Abstract
Populations at the edges of their geographical range tend to have lower genetic diversity, smaller effective population sizes and limited connectivity relative to centre of range populations. Range edge populations are also likely to be better adapted to more extreme conditions for future survival and resilience in warming environments. However, they may also be most at risk of extinction from changing climate. We compare reproductive and genetic data of the temperate seagrass, Posidonia australis on the west coast of Australia. Measures of reproductive effort (flowering and fruit production and seed to ovule ratios) and estimates of genetic diversity and mating patterns (nuclear microsatellite DNA loci) were used to assess sexual reproduction in northern range edge (low latitude, elevated salinities, Shark Bay World Heritage Site) and centre of range (mid-latitude, oceanic salinity, Perth metropolitan waters) meadows in Western Australia. Flower and fruit production were highly variable among meadows and there was no significant relationship between seed to ovule ratio and clonal diversity. However, Shark Bay meadows were two orders of magnitude less fecund than those in Perth metropolitan waters. Shark Bay meadows were characterized by significantly lower levels of genetic diversity and a mixed mating system relative to meadows in Perth metropolitan waters, which had high genetic diversity and a completely outcrossed mating system. The combination of reproductive and genetic data showed overall lower sexual productivity in Shark Bay meadows relative to Perth metropolitan waters. The mixed mating system is likely driven by a combination of local environmental conditions and pollen limitation. These results indicate that seagrass restoration in Shark Bay may benefit from sourcing plant material from multiple reproductive meadows to increase outcrossed pollen availability and seed production for natural recruitment.
Keywords: Environmental gradient, mating system, microsatellite DNA loci, monoecy, outcrossing rate, Posidonia australis, restoration, seed abortion
Plant species including seagrasses reproduce using sexual (seeds) and asexual (vegetative) means which can vary across a species range. We compare sexual reproduction, genetic diversity and the mating system in Posidonia australis seagrass meadows from Western Australia. Flower and fruit production were two orders of magnitude lower in Shark Bay meadows which also had lower genetic diversity and a mixed mating system when compared with meadows in Perth metropolitan waters. These results indicate that seagrass restoration in Shark Bay may benefit from sourcing plant material from multiple reproductive meadows to increase outcrossed pollination and seed production for natural recruitment.
Introduction
Populations at their geographic range edges tend to have smaller effective population sizes, reduced sexual reproduction and limited connectivity relative to centre of range populations (Eckert et al. 2008; Sexton et al. 2011). These patterns have been established through decades of theoretical and empirical studies in population genetics and integration with mating systems (reviewed in Charlesworth and Charlesworth 2017), although the pattern is less clear in marine species (e.g. Diekmann and Serrão 2012; Assis et al. 2013; Liggins et al. 2014). Renewed interest in understanding the drivers of biogeographic ranges has been reignited by the profound influence of climate change on distributional patterns of taxa and the ecosystems they inhabit (Chen et al. 2011; Nicastro et al. 2013; Wernberg et al. 2016). Distributional changes by species in the marine environment, particularly those inhabiting inshore coastal shelves, occur through sea level changes (Miller et al. 2011), as well as in response to changing climate (e.g. Pecl et al. 2017). The persistence of a species during these periods of change is ultimately influenced by available habitat and a species’ ability to respond to change. Some species’ ranges have not (yet) shifted, and their declines in demographic processes (e.g. survival or reproduction) are offset by increases in others (e.g. self-fertilization), potentially buffering populations from extinction (Sheth and Angert 2018). This may especially be the case for plant species with the ability to reproduce through sexual and asexual means (e.g. seagrasses), as adult plants may persist through extreme climate events over extended periods even when sexual reproduction fails.
Natural variation in traits, such as those associated with sexual reproduction, occur among populations across a species range; however, range edge populations may evolve physiological, morphological and life-history attributes that better attune them to warming environments (Levin 2012). These populations are also regarded as most threatened under climate change (Hampe and Petit 2005; Zardi et al. 2015). The extent to which individuals and populations have an outcrossed mating system can influence genetic structure, extent of gene flow, effective population size and expression of inbreeding depression (Barrett and Harder 2017). A recent meta-analysis by Whitehead et al. (2018) highlights the substantial variation in outcrossing rates across 105 species in which mating system analyses were obtained in more than three populations. Examination of mating systems in multiple populations provides an opportunity to assess links between specific influences—with a suggestion that abiotic pollination factors (e.g. wind, water) provide a greater opportunity for consistency in outcrossing rates (Whitehead et al. 2018). Goodwillie et al. (2005) reported elevated levels of environmental or genetic-based self-pollination also afford populations a measure of reproductive assurance, despite the genetic costs associated with inbreeding. We explore these hypotheses further in the marine environment where hydrophilous pollination is common.
Seagrasses are ancient marine flowering plants, of which most species complete their life cycle entirely underwater (Ackerman 1995). Globally, 24 % of species are classified as ‘threatened’ or ‘near-threatened’ on the IUCN’s Red List (Short et al. 2011), with the rate of decline continuing to increase due to anthropogenic activities including climate change (Orth et al. 2006; Waycott et al. 2009). Seagrasses play a central role in ecosystem services (Costanza et al. 1997; Lamb et al. 2017) and in mitigating climate change (Fourqurean et al. 2012; Duarte et al. 2013). Genetic data showing high outcrossing rates are common among monoecious species (summarized in Sinclair et al. 2014a), providing support for Ackerman’s hydrophilous pollination syndrome (Ackerman 2000). Here, we assessed reproductive (flower and seed production) and genetic data (diversity, population structure and mating system) for range edge and centre of range meadows of the Australian temperate seagrass, Posidona australis, a species with high seed dispersal capabilities (Kendrick et al. 2012, McMahon et al. 2014). We test the following hypotheses: (i) sexual reproduction is higher in Perth metropolitan waters than Shark Bay meadows; (ii) there is a shift from complete outcrossing in Perth metropolitan waters meadows to a mixed mating system in Shark Bay meadows; and (iii) the variance in outcrossing rates among P. australis ‘families’ within meadows is higher in mixed mating meadows than completely outcrossed meadows. The combination of reproductive and genetic data enables a more comprehensive understanding of seed production and the long-term implications for resilience and restoration of range edge seagrass meadows.
Materials and Methods
Study species
Posidonia australis is a perennial, marine angiosperm endemic to temperate Australian waters from Shark Bay at the northern range edge on the west coast to Wallis Lake on the east coast (Edgar 2000). It occurs in protected coastal waters and estuaries, just below the low water mark to 15 m water depth (Carruthers et al. 2007) and reproduces both vegetatively (clonal rhizome extension) and sexually (pollen and seed production). Thus, this long-lived species can persist through prolonged times of sexual reproductive failure. Posidonia australis is diploid (somatic chromosome number 2n = 20; Kuo et al. 1990). It has perfect or hermaphroditic flowers (den Hartog 1970), in which anthers mature and release pollen ahead of stigma receptivity (protandrous; McConchie and Knox 1989). Initiation of inflorescence development occurs in May (Austral autumn), with pollination occurring in July/August (Austral winter) and fruit release in November–January (Austral spring–summer). Timing of fruit release varies with latitude; fruits ripen 1 month earlier in Shark Bay (25–26°S) than Perth metropolitan waters (32°S). Inflorescences are positioned above the leaf canopy, with up to 20 fruits being produced per inflorescence. Flower and seed production are temporally and spatially variable across the species range, but typically annual and prolific in Perth metropolitan waters (Cambridge and Hocking 1997). Flowering meadows in Shark Bay cover a few km2 compared with 10s to 100s km2 in Perth metropolitan waters.
Field sites
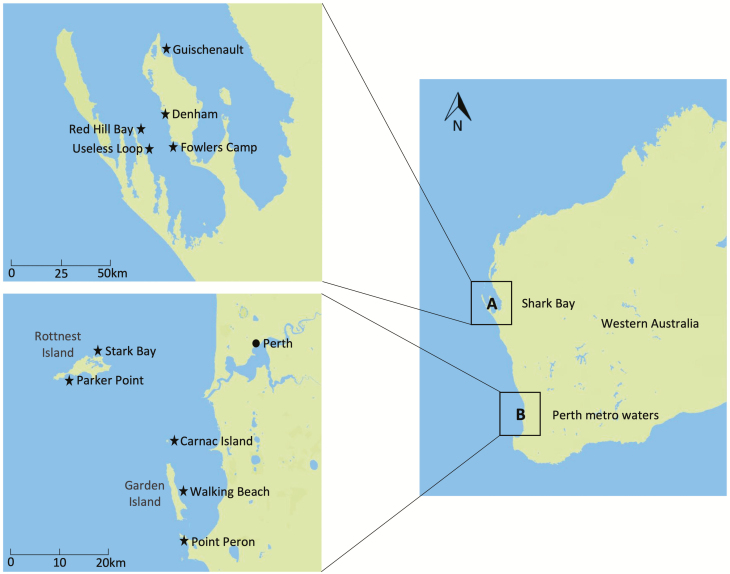
Flowering meadows were sampled from northern range edge meadows in Shark Bay and mid-latitude meadows in Perth metropolitan waters (Fig. 1; Table 1). All sampled meadows were situated in shallow waters (<3 m) on the broad continental shelf off the Western Australian coastline. Palaeo sea level records from Western Australia, including tubeworm data from Rottnest Island (Playford 1988; Baker et al. 2005), indicate that sea level was within 2 m of its modern level by mid-Holocene (~7100 ± 70 cal. years BP); thus, seagrass meadows are likely to have been broadly stable since that time.
Figure 1.
Map of Western Australia showing the location of sampled meadows in Shark Bay, inset A: Guischenault, Red Hill Bay, Denham, Useless Loop, Fowlers Camp, and meadows in Perth metropolitan waters, inset B: Stark Bay and Parker Point, Rottnest Island, Carnac Island, Walking Beach, Garden Island, Point Peron.
Table 1.
Location and characteristics for Posidonia australis meadows.
| Location (north–south) | Abbrev. | Latitude (S) | Longitude (E) | Salinity (PSU) | Depth (m) | Meadow characteristics | Hydrodynamics |
|---|---|---|---|---|---|---|---|
| Shark Bay (northern range edge): | |||||||
| Guischenault | GU | −25.61895 | 113.58918 | 36–38 | <2 | Expansive meadow | Large tides at time of pollen release, water tends to spill off the banks |
| Red Hill Bay | HP | −26.03051 | 113.37399 | 36–38 | <2 | Fringing seagrass, highly fragmented | Strong tidal movement |
| Fowlers Camp | FC | −26.10549 | 113.61285 | >40 | <2 | Fringing seagrass near expansive meadow | Large tides at time of pollen release, sheltered from waves |
| Perth metropolitan waters (centre of range): | |||||||
| Stark Bay, Rottnest Island | RST | −32.00604 | 115.48488 | Oceanic | <3 | Expansive meadow mixed with rocky reef | Exposed to waves from the north |
| Parker Point, Rottnest Island | RPP | −32.02242 | 115.53092 | Oceanic | <2 | Fringing seagrass, highly fragmented | Sheltered with weak current |
| Carnac Island, Cockburn Sound | CI | −32.12040 | 115.66547 | Oceanic | <3 | Fringing seagrass, fragmented | Sheltered meadow with strong water movement from swell and wind refracting around the island |
Shark Bay, to the north, was formed by a marine transgression ~7000–8000 years ago (Bufarale and Collins 2015). Radiocarbon dating of sediment cores indicates seagrass has been present in the Bay throughout the Holocene (not earlier than 8.5–8.0 ka BP, Bufarale and Collins 2015; 3000 years, Serrano et al. 2016). Shark Bay is an inverse estuary with a permanent salinity gradient—from oceanic in the north to hypersaline conditions in the southern reaches (35–70 practical salinity unit (PSU)). The salinity gradient has been maintained since the last sea level adjustments (~4500 years ago) by the formation of seagrass-dominated sills and banks that have restricted water movement and nutrients (Fraser et al. 2012; Bufarale and Collins 2015). Shark Bay is recognized as a UNESCO World Heritage Site (WHS) for its unique, highly biodiverse ecosystem at the interface of warm tropical and southern temperate zone ecosystems and home to the largest reported seagrass meadows in the world (Walker et al. 1988; Kendrick et al. 2012). The mostly pristine nature and legal protection afforded to the marine environments around Rottnest and Carnac Islands and Shark Bay WHS, within which P. australis meadows inhabit, provide an opportunity to understand contemporary processes relatively free from localized anthropogenic threats.
Sexual reproduction
Flower and fruit production were measured in situ for two meadows in Shark Bay (Guischenault, Red Hill Bay) and two meadows in Perth metropolitan waters (Stark Bay and Parker Point, Rottnest Island) during Spring 2016. Inflorescence density was estimated by using five replicate 10 m × 1 m (10 m2) belt transects. Flower and fruit production per inflorescence were estimated from the random collection of 12 inflorescences from transects at each site. Inflorescences consisted of a stem (petiole) bearing several spikes (3–12) with 3–5 hermaphrodite flowers. Following successful pollination, fruit development takes ~12 weeks. The number of fully developed fruit, undeveloped fruit and remains of flowers that had not been pollinated were counted on each inflorescence spike. The total number of flowers per inflorescence was derived from the sum of all fruit, undeveloped fruit and remains of flowers. Floral (number of flowers per m2) and fruit density (number of fruits per m2) were determined before fruit release. Production at Fowlers Camp was estimated based on fruit scars, as fruit had released prior to sampling. Seed to ovule ratio was determined from the total number of mature fruit (1 fruit = 1 seed) divided by the total numbers of flowers (1 flower = 1 ovule). The floral and fruit density data and seed to ovule ratios were assessed for heteroscedasticity and normality. A Tukey Ladder of Powers approach (Tukey 1977) was used to power transform the data to maximize normality of residuals. The normality of residuals was visualized and assessed with a Shapiro–Wilk tests for normality. A one-way ANOVA (meadow as fixed factor) and Tukey’s HSD test were performed for multiple comparisons of means between sites and regions, with a 95 % confidence level.
Genetic sampling, DNA extraction and genotyping
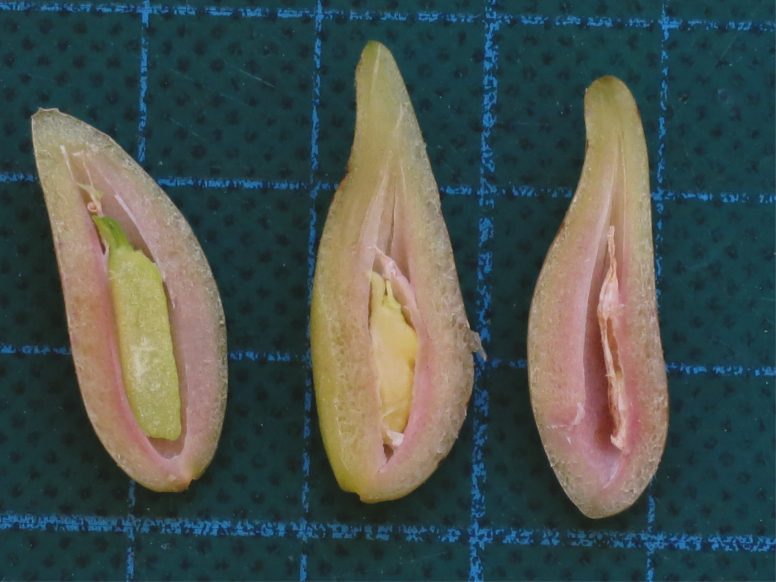
Opportunistic collections of adult shoots and associated inflorescences were made on SCUBA between 2014 and 2018 at three meadows in Shark Bay (Guischenault, Red Hill Bay, Fowlers Camp) and three meadows in Perth metropolitan waters (Stark Bay and Parker Point, Rottnest Island, and Carnac Island, Cockburn Sound; Fig. 1). Approximately 30 individual maternal shoots were collected from each meadow just prior to fruit dehiscence (with the exception of Fowlers Camp) using methods described in Sinclair et al. (2014a). A visual assessment of seed viability (viable, mutant or aborted; Fig. 2) was performed prior to DNA extraction. Aborted embryos were discarded as there was not sufficient tissue to genotype.
Figure 2.
Transverse section of mature Posidonia fruit showing (left to right) viable, ‘mutant’ and aborted embryos. Scale: one square = 1 cm. Photo by E. A. Sinclair.
DNA was extracted from shoot meristem and embryos using methods previously described (Sinclair et al. 2009, 2014a). Seven polymorphic microsatellite loci were used to generate multilocus genotypes (MLGs) using two multiplex mixes containing 5.2 µL of 2× Multimix and 1.98 µL of 5× Q sol (Type-It Microsatellite PCR kit; Qiagen, Hinden, Germany), 1.0 µL of primer mix (PM) and 2.0 µL of 5–10 ng DNA in a 10 µL reaction. Primer mix 1 contained the primers PaA1, PaA105, PaA120; primer mix 2 contained PaB6, PaB8, PaB112, PaD113. Forward primers were fluorescently labelled (FAM, VIC or PET; seeSupporting Information—Table S1) and microsatellite regions were amplified for all individuals by PCR using a Veriti thermocycler (Thermo Fisher Scientific, Waltham, MA, USA) using the following PCR conditions: an initial 1-min denaturation at 95 °C, 35 cycles of 94 °C for 10 s, 60 °C for 30 s and 72 °C for 45 s followed by a final extension of 15 min at 60 °C. Electrophoresis was run on an ABI 3500 sequencer (Life Technologies) with size standard LIZ. Allele sizes were scored using Geneious version 7.1 (Biomatters Ltd, Auckland, New Zealand). Replicate PCRs were performed to ensure the accuracy of the final data set. There was no evidence of linkage disequilibrium or null alleles at these loci based on previously obtained diploid genotypes (Sinclair et al. 2009, 2014a, b, 2016a); however, we ran all new diploid data (see below) through Micro-Checker v2.2.3 to assess for the presence of scoring error due to stuttering, large allele dropout or null alleles (van Oosterhout et al. 2004; http://www.nrp.ac.uk/nrp-strategic-alliances/elsa/software/microchecker/).
Genetic diversity, clonal diversity and genetic structure
Tri-allelic genotypes were observed in six out of the seven loci that typically give di-allelic genotypes. Allele frequencies were therefore calculated using GENODIVE version 2.0b27 (Meirmans and Van Tienderen 2004), which handles genotypes with more than two alleles per locus and permits mixed (diploid and triple allelic) genotypes to be included within the same analyses. The summary statistics calculated on the complete data set were: total number of alleles (Na), private alleles (p[i]), mean number of alleles (Num), effective number of alleles (Eff Num), observed (Ho) and expected (Hs) heterozygosity within sampled meadows and seed ‘populations’. The ‘Assign clones’ option was used to identify unique clones or MLGs and their frequency due to the high chance of sampling multiple flowering ramets from the same plant within a meadow. We implemented a stepwise mutation model (SMM), with a threshold of 1. This was used due the unusually large number of MLGs differing by a single allele at Carnac Island (see Sinclair et al. 2014b). Clonal richness (R = (G − 1)/(N − 1)), where G = number of MLGs, and N = number of samples, was estimated for each meadow (Dorken and Eckert 2001) and clonal evenness (ED), a measure of abundance of MLGs. Deviations from Hardy–Weinberg equilibrium, described as an inbreeding coefficient (Gis), were calculated based on MLGs with 10 000 permutations, with positive values indicating a heterozygote deficit and negative values indicating a heterozygote excess. We conducted a t-test to determine whether there was a significant difference between clonal diversity in embryo ‘populations’ in Shark Bay and Perth metropolitan waters. Genetic differentiation (FST) was generated for all pairs of ‘populations’ using the complete data set, with significance between populations assessed using with 10 000 permutations.
Mating system
Many of the standard population genetic tools have been developed for diploid data sets and therefore not feasible for polyploid or mixed data sets (Dufresne et al. 2014). In the absence of any evidence of polyploidy in P. australis (Sinclair et al. 2016b), individuals containing tri-allelic genotypes were reduced to diploid genotypes to enable estimation of mating system parameters using the software program MLTR v.3.4 (Ritland 2002; http://kermitzii.com/softwares/). Alleles that were not detected in a homozygous form, or were rare (where f < 0.05), were removed. Identical, commonly occurring tri-allelic genotypes were reduced to the same diploid genotype so as not to alter the total number of MLGs. All genotypes were manually checked to ensure each embryo contained at least one maternal allele. We acknowledge that this may introduce bias through an artificial increase in estimated selfing rates. Mating system parameters were estimated for each meadow, as well as by families within each meadow, using a maximum likelihood approach. These estimates were based on the multilocus mixed-mating model that assumes plants are randomly mating and the level of outcrossing is inversely proportional to the level of selfing (Shaw et al. 1981; Ritland 2002). The program was used to derive both single (ts) and multilocus (tm) outcrossing rates, biparental inbreeding (tm–ts, proportion of embryos due to mating between closely related parents) and multilocus correlation of P (proportion of full siblings) (Ritland 2002). Outcrossed embryos were unambiguously identified by the presence of a non-maternal allele. The level of inbreeding among maternal plants was characterized by the inbreeding coefficient (f), where a positive value indicates an excess of homozygotes and a negative value indicates an excess of heterozygotes as a result of outcrossing, as compared to expectations under Hardy–Weinberg equilibrium. Standard errors for all estimates were derived using a bootstrap method with 1000 bootstraps. The effective number of pollen donors (Nep(w)) per inflorescence (= family) was estimated by taking the inverse of the rp (Smouse et al. 2001). We conducted a t-test to determine whether there was a significant difference between outcrossing rates in meadows from Shark Bay and Perth metropolitan waters.
Results
Sexual reproduction
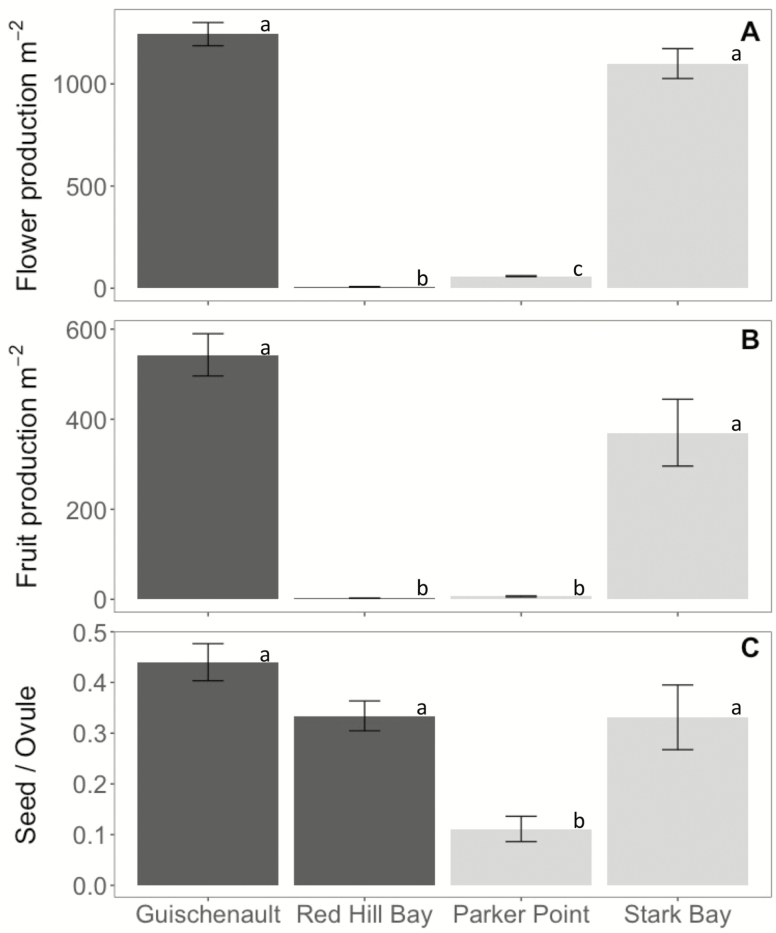
There was a significant difference in sexual reproduction among meadows, as measured by flower production, fruit production and seed to ovule ratio (Fig. 3A–C). Flower production was very high (>1000 m−2) at Guischenault and Stark Bay, with no statistically significant differences in densities recorded (Fig. 3A). They were both significantly higher than Parker Point (<50 m2) and Red Hill Bay (<10 m−2) (Tukey’s HSD test, P < 0.0001). A similar pattern was observed in fruit production (Fig. 3B). The only difference being that Parker Point and Red Hill Bay had an extremely low number of fruit (<10 m−2) and they were not significantly different from each other. Parker Point had a significantly lower seed to ovule ratio than the other three sites (Tukey’s HSD test, P < 0.0001). Thirty-two to 45 % of all ovules produced seed in the other three sites and differences in seed to ovule ratio were not significant (Fig. 3C).
Figure 3.
Sexual reproductive output between four sampled P. australis meadows, as measured by (A) flower production, (B) fruit production and (C) seed to ovule ratio. The letters above the graphs (a–c) represent significant statistical outcomes from a one-way ANOVA and Tukey’s HSD test, P < 0.0001. Shark Bay meadows are Guischenault and Red Hill Bay, Perth metropolitan waters are Stark Bay and Parker Point, Rottnest Island.
Genetic diversity, clonal diversity and genetic structure
There was no systematic evidence of scoring error due to stuttering, large allele dropout or null alleles across loci and populations. However, the presence of null alleles due to high homozygosity was suggested in embryo populations from Guischenault (three loci) and Red Hill Bay (one locus). Overall genetic diversity estimates were higher in Perth metropolitan waters relative to Shark Bay meadows (Table 2; seeSupporting Information—Table S2). Clonal diversity among maternal shoots was similar (R = 0.17–0.41; Table 2), with the exception of Carnac Island which was high (R = 0.94). Centre of range embryo populations had significantly higher clonal diversity (R = 0.66–0.96) relative to embryos from range edge meadows (R = 0.30, 0.26) (t-test: −4.66, P-value = 0.009). Clonal evenness was lower in embryo populations than meadows for range edge meadows, with 47.8 % (45/94) of embryos from Guischenault sharing the same MLG. The pattern was reversed in centre of range meadows, with the exception of Carnac Island (Table 2). One to three private alleles were detected in all embryo populations. Significant deviations from Hardy–Weinberg equilibrium were detected in most maternal and embryo populations, likely as a result of high clonality due to selective sampling for reproductive shoots.
Table 2.
Summary of genetic diversity and clonal indices for sampled P. australis shoots and embryos; N = number of shoot samples; MLG = multilocus genotypes; R = number of unique clonal richness; ED = clonal evenness; 3× = number of samples with an additional allele in at least one locus; Na = total number of alleles; p[i] = private alleles; Num = mean number of alleles; Eff Num = effective number of alleles; Ho = observed heterozygosity; Hs = expected heterozygosity; Gis = inbreeding coefficient; *significant deviation from Hardy–Weinberg equilibrium at P < 0.05.
| Sampling location and type | Abbrev. | Coll. year | N | MLG | R | ED | 3× | N a | p [i] | Num | Eff Num | H o (%) | H s (%) | G is |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shark Bay (northern range edge): | ||||||||||||||
| Guischenault | ||||||||||||||
| Shoots (maternal) | GU | 2014 | 28 | 11 | 0.37 | 0.54 | 6 | 18 | 1 | 2.57 | 2.57 | 17.9 | 16.9 | −0.058* |
| Embryos (offspring) | GUe | 2014 | 123 | 37 | 0.30 | 0.17 | 47 | 21 | 2 | 3.00 | 1.39 | 16.7 | 24.1 | 0.307* |
| Red Hill Bay | ||||||||||||||
| Shoots (maternal) | HP | 2016 | 15 | 4 | 0.21 | 0.62 | 0 | 11 | 0 | 1.57 | 1.37 | 35.7 | 20.5 | −0.722* |
| Embryos (offspring) | HPe | 2016 | 94 | 25 | 0.26 | 0.43 | 2 | 15 | 1 | 2.14 | 1.36 | 19.3 | 19.3 | −0.001 |
| Fowlers Camp (maternal) | FC | 2018 | 27 | 6 | 0.19 | 0.39 | 27 | 20 | 2 | 2.86 | 1.92 | 47.1 | 34.6 | −0.360* |
| Perth metropolitan waters (centre of range): | ||||||||||||||
| Stark Bay, Rottnest Isl. | ||||||||||||||
| Shoots (maternal) | RST | 2014 | 28 | 12 | 0.41 | 0.59 | 7 | 24 | 0 | 3.43 | 2.48 | 59.7 | 50.6 | −0.180* |
| Embryos (offspring) | RSTe | 2014 | 106 | 102 | 0.96 | 0.97 | 26 | 35 | 3 | 5.00 | 2.82 | 57.1 | 53.5 | −0.069* |
| Parker Point, Rottnest Isl. | ||||||||||||||
| Shoots (maternal) | RPP | 2014 | 30 | 6 | 0.17 | 0.45 | 0 | 21 | 0 | 3.00 | 1.58 | 36.2 | 31.3 | −0.156* |
| Embryos (offspring) | RPPe | 2014 | 144 | 115 | 0.80 | 0.83 | 0 | 27 | 1 | 3.86 | 1.82 | 50.1 | 41.8 | −0.198* |
| Carnac Island | ||||||||||||||
| Shoots (maternal) | CI | 2014 | 32 | 30 | 0.94 | 0.95 | 1 | 29 | 0 | 4.14 | 1.63 | 30.4 | 29.6 | −0.025 |
| Embryos (offspring) | CIe | 2014 | 274 | 182 | 0.66 | 0.46 | 9 | 36 | 2 | 5.14 | 1.59 | 29.8 | 28.9 | −0.031* |
Shoot samples with identical MLGs within a meadow were assumed to belong to the same flowering genet (or clone), while shared embryo MLGs were likely a result of low genetic diversity, self-pollination and/or apomixis. Twenty-six MLGs were shared among shoot and embryo ‘populations’ within Guischenault and Red Hill Bay. A single MLG was shared among two embryos from Guischenault and shoots from Red Hill Bay (n = 1), Useless Loop (n = 2) and Fowlers Camp (n = 17). Overall, there was significant genetic differentiation among meadows (adult shoots FST = 0.149, P < 0.001), with no significant differentiation between maternal shoot and embryo ‘populations’ from Red Hill Bay, Stark Bay and Carnac Island [seeSupporting Information—Table S3]. Maternal shoot and embryo ‘populations’ were weakly differentiated at Guischenault and Parker Point.
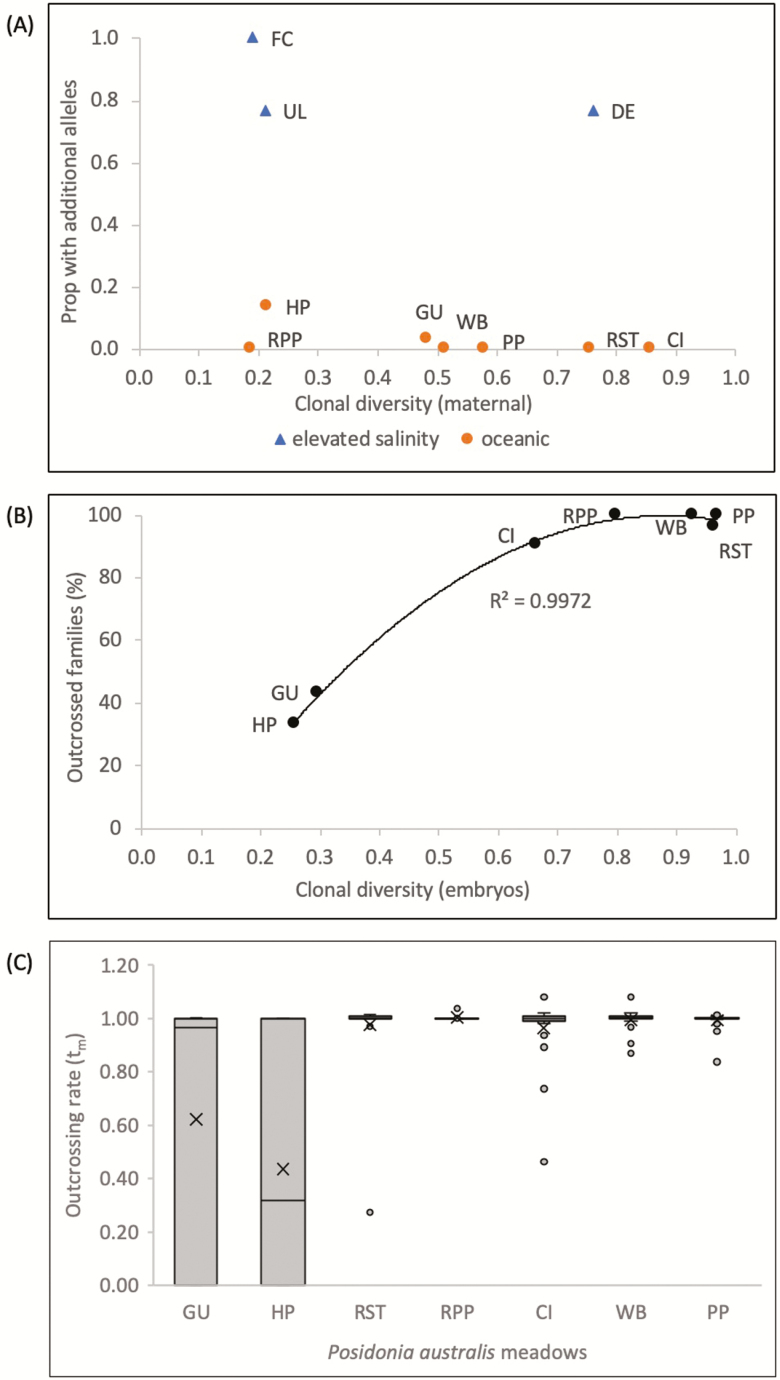
Additional alleles (tri-allelic genotypes) were present in at least one locus for every shoot genotype at Fowlers Camp, contributing to elevated observed heterozygosity (Ho) relative to Guischenault. Tri-allelic genotypes were also observed in some embryos (Table 2). The proportion of samples with additional alleles is much higher in Shark Bay meadows with elevated salinities, regardless of clonal diversity (Fig. 4A).
Figure 4.
Genetic diversity: relationship between (A) clonal diversity and proportion of samples with 3× alleles within a meadow relative to salinity, (B) clonal diversity in embryos and percentage of outcrossed families by meadow and (C) variance in outcrossing rates (tm) among P. australis ‘families’ by seagrass meadow. The box corresponds to the lower and upper quartiles (25 and 75th percentiles), internal horizontal bars indicate the median and vertical whiskers extend to the lowest and highest values no further than 1.5 interquartile range. Points outlying this are represented as dots. Abbreviations for range edge meadows in Shark Bay are Guischenault (GU), Red Hill Bay (HP), Denham (DE), Useless Loop (UL), Fowlers Camp (FC) and Perth metropolitan waters are Stark Bay (RST) and Parker Point (RPP) on Rottnest Island, Carnac Island (CI), Walking Beach, Garden Island (WB), Point Peron (PP).
No significant relationship was observed between seed to ovule ratios and shoot clonal diversity using maternal genotypes and previously collected population genetics data (summarized in Table 4) from meadows on the west coast of Australia (Pearson r (maternal) = 0.763, P = 0.133; Pearson r (population): 0.597, P = 0.287).
Table 4.
Summary of all genetic data from Shark Bay meadows and mating system studies for Posidonia australis meadows. Meadow abbrev. = random shoot sample from meadow; meadow abbrev. with ‘m’ denotes maternal genotypes only; meadow abbrev. with ‘e’ denotes embryo genotypes only; N = number of samples; MLG = number of unique multilocus genotypes; R = clonal richness; Ho = observed heterozygosity; 3× = number of samples with an additional allele in at least one locus; *fruit were not collected, so it was not possible to determine whether the embryos were viable.
| Meadow abbrev. | Salinity (PSU) | Coll. year | N | MLG | R | H o (%) | 3× | Fruit | Seed: ovule | Outcrossing rate | Pollen donors | Prop of full sibs | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shark Bay (northern range edge): | |||||||||||||
| GU | 35–38 | 2012 | 30 | 15 | 0.48 | 25.9 | 1 | Unpubl. data | |||||
| GUm | 2014 | 28 | 11 | 0.37 | 17.9 | 6 | This paper | ||||||
| GUe | 2014 | 123 | 37 | 0.30 | 16.7 | 47 | Yes | 0.45 | 50 % | 1.1 | 0.90 | This paper | |
| HP | 35–38 | 2016 | 29 | 7 | 0.21 | 42.9 | 4 | This paper | |||||
| HPm | 2016 | 15 | 3 | 0.14 | 35.7 | 0 | This paper | ||||||
| HPe | 2016 | 94 | 25 | 0.26 | 19.3 | 2 | Yes | 0.32 | 57 % | 1.0 | 0.93 | This paper | |
| UL | >38 | 2011 | 37 | 13 | 0.33 | 62.9 | 29 | Yes | – | – | – | – | Sinclair et al. (2016b) |
| DE | >38 | 2012 | 30 | 16 | 0.52 | 55.2 | 23 | – | – | – | – | – | Unpubl. data |
| FC | >40 | 2018 | 27 | 6 | 0.19 | 47.1 | 27 | Yes* | 0.07 | – | – | – | This paper |
| Perth metropolitan waters (centre of range): | |||||||||||||
| RST | Oceanic | 2015 | 50 | 38 | 0.76 | 54.1 | 0 | Unpubl. data | |||||
| RSTm | 2014 | 28 | 12 | 0.41 | 59.7 | 7 | This paper | ||||||
| RSTe | 2014 | 106 | 102 | 0.96 | 57.1 | 26 | Yes | 0.32 | 100 % | 8.8 | 0.11 | This paper | |
| RPP | Oceanic | 2009 | 49 | 10 | 0.19 | 54.3 | 0 | Sinclair et al. (2014b) | |||||
| RPPm | 2014 | 30 | 6 | 0.17 | 36.2 | 0 | This paper | ||||||
| RPPe | 2014 | 144 | 115 | 0.80 | 50.1 | 0 | Yes | 0.10 | 100 % | 4.8 | 0.21 | This paper | |
| CI | Oceanic | 2009 | 50 | 43 | 0.86 | 30.6 | 0 | Sinclair et al. (2014b) | |||||
| CIm | 2014 | 32 | 30 | 0.94 | 30.4 | 1 | This paper | ||||||
| CIe | 2014 | 274 | 182 | 0.66 | 29.8 | 9 | Yes | – | 100 % | 1.5 | 0.68 | This paper | |
| PP | Oceanic | 2010 | 46 | 27 | 0.58 | 54.8 | 0 | Sinclair et al. (2014b) | |||||
| PPm | 2010 | 32 | 15 | 0.45 | 0.49 | 0 | Sinclair et al. (2014a) | ||||||
| PPe | 2010 | 213 | 206 | 0.97 | 0.51 | 0 | Yes | – | 100 % | 11.1 | 0.09 | Sinclair et al. (2014a) | |
| WB | Oceanic |
2010 | 48 | 25 | 0.51 | 52.0 | 0 | Sinclair et al. (2014b) | |||||
| WBm | 2010 | 34 | 22 | 0.64 | 0.46 | 0 | Sinclair et al. (2014a) | ||||||
| WBe | 2010 | 208 | 193 | 0.93 | 0.47 | 0 | Yes | – | 100 % | 6.6 | 0.15 | Sinclair et al. (2014a) |
Mating system
Mating system analyses were conducted on samples from five meadows which produced viable embryos. Complete outcrossing was seen in the three meadows from Perth metropolitan waters (Stark Bay, Parker Point, Carnac Island; Table 3). Mixed mating (i.e. self- and cross-fertilized embryos) was observed in the two Shark Bay meadows that produced viable embryos, with multilocus outcrossing rates of 0.50 at Guischenault and 0.57 at Red Hill Bay (Table 3). There was a significant difference in outcrossing rates between meadows in Shark Bay and Perth metropolitan waters (t-test: −7.75, P-value < 0.01). Correlated paternity (or proportion of full siblings) was much higher in Shark Bay meadows (rp): range edge = 0.90, 0.93 than Perth metropolitan waters = 0.11–0.21, indicating a much higher effective number of pollen donors per inflorescence for Perth metropolitan waters (4.8–8.8) compared with Shark Bay (~1.0) meadows. Carnac Island was the exception, with the highest proportion of full sibs from all sampled meadows in Perth metropolitan waters (0.68). Outcrossing rates within individual families were much more variable in Shark Bay meadows than Perth metropolitan waters meadows (Table 3), with a significant correlation between clonal diversity in embryos and percentage of outcrossed families by meadow, regardless of location (Fig. 4B, Pearson r = 0.956, P-value < 0.001). A visual inspection of Fig. 4C shows a much larger variance in family outcrossing rates in meadows with mixed mating systems in Shark Bay relative to completely outcrossed meadows across Perth metropolitan waters.
Table 3.
Mating system parameters for Posidonia australis meadows and by ‘families’ within meadows. Values are based on genotypes for seven microsatellite loci. *See Fig. 2.
| Parameter | Shark Bay meadows | Perth metropolitan waters | |||
|---|---|---|---|---|---|
| GU (±SD) | HP (±SD) | RST (±SD) | RPP (±SD) | CI (±SD) | |
| No. mature embryos genotyped/infructescence | 2–8 | 2–10 | 2–8 | 3–8 | 3–14 |
| Mean family size | 4.4 (±1.6) | 6.3 (±2.4) | 3.8 (±1.3) | 4.8 (±1.4) | 8.6 (±2.5) |
| Total number of aborted embryos | 9 | 15 (+21 mutant*) | 1 | 2 | 5 |
| Percentage of aborted embryos | 0.40 % | 13.60 % | 0.08 % | 1.30 % | 1.70 % |
| Number of families genotyped | 28 | 15 | 28 | 30 | 32 |
| Number of embryos genotyped | 123 | 94 | 106 | 144 | 274 |
| Mating system parameters by meadow: | |||||
| Parental inbreeding f | 0.19 (±0.15) | −0.20 (±0.01) | −0.09 (±0.02) | −0.11 (±0.06) | −0.20 (±0.05) |
| Multilocus outcrossing rate tm | 0.50 (± 0.09) | 0.57 (± 0.26) | 1.03 (±0.02) | 1.20 (±0.03) | 1.06 (±0.04) |
| Single-locus outcrossing rate ts | 0.54 (±0.10) | 0.59 (±0.27) | 1.13 (±0.03) | 1.20 (±0.00) | 1.04 (±0.04) |
| Biparental inbreeding tm–ts | −0.04 (±0.03) | −0.02 (±0.04) | −0.10 (±0.04) | 0.00 (±0.04) | 0.02 (±0.04) |
| Multilocus correlation of P within genets rp(w) | 0.90 (±0.19) | −0.93 (±0.33) | 0.11 (±0.13) | 0.21 (±0.06) | 0.68 (±0.02) |
| Effective number of pollen donors Nep(w) | 1.1 | 1.0 | 8.8 | 4.8 | 1.5 |
| Mating system parameters within family: | |||||
| Percentage (%) outcrossed families | 43.3 | 33.3 | 96.4 | 100.0 | 90.6 |
| Single-locus outcrossing rate—range ts (±SE) | −0.41–1.99 (±0.25) | −0.72–2.40 (±0.40) | 0.10–1.73 (±0.16) | 1.04–1.93 (±0.16) | 0.38–1.32 (±0.11) |
| Multilocus outcrossing rate—range tm (±SE) | −0.41–1.49 (±0.16) | −0.75–2.16 (±0.48) | 0.27–1.06 (±0.00) | 1.00–1.10 (±0.00) | 0.46–1.38 (±0.04) |
Discussion
Posidonia australis meadows were completely outcrossed in Perth metropolitan waters while range edge meadows in Shark Bay had a mixed mating system. We also observed lower levels of genetic diversity and higher clonality in meadows within Shark Bay relative to Perth metropolitan waters, which together support our population genetic hypotheses, despite limited replication to distinguish between an edge effect and the potentially confounding effect of elevated salinity. Low levels of genetic diversity within individual meadows with low to no sexual reproduction were also reported in several northern range edge P. australis meadows on the east coast of Australia (Evans et al. 2014). However, the considerable diversity retained across P. australis meadows in Shark Bay may be the result of genetic connectivity over larger spatial areas and longer time periods, as well as additional alleles. A regional assessment of eelgrass, Zostera marina, showed that genetic diversity was retained across multiple (southern) range edge meadows relative to within individual meadows which had small effective population size, reduced habitat area, low sexual reproduction and gene flow (Diekmann and Serrão 2012). Marine seascape patterns are often complex, with temperature, oceanography and geography showing equal prevalence of influence on spatial genetic patterns (reviewed in Selkoe et al. 2016).
Our hypotheses appear less well supported by productivity data on flowering and seed production and ratio of seed set to flowering (seed to ovule ratio). Sampling was however focussed on seed-producing meadows to obtain data on outcrossing rates. The spatial extent of the Shark Bay meadows was two orders of magnitude lower than Perth metropolitan waters where there are 10s to 100s km of reproductively fecund meadows. There are many more meadows in Shark Bay that have low densities of flowers, with no viable seeds being produced (see Kendrick et al. 2019).
Our combined P. australis data for seven meadows (Sinclair et al. 2014a; this study) are consistent with patterns observed in terrestrial plant species, regardless of pollination method whereby range edge populations tend to have mixed mating. The potentially false increases to selfing rates introduced by reducing tri- to bi-allelic genotypes to estimate outcrossing rates are unlikely to account for such significant differences observed. The high frequency of additional alleles (tri-allelic genotypes) present in three non-reproductive Shark Bay meadows genotyped (Table 4, Denham (DE), Fowlers Camp (FC) and Useless Loop (UL)) contributed to elevated heterozygosity (Ho) relative to Guischenault (GU), which was the most fecund meadow. Alternative hypotheses proposed to explain the presence of additional alleles across multiple meadows include ancient hybridization, putative aneuploidy and somatic mutations leading to genetic mosaicism, all of which have been reported in seagrasses (Reusch and Boström 2011; Sinclair et al. 2019; Digiantonio et al. 2020). The accumulation of somatic mutations (leading to genetic mosaicism) could explain the additional alleles; however, it is unlikely to account for the high frequency and widespread observations across Shark Bay and beyond. The more widespread occurrence of additional alleles suggests they made be a legacy of hybridization event(s), whereby a diploid F1 hybrid plant is fertile and able to backcross to a parental species (Sinclair et al. 2016b). Additional alleles in the backcross hybrid may be caused by unreduced (diploid) pollen combining with the haploid pollen from either parental species. These backcross hybrids persist through vegetative growth, but are probably sterile, and consistent with reduced or complete failure to produce viable seed in these meadows. Such an explanation is unlikely in the absence of polyploidy and/or whole-genome duplication (see ploidy cycling in Wendel 2015). Additional research with appropriately designed sampling using genomic approaches may determine the true origin of additional alleles in the future, as demonstrated in another seagrass genus, Zostera (Yu et al. 2020).
Role of the local environment on sexual reproduction
Understanding the relative influence of geographic location and environmental conditions on sexual reproduction is challenging. The relationship between reproductive output and genetic diversity measures may also be affected by local environmental conditions (e.g. hydrology and salinity). The Shark Bay meadows had fewer reproductive clones and a mixed mating system, although flower and fruit production and seed to ovule ratios can be comparable to meadows in Perth metropolitan waters. The Guischenault meadow has some of the highest recorded flowering and fruit densities in P. australis with a high seed to ovule ratio, yet overall levels of genetic diversity were low in maternal plants and embryos, with only 50 % outcrossing. In contrast, Red Hill Bay has some of the lowest recorded flowering and fruit densities in P. australis with very low genetic and clonal diversity (similar to Parker Point) yet has a similarly high seed to ovule ratio and mixed mating. Both these reproductive range edge meadows have high water movement (i.e. strongly tidal, linear movement) at close to oceanic salinity, thus likely to promote pollination success for the available pollen, leading to higher seed production than anticipated. This is in contrast to an exceptionally low seed to ovule ratio at Fowlers Camp, a sheltered meadow with weak tidal current, and exceptionally high flowering in elevated salinities. No reproductive data were collected for Carnac Island; however, large numbers of viable fruit are observed annually. Carnac Island appears to be a special case where high clonal diversity, parental inbreeding, complete outcrossing and high proportion of full sibs are consistent with pollination and recruitment occurring within this highly sheltered meadow (as proposed in Sinclair et al. 2014a).
The magnitude of pollen limitation observed in natural populations depends on both historical constraints and contemporary ecological factors (Knight et al. 2005). Pollen limitation has been reported in several seagrass genera Phyllospadix spp. (Shelton 2008; Buckel et al. 2012), Thalassia testudinum (Van Tussenbroek et al. 2016b) and Zostera spp. (Reusch 2003; Van Tussenbroek et al. 2016a), where there is dominance of a few large clones and/or high spatial and temporal heterogeneity in flowering. Stigmas on flowers in Guischenault and Red Hill Bay may be exposed to a large amount of local pollen through strong tidal water movement, but outcrossing rates were lower because pollen was produced by a few clones, leading to selfing and/or apomixis.
Levin (2012) and Breed et al. (2015) highlight declining outcrossing rates in range edge and disturbed environments as a result of environmental changes. Increased selfing may be advantageous in range edge populations due to the possible advantages of reproductive assurance and through maintaining locally adapted genotypes (Arnaud-Haond et al. 2006; Levin 2012), despite the risks of increased mutational load that reduces fitness (Willi et al. 2018). Substantial increases in self-fertilization rates may also occur via plastic responses to stress (Levin 2012). An experimental study of an annual succulent halophyte Cakile maritima reported significant decline in plant biomass, as well as the number and size of fruit, with elevated salinity (Debez et al. 2004). The accumulation of Na+ and Cl− in pollen and stigmas is known to be strongly implied in salt-induced sterility in rice (Oryza sativa, Khatun et al. 1995). Sparse flowering records in P. australis with low seed to ovule ratio and no viable fruit recovered from meadows growing at elevated salinities (>38 PSU) are consistent with this finding. Pseudoviviparous plantlets in unfertilized inflorescences have also been observed following lower-than-usual water temperature and complete seed abortion when plants were under thermal stress from an extreme marine heat wave (Sinclair et al. 2016b). This suggests a trade-off between sexual and asexual reproduction which may also be driven by both salinity and temperature (e.g. Salter et al. 2010). However, additional information on the spatial extent of fecund meadows is required to interpret ecological comparisons of sexual reproduction.
Implications for long-term resilience and restoration
Strongly clonal species are known to survive for very long periods of time (de Witte and Stocklin 2010). Individual clones can persist thousands of years, surviving through significant climatic events, and essentially buffering species or populations against short-term or localized stress (e.g. Reusch et al. 1999; May et al. 2009; Arnaud-Haond et al. 2012). Surviving such events requires the genetic capacity to adapt and/or the propensity to shift geographical ranges (i.e. associated with sea level change). Honnay and Bossuyt (2005) argue that prolonged and nearly exclusive clonal growth through environmental suppression of sexual reproduction can ultimately lead to local sexual extinction and to monoclonal populations, with significant consequences for population viability.
Shark Bay was impacted by an extreme marine heatwave in 2010/11 which caused significant loss of seagrass (Fraser et al. 2014; Thomson et al. 2015) and sexual reproductive failure in P. australis (Sinclair et al. 2016b). A recent review of the impacts of this heatwave showed it has taken 6 years to observe natural recovery of shoot density (Kendrick et al. 2019). However, this recovery is likely driven through rhizome expansion rather than sexual recruitment (Kendrick et al. 2019), as seed production is poor and patchy.
Conservation and mitigation of disturbance have typically been the first line of defence for seagrass loss, but ecological restoration is becoming increasingly necessary in a rapidly changing environment (Statton et al. 2018). It is potentially a more effective management strategy where seagrass habitat has been recently lost or heavily impacted and sexual reproduction is sporadic, as natural recruitment events are rare. Tackling restoration in warming range edge populations across environmental gradients may present additional challenges. Sexton et al. (2011) manipulated patterns of gene flow in an annual plant to experimentally show that offspring fitness improved with outcrossing, but that lifetime reproductive success only increased significantly when pollen originated from other warm edge populations. They emphasized the overlooked importance of gene flow among populations occurring near the same range edge, highlighting the potential for prescriptive gene flow as a conservation/restoration option. Restoration of marine ecosystems will benefit from vigorous debates around the use of local (maintain local adaptation) versus non-local (mitigate against climate change) plant material (Breed et al. 2018). Other alternatives include the use of population genomics to understand the genetic basis of adaptation to inform seed sourcing (Breed et al. 2019).
Seagrasses have persisted for thousands of years through multiple climate cycles, with no recent evidence of latitudinal range contraction in P. australis. The range edge meadows of Shark Bay have retained (neutral) genetic diversity and the ability to reproduce sexually (albeit lower). The use of vegetative (clonal) and seed material from multiple P. australis meadows across Shark Bay to assist recovery may artificially enhance meadow diversity and outcrossing rates for better quality seed production in the future. Ongoing research into the role of adaptation, acclimation and plasticity in range edge meadows may shed light on how these meadows with reduced sexual reproduction and outcrossing rates may overcome additional challenges across a salinity gradient under changing climates.
Supporting Information
The following additional information is available in the online version of this article—
Table S1. Seven labelled polymorphic microsatellite loci isolated from Posidonia australis.
Table S2. Allele frequencies for all genotyped samples by meadow and tissue type (maternal shoots and embryos). Based on tri-allelic genotypes and calculated in Genodive.
Table S3. Genetic differentiation as estimated using FST (above diagonal) and P-values (below diagonal) between all pairs of maternal shoots and embryo ‘populations’.
Data Availability
All raw microsatellite genotype data are available from the UWA Research Repository at: 10.26182/5f0bfbc877c60.
Acknowledgements
Thank you to A. Brearley, S. Gustin-Craig, R. Hovey, L. Ruiz-Montoya, A. Zavala for help with field collections; reviewers for their constructive comments to improve this manuscript; the Malaga Aboriginal Corporation for their permission to conduct research on Gathaagudu Country.
Sources of Funding
This project was funded by the Australian Research Council (LP130100918 to G.A.K., LP160101011 to G.A.K., DP180100668 to G.A.K. and M.F.B.). J.M.E. was supported by a Friends of Kings Park Summer Scholarship.
Contributions by the Authors
E.A.S. and G.A.K. conceived the study; G.A.K. and J.S. collected and analysed the reproductive data; G.A.K., J.S. and E.A.S. collected genetic material; J.M.E. J.M.A. and E.A.S. collected the genetic data; E.A.S. and M.F.B. analysed the genetic data; E.A.S., G.A.K., M.F.B. and J.S. interpreted results; E.A.S. led the writing with contributions from all authors.
Conflict of Interest
None declared.
Literature Cited
- Ackerman JD. 1995. Convergence of filiform pollen morphologies in seagrasses: functional mechanisms. Evolutionary Ecology 9:139–153. [Google Scholar]
- Ackerman JD. 2000. Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives. Plant Systematics and Evolution 222:167–185. [Google Scholar]
- Arnaud-Haond S, Duarte CM, Diaz-Almela E, Marbà N, Sintes T, Serrão EA. 2012. Implications of extreme life span in clonal organisms: millenary clones in meadows of the threatened seagrass Posidonia oceanica. PLoS One 7:e30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud-Haond S, Teixeira S, Massa SI, Billot C, Saenger P, Coupland G, Duarte CM, Serrão EA. 2006. Genetic structure at range edge: low diversity and high inbreeding in Southeast Asian mangrove (Avicennia marina) populations. Molecular Ecology 15:3515–3525. [DOI] [PubMed] [Google Scholar]
- Assis J, Castilho Coelho N, Alberto F, Valero M, Raimondi P, Reed D, Serrão EA. 2013. High and distinct range-edge genetic diversity despite local bottlenecks. PLoS One 8:e68646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RGV, Haworth R, Flood PG. 2005. An oscillating holocene sea-level? Revisiting Rottnest Island, Western Australia, and the Fairbridge eustatic hypothesis. Journal of Coastal Research 21:3–14. [Google Scholar]
- Barrett SCH, Harder L. 2017. The ecology of mating and its evolutionary consequences in seed plants. Annual Review of Ecology and Systematics 48:135–157. [Google Scholar]
- Breed MF, Harrison PA, Bischoff A, Durruty P, Gellie NJC, Gonzales EK, Havens K, Karmann M, Kilkenny FF, Krauss SL, Lowe AJ, Marques P, Nevill PG, Vitt PL, Bucharova A. 2018. Priority actions to improve provenance decision-making. BioScience 68:510–516. [Google Scholar]
- Breed MF, Harrison PA, Blyth C, Byrne M, Gaget V, Gellie NJC, Groom SVC, Hodgson R, Mills JG, Prowse TAA, Steane DA, Mohr JJ. 2019. The potential of genomics for restoring ecosystems and biodiversity. Nature Reviews Genetics 20:615–628. [DOI] [PubMed] [Google Scholar]
- Breed MF, Ottewell KM, Gardner MG, Marklund MHK, Dormontt EE, Lowe AJ. 2015. Mating patterns and pollinator mobility are critical traits in forest fragmentation genetics. Heredity 115:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckel CA, Blanchette CA, Warner RR, Gaines SD. 2012. Where a male is hard to find: consequences of male rarity in the surfgrass Phyllospadix torreyi. Marine Ecology Progress Series 449:121–132. [Google Scholar]
- Bufarale G, Collins LB. 2015. Stratigraphic architecture and evolution of a barrier seagrass bank in the mid-late Holocene, Shark Bay, Australia. Marine Geology 359:1–21. [Google Scholar]
- Cambridge ML, Hocking PJ. 1997. Annual primary production and nutrient dynamics of the seagrasses Posidonia sinuosa and Posidonia australis in south-western Australia. Aquatic Botany 59:277–295. [Google Scholar]
- Carruthers TJB, Dennison WC, Kendrick GA, Waycott M, Walker DI, Cambridge ML. 2007. Seagrasses of south-west Australia: a conceptual synthesis of the world’s most diverse and extensive seagrass meadows. Journal of Experimental Marine Biology and Ecology 350:21–45. [Google Scholar]
- Charlesworth B, Charlesworth D. 2017. Population genetics from 1966 to 2016. Heredity 118:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026. [DOI] [PubMed] [Google Scholar]
- Costanza R, dArge R, deGroot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, Oneill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M. 1997. The value of the world’s ecosystem services and natural capital. Nature 387:253–260. [Google Scholar]
- de Witte LC, Stöcklin J. 2010. Longevity of clonal plants: why it matters and how to measure it. Annals of Botany 106:859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debez A, Ben Hamed K, Grignon C, Abdelly C. 2004. Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima. Plant and Soil 262:179–189. [Google Scholar]
- den Hartog C. 1970. The seagrasses of the world, Vol. 59 Amsterdam: North-Holland Publishing Company, 275pp. [Google Scholar]
- Diekmann OE, Serrão EA. 2012. Range-edge genetic diversity: locally poor extant southern patches maintain a regionally diverse hotspot in the seagrass Zostera marina. Molecular Ecology 21:1647–1657. [DOI] [PubMed] [Google Scholar]
- Digiantonio G, Blum L, McGlathery KJ, van Dijk K-J, Waycott M. 2020. Genetic mosaicism and population connectivity of edge-of-range Halodule wrightii populations. Aquatic Botany 161:103161. [Google Scholar]
- Dorken ME, Eckert CG. 2001. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). Journal of Ecology 89:339–350. [Google Scholar]
- Duarte CM, Losada IJ, Hendricks IE, Mazarrasa I, Marbà N. 2013. The role of coastal plant communities for climate change mitigation and adaptation. Nature Climate Change 3:961–968. [Google Scholar]
- Dufresne F, Stift M, Vergilino R, Mable BK. 2014. Recent progress and challenges in population genetics of polyploid organisms: an overview of current state-of-the-art molecular and statistical tools. Molecular Ecology 23:40–69. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Lougheed SC. 2008. Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Molecular Ecology 17:1170–1188. [DOI] [PubMed] [Google Scholar]
- Edgar GJ. 2000. Australian marine life; the plants and animals of temperate waters. Sydney, Australia: New Holland Publishers. [Google Scholar]
- Evans SM, Sinclair EA, Poore AGB, Steinberg PD, Kendrick GA, Vergés A. 2014. Genetic diversity in threatened Posidonia australis seagrass meadows. Conservation Genetics 15:717–728. [Google Scholar]
- Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, Mateo MA, Apostolaki ET, Kendrick GA, Krause-Jensen D, McGlathery KJ, Serrano O. 2012. Seagrass ecosystems as a globally significant carbon stock. Nature Geosciences 5:505–509. [Google Scholar]
- Fraser MW, Kendrick GA, Grierson PF, Fourqurean JW, VanderKlift MA, Walker DI. 2012. Nutrient status of seagrasses cannot be inferred from system-scale distribution of phosphorus in Shark Bay, Western Australia. Marine and Freshwater Research 63:1015–1026. [Google Scholar]
- Fraser MW, Kendrick GA, Statton J, Hovey RK, Zavala-Perez A, Walker DI. 2014. Extreme climate events lower resilience of foundation seagrass at edge of biogeographical range. Journal of Ecology 102:1528–1536. [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. 2005. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology and Systematics 36:47–79. [Google Scholar]
- Hampe A, Petit RJ. 2005. Conserving biodiversity under climate change: the rear edge matters. Ecology Letters 8:461–467. [DOI] [PubMed] [Google Scholar]
- Honnay O, Bossuyt B. 2005. Prolonged clonal growth: escape route or route to extinction? Oikos 108:427–432. [Google Scholar]
- Kendrick GA, Nowicki RJ, Olsen YS, Strydom S, Fraser MW, Sinclair EA, Statton J, Hovey RK, Thomson JA, Burkholder DA, McMahon K, Kilminster K, Hetzel Y, Fourqurean JW, Heithaus MR, Orth RJ. 2019. A systematic review of how multiple stressors from an extreme event drove ecosystem-wide loss of resilience in an iconic seagrass community. Frontiers in Marine Science 6:455. [Google Scholar]
- Kendrick GA, Waycott M, Carruthers T, Cambridge ML, Hovey RK, Krauss SL, Lavery P, Les DH, Lowe R, Mascaró O, Ooi Lean Sim J, Orth RJ, Rivers D, Ruiz-Montoya L, Sinclair EA, Statton J, van Dijk K-J, Verduin JJ. 2012. The central role of dispersal in the maintenance and persistence of seagrass populations. BioScience 62:56–65. [Google Scholar]
- Khatun S, Rizzo CA, Flowers TJ. 1995. Genotypic variation in the effect of salinity on fertility in rice. Plant and Soil 173:239–250. [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman T-L. 2005. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology Evolution and Systematics 36:467–497. [Google Scholar]
- Kuo J, James SH, Kirkman H, den Hartog CKJ. 1990. Chromosome numbers and their systematic implications in Australian marine angiosperms the Posidoniaceae. Plant Systematics and Evolution 171:199–204. [Google Scholar]
- Lamb JB, van de Water JA, Bourne DG, Altier C, Hein MY, Fiorenza EA, Abu N, Jompa J, Harvell CD. 2017. Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science 355:731–733. [DOI] [PubMed] [Google Scholar]
- Levin DA. 2012. Mating system shifts on the trailing edge. Annals of Botany 109:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins L, Gleeson L, Riginos C. 2014. Evaluating edge-of-range genetic patterns for tropical echinoderms, Acanthaster planci and Tripneustes gratilla, of the Kermadec Islands, southwest Pacific. Bulletin of Marine Science 90:379–397. [Google Scholar]
- May MR, Provance MC, Sanders AC, Ellstrand NC, Ross-Ibarra J. 2009. A Pleistocene clone of Palmer’s oak persisting in Southern California. PLoS One 4:e8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConchie CA, Knox RB. 1989. Pollen–stigma interaction in the seagrass Posidonia australis. Annals of Botany 63:235–248. [Google Scholar]
- McMahon K, van Dijk K-J, Ruiz-Montoya L, Kendrick GA, Krauss SL, Waycott M, Verduin J, Lowe R, Statton J, Brown E, Duarte CM. 2014. The movement ecology of seagrasses. Proceedings of the Royal Society London B 281:20140878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirmans PG, van Tienderen PH. 2004. GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Molecular Ecology Notes 4:792–794. [Google Scholar]
- Miller KG, Mountain GS, Wright JD, Browning JV. 2011. A 180-million-year record of sea level and ice volume variations from continental margin and deep-sea isotopic records. Oceanography 24:40–53. [Google Scholar]
- Nicastro KR, Zardi GI, Teixeira S, Neiva J, Serrão EA, Pearson GA. 2013. Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biology 11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck Jr KL, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, Olyarnik S, Short FT, Waycott M, Williams SL. 2006. A global crisis for seagrass ecosystems. BioScience 56:987–996. [Google Scholar]
- Pecl GT, Araújo MB, Bell JD, Blanchard J, Bonebrake TC, Chen IC, Clark TD, Colwell RK, Danielsen F, Evengård B, Falconi L, Ferrier S, Frusher S, Garcia RA, Griffis RB, Hobday AJ, Janion-Scheepers C, Jarzyna MA, Jennings S, Lenoir J, Linnetved HI, Martin VY, McCormack PC, McDonald J, Mitchell NJ, Mustonen T, Pandolfi JM, Pettorelli N, Popova E, Robinson SA, Scheffers BR, Shaw JD, Sorte CJB, Strugnell JM, Sunday JM, Tuanmu M-N, Vergés A, Villanueva C, Wernberg T, Wapstra E, Williams SE. 2017. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355:eaai9214. [DOI] [PubMed] [Google Scholar]
- Playford PE. 1988. Guidebook to the geology of Rottnest Island. Perth, Australia: Geological Society of Australia & Geological Survey of Western Australia. [Google Scholar]
- Reusch TBH. 2003. Floral neighbourhoods in the sea: how floral density, opportunity for outcrossing and population fragmentation affect seed set in Zostera marina. Journal of Ecology 91:610–615. [Google Scholar]
- Reusch TBH, Boström C. 2011. Widespread genetic mosaicism in the marine angiosperm Zostera marina is correlated with clonal reproduction. Evolutionary Ecology 25:899–913. [Google Scholar]
- Reusch TBH, Boström C, Stam WT, Olsen JL. 1999. An ancient eelgrass clone in the Baltic Sea. Marine Ecology Progress Series 183:301–304. [Google Scholar]
- Ritland K. 2002. Extensions of models for the estimation of mating systems using n independent loci. Heredity 88:221–228. [DOI] [PubMed] [Google Scholar]
- Salter J, Morris K, Read JF, Broon PI. 2010. Impact of long-term, saline flooding on condition and reproduction of the clonal wetland tree, Melaleuca ericifolia (Myrtaceae). Plant Ecology 206:41–57. [Google Scholar]
- Selkoe KA, D’Aloia CC, Crandall ED, et al. 2016. A decade of seascape genetics: contributions to basic and applied marine connectivity. Marine Ecology Progress Series 554:1–19. [Google Scholar]
- Serrano O, Lavery PS, López-Merino L, Ballesteros E, Mateo MA. 2016. Location and associated carbon storage of erosional escarpments of seagrass Posidonia mats. Frontiers and Marine Science 3:42. [Google Scholar]
- Sexton JP, Strauss SY, Rice KJ. 2011. Gene flow increases fitness at the warm edge of a species’ range. Proceedings of the National Academy of Sciences of the United States of America 108:11704–11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DV, Kahler AL, Allard RW. 1981. A multilocus estimator of mating system parameters in plant populations. Proceedings of the National Academy of Sciences of the United States of America 78:1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton AO. 2008. Skewed sex ratios, pollen limitation, and reproductive failure in the dioecious seagrass Phyllospadix. Ecology 89:3020–3029. [DOI] [PubMed] [Google Scholar]
- Sheth SN, Angert AL. 2018. Demographic compensation does not rescue populations at a trailing range edge. Proceedings of the National Academy of Sciences of the United States of America 115:2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short FT, Polidoro B, Livingstone SR, Carpenter KE, Bandeira S, Bujang JS, Calumpong HP, Carruthers TJB, Coles RG, Dennison WC, Erftemeijer PLA, Fortes MD, Freeman AS, Jagtap TG, Kamal AHM, Kendrick GA, Kenworthy JW, La Nafie YA, Nasution IM, Orth RJ, Prathep A, Sanciangco JC, Tussenbroek Bv, Vergara SG, Waycott M, Zieman JC. 2011. Extinction risk assessment of the world’s seagrass species. Biological Conservation 144:1961–1971. [Google Scholar]
- Sinclair EA, Anthony JM, Coupland GT, Waycott M, Barrett MD, Barrett RL, Cambridge ML, Wallace MJ, Dixon KW, Krauss SL, Kendrick GA. 2009. Characterisation of polymorphic microsatellite markers in the widespread Australian seagrass, Posidonia australis Hook. f. (Posidoniaceae), with cross-amplification in the sympatric P. sinuosa. Conservation Genetics Resources 1:273–276. [Google Scholar]
- Sinclair EA, Anthony JM, Greer D, Ruiz-Montoya L, Evans SM, Krauss SL, Kendrick GA. 2016a. Genetic signatures of Bassian glacial refugia and contemporary connectivity in a marine foundation species Journal of Biogeography 43:2209–2222. [Google Scholar]
- Sinclair EA, Cambridge ML, Kendrick GA. 2019. First report of hybridisation in the marine plant genus Posidonia. Aquatic Botany 156:10–13. [Google Scholar]
- Sinclair EA, Gecan I, Krauss SL, Kendrick GA. 2014a. Against the odds: complete outcrossing in a monoecious clonal seagrass Posidonia australis (Posidoniaceae). Annals of Botany 113:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair EA, Krauss SL, Anthony JM, Hovey RK, Kendrick GA. 2014b. The interaction of environment and genetic diversity within meadows of the seagrass Posidonia australis (Posidoniaceae). Marine Ecology Progress Series 506:87–98. [Google Scholar]
- Sinclair EA, Statton J, Hovey R, Anthony JM, Dixon KW, Kendrick GA. 2016b. Reproduction at the extremes: pseudovivipary, hybridization and genetic mosaicism in Posidonia australis (Posidoniaceae). Annals of Botany 117:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smouse PE, Dyer RJ, Westfall RD, Sork VL. 2001. Two-generation analysis of pollen flow across a landscape. I. Male gamete heterogeneity among females. Evolution 55:260–271. [DOI] [PubMed] [Google Scholar]
- Statton J, Dixon KW, Irving AD, Jackson EL, Kendrick GA, Orth RJ, Sinclair EA. 2018. Decline and restoration ecology of Australian seagrasses. In: Larkum AWD, Ralph P, Kendrick GA, eds. Seagrasses of Australia: structure, ecology and conservation. Cham, Switzerland: Springer International Publishing, 665–704. [Google Scholar]
- Thomson JA, Burkholder DA, Heithaus MR, Fourqurean JW, Fraser MW, Statton J, Kendrick GA. 2015. Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Global Change Biology 21:1463–1474. [DOI] [PubMed] [Google Scholar]
- Tukey JW. 1977. Exploratory data analysis. Reading, MA: Addison-Wesley Publishing Company. [Google Scholar]
- van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P. 2004. Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes 4:535–538. [Google Scholar]
- Van Tussenbroek BI, Soissons LM, Bouma TJ, Asmus R, Auby I, Brun FG, Cardoso PG, Desroy N, Fournier J, Ganthy F, Garmendia JM, Godet L, Grilo TF, Kadel P, Ondiviela B, Peralta G, Recio M, Valle M, Van der Heide T, Van Katwijk MM. 2016a. Pollen limitation may be a common Allee effect in marine hydrophilous plants: implications for decline and recovery in seagrasses. Oecologia 182:595–609. [DOI] [PubMed] [Google Scholar]
- Van Tussenbroek BI, Valdivia-Carrillo T, Rodríguez-Virgen IT, Sanabria-Alcaraz SN, Jiménez-Durán K, Van Dijk KJ, Marquez-Guzmán GJ. 2016b. Coping with potential bi-parental inbreeding: limited pollen and seed dispersal and large genets in the dioecious marine angiosperm Thalassia testudinum. Ecology and Evolution 6:5542–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DI, Kendrick GA, McComb AJ. 1988. The distribution of seagrass species in shark bay Western Australia with notes on their ecology. Aquatic Botany 30:305–318. [Google Scholar]
- Waycott M, Duarte CM, Carruthers TJ, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL Jr, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106:12377–12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel JF. 2015. The wondrous cycles of polyploidy in plants. American Journal of Botany 102:1753–1756. [DOI] [PubMed] [Google Scholar]
- Wernberg T, Bennett S, Babcock RC, de Bettignies T, Cure K, Depczynski M, Dufois F, Fromont J, Fulton CJ, Hovey RK, Harvey ES, Holmes TH, Kendrick GA, Radford B, Santana-Garcon J, Saunders BJ, Smale DA, Thomsen MS, Tuckett CA, Tuya F, Vanderklift MA, Wilson S. 2016. Climate-driven regime shift of a temperate marine ecosystem. Science 353:169–172. [DOI] [PubMed] [Google Scholar]
- Whitehead MR, Lanfear R, Mitchell RJ, Karron JD. 2018. Plant mating systems often vary widely among populations. Frontiers in Ecology and Evolution 6:38. [Google Scholar]
- Willi Y, Fracassetti M, Zoller S, Van Buskirk J. 2018. Accumulation of mutational load at the edges of a species range. Molecular Biology and Evolution 35:781–791. [DOI] [PubMed] [Google Scholar]
- Yu L, Boström C, Franzenburg S, Bayer T, Dagan T, Reusch TBH. 2020. Somatic genetic drift and multilevel selection in a clonal seagrass. Nature Ecology & Evolution 4:952–962. [DOI] [PubMed] [Google Scholar]
- Zardi GI, Nicastro KR, Serrao EA, Jacinto R, Monteiro CA, Pearson GA. 2015. Closer to the rear edge: ecology and genetic diversity down the core-edge gradient of a marine macroalga. Ecosphere 6:23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw microsatellite genotype data are available from the UWA Research Repository at: 10.26182/5f0bfbc877c60.