INTRODUCTION
Computed tomography (CT) has been extensively leveraged in research investigations of chronic obstructive pulmonary disease (COPD). Computer-aided tools can provide objective characterization of lung disease, yet advances in clinical care lag behind discoveries made in research. Although this phenomenon is generally true of all medical conditions, the gulf between imaging-based research and COPD treatment is striking. This article provides an overview of the advances made in COPD-based image processing over the past 4 decades, describes the current standard of practice for COPD care, and then provides a brief description of the path forward where the two may meet.
BACKGROUND
Lung Parenchyma
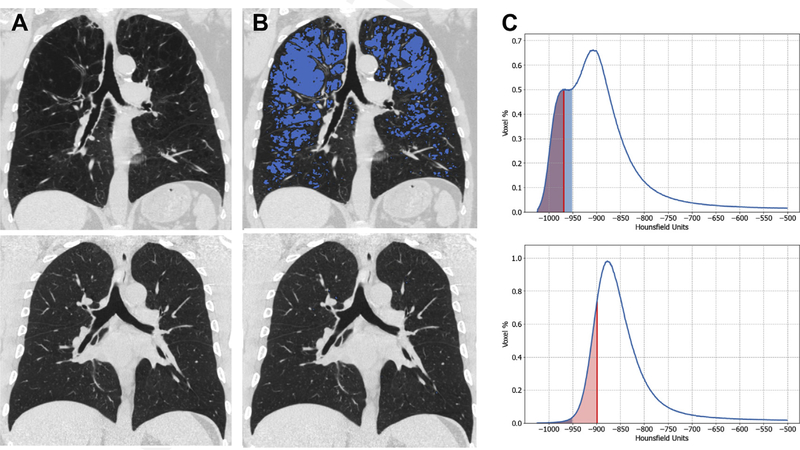
CT was introduced into clinical care in the 1980s, and since that time there have been extensive efforts to identify and quantify features that may be used to improve understanding of chronic respiratory conditions such as COPD. One of the pathologic bases for COPD is emphysematous destruction of the lung parenchyma. This process, defined as abnormal and permanent dilation of the distal airspaces due to destruction of alveolar walls, results in reduced lung elastic recoil leading to hyperinflation, expiratory airflow obstruction, and breathlessness.1 On CT, emphysema appears as holes in the lung that can be readily quantified by measuring the density or attenuation (in Hounsfield units, HU) of the parenchyma.2–4 While normal lung tissue may have an attenuation of approximately −856 HU, emphysema has attenuation values less than −910 to −960 HU depending on the parameters used for image acquisition and reconstruction. Thus, an HU threshold can be selected that differentiates nonemphysematous and emphysematous lung, and the percent of low attenuation areas (LAA%: volume of low attenuating lung/total lung volume*100) can be calculated as the proportion of emphysematous lung tissue (Fig. 1).3 A second way to objectively quantify emphysema is by calculating the HU value that demarcates the lowest 15% of the lung histogram from the remaining 85%, called the percent density (PD) 15.5 Both of these measures from the density histogram are obtained at suspended full inspiration, and a limitation of the test is suboptimal inspiration during the CT scan. One way to overcome this limitation is to adjust PD15 for differences in lung volume,6 although as hyperinflation is part of the disease process in COPD, it is possible this may cause some overcorrection in some cases.
Fig. 1.
Imaging of emphysema for an individual with COPD (top panels) and without COPD (bottom panels) showing (A) coronal CT image, (B) coronal CT image with blue shading to indicate low attenuation areas (LAA, or emphysema-like lung) with attenuation of no more than −950 HU and (C) lung density distribution of all voxels for each CT showing the percent LAA (shaded blue) and density at the lower 15th percentile (PD15, shaded red), y-axis is the percent density at each HU. (Top panel: %LAA 23.9, PD15 −968 HU. Bottom panel: %LAA 0.5, PD15 −898 HU.)
The initial efforts to objectify emphysema were performed with density mask or densitometric analyses, which demonstrated that there was a direct relationship between the degree of emphysema evident on CT and both the severity of lung disease and histopathologically determined degree of airspace dilation.3,4 Multiple subsequent investigations have replicated these findings, and densitometry has become the standard method for quantifying airspace dilation in clinical, epidemiologic, genetic, and therapeutic investigation. The evolution of computational capacity has fostered the growth of more advanced postprocessing machine learning and deep learning techniques. These algorithms have several advantages over conventional densitometry, as they incorporate information on the distribution of emphysema within the lung and quantifying the admixture of centrilobular, para-septal, and panlobular emphysema present in an individual.
CT-based investigation has also demonstrated that the lung manifests divergent responses to noxious insults such as chronic tobacco smoke exposure. Smokers may appear resilient to the injurious effects of tobacco smoke or may develop emphysema and even pulmonary fibrosis. Recent investigation suggests approximately 6% to 8% of smokers over the age of 50 have some degree of interstitial remodeling of the lung parenchyma.7 These processes have collectively been termed interstitial lung abnormalities (ILAs) and have been shown to have similar genetic associations as advanced pulmonary fibrosis. The presence of ILA is independently associated with all-cause and respiratory-specific mortality in population- based studies.8–10 Extensive work is ongoing to determine which subset of these ILAs progress to classic interstitial lung disease and potentially when to initiate antifibrotic therapy.
AIRWAYS
A second area of focused investigation in smokers is the CT-based assessment of airway disease. Studies using these techniques initially reported that smokers with thicker airway walls on CT scan were more likely to have more compromised lung function.11,12 Histopathologic studies have repeatedly demonstrated that remodeling of the small airways is the primary event in the development of expiatory airflow obstruction in smokers.13,14 Using retrograde catheterization and explanted human lungs, Hogg and colleagues15 demonstrated that those airways less than 2 mm in diameter were the site of greatest resistance to airflow in COPD. Subsequent work has explored the pathologic changes to the airways and discovered that not only does the remodeling process manifest as inflammation, fibrosis, and mural plugging, but these processes may culminate in destruction and ultimately an absence of these small airways.16 More recent work has demonstrated that the reduction in numbers of terminal bronchioles was highly correlated with disease stage but was also associated with reductions of the more proximal airway count collected from clinical CT scanning, suggesting that objective assessments of the airways on conventional CT may provide insight into the distal lung.17
BEYOND LUNG PARENCHYMA AND AIRWAYS
Another quantitative method for assessing the airways on CT is parametric response mapping (PRM).18 This technique utilizes the difference in lung density between inspiratory and expiratory scans to calculate gas trapping caused by small airway disease. Small airway obstruction does not allow full deflation of the lung parenchyma; this results in a lower attenuation of these areas on expiration when compared with lung parenchyma supplied by normal airways. PRM has been used extensively in the clinical characterization of COPD and has been recently validated against micro-CT based assessments of distal lung architecture.19
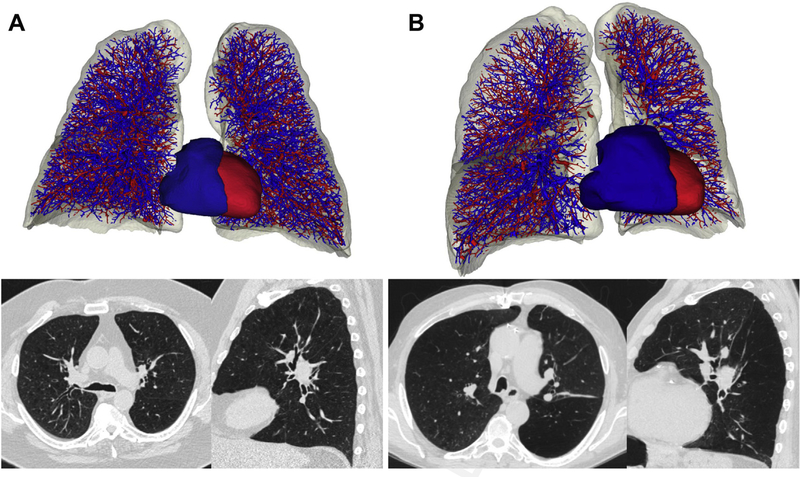
The increasing utilization of imaging in COPD and the multidisciplinary approaches to research have accelerated the refinement of techniques focused on the parenchyma and airways while facilitating the collection of imaging data that have provided more full characterization of the breadth of thoracic pathology evident in smokers. Examples of this work include morphologic assessments of the pulmonary vasculature, both the intraparenchymal vessels and central pulmonary artery and aorta. Vascular measures, including arterial and venous segmentation on CT, can be used to identify people at greatest risk for disease progression, improve the understanding of the interdependence of heart and lung, and be used to predict acute respiratory exacerbations and response to therapeutic interventions such as bronchoscopic lung volume reduction (Fig. 2).20–23
Fig. 2.
Imaging from individuals without vascular pruning (left) and with vascular pruning (right) showing (A) reconstructed CT imaging of pulmonary vasculature, segmented into arteries (blue) and veins (red) and (B) axial and sagittal CT images from the same individuals. (Left: forced expiratory volume in the first second of expiration [FEV1] 1.3 L, FEV1/forced vital capacity [FVC] 0.38, %LAA 19.9, PD15–959 HU. Right: FEV1 1.2 L, FEV1/FVC 0.34, % LAA 18.7, PD15 −955.)
CT is also being leveraged to expand appreciation of the extracardiopulmonary effects of COPD. Examples of this include detailed assessments of body composition and the recognition that sarcopenia is a major contributor to the morbidity and mortality of COPD and even smokers with normal lung function.24,25 Similarly, objective assessments of volumetric bone mineral density demonstrated that the pathologic loss of bone density is highly prevalent in smokers, even those without COPD.26 Further, contrary to standard screening practices, these losses and subsequent vertebral compression fractures are equally if not more common in men than women.26
The next section of this document provides a description of the current use of imaging in the clinical care of patients with COPD in the ambulatory setting. It will become quickly evident to the reader that much of what was just described, the advanced postprocessing techniques enabling detection and quantification of parenchymal, airway, cardiovascular and metabolic conditions in COPD, are not utilized in clinical practice. The reasons for this are detailed after the section describing the current use of imaging in the clinical care of COPD in the ambulatory setting.
CLINICAL UTILIZATION OF IMAGING IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD should be considered in any patient with chronic dyspnea or productive cough, or those with recurrent exacerbations of these symptoms, particularly in the context of risk factors such as family history, tobacco smoke, or other inhalational exposures.27 Imaging is not considered in the diagnosis of COPD, which requires the finding of an incompletely reversible obstructive deficit on spirometry. However, in clinical practice, chest imaging is often helpful to exclude other causes of cough or dyspnea, such as lung cancer, tuberculosis, bronchiectasis, interstitial lung disease, or pulmonary embolism.
In established COPD, imaging is used as needed to evaluate for coexisting pulmonary processes. Because COPD is so common, it has considerable overlap with other pulmonary diseases, particularly bronchiectasis, interstitial lung disease, and vascular abnormalities, including pulmonary embolism and pulmonary hypertension. In the authors’ opinion, further evaluation is warranted when patients have disproportionate symptoms, more advanced disease than expected given their age and exposure history, when a restrictive deficit is also present on pulmonary function testing, or after recurrence of respiratory events.
Disproportionate symptoms refer to dyspnea that is disproportionate to the functional impairment observed in pulmonary function testing in obstructed patients, particularly if the symptom does not respond to bronchodilator therapy, or a cough that is more tenacious and purulent than usually seen, suggestive of bronchiectasis. More advanced disease than expected includes younger patients (those younger than 50) and those with minimal smoking history or other known exposures. On spirometry, a low vital forced vital capacity in COPD may be caused by gas trapping, but a true restrictive deficit by lung volumes may indicate coexisting interstitial lung disease. A significantly reduced DLCO without extensive emphysema or air trapping may indicate coexisting pulmonary vascular disease. Repeated hospitalizations or respiratory events may also indicate an additional process such as bronchiectasis. Thus, although CT is not routinely performed in COPD and may not alter treatment, there are many scenarios in which it is helpful to evaluate for coexisting diseases.
In severe COPD, chest imaging is recommended to evaluate for the distribution of emphysema as part of an assessment for lung volume reduction by surgical or bronchoscopic approach. Lung volume reduction surgery is one of the few treatments with proven mortality benefit in COPD, in selected patients.28 Newer broncho- scopic approaches to lung volume reduction have also proven to be of clinical benefit in advanced emphysema, and longer term studies are ongoing.29,30 Thus, patients with significant hyperinflation should be referred to an experienced hospital center with both thoracic surgery and interventional pulmonary expertise in order to determine the treatment options available.
THE PATH FORWARD
The gulf between the ability to objectively characterize the multifaceted nature of COPD on thoracic CT and what is used for the clinical management of COPD is clear. There are several reasons for this, which can be largely ascribed to the understandable lag between enhanced understanding of disease and the development of new therapies for that disease. The years spanning the 1980s to 2000s were characterized by the belief that objective metrics of emphysema and airway disease would allow clinicians to understand disease and optimize therapy. Although the heterogeneity of COPD quickly became visually apparent with the advent of CT, this enhanced understanding has as of yet had minimal impact on clinical practice. Short- and long-acting bronchodilators and antiinflammatory agents, the long-standing backbone of COPD treatment, require little to no radiologic insight to initiate or monitor the effects of therapy. Improvements in symptoms, lung function or both were and are still deemed a success. Advances in CT technology over the past 40 years have also improved image resolution while lowering radiation exposure, and the risk-benefit profile makes them more palatable for research and increasing clinical applications.
Pharmaceutical companies are now developing therapies targeting the pathologic basis of disease and attempting to slow the progression of emphysema, all of which are of great interest. Although the relationship between emphysema and reduced lung function suggests that one could simply use lung function as the primary outcome, the sample sizes and study duration required to impact on lung function are prohibitive, and given the heterogeneity of disease and its progression, there is reason to believe that some interventions may work on only the airways or emphysema, not both simultaneously. CT is being used to detect patients most likely to respond to such treatment (ie, those with some degree of existing emphysema). The technique is also being used to monitor their response to treatment in placebo-controlled studies. The abundance of cross-sectional and longitudinal imaging and clinical data collected over the past 40 years has begun to foster a level of comfort in the biomedical community about using imaging to identify potential new therapies and has provided the foundation to prepare and submit an application to the US Food and Drug Administration (FDA) to qualify CT measures of emphysema progression as an endpoint for clinical investigation. This work is being led by the COPD Biomarkers Qualification Consortium similar to what has been done for other biomarkers like fibrinogen.31
There is another pragmatic utilization of CT data that can have immediate clinical implications related to disease diagnosis. Thoracic CT scans are among the most common types of imaging obtained during routine clinical care. The indications for such testing are broad and include acute-onset dyspnea or pleuritic chest discomfort. Such tests often rule out processes such as thromboembolism but are less often leveraged for more comprehensive assessments of lung heath and the presence of characteristics suggestive of chronic lung disease. As described earlier, there are multiple radiologic features evident on thoracic CT that may be used to detect and stratify smoking- related lung disease. The presence of emphysema, parenchymal fibrosis, or bronchiectatic dilation of the airways all merit further evaluation in patients with and without COPD. These features are also sometimes evident in the lung bases and thus can be an incidental finding on abdominal CT as well.
The first step in optimizing the clinical use of existing CT data is making sure that these features are noted by the interpreting radiologist and recorded in their report in a standardized fashion. Particularly with the evolution of smart electronic medical record systems, such documentation can then trigger further clinical evaluation such as a detailed review of exposure history, family history, spirometry, and even referral to a respiratory specialist depending upon practice location and physician availability. Even in the absence of obstructive or restrictive physiology, emphysema, fibrosis, and bronchiectasis are associated with a poorer prognosis and warrant clinical evaluation and often times therapeutic intervention. Although uncommon, emphysema in younger or nonsmoking patients should raise the possible diagnosis of alpha-1 antitrypsin deficiency, a diagnosis that has important implications for disease prevention (including in potentially affected family members) and therapy. There are also new promising therapies for fibrosis, but patients who are undiagnosed cannot access such care.
The goals of imaging-based detection of parenchymal and airway abnormalities are early diagnosis and intervention leading to disease prevention. If the goal is to identify treatments that work in early disease, research efforts must include those patients who do not yet meet the spirometric definition of COPD but who are at high risk. It is hoped that at some point, one will not wait for a patient to develop expiratory airflow obstruction before initiating therapies that may improve symptoms or reduce the risk of future respiratory events and hospitalization in chronic lung disease. CT imaging provides an important tool that can identify these patients, and one can expect that it will be increasingly incorporated into routine clinical care.
SUMMARY
CT imaging has been extensively utilized in research, as it provides qualitative and quantitative information relevant to parenchymal, airway, vascular, and extrapulmonary manifestations of COPD. However, there has been a delay in translating this knowledge to the bedside, as the current clinical application is primarily in evaluating the extent of emphysema in candidates for surgical or bronchoscopic lung volume reduction. Imaging is also helpful in evaluating for alternate or additional causes of dyspnea and/or cough, including coexisting diseases such as bronchiectasis, interstitial lung disease, lung cancer, pulmonary embolism, and pulmonary hypertension. Although much work remains, the authors believe imaging has the near-term potential to lead to earlier diagnosis, better disease prognostication, and more individualized therapeutic intervention.
KEY POINTS.
Computed tomography provides information relevant to parenchymal, airway, vascular, and extrapulmonary manifestations of chronic obstructive pulmonary disease (COPD).
Currently, the primary clinical application of imaging in COPD is to evaluate emphysema in candidates for surgical or bronchoscopic lung volume reduction.
At the time of diagnosis and in management of COPD, imaging is also utilized to look for alternative or additional causes of dyspnea and cough.
There has been a lag between research investigation and clinical applications of imaging in COPD; however, promising studies are ongoing with near-term potential to impact disease diagnosis, prognosis, and therapy.
Acknowledgments
Competing interests: C.L. Pistenmaa received research grants from the Alpha-1 Foundation and Boehringer Ingelheim. G.R. Washko received research grants from Boehringer Ingelheim, BTG Interventional Medicine and Janssen Pharmaceuticals, and personal fees from Boehringer Ingelheim, PulmonX, Janssen Pharmaceuticals, GlaxoSmithKline, Novartis, and Vertex and is a cofounder and co-owner of Quantitative Imaging Solutions, a company that provides image-based consulting and develops software to enable data sharing. Funded by: NIHHYB. Grant number(s): K23 HL141651; R01 HL116473.
REFERENCES
- 1.Snider GL. Emphysema: the first two centuries-and beyond. A historical overview, with suggestions for future research: part 1. Am Rev Respir Dis 1992; 146(5 Pt 1):1334–44. [DOI] [PubMed] [Google Scholar]
- 2.Hayhurst MD, MacNee W, Flenley DC, et al. Diagnosis of pulmonary emphysema by computerised tomography. Lancet 1984;2(8398):320–2. [DOI] [PubMed] [Google Scholar]
- 3.Muller NL, Staples CA, Miller RR, et al. “Density mask.” An objective method to quantitate emphysema using computed tomography. Chest 1988; 94(4):782–7. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella M, Muller NL, Abboud RT, et al. Quantitation of emphysema by computed tomography using a “density mask” program and correlation with pulmonary function tests. Chest 1990;97(2):315–21. [DOI] [PubMed] [Google Scholar]
- 5.Parr DG, Sevenoaks M, Deng C, et al. Detection of emphysema progression in alpha 1-antitrypsin deficiency using CT densitometry; methodological advances. Respir Res 2008;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoel BC, Putter H, Bakker ME, et al. Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc 2008;5(9):919–24. [DOI] [PubMed] [Google Scholar]
- 7.Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 2011; 364(10):897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunninghake GM, Hatabu H, Okajima Y, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med 2013;368(23): 2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle TJ, Washko GR, Fernandez IE, et al. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med 2012;185(7):756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putman RK, Hatabu H, Araki T, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA 2016;315(7):672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000;162(3 Pt 1): 1102–8. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173(12):1309–15. [DOI] [PubMed] [Google Scholar]
- 13.Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978; 298(23):1277–81. [DOI] [PubMed] [Google Scholar]
- 14.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med 1974;291(15):755–8. [DOI] [PubMed] [Google Scholar]
- 15.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968;278(25):1355–60. [DOI] [PubMed] [Google Scholar]
- 16.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350(26): 2645–53. [DOI] [PubMed] [Google Scholar]
- 17.McDonough JE, Yuan R, Suzuki M, et al. Smallairway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365(17):1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galban CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012;18(11): 1711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasilescu DM, Martinez FJ, Marchetti N, et al. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2019;200(5): 575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367(10):913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estepar RS, Kinney GL, Black-Shinn JL, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med 2013; 188(2):231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washko GR, Nardelli P, Ash SY, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease. A longitudinal observational study. Am J Respir Crit Care Med 2019;200(4):454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuhmann M, Raffy P, Yin Y, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. Am J Respir Crit Care Med 2015;191(7): 767–74. [DOI] [PubMed] [Google Scholar]
- 24.Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166(6):809–13. [DOI] [PubMed] [Google Scholar]
- 25.Diaz AA, Martinez CH, Harmouche R, et al. Pectoralis muscle area and mortality in smokers without airflow obstruction. Respir Res 2018;19(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaramillo JD, Wilson C, Stinson DS, et al. Reduced Bone Density and vertebral fractures in smokers. men and COPD patients at increased risk. Ann Am Thorac Soc 2015;12(5):648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J 2019;53(5): 1900164. [DOI] [PubMed] [Google Scholar]
- 28.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume- reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348(21):2059–73. [DOI] [PubMed] [Google Scholar]
- 29.Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373(24): 2325–35. [DOI] [PubMed] [Google Scholar]
- 30.Criner GJ, Delage A, Voelker K, et al. Improving lung function in severe heterogenous emphysema with the spiration valve system (EMPROVE). a multicenter, open-label randomized controlled clinical trial. Am J Respir Crit Care Med 2019;200(11): 1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller BE, Tal-Singer R, Rennard SI, et al. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease. perspective of the chronic obstructive pulmonary disease biomarker qualification consortium. Am J Respir Crit Care Med 2016;193(6):607–13. [DOI] [PubMed] [Google Scholar]