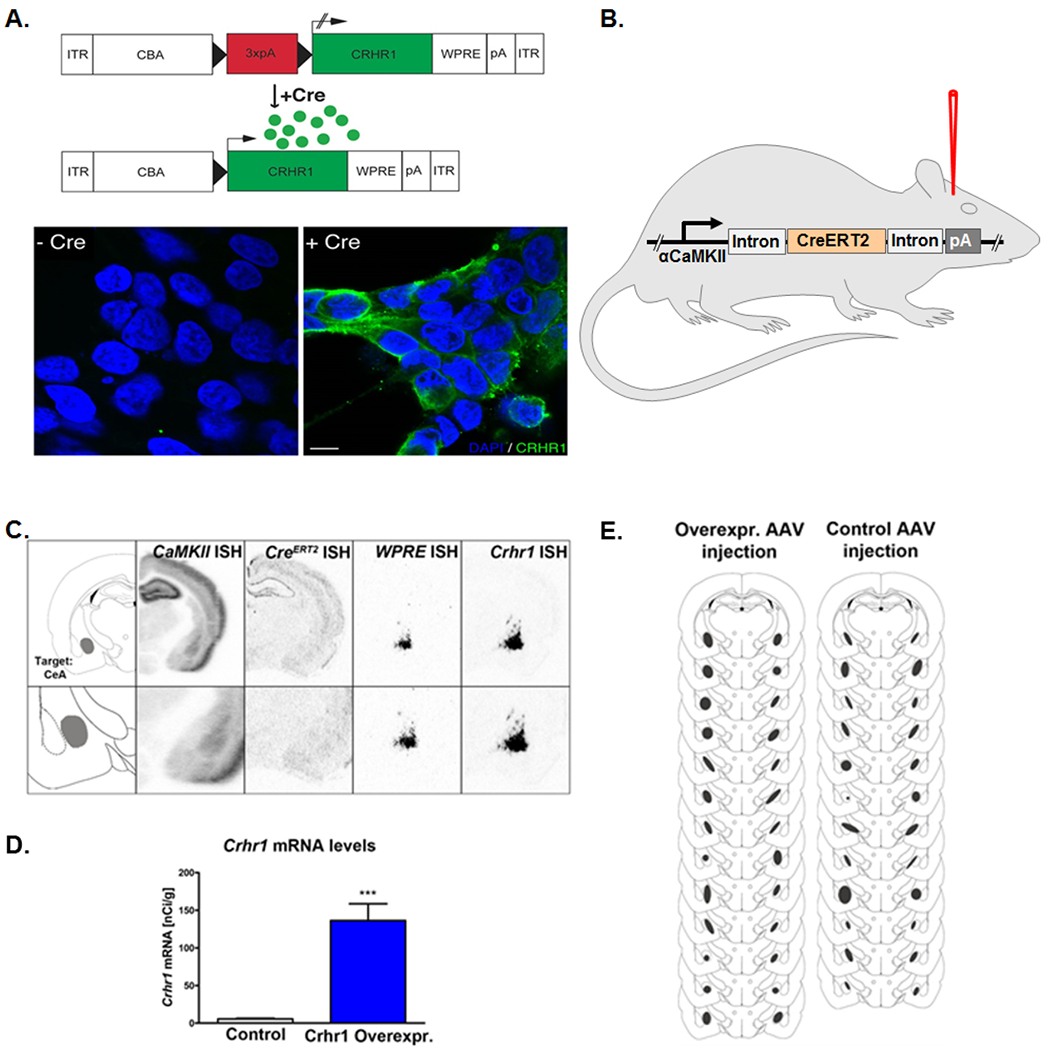
(A.) Cre-recombinase-activated overexpression of CRHR1 in vitro. Co-transfection of HEK cells with pAAV-Stop-Crhr1/control and pAAV-Cre resulted in strong CRHR1 at the cell surface, while no transgene expression could be detected in the absence of Cre expression. Blue color; cell nucleus staining with DAPI; green color: CRHR1-ir; scale bar 10 μm. (B.) Crhr1 overexpression virus or control virus were bilaterally injected into the CeA of αCaMKII-CreERT2 transgenic rats. (C.) Schematic outline of the target region (left, Bregma −2.8 mm according to (Paxinos and Watson 1998), and representative images of in situ hybridization for αCaMKII, CreERT2, WPRE and Crhr1mRNA. (D.) AAV overexpression induced a 24-fold increase of Crhr1mRNA as compared to control AAV injected rats. (E.) Schematic illustration showing quality of intra-amygdala injected AAV. Virus injection sites were confirmed by WPRE in situ hybridization of each injected rat. For more details, see Material and Methods.
In vivo validation of Cre-dependent Crhr1 overexpression.
After behavioral experiments, Crhr1 mRNA expression levels were determined in the CeA of active and control AAV injected αCaMKII-CreERT2 rats (Figure 3B). In situ hybridization for Crhr1 mRNA in the amygdala (Figure 3C) showed a strong, 24-fold increase in the overexpression vector injected rats compared to control vector injected rats (F[1,23]=31.6; p<.001, Figure 3D). Placement of intra-amygdala injections were estimated by localization of the viral WPRE-sequence via in situ hybridization (Figure 3C), and a map of the injection site was generated for each rat (Figure 3E). Animals in which the injection did not accurately target the CeA were excluded from the entire study. Thus, 13 overexpression and 12 control AAV rats were used for the analysis.
Discussion
Stress is an important precipitating factor in the reinstatement of alcohol-seeking after prolonged abstinence. As the CRH system is one of the major stress response systems in the brain, many studies have investigated the effects of alcohol on CRH and its receptors, as well as the role of the CRH system in alcohol consumption and dependence. In the present study we show for the first time that overexpression of CRHR1 in a distinct population of αCaMKII- positive CeA cells is sufficient to drive increased yohimbine-induced reinstatement. This was accomplished using a transgenic αCaMKII-CreERT2 rat driver line and AAV-mediated gene transfer. We demonstrated strong co-expression of CRHR1 in αCaMKII neurons of the CeA, and validated the Crhr1 overexpression induced by our conditional gene transfer approach both in vitro and in vivo. When analyzing reinstatement behavior, we found that Crhr1 overexpression in the CeA increased yohimbine-induced reinstatement, but not alcohol self-administration or cue-induced reinstatement of alcohol-seeking.
Defining the distinct role of the CRH system in alcohol dependence is challenging. Although there are many reports dedicated to this topic, study designs vary widely, as well as the interpretation of results (Phillips et al. 2015). Crhr1 knockout mice have been reported to be more sensitive to alcohol concentration (Pastor et al. 2011), and voluntary alcohol consumption is not altered in Crhr1 knockout mice in a long-term continuous two-bottle, free-choice access paradigm (Molander et al. 2012; Sillaber et al. 2002). However, alcohol consumption was reduced in female Crhr1 knockout mice when assayed using a short-term, single-bottle drinking-in-the-dark paradigm (Kaur et al. 2012). Moreover, stress-induced alcohol intake was increased in global Crhr1 knockout mice (Sillaber et al. 2002), but was decreased in conditional brain-specific knockouts (Molander et al. 2012). These latter experiments suggest opposite effects of peripheral and central CRHR1 on stress responses. In alcohol dependent animals, CRH and CRHR1 expression is chronically increased in the amygdala, and dependence-induced behavioral sensitivity to stress was can be blocked by CRHR1 antagonism (Sommer et al. 2008). More importantly, administration of the CRHR1/2 antagonist D-Phe-CRH into the CeA significantly decreased alcohol self-administration in dependent but not in control rats (Funk et al. 2006), pointing to the CeA as a major site of signaling adaptation to alcohol.
An increase in Crhr1 expression has also been found in a rat line genetically selected for high alcohol consumption, i.e. the alcohol preferring Marchigian-Sardinian Preferring (msP) rats (Ciccocioppo et al. 2006). These animals show an increase of CRHR1 in the CeA that likely triggers their excessive drinking and high stress vulnerability (Hansson et al. 2006; Kirson et al. 2018; Natividad et al. 2017; Stopponi et al. 2017). An intriguing observation is that in msP rats given ad lib access to alcohol, many differentially-expressed genes (e.g. CRHR1) are regulated similarly to control levels, suggesting high alcohol consumption resolves aberrant expression, perhaps serving as a self-medication strategy for alcohol-preferring animals (Hansson et al. 2007). CeA injection of the CRHR1 antagonist antalarmin has been shown to attenuate binge-like alcohol intake in mice (Lowery-Gionta et al. 2012). CRHR1 antagonism also diminishes stress-escalated alcohol consumption in mice with a history of chronic alcohol intake (Albrechet-Souza et al. 2017), and in mice subjected to social defeat induced elevated alcohol drinking (Norman et al. 2015) (Hwa et al. 2016). These experimental findings suggest the importance of CRHR1 in alcohol consumption behavior. However, here we found no changes on baseline alcohol self-administration after intra-CeA Crhr1 mRNA overexpression in αCaMKII neurons. One possible explanation for this negative result is that CRHR1 signaling in non-αCaMKII-containing CeA CRHR1 neurons is crucial for alcohol drinking escalation.
We found a significant increase in baseline self-administration after surgery during the recovery period. It is currently unclear whether this effect is due to surgery, anesthesia, or some interaction between these or other perioperative variables. Studies in rodents suggest that increased operant performance may be related directly to anesthesia-induced neural activity, which may be selective to tasks that primarily measure spatial learning and memory processes (Walters et al. 2016).
For reinstatement of alcohol-seeking behavior, many studies found decreased active lever pressing after administration of CRHR antagonists, although these results depend highly on the particular animal strain and paradigm used. It has been shown that the CRHR1/2 antagonist D-Phe-CRH and the CRHR1 antagonist CP-154,526 attenuate footshock-induced reinstatement in Wistar rats (Le et al. 2000). In alcohol dependent rats, D-Phe-CRH was effective in reducing active lever pressing in footshock-induced reinstatement and a combination of footshock- and cue-induced reinstatement, while having no effect on cue-induced reinstatement alone (Liu and Weiss 2002). Another study found that the CRHR1 antagonist antalarmin blocked footshock-induced reinstatement of alcohol-seeking in msP rats, with no effect in Wistar rats (Hansson et al. 2006). Likewise, the CRHR1 antagonist MTIP reduced footshock-induced reinstatement in msP rats and alcohol dependent rats, while not affecting Wistar control rats (Gehlert et al. 2007). Antalarmin (ip) and D-Phe-CRH (intramedian raphe nucleus) were also found to reduce yohimbine-induced reinstatement of alcohol drinking in Wistar rats (Le et al. 2013; Marinelli et al. 2007), which has been proven to be a stable stressor that reliably induces reinstatement of drug-seeking (Le et al. 2005; Shepard et al. 2004). Overall, our findings are consistent with these studies. As CRHR1 antagonism decreases stress-induced reinstatement, we show that overexpression of Crhr1 mRNA in the CeA increases stress- but not cue-induced reinstatement.
In contrast to most previous pharmacological studies that applied CRHR1 antagonists either systemically or by i.c.v. injection, we used local overexpression of CRHR1 in the CeA, a region critical for stress and anxiety-like behavior (reviewed in (Ehrlich et al. 2009)), alcohol intake in dependent rats (Funk et al. 2006) and stress-induced alcohol seeking (Simms et al. 2012), and assayed reinstatement of alcohol self-administration. Two subregions of the CeA can be distinguished, the CeL and the medial (CeM) division of the CeA (McDonald 1982), which both use GABA as a neurotransmitter, but play different functional roles in determining amygdalar output (Duvarci and Pare 2014; Lopez de Armentia and Sah 2004). The CeL acts as the major input region of the CeA, receiving projections from the lateral amygdala and from other regions, such as sensory and higher-order cortical areas (Ehrlich et al. 2009; McDonald 1998; Savander et al. 1995). In addition to sending GABAergic projections to the BNST, the lateral hypothalamus, and the parabrachial nucleus (Veinante and Freund-Mercier 1998), the CeL is thought to inhibit the CeM, the major output region of the CeA, through GABAergic projections (LeDoux et al. 1988; Petrovich and Swanson 1997; Pitkanen et al. 2000; Sun et al. 1994; Veening et al. 1984). An important difference between these two subdivisions of the CeA is the high density of CRH peptide in the CeL, while significantly less CRH peptide is present in the CeM (Cassell et al. 1999; Cassell et al. 1986; Veening et al. 1984). In addition, the CeL is enriched for expression of the dopamine receptor D2, another system that contributes to the generation of anxiety-like behavior (Perez de la Mora et al. 2012) via action in the CeA. In line with these findings, we report immunoreactivity for the CRHR1 in the CeL. Strong CRHR1-ir was also present in the ITCs. Although, not part of the CeA, the ITCs project to the CeL and CeM, as well as other nuclei that mediate anxiety- and stress-related behavior (Amir et al. 2011; Koob and Le Moal 2008). Our targeting approach did not distinguish between these different CRHR1 expressing amygdala subregions. Thus, an alternative explanation of our results is that overexpression occurs in cells that do not normally express the receptor, perhaps accounting for the behavioral effects observed here, which potentially represents a pathologic mechanism in stress related disorders including alcohol addiction.
We used a Crhr1 reporter mouse line to identify CRHR1 expressing cells in the CeA (Justice et al. 2008). We found that 48% of CRHR1-GFP positive cells co-express αCaMKII in the CeA. The available data on Crhr1 mRNA and CRHR1 protein distribution in the CeA does not suggest substantial differences between mouse and rat (Treweek et al. 2009; Van Pett et al. 2000). We therefore used Crhr1-GFP reporter mice to guide our behavioral experiments in rats using the αCaMKII-CreERT2 driver line (Schonig et al. 2012) to conditionally overexpression Crhr1. αCaMKII is considered a marker of glutamatergic neurons within the hippocampus and cortical regions (Benson et al. 1991). This kinase is expressed throughout the forebrain and plays a crucial role in the modulation of synaptic function due to its calcium-dependent activation (Benson et al. 1992; Benson et al. 1991; Jones et al. 1994; Liu and Murray 2012). Based on in situ hybridization studies, αCaMKII is also expressed in GABAergic neurons of the thalamic reticular nucleus, the globus pallidus, cerebellar Purkinje cells, and the commissural nucleus of the stria terminalis (Benson et al. 1992). Furthermore, recent studies suggest αCaMKII is expressed in GABAergic cells of the amygdala, in particular in the ITCs (Huang et al. 2014; Meins et al. 2010) and in a subset of CeA neurons (Beckerman et al. 2013). Thus, αCaMKII is expressed both in excitatory and inhibitory neurons. The high degree of overlap in CRHR1 and αCaMKII expression found in CRHR1-GFP mice suggests that the αCaMKII rat line is a useful tool non-ectopic, cell-specific overexpression of Crhr1. Injecting Cre-induciblee-Crhr1 encoding AAV into this region increased expression of Crhr1 mRNA approximately 24-fold, without causing nonspecific behavioral effects as tested in the open field paradigm. Because the CRH system is considered an ‘alarm’-system and is thought to be silent under non-stimulated conditions (reviewed in (Heilig and Koob 2007)), we expected and confirmed that Crhr1 overexpression had no effect on non-stimulated, i.e. non-stressed, behaviors such as locomotion and alcohol self-administration. Several brain regions, including the BNST, the CeA, and the nucleus accumbens shell, are key regions involved in stress-induced reinstatement (reviewed in (Shaham et al. 2003). Here, we manipulated CRHR1 expression in only one of these regions (CeA), perhaps explaining the relatively low effect size observed.
A key consideration is that CRHR1 colocalization with αCaMKII was assessed in healthy animals in a basal state. Under condition of long-term activation (e.g. in alcohol addiction), there is a long-term increase of Crhr1 expression in the amygdala, and this likely includes an increased expression in a subset of αCaMKII neurons. In addition, recent studies identified distinct sets of neurons, so called neuronal ensembles within the amygdala region (de Guglielmo et al. 2016) and the prefrontal cortex area (George and Hope 2017; Pfarr et al. 2015; Pfarr et al. 2018) that are functionally involved in the reinstatement to alcohol-seeking behavior. Thus during the transition into dependence, we hypothesize that neuroadaptations occur in these ensembles similar to those experimentally-induced by overexpression of CRHR1. Future studies are needed to more thoroughly characterize the role of CRHR1 in specific neuronal ensembles involved in dependence-induced drinking and reinstatement behavior.
A potential limitation of the study is that repeated reinstatement sessions were performed without randomizing the sequence of session (i.e. cue-induced, yohimbine-induced, cue+yohimbine-induced). Although we have used a similar experimental design in the past without noticeable effects on behavioral performance (Pfarr et al. 2015; Pfarr et al. 2018) we cannot exclude potentially carry-over effects between trials. However, the goal here was not to compare different aspects of reinstatement behaviors, but to demonstrate the utility of a novel technical tool that allows to interfere with CRHR1 expression in a time, region and cellular manner.
Overall, our study further supports the importance of CRHR1 in stress-induced relapse to alcohol seeking. Using a new technical approach to drive CRHR1 overexpression in specific neuronal populations of the amygdala, we demonstrated that increased expression of this receptor is sufficient to cause exaggerated stress dependent alcohol consumption, which serves as a potential mechanism that determines higher vulnerability to excessive alcohol consumption in humans. This virus-based conditional expression approach employing available transgenic Cre-driver rat lines will be key to assessing the function of different populations of amygdalar CRHR1 neurons in alcohol-seeking and relapse-like behavior.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Elisabeth Röbel and Sabrina Koch for their technical support.
FUNDING AND DISCLOSURES
Our work is supported by the Bundesministerium für Bildung und Forschung (e:Med program, FKZ: 01ZX1311A (Spanagel et al. 2013)) and the Deutsche Forschungsgemeinschaft (SFB1134, DFG HA6102/1-1). N.J. was supported by funding from the NIH R56MH114032 (N.J.), and R01MH112768 (N.J.). The authors declare that they have no competing or conflicting interest and no financial disclosures to include.
References
- Albrechet-Souza L, Viola TW, Grassi-Oliveira R, Miczek KA, de Almeida RMM (2017) Corticotropin Releasing Factor in the Bed Nucleus of the Stria Terminalis in Socially Defeated and Non-stressed Mice with a History of Chronic Alcohol Intake. Front Pharmacol 8: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D (2011) Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol 105: 3054–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Amur Rev Pharmacol Toxicol 44: 525–57. [DOI] [PubMed] [Google Scholar]
- Beckerman MA, Van Kempen TA, Justice NJ, Milner TA, Glass MJ (2013) Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Experimental neurology 239: 120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Isackson PJ, Gall CM, Jones EG (1992) Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience 46: 825–49. [DOI] [PubMed] [Google Scholar]
- Benson DL, Isackson PJ, Hendry SH, Jones EG (1991) Differential gene expression for glutamic acid decarboxylase and type II calcium-calmodulin-dependent protein kinase in basal ganglia, thalamus, and hypothalamus of the monkey. J Neurosci 11: 1540–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Broccoli L, Hirth N, Justice NJ, Deussing JM, Hansson AC, Spanagel R (2017) Dissociable Role of Corticotropin Releasing Hormone Receptor Subtype 1 on Dopaminergic and D1 Dopaminoceptive Neurons in Cocaine Seeking Behavior. Front Behav Neurosci 11: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Uhrig S, Spanagel R, Hansson AC (2014) Transcriptional regulation of L-type calcium channel subtypes Cav1.2 and Cav1.3 by nicotine and their potential role in nicotine sensitization. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 16: 774–85. [DOI] [PubMed] [Google Scholar]
- Bjork K, Hansson AC, Sommer WH (2010) Genetic variation and brain gene expression in rodent models of alcoholism implications for medication development. Int Rev Neurobiol 91: 129–71. [DOI] [PubMed] [Google Scholar]
- Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S (2011) The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology 94: 12–20. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C (1999) The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci 877: 217–41. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ (1986) Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol 246: 478–99. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M (2006) Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addiction biology 11: 339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F (2003) Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 168: 208–215. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F (2002) Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27: 391–9. [DOI] [PubMed] [Google Scholar]
- Coste SC, Heard AD, Phillips TJ, Stenzel-Poore MP (2006) Corticotropin-releasing factor receptor type 2-deficient mice display impaired coping behaviors during stress. Genes Brain Behav 5: 131–8. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C (2000) The amygdala. Curr Biol 10: R131. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Crawford E, Kim S, Vendruscolo LF, Hope BT, Brennan M, Cole M, Koob GF, George O (2016) Recruitment of a Neuronal Ensemble in the Central Nucleus of the Amygdala Is Required for Alcohol Dependence. J Neurosci 36: 9446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW (2001) Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38: 192–246. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D (2014) Amygdala microcircuits controlling learned fear. Neuron 82: 966–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A (2009) Amygdala inhibitory circuits and the control of fear memory. Neuron 62: 757–71. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P (1997) Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237: 752–7. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell FE, Crawford EF, Koob GF (2006) Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26: 11324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Fe AD, Hipskind PA, Hamdouchi C, Fu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M (2007) 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci 27: 2718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Hope BT (2017) Cortical and amygdalar neuronal ensembles in alcohol seeking, drinking and withdrawal. Neuropharmacology 122: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenhuber S, Monory K, Futz B, Klugmann M (2010) AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS One 5: e15707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M (2007) Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addiction biology 12: 30–4. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia F, Terasmaa A, Massi M, Heilig M, Ciccocioppo R (2006) Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A 103: 15236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Koopmann A, Uhrig S, Buhler S, Domi E, Kiessling E, Ciccocioppo R, Froemke RC, Grinevich V, Kiefer F, Sommer WH, Vollstadt-Klein S, Spanagel R (2017) Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM (2006) Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets 5: 453–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF (2007) A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M (2013) Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci 33: 3284–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Broccoli F, Vengeliene V, Rossmanith M, Perreau-Fenz S, Kohr G, Sommer WH, Spanagel R, Hansson AC (2016) Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Chen CC, Liang YC, Hsu KS (2014) Long-term potentiation at excitatory synaptic inputs to the intercalated cell masses of the amygdala. Int J Neuropsychopharmacol 17: 1233–42. [DOI] [PubMed] [Google Scholar]
- Hwa FS, Holly EN, DeBold JF, Miczek KA (2016) Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology (Berl) 233: 681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D, Kozicz T (2013) Is it really a matter of simple dualism? Corticotropin-releasing factor receptors in body and mental health. Front Endocrinol (Lausanne) 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Huntley GW, Benson DF (1994) Alpha calcium/calmodulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: comparison with GAD-67 expression. J Neurosci 14: 611–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W (2008) Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol 511: 479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Li J, Stenzel-Poore MP, Ryabinin AE (2012) Corticotropin-releasing factor acting on corticotropin-releasing factor receptor type 1 is critical for binge alcohol drinking in mice. Alcohol Clin Exp Res 36: 369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D, Oleata CS, Parsons LH, Ciccocioppo R, Roberto M (2018) CB1 and ethanol effects on glutamatergic transmission in the central amygdala of male and female msP and Wistar rats. Addiction biology 23: 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ (2005) AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci 28: 347–60. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2008) Addiction and the brain antireward system. Amur Rev Psychol 59: 29–53. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y (2013) Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addiction biology 18: 448–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y (2002) The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci 22: 7844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y (2005) Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 179: 366–73. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y (2000) The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 150: 317–24. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000) Emotion circuits in the brain. Amur Rev Neurosci 23: 155–84. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988) Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8: 2517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Selvage D, Hansen K, Rivier C (2004) Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebro ventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology 145: 4470–9. [DOI] [PubMed] [Google Scholar]
- Lee S, Smith GW, Vale W, Lee KF, Rivier C (2001) Mice that lack corticotropin-releasing factor (CRF) receptors type 1 show a blunted ACTH response to acute alcohol despite up-regulated constitutive hypothalamic CRF gene expression. Alcohol Clin Exp Res 25: 427–33. [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE (2012) Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490: 402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F (2002) Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci 22: 7856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Murray KD (2012) Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia 53 Suppl 1: 45–52. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P (2004) Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol 92: 1285–94. [DOI] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pled KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE (2012) Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci 32: 3405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD (2007) The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 195: 345–55. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1982) Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol 212: 293–312. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1998) Cortical pathways to the mammalian amygdala. Prog Neurobiol 55: 257–332. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH (2013) Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience 33: 2794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Sommer WH (2015) Postdependent state in rats as a model for medication development in alcoholism. Addiction biology 20: 1–21. [DOI] [PubMed] [Google Scholar]
- Meins M, Herry C, Muller C, Ciocchi S, Moreno E, Luthi A, Monard D (2010) Impaired fear extinction in mice lacking protease nexin-1. The European journal of neuroscience 31: 2033–42. [DOI] [PubMed] [Google Scholar]
- Millhouse OE (1986) The intercalated cells of the amygdala. J Comp Neurol 247: 246–71. [DOI] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R (2012) Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology 37: 1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Herman MA, Kirson D, Oleata CS, Irimia C, Polis I, Ciccocioppo R, Roberto M, Parsons LH (2017) Constitutive Increases in Amygdalar Corticotropin-Releasing Factor and Fatty Acid Amide Hydrolase Drive an Anxious Phenotype. Biological psychiatry 82: 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR (2004) Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science 303: 1512–4. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR (2009) Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal 9: 68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Helinski S, Spanagel R (2014) Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addiction biology 19: 225–32. [DOI] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, Miczek KA (2015) Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology (Berl) 232: 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C (1998) Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res 22: 243S–247S. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE (2004) New vistas on amygdala networks in conditioned fear. J Neurophysiol 92: 1–9. [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ (2008) Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci U S A 105: 9070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R, Reed C, Burkhart-Kasch S, Li N, Sharpe AL, Coste SC, Stenzel-Poore MP, Phillips TJ (2011) Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice. Psychopharmacology (Berl) 218: 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates. [Google Scholar]
- Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates, Fourth edn. Academic Press [Google Scholar]
- Perez de la Mora M, Gallegos-Cari A, Crespo-Ramirez M, Marcellino D, Hansson AC, Fuxe K (2012) Distribution of dopamine D(2)-like receptors in the rat amygdala and their role in the modulation of unconditioned fear and anxiety. Neuroscience 201: 252–66. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW (1997) Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res 763: 247–54. [DOI] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, Sommer WH (2015) Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci 35: 10750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr S, Schaaf L, Reinert JK, Paul E, Herrmannsdorfer F, Rossmanith M, Kuner T, Hansson AC, Spanagel R, Korber C, Sommer WH (2018) Choice for drug or natural reward engages largely overlapping neuronal ensembles in the infralimbic prefrontal cortex. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Reed C, Pastor R (2015) Preclinical evidence implicating corticotropin-releasing factor signaling in ethanol consumption and neuroadaptation. Genes Brain Behav 14: 98–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A (2000) Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci 911: 369–91. [DOI] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM (2011) Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 333: 1903–7. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH (2010) Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biological psychiatry 67: 831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, Agoglia AE, Kash TF, Hodge CW (2016) Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biological psychiatry 79: 430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savander V, Go CG, FeDoux JE, Pitkanen A (1995) Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol 361: 345–68. [DOI] [PubMed] [Google Scholar]
- Schonig K, Weber T, Frommig A, Wendler F, Pesold B, Djandji D, Bujard H, Bartsch D (2012) Conditional gene expression systems in the transgenic rat brain. BMC Biol 10: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Fu F, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biological psychiatry 55: 1082–9. [DOI] [PubMed] [Google Scholar]
- Shinnick-Gallagher P, McKeman MG, Xie J, Zinebi F (2003) L-type voltage-gated calcium channels are involved in the in vivo and in vitro expression of fear conditioning. Ann N Y Acad Sci 985: 135–49. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Fetterly TF, Awad EK, Milano EJ, Usdin TB, Winder DG (2015) Ethanol produces corticotropin-releasing factor receptor-dependent enhancement of spontaneous glutamatergic transmission in the mouse central amygdala. Alcohol Clin Exp Res 39: 2154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Winder DG (2015) Ethanol and corticotropin releasing factor receptor modulation of central amygdala neurocircuitry: An update and future directions. Alcohol 49: 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R (2002) Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science 296: 931–3. [DOI] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CF, Bito-Onon J, Li R, Bartlett SE (2012) Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology 37: 906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA (2008) Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological psychiatry 63: 139–45. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Durstewitz D, Hansson A, Heinz A, Kiefer F, Kohr G, Matthaus F, Nothen MM, Noori HR, Obermayer K, Rietschel M, Schloss P, Scholz H, Schumann G, Smolka M, Sommer W, Vengeliene V, Walter H, Wurst W, Zimmermann US, Addiction GRG, Stringer S, Smits Y, Derks EM (2013) A systems medicine research approach for studying alcohol addiction. Addiction biology 18: 883–96. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M (2014) Stress and alcohol interactions: animal studies and clinical significance. Trends Neurosci 37: 219–27. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Fotio Y, Domi A, Borruto AM, Natividad F, Roberto M, Ciccocioppo R, Cannella N (2017) Inhibition of fatty acid amide hydrolase in the central amygdala alleviates co-morbid expression of innate anxiety and excessive alcohol intake. Addiction biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Yi H, Cassell MD (1994) Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J Comp Neurol 340: 43–64. [DOI] [PubMed] [Google Scholar]
- Swanson FW, Petrovich GD (1998) What is the amygdala? Trends Neurosci 21: 323–31. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Sadeghi KG, Samson HH (1988) Suppressed ethanol intake by CER following the sucrose-fading initiation procedure. Pharmacol Biochem Behav 31: 949–52. [DOI] [PubMed] [Google Scholar]
- Treweek JB, Jaferi A, Colago EE, Zhou P, Pickel VM (2009) Electron microscopic localization of corticotropin-releasing factor (CRF) and CRF receptor in rat and mouse central nucleus of the amygdala. J Comp Neurol 512: 323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrig S, Vandael D, Marcantoni A, Dedic N, Bilbao A, Vogt MA, Hirth N, Broccoli E, Bernardi RE, Schonig K, Gass P, Bartsch D, Spanagel R, Deussing JM, Sommer WH, Carbone E, Hansson AC (2017) Differential Roles for L-Type Calcium Channel Subtypes in Alcohol Dependence. Neuropsychopharmacology 42: 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE (2000) Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE (1984) The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res 303: 337–57. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ (1998) Intrinsic and extrinsic connections of the rat central extended amygdala: an in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res 794: 188–98. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Spanagel R (2014) The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol 48: 313–20. [DOI] [PubMed] [Google Scholar]
- Walters JL, Chelonis JJ, Fogle CM, Orser BA, Paule MG (2016) Single and repeated exposures to the volatile anesthetic isoflurane do not impair operant performance in aged rats. Neurotoxicology 56: 159–169. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang C, Szabo G, Sun QQ (2013) Distribution of CaMKIIalpha expression in the brain in vivo, studied by CaMKIIalpha-GFP mice. Brain Res 1518: 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T (2005) Neuronal Ca2+/calmodulin-dependent protein kinase II--discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biol Pharm Bull 28: 1342–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


