Abstract
Purpose of review
Despite improvement in short-term renal allograft survival in recent years, renal transplant recipients (RTR) have poorer long-term allograft outcomes. Allograft function slowly declines with periods of stable function similar to natural progression of chronic kidney disease (CKD) in non-transplant population. Nearly all RTR transitions to failing renal allograft (FRG) period and require transition to dialysis. Conservative CKD management before transition to end-stage renal disease (ESRD) is an increasingly important topic; however, there is limited data in RTR regarding how to delay dialysis initiation with conservative management.
Recent findings
Since immunological and non-immunological factors unique to RTR contribute to decline in allograft function, therapies to slow progression of FRG should take both sets of factors into account. Renal replacement therapy (RRT) either incremental dialysis or re-kidney transplantation should be explored. This required taking benefits and risks of continuing immunosuppressive medications into account when allograft nephrectomy may be necessary.
Summary
FRG may benefit from various interventions to slow progression of worsening allograft function. Until there are stronger evidence to guide interventions to preserve renal function, extrapolating evidence from non-transplant patients and clinical judgement are necessary. The goal is to provide individualized care for conservative management of RTR with FRG.
Keywords: dialysis after allograft loss, failing renal allograft, immunosuppression, kidney transplantation, residual renal allograft function
1. Introduction
Kidney transplantation (KT) is a treatment of choice for an appropriate advanced chronic kidney disease (CKD) and end-stage renal disease (ESRD) [1]. After the introduction of cyclosporine (CsA) in the early 1980s, short-term renal allograft outcomes were improved [2, 3], but long-term outcomes remains poor [4]. Renal transplant recipients (RTR) will ultimately develop allograft failure with time, requiring dialysis initiation or re-initiation [5].
Allograft survival varies according to multiple factors, as such the pattern and time course deterioration of allograft function can be unpredictable. We define failing renal allograft (FRG) as “a process of progressive decline in renal allograft function after a successful kidney transplantation with a previously established baseline renal allograft function”.
Common causes of CKD and ESRD occur in kidney recipients with allograft failure (KRAF); but immunological factors are the specific causes of worsening allograft function. Table 1 summarizes common etiologies of FRG.
Table 1:
Common etiologies of failing renal allograft.
| Groups of diseases or conditions | Common diseases or conditions | |
|---|---|---|
| Non-immunological-related causes | ||
| Prerenal causes | • Systemic diseases • Reno-vascular disease |
• Orthostatic hypotension commonly in long-standing diabetes mellitus, cardio-renal syndrome |
| Intrinsic renal causes | • Recurrent native renal disease in a transplant renal allograft • Interstitial disease |
• E.g. FSGS, MGN, IgAN, LN • E.g. recurrent transplant pyelonephritis |
| Post-renal causes | • Transplant nephrolithiasis | |
| Immunological-related causes | ||
| Acute renal allograft rejection | • Unintended lowering immunosuppression • Intended lowering immunosuppression |
• Non-medication adherence • E.g. Lowering immunosuppression during over immunosuppressed stage when the following complications occur: CMV, BK, GI side effects, malignancy |
| Chronic renal allograft dysfunction or the old term “transplant glomerulopathy” | • ChronicABMR | • Untreated, inadequately treated, or even unsuccessfully treated acute renal allograft rejections leading to ongoing inflammatory process and ultimately chronic scarring |
| Immunosuppressive medications | • Chronic CNI nephrotoxicity | • Long-standing exposure to high level of immunosuppressive medications |
| Opportunistic infection | • BKVAN | • Leading to interstitial disease or post-renal obstruction |
ABMR, antibody-mediated rejection
BKVAN, BK virus-associated nephropathy
CMV, cytomegalovirus
CNI, calcineurin inhibitor
FSGS, focal segmental glomerulosclerosis
GI, gastrointestinal
IgAN, immunoglobulin A nephropathy
LN, lupus nephritis
Given the RTR undergo “for cause” allograft biopsy rather than protocol biopsy, prevalence of each cause of FRG is unclear. Moreover, transplant providers may forego renal biopsy since a biopsy of a FRG may not change management or the outcome.
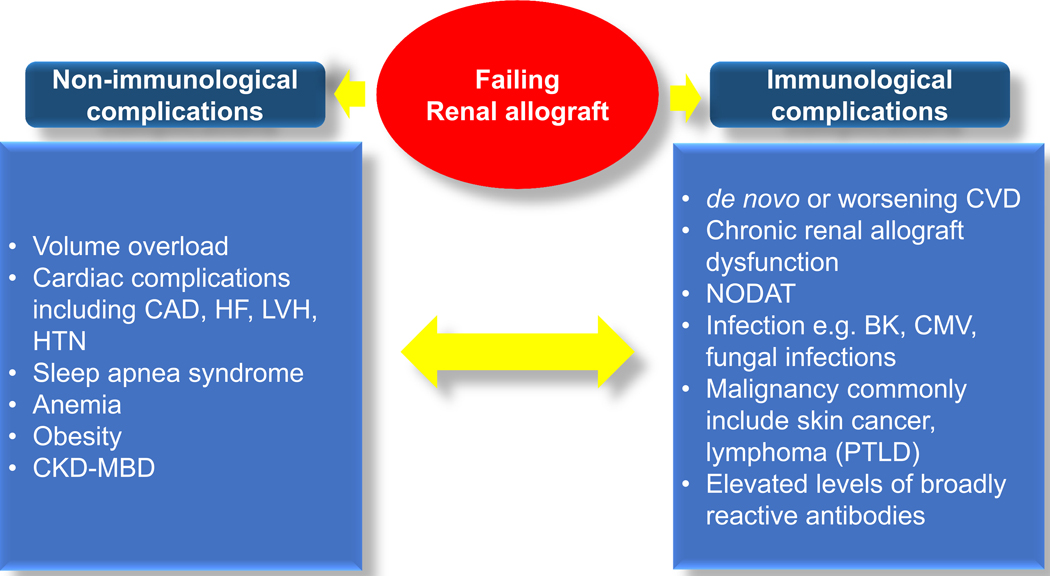
In addition to complications from worsening allograft function, the level of immunosuppression causes pathogenesis and clinical manifestations FRG, which are different from non-transplant advanced CKD patients (Figure 1).
Figure 1:
Consequences of failing renal allograft both non-immunological and immunological complications and their interconnection.
CAD, coronary artery disease
CKD-MBD, Chronic Kidney Disease-Mineral Bone Disorder
CVD, cardiovascular disease
HF, heart failure
HTN, hypertension
LVH, left ventricular hypertrophy
NODAT, new-onset diabetes after transplantation
PTLD, post-transplant lymphoproliferative disorder
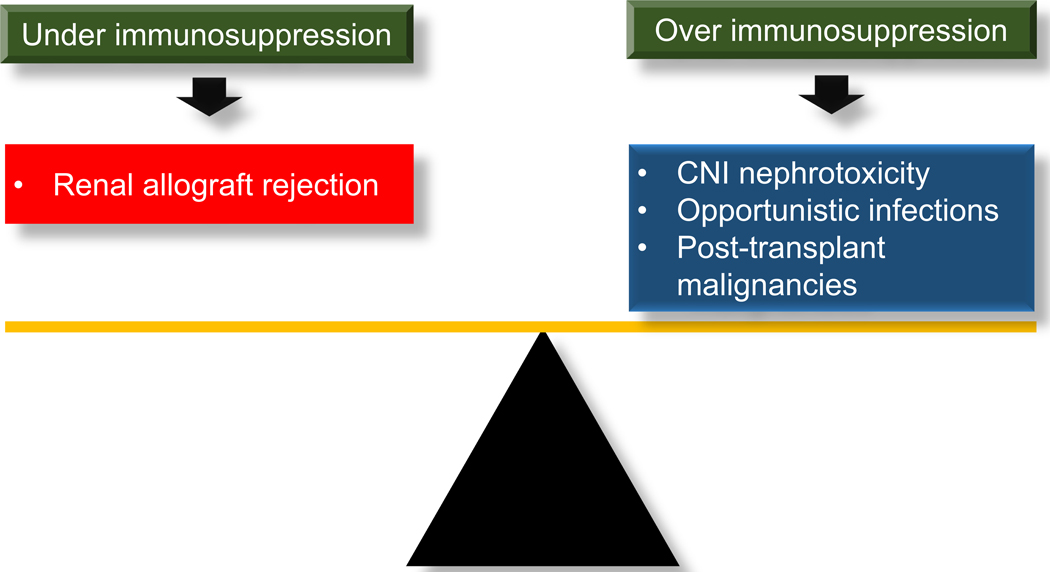
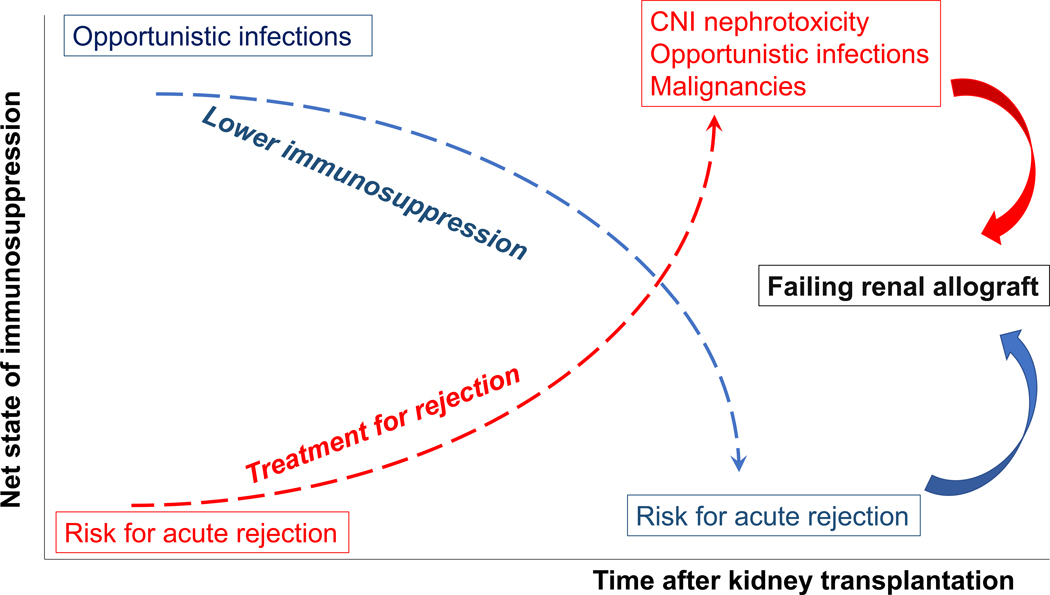
Over immunosuppression causes chronic calcineurin inhibitor (CNI) nephrotoxicity, opportunistic infections, and malignancies; whereas, under immunosuppression increases risks of acute rejection, and sensitization which decreases possibility of receiving compatible donors for subsequent KT (Figure 2). The expected clinical course after imbalance of immunosuppression in RTR will ultimately lead to FRG (Figure 3).
Figure 2:
Balance between continuing immunosuppressive medications to prevent renal allograft rejection and being at risk for complications from net stage of over immunosuppression in kidney transplant recipients with failing renal allograft
CNI, calcineurin inhibitor
Figure 3:
Common clinical course from imbalance of immunosuppression in kidney transplant recipients. Both over and under immunosuppression will ultimately lead to failing renal allograft.
In this articles, we review therapies which delay allograft function decline, and propose an approach for transiting from FRG to subsequent renal replacement therapy (RRT). Given the recent focus of delaying or preventing renal function decline, therapies that delay need for RRT, and utilizing an incremental approach to RRT are recommended in the review.
2. Therapy to slow progression of failing renal allograft
Available evidence and controversies of non-pharmacological and pharmacological approaches to slow progression of FRG are reviewed (Table 2).
Table 2:
General therapeutic interventions to slow progression of failing renal allograft by divided into non-pharmacological and pharmacological approaches
| Non-pharmacological interventions | ||
| Low dietary protein intake Plant-based protein diet | • Avoid glomerular hyperfiltration | |
| Low dietary sodium intake | • Avoid direct vascular injury or indirect injury from elevated blood pressure • Avoid glomerular hyperfiltration • Maximize effect of antihypertensive medications especially in salt-sensitive hypertension |
|
| Weight control and nutrition management | • Malnutrition-inflammation complex syndrome (MICS) • Protein energy wasting (PEW) • Reversal of the reverse epidemiology |
|
| Smoking cessation | • Acute hemodynamic e.g. elevated blood pressure and intraglomerular pressure • Chronic effects e.g. endothelial cell dysfunction |
|
| Pharmacological interventions | ||
| Antiproteinuria | • Angiotensin-converting enzyme inhibitor • Angiotensin receptor blocker • Non-dihydropyridine calcium channel blocker |
|
| Blood pressure control* | • Dihydropyridine calcium channel blocker counteracts with vasoconstrictive effect of CNI by causing renal artery vasodilation • Decrease interstitial volume by calcium channel blocker (nifedipine) |
|
| Glycemic control | • Lower severe arteriolar hyalinosis lesions | |
| Cholesterol control | • Inconclusive for renoprotective effect • Cardiovascular morbidity and mortality |
|
| Volume control | • Salt-sensitive hypertension • Decreased residual renal allograft function |
|
| Chronic Kidney Disease-Mineral Bone Disorder (CKD-MBD) management | • Calcitriol ? | |
| Post-transplant metabolic acidosis | • Correcting metabolic acidosis may not improve renal allograft function but there may improve muscle and bone health and anemia • Sodium bicarbonate • Low animal protein diet and high plant-based diet |
|
| Post-transplant anemia | • Possible protection from renal ischemia | |
| Aspirin | • Possible prevention of endothelial damage which can lead to chronic renal allograft dysfunction | |
| Anti-oxidants | • Vitamin C • Vitamin E • Nutritional antioxidants • N-acetyl-cysteine • ? Supraphysiologic dose of folate, vitamin B6, and vitamin B12 |
Antihypertensive medications should be used with low sodium diet for maximize antihypertensive effect of medications.
2.1. Non-pharmacological management
2.1.1. Low dietary protein intake
High protein intake increases renal blood flow (RBF), intraglomerular pressure, and ultimately causes increased glomerular filtration rate (GFR). This appears to be the physiologic mechanism to allow the kidneys to increase excretion of nitrogen waste products from high dietary protein [6]. This renal hemodynamic change, also known as glomerular hyperfiltration [7], may lead to glomerular injury and nephrosclerosis. These occur as a long-term consequence of higher protein intake in both non-dialysis CKD patients or person with a solitary kidney including living kidney donors [8, 9*].
The RTR with FRG are in the later stages of non–dialysis-dependent CKD (CKD-T) [10] Decreased functioning nephron mass leads to pathophysiological processes of glomerular hyperfiltration and glomerulomegaly, and ultimately secondary focal segmental glomerulosclerosis (FSGS) manifesting as proteinuria. One prospective observational study demonstrated effect of high protein intake and progression of allograft function. Patients with moderate protein (0.8 g/kg/day) and sodium (3 g/day) intake had no change in allograft function during a 12-year follow-up; whereas, allograft function declined >40% of excretion efficiency in patients with higher protein (1.4 g/kg/day) and sodium intake (5 g/day) [11].
Although there is not much evidence to confirm the advantage of low-protein diet (LPD) in RTR, from evidence of reno-protective effect of LPD in CKD patients, protein intake of 1 g/kg/day if estimated glomerular filtration rate (eGFR) >45 ml/min/1.73 m2 or albumin-to-creatinine ratio (ACR) <30 mg/g of creatinine, and 0.6–0.8 g/kg/day if eGFR <45 ml/min/1.73 m2 or ACR >30 mg/g of creatinine is suggested [12].
2.1.2. Low dietary sodium intake
High sodium intake causes renal damage directly from vascular injury and indirectly from elevated blood pressure (BP) and proteinuria. The same mechanism of renal injury is via glomerular hyperfiltration that is seen with a high protein diet.
The above-mentioned study revealed that moderate sodium intake slows progression of allograft function compared to high sodium intake. However, this effect may be attributable to lower protein intake in the former group [11].
Another study including 38 RTR participating in low sodium intake of <80 mmol/day demonstrated that systolic blood pressure (SBP), diastolic blood pressure (DBP), average and night time SBP and DBP from a 24-hour ambulatory blood pressure measurements decreased after 14 days of low dietary sodium intake; however, urinary protein excretion was unchanged [13].
Evidence of renoprotective effect of low sodium diet in RTR remains scant. We suggest sodium intake <3 g/day if eGFR >45 ml/min/1.73 m2 or ACR <30 mg/g of creatinine and <2.3 g/day if eGFR <45 ml/min/1.73 m2 or ACR >30 mg/g of creatinine [12].
2.2. Pharmacological management
2.2.1. Antiproteinuria
Proteinuria in RTR is associated with poor allograft outcomes [14–16]. Angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) lower proteinuria and slow progression of allograft function in patients with chronic allograft nephropathy (CAN) [17–19]. One retrospective observational study followed 56 patients with biopsy-proven CAN for 5 years. Compared to rate of rising serum creatinine before ACEI or ARB initiation, the rate was decreased after initiation of these medications [20]. Although there is inadequate evidence that this would prolong allograft survival in advanced allograft dysfunction in the long-term follow-ups, ACEI or ARB may be considered as antiproteinuric agents in patients with FRG unless there is side effects such as hypotension, hyperkalemia, anemia, or rising serum creatinine.
2.2.2. Blood pressure control
Hypertension is associated with poor allograft and patient outcomes [21–24]. Calcium channel blockers (CCB) appears to mitigate nephrotoxic effect of CNI from its vasodilatory property [25] and delays chronic histological changes including interstitial fibrosis in RTR taking CsA [26]; however, this protective effect of CCB in RTR with chronic renal allograft dysfunction (CGD) is unclear. Moreover, dihydropyridine CCB (DCCB) cause afferent arteriolar vasodilatation, increased intraglomerular pressure, filtration fraction, and ultimately proteinuria [27].
A systematic review with meta-analysis including 28 randomized clinical trials (RCT) showed that non-dihydropyridine CCB (NDCCB) had antiproteinuric effect in non-kidney transplant adult regardless presence or absence of diabetes [28]. Although there is no strong evidence of antiproteinuric effect of NDCCB in RTR, NDCCB allows lowering CNI dose without interfering CNI hepatic metabolism, this results in lower CNI exposure. The combination of NDCCB, low-dose CNIs, and ACEI/ARB in RTR may be considered in the setting of proteinuria.
Low sodium intake is required to maximize the effect of antihypertensive medications [13, 29]. Since high sodium intake causes increased intraglomuerular pressure and proteinuria and salt-sensitive hypertension is common in patients with a declined renal function, ACEI/ARB and NDCCB should be used with low sodium diet.
2.2.3. Chronic Kidney Disease-Mineral Bone Disorder management
Medial arterial calcification (MAC), which is a risk for cardiovascular disease (CVD), is common in CKD and ESRD [30]. After successful KT, bone-mineral metabolism derangement may return to lower values or persist depending on pre-transplant levels. Though MAC appear to be irreversible [31]. One single-center case-control study was conducted in RTR with biopsy-proven CAN or elevated serum Cr ≥0.3 mg/dL from baseline serum creatinine at transplant discharge. Patient in these groups treated with calcitriol for secondary hyperparathyroidism after KT had a significant improvement in allograft function compared to age- and gender-matched control RTR [32]. Although RTR with FRG likely require therapy to control mineral and bone metabolism abnormalities, additional evidence are required to confirm the benefit of chronic kidney disease-mineral bone disorder (CKD-MBD) therapy in the RTR population.
2.2.4. Post-transplant metabolic acidosis
In RTR with FRG, CNI both CsA and tacrolimus cause metabolic acidosis (MA) via non-CNI pathway. High protein intake may cause MA. MA alters muscle protein catabolism [33] leading to muscle protein loss, renal osteodystrophy in CKD [34], and post-transplant anemia (PTA) [35]. However, MA induces renal hypertrophy which increases GFR as a compensatory mechanism of low nephron mass [36]. It is unclear that treating MA improves allograft function. Correcting MA is a benign intervention that improves muscle and bone health, PTA, and frailty. Therefore, sodium bicarbonate may be used in RTR with FRG. Additionally, low animal protein and high plant-based diet can improve MA [37].
2.2.5. Post-transplant anemia
PTA is associated with allograft loss [38, 39]. Correction of anemia with erythropoiesis-stimulating agent (ESA) in RTR with FRG may protect renal ischemia and avoid complications of PTA. Two recent RCT showed a renoprotective effect of correcting anemia by ESA in RTR with CAN. RTR in high hemoglobin group treated with ESA to target hemoglobin of 12.5–15 g/dL had a lower rate of eGFR decline compared to patients with a low hemoglobin of 10.5–11.5 g/dL [40, 41**].
2.2.6. Aspirin
CGD includes several histological features including vascular damage, narrowing of small and glomerular vessels, interstitial fibrosis and tubular atrophy, and glomerulosclerosis [42]. These histological changes resulted from factors causing endothelial cell activation, adhesion and activation of platelets and leukocytes [43–45], which are similar in CVD. Therefore, aspirin may slow progression of CGD. A retrospective cohort study revealed that RTR receiving low-dose aspirin (100 mg/day) had a lower rate of rising serum creatinine, proteinuria, and microscopic hematuria both short- and long-term aspirin therapy (aspirin use <50% and >50% of overall graft survival time, respectively) [45]. Although, additional evidence is required, aspirin may be considered in RTR with FRG who have CVD or CV co-morbidity if there is no contraindications.
2.2.7. Anti-oxidants
Inflammation begins at the time of KT through allograft loss and continues until allograft nephrectomy [46–53]. Increased oxidative stress in CKD causes increased proinflammatory cytokines which lead to interstitial inflammation and CKD progression [54]. Oxidative stress also occurs in RTR with CAN especially from CNI use [55, 56].
Vitamin C and E, nutritional antioxidants, neutralize some effects of CsA. Additionally, antioxidants improve histological injury from CsA and renal function [57]. N-acetyl-cysteine (NAC) improves markers of oxidative stress and histologic changes from CsA in rat [58]. Although there is no enough evidence in RTR with FRG and clinical studies are required, antioxidants may be considered in these patients.
Hyperhomocystenemia is highly prevalent in ESRD patients and RTR [59, 60]. Supraphysiologic dose of folate, vitamin B6, and B12 normalizes homocysteine in chronic stable RTR and mild to moderate CKD patients; however, this treatment do not lower homocysteine in >90% of ESRD patients [61].
Since homocysteine lowering therapy is not associated with CV outcomes and all-cause mortality in CKD, ESRD, and kidney transplant patients or rate of commencement in dialysis initiation, we do not recommended for RTR with FRG [62*].
3. Preparation for subsequent kidney transplantation
Once allograft begins the FRG period, the plan should be to slow the rate of decline in allograft function, while the next RRT with dialysis or subsequent KT is being prepared. The feasibility of receiving a subsequent KT is one of the main factors that dictates the choice of RRT and immunosuppressive medication management. At this phase, immunosuppressive medication and dialysis management can be therapeutic strategies to slow progression of the FRG.
3.1. Immunosuppressive medication management during failing renal allograft
General considerations
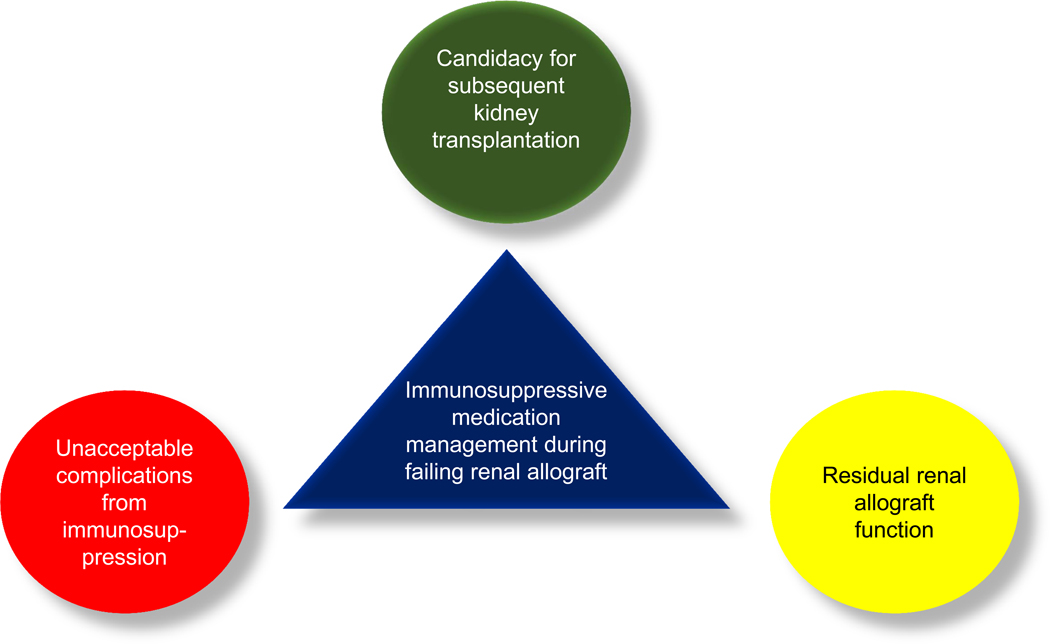
Once allograft function begins to progressively declines, three main factors that should be taken into the consideration for immunosuppressive medication management are candidacy for the next KT, potential unacceptable complications from maintaining immunosuppressive medications, and residual renal allograft function (RGF) (Figure 4).
Figure 4:
Three main factors to consider for immunosuppressive medication management during failing renal allograft
Clinical significance of residual renal allograft function
There are several benefits of preserving residual kidney function (RKF) in non-kidney transplant populations including solute clearance, volume control, reduced inflammation, and survival benefits [63*]. Table 3 summarizes possible mechanism leading to the benefits from preserving RKF including solute control [64], volume control [65–67], inflammatory reduction [68], improvement in anemia [68], nutrition [69, 70], and QoL [71].
Table 3.
Possible mechanism leading to the benefits from residual renal function in dialysis-dependent patients
| Benefits | Mechanism |
|---|---|
| Solute clearance | • B2-microglobulin • protein bound solutes |
| Volume control | • Lower ultrafiltration volumes in each hemodialysis session • Less intradialytic hypotension • Less myocardial stunning • reduction in cardiovascular mortality |
| Inflammatory reduction | • C-reactive protein and interleukin-6 |
| Anemia management | • Less anemia with less use of epoetin alpha |
| Nutritional improvement | • Better overall nutritional status • Better control of serum phosphorus. |
| Quality of life and survival | • Better quality of life |
For RTR returning to dialysis after allograft loss (DAGL), preservation of RGF is also crucial. A study using a decision analytic model (Markov model) among a theoretical cohort of patients with chronic allograft failure demonstrated survival benefit of higher RGF from maintaining immunosuppressive medications in RTR returning to peritoneal dialysis (PD) compared to those who were in FRG and withdrew immunosuppressive medications [72].
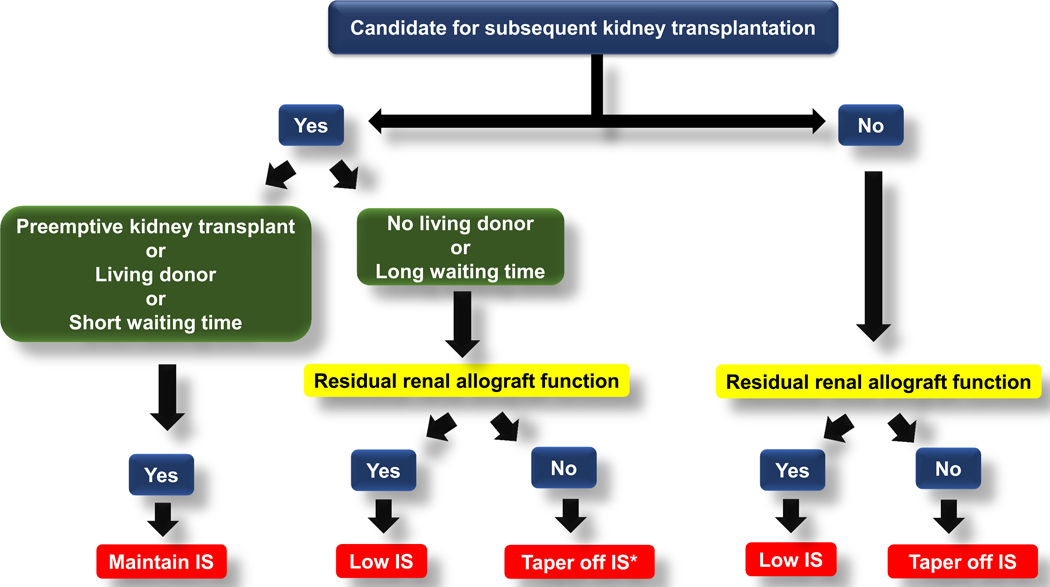
The following is a proposed immunosuppressive medication management in RTR with FRG (Figure 5).
Figure 5:
Proposed algorithm for immunosuppressive medication management in kidney transplant recipients with failing renal allograft
IS, immunosuppressive medications
• if the patients can receive KT sooner such in the case of available potential living kidney donors or being also listed in other transplant programs with a short waiting time, maintaining low does, single agent immunosuppressive medication should be considered unless their immunosuppressive medications are already tapered off
3.1.1. Candidacy for the next kidney transplantation
RTR with FRG, who are potential candidates for the subsequent KT, should maintain immunosuppressive medications at the lowest dose. The balance should aim to suppress alloreactivation and preserve RGF, while avoiding complications from immunosuppression. The anticipated time to receive the next KT and RGF can guide the appropriate level of immunosuppression.
3.1.1.1. Patients with an anticipated short waiting time
RTR with FRG who are anticipated to receive the subsequent KT soon such as those having potential living kidney donors or listed in transplant centers with a short waiting time e.g. <2 years, should continue immunosuppressive medications that adequately suppress alloreactivation regardless their RGF. However, complications from immunosuppression need to be monitored to avoid unnecessarily over immunosuppression.
3.1.1.2. Patients with an anticipated long waiting time
For RTR with FRG and low likelihood to receive subsequent KT in a short period of time, RGF can guide immunosuppressive medication management. Patients who still have RGF, immunosuppressive medications should be tapered to the lowest levels possible but can maintain RGF and avoid acute rejection in a failed allograft. This strategy avoids escalation of immunosuppression or transplant renal allograft nephrectomy. Generally, mycophenolate is first tapered off followed by steroids. CNI is then tapered to the lowest dose possible.
In patients who lose their RGF, all immunosuppressive medications should be tapered until completely off generally after 6 months of allograft loss to avoid acute rejection in a failed allograft.
The degree of sensitization at the time of FRG can also guide the strategy to taper immunosuppressive medications. Highly sensitized RTR have lower risks of further sensitization compared to non-sensitized patients because immunosuppressive medication tapering or withdrawal likely increases the possibility to broaden reactive antibodies and make non-sensitized patients become highly sensitized [73].
3.1.2. Non-candidate for the next kidney transplantation
In patients who are not a candidate for re-transplantation, further tapering of immunosuppressive medications may be a better approach.
Immunosuppressive medication management in patients who are not candidates for re-transplantation is similar to RTR with FRG and acceptable transplant candidacy, except for non-transplant candidate patients who lose their RGF, all immunosuppressive medications should be tapered until completely off after 6 months of allograft loss regardless availability of potential living kidney donors.
3.2. Dialysis care during failing renal allograft
Patients with FRG may ultimately require dialysis initiation either temporary dialysis while waiting for subsequent KT or permanent dialysis in patients who are not a candidate for the next KT. Transition care for advanced CKD patients to ESRD involves in factors contributing to outcomes such as time of dialysis initiation, type of RRT, prelude conditions and comorbidities [74]. For RTR who return to DAGL, conditions during functioning allograft and FRG periods and immunological factors contribute to outcomes post-transition to dialysis. Compared to transplant naïve HD patients, RTR who re-initiated HD after allograft loss had similar survival [75].
There are benefits of RGF, and a potential risk of a rapidly decline in RGF once dialysis is initiated. Utilizing incremental HD strategy with 1–2 times-per-week HD is one strategy to preserve RGF.
Several observational cohort studies demonstrated a slower decline in RKF in non-transplant dialysis patients having twice-weekly HD compared to those having thrice-weekly HD [76–79].
A pilot study examined the effect of oral NAC, as an antioxidant, on RKF in non-transplant incidental PD patients. After 1 months of oral NAC 1,200 mg twice daily, urine output, residual Kt/V, and residual urea and creatinine clearance were significantly increased [80]. Hence, NAC may be also considered in RTR returning to PD.
Although there is no evidence showing survival benefit of incremental dialysis in RTR returning to DAGL, preservation of RGF by incremental HD remains beneficial and should be utilized in these patients.
Dialysis modality
In transplant naïve ESRD patients, PD provides better survival during the early post-dialysis transition compared to HD, but the survival benefit does not persist in the long-term [81, 82]. For RTR returning to DAGL, both PD and HD lead to similar short- and long-term survivals. A study including 2,111 RTR who underwent DAGL demonstrated no difference in survival between patients who underwent PD and HD at early (2 years) and late (>2 years) after dialysis initiation [82].
Time to initiate dialysis after allograft loss
Mortality is not different between early and late dialysis initiations in transplant naïve patients, but more evidence showed that late dialysis initiation is associated with better outcomes. There is little evidence showing benefit of early dialysis initiation in RTR with allograft loss, but survival outcome may be worsened in some populations such as women and healthy young patients [83]. eGFR is not the best marker to determine the time of dialysis initiation since some factors such as predialysis care, late referral, dialysis dose, timing of immunosuppression reduction and RGF may contribute to outcomes after re-initiation of dialysis [84].
3.3. Transplant renal allograft nephrectomy and blood transfusion
Failed allograft is thought to be “antibody sink” that prevents a higher level of broadly reactive antibodies [73]. In CsA era, transplant nephrectomy increased panel reactive antibodies (PRA) [85, 86] especially when patients were unsensitized or nephrectomy was performed within 6 months after allograft failure [86]. One study showed that PRA levels significantly effect subsequent allograft survival regardless transplant nephrectomy of the prior failed renal allograft [87]. Although transplant nephrectomy is associated with the development of antigenicity, it did not affect rate of re-transplantation, allograft or patient survivals [85, 88].
Generally, transplant nephrectomy is indicated in case of acute complications such as allograft rejection or infection. One single study demonstrated that patients with vascular thrombosis or non-compliance were more likely to underwent nephrectomy; whereas, those with chronic rejection were less likely to require nephrectomy [88, 89].
Given its role of persistent inflammation, failed allograft may contribute to anemia and ESA resistant [90]. Apart from prior transplantation and pregnancy, blood transfusion is among the most common cause of sensitization [91]. A leukocyte-reduced blood cannot prevent sensitization [91]. Donor specific transfusion (DST) by using human leukocyte antigen (HLA)-matched blood lowers risk of sensitization. In patients with functioning allograft and maintaining immunosuppressive medications, blood transfusion was not related to increased HLA antibodies [92]. Using CsA (1 day prior through 1 week post-transfusion) [93] or azathioprine (concomitant with transfusion) [94] in failed allograft patients who are not on immunosuppressive medication was effective in lowering levels of sensitization. Graft-versus host disease can be prevented by using irradiated blood [91].
Special consideration to prevent and mitigate failing renal allograft
Apart from death with a functioning graft, chronic CNI nephrotoxicity is one of the most common causes of CGD. Minimizing CNI exposure at the early or even later stage post-transplantation is one of the strategies that have been utilized for several decades. These strategies include CNI minimization, conversion, avoidance, and withdrawal by using mammalian target of rapamycin (mTOR) inhibitor or belatacept. Systematic reviews and meta-analyses consistently demonstrated that mTOR inhibitors and belatacept are associated with favorable renal allograft function but associated higher risk of rejection [95, 96].
Tolerance induction is another elegant strategic approach to withdraw CNI and essentially all immunosuppressive medications. There are several protocols used in different research transplant centers with various populations and outcomes [97]. Although tolerance induction remains a research technique, cumulative evidences of promising outcomes provides hope in improving allograft outcomes. One must weight the potential infectious and hematological risks from possible over immunosuppression from the tolerance induction protocols, however.
Conclusion
Care for RTR with FRG is complex and both immunological and non-immunological factors are required. Several therapeutic strategies are not from strong evidence and some are extrapolated from non-transplant CKD or ESRD patients. Preparation for the next RRT either dialysis or subsequent KT should be planned by adjusting immunosuppressive medications as per individual conditions. The goal is to maximize the possibility of receiving subsequent KT and avoid complications from unnecessarily over immunosuppression [98*]. Lastly, despite strategies to slow progression of FRG may delay worsening allograft function, the ultimate outcome is allograft loss. Patients should be counseled and those who are candidates for a subsequent KT, should be referred to transplant center early. Apart from medical consideration, psychological management is also required since successful therapy for FRG depends heavily on the patients’ cooperation. Transplant teams, primary nephrologists, and primary care physicians need to collaborate in the care for such these complex patients.
Key points.
Long-term allograft outcomes remains poor from CGD secondary to various pathogenesis and risk factors.
FRG is a continuous process and involves in both immunological and non-immunological factors.
Several therapeutic interventions to slow FRG should be an integration of both non-pharmacological and pharmacological approaches.
Preparation for the next RRT either dialysis or re-KT for RTR with FRG should be planned and individualized to provide appropriate immunosuppressive medication management, maximize possibility of the subsequent KT, and avoid complications from unnecessarily over immunosuppression.
CNI minimization or withdrawal either by using mTOR inhibitor or belatacept and tolerance induction can avoid effect of CNI nephrotoxicity, but increased risk for rejection or complications from tolerance induction protocols should be considered.
Acknowledgements:
Authors would like to thank our kidney transplant patients to motivate us to research and expand our knowledge in the field of kidney transplantation during failing renal allograft.
Financial support and sponsorship:
Supported by research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health K24-DK091419 and philanthropic grants from Mr. Louis Chang and Dr Joseph Lee.
Conflicts of interest:
KKZ has received honoraria and/or grants from Abbott, Abbvie, Alexion, Amgen, DaVita, Fresenius, Genzyme, Keryx, Otsuka, Shire, Rockwell, and Vifor, the manufacturers of drugs or devices and/or providers of services for CKD patients. KKZ serves as a physician in a US Department of Veterans Affairs medical centers with part-compensation and is a part-time employees of a US Department of Veterans Affairs medical centers. Opinions expressed in this paper are those of the authors’ and do not represent the official opinion of the US Department of Veterans Affairs. RMH is a paid consultant and a member of the 2019–2020 speaker’s bureau for Alexion pharmaceuticals for eculizumab (Soliris) and Ravulizumab (Ultomiris).
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- ACR
albumin-to-creatinine ratio
- ARB
angiotensin receptor blocker
- BP
blood pressure
- CAN
chronic allograft nephropathy
- CCB
calcium channel blocker
- CGD
chronic renal allograft dysfunction
- CKD
chronic kidney disease
- CKD-MBD
chronic kidney disease-mineral and bone disorder
- CKD-T
non–dialysis-dependent CKD
- CNI
calcineurin inhibitor
- CsA
cyclosporine A
- CVD
cardiovascular disease
- DAGL
dialysis after allograft loss
- DBP
diastolic blood pressure
- DCCB
dihydropyridine calcium channel blocker
- DST
donor specific transfusion
- GFR
glomerular filtration rate
- eGFR
estimated glomerular filtration rate
- ESA
erythropoiesis-stimulating agent
- ESRD
end-stage renal disease
- FRG
failing renal allograft
- FSGS
focal segmental glomerulosclerosis
- GFR
glomerular filtration rate
- HLA
human leukocyte antigen
- KRAF
kidney recipients with allograft failure
- KT
kidney transplantation
- LPD
low-protein diet
- MA
metabolic acidosis
- MAC
medial arterial calcification
- mTOR
mammalian target of rapamycin
- NAC
N-acetyl-cysteine
- NDCCB
nondihydropyridine calcium channel blocker
- PD
peritoneal dialysis
- PRA
panel reactive antibody
- PTA
post-transplant anemia
- QoL
quality of life
- RBF
renal blood flow
- RCT
randomized clinical trial
- RGF
residual renal allograft function
- RKF
residual kidney function
- RRT
renal replacement therapy
- RTR
renal transplant recipient
- SBP
systolic blood pressure
References:
- 1.Suthanthiran M, Strom TB. Renal transplantation. N Engl J Med. 1994;331(6):365–76. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Klintmalm GB, Weil R 3rd, et al. Cyclosporin A and steroid therapy in sixty-six cadaver kidney recipients. Surg Gynecol Obstet. 1981;153(4):486–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Terasaki PI, Cecka JM, Gjertson DW, et al. A ten-year prediction for kidney transplant survival. Clin Transpl. 1992:501–12. [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4(3):378–83. [DOI] [PubMed] [Google Scholar]
- 5.Perl J Kidney transplant failure: failing kidneys, failing care? Clin J Am Soc Nephrol. 2014;9(7):1153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouque D, Aparicio M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat Clin Pract Nephrol. 2007;3(7):383–92. [DOI] [PubMed] [Google Scholar]
- 7.Ko GJ, Obi Y, Tortorici AR, Kalantar-Zadeh K. Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2017;20(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Moore LW, Tortorici AR, et al. North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016;17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tantisattamo E, Dafoe DC, Reddy UG, et al. Current Management of Patients With Acquired Solitary Kidney. Kidney Int Rep. 2019;4(9):1205–18.* This is a review pathophysiology of glomerular hyperfiltration as a cosequence of acquired solitary kidney in human mainly focusing on living kidney donation as well as care for these patients.
- 10.National Kidney F KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–86. [DOI] [PubMed] [Google Scholar]
- 11.Bernardi A, Biasia F, Pati T, et al. Long-term protein intake control in kidney transplant recipients: effect in kidney graft function and in nutritional status. Am J Kidney Dis. 2003;41(3 Suppl 1):S146–52. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Fouque D. Nutritional Management of Chronic Kidney Disease. N Engl J Med. 2017;377(18):1765–76. [DOI] [PubMed] [Google Scholar]
- 13.Soypacaci Z, Sengul S, Yildiz EA, et al. Effect of daily sodium intake on post-transplant hypertension in kidney allograft recipients. Transplant Proc. 2013;45(3):940–3. [DOI] [PubMed] [Google Scholar]
- 14.First MR, Vaidya PN, Maryniak RK, et al. Proteinuria following transplantation. Correlation with histopathology and outcome. Transplantation. 1984;38(6):607–12. [PubMed] [Google Scholar]
- 15.Vathsala A, Verani R, Schoenberg L, et al. Proteinuria in cyclosporine-treated renal transplant recipients. Transplantation. 1990;49(1):35–41. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Fresnedo G, Plaza JJ, Sanchez-Plumed J, et al. Proteinuria: a new marker of long-term graft and patient survival in kidney transplantation. Nephrol Dial Transplant. 2004;19 Suppl 3:iii47–51. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Valeri AM, Markowitz GS, et al. Angiotensin converting enzyme inhibition in chronic allograft nephropathy. Transplantation. 2002;73(5):783–8. [DOI] [PubMed] [Google Scholar]
- 18.Inigo P, Campistol JM, Saracho R, et al. Renoprotective effects of losartan in renal transplant recipients. Results of a retrospective study. Nephron Clin Pract. 2003;95(3):c84–90. [DOI] [PubMed] [Google Scholar]
- 19.Muirhead N, House A, Hollomby DJ, Jevnikar AM. Effect of valsartan on urinary protein excretion and renal function in patients with chronic renal allograft nephropathy. Transplant Proc. 2003;35(7):2412–4. [DOI] [PubMed] [Google Scholar]
- 20.Zaltzman JS, Nash M, Chiu R, Prasad R. The benefits of renin-angiotensin blockade in renal transplant recipients with biopsy-proven allograft nephropathy. Nephrol Dial Transplant. 2004;19(4):940–4. [DOI] [PubMed] [Google Scholar]
- 21.Rigatto C, Foley R, Jeffery J, et al. Electrocardiographic left ventricular hypertrophy in renal transplant recipients: prognostic value and impact of blood pressure and anemia. J Am Soc Nephrol. 2003;14(2):462–8. [DOI] [PubMed] [Google Scholar]
- 22.Mange KC, Feldman HI, Joffe MM, et al. Blood pressure and the survival of renal allografts from living donors. J Am Soc Nephrol. 2004;15(1):187–93. [DOI] [PubMed] [Google Scholar]
- 23.de Vries AP, Bakker SJ, van Son WJ, et al. Metabolic syndrome is associated with impaired long-term renal allograft function; not all component criteria contribute equally. Am J Transplant. 2004;4(10):1675–83. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Fresnedo G, Escallada R, Martin de Francisco AL, et al. Association between pulse pressure and cardiovascular disease in renal transplant patients. Am J Transplant. 2005;5(2):394–8. [DOI] [PubMed] [Google Scholar]
- 25.Merkus JW, Hilbrands LB, Hoitsma AJ, et al. Haemodynamic changes in human kidney allografts following administration of nifedipine: assessment with Doppler spectrum analysis. Transpl Int. 1992;5 Suppl 1:S17–20. [DOI] [PubMed] [Google Scholar]
- 26.McCulloch TA, Harper SJ, Donnelly PK, et al. Influence of nifedipine on interstitial fibrosis in renal transplant allografts treated with cyclosporin A. J Clin Pathol. 1994;47(9):839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inigo P, Campistol JM, Lario S, et al. Effects of losartan and amlodipine on intrarenal hemodynamics and TGF-beta(1) plasma levels in a crossover trial in renal transplant recipients. J Am Soc Nephrol. 2001;12(4):822–7. [DOI] [PubMed] [Google Scholar]
- 28.Bakris GL, Weir MR, Secic M, et al. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004;65(6):1991–2002. [DOI] [PubMed] [Google Scholar]
- 29.van den Berg E, Geleijnse JM, Brink EJ, et al. Sodium intake and blood pressure in renal transplant recipients. Nephrol Dial Transplant. 2012;27(8):3352–9. [DOI] [PubMed] [Google Scholar]
- 30.Abou-Hassan N, Tantisattamo E, D’Orsi ET, O’Neill WC. The clinical significance of medial arterial calcification in end-stage renal disease in women. Kidney Int. 2015;87(1):195–9. [DOI] [PubMed] [Google Scholar]
- 31.Tantisattamo E, Han KH, O’Neill WC. Increased vascular calcification in patients receiving warfarin. Arterioscler Thromb Vasc Biol. 2015;35(1):237–42. [DOI] [PubMed] [Google Scholar]
- 32.O’Herrin JK, Hullett DA, Heisey DM, et al. A retrospective evaluation of 1,25-dihydroxyvitamin D(3) and its potential effects on renal allograft function. Am J Nephrol. 2002;22(5–6):515–20. [DOI] [PubMed] [Google Scholar]
- 33.Franch HA, Mitch WE. Catabolism in uremia: the impact of metabolic acidosis. J Am Soc Nephrol. 1998;9(12 Suppl):S78–81. [PubMed] [Google Scholar]
- 34.Coen G, Mazzaferro S, Ballanti P, et al. Renal bone disease in 76 patients with varying degrees of predialysis chronic renal failure: a cross-sectional study. Nephrol Dial Transplant. 1996;11(5):813–9. [DOI] [PubMed] [Google Scholar]
- 35.Yorgin PD, Scandling JD, Belson A, et al. Late post-transplant anemia in adult renal transplant recipients. An under-recognized problem? Am J Transplant. 2002;2(5):429–35. [DOI] [PubMed] [Google Scholar]
- 36.Preisig P A cell cycle-dependent mechanism of renal tubule epithelial cell hypertrophy. Kidney Int. 1999;56(4):1193–8. [DOI] [PubMed] [Google Scholar]
- 37.van den Berg E, Engberink MF, Brink EJ, et al. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol. 2012;7(11):1811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molnar MZ, Czira M, Ambrus C, et al. Anemia is associated with mortality in kidney-transplanted patients--a prospective cohort study. Am J Transplant. 2007;7(4):818–24. [DOI] [PubMed] [Google Scholar]
- 39.Schjelderup P, Dahle DO, Holdaas H, et al. Anemia is a predictor of graft loss but not cardiovascular events and all-cause mortality in renal transplant recipients: follow-up data from the ALERT study. Clin Transplant. 2013;27(6):E636–43. [DOI] [PubMed] [Google Scholar]
- 40.Choukroun G, Kamar N, Dussol B, et al. Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol. 2012;23(2):360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsujita M, Kosugi T, Goto N, et al. The effect of maintaining high hemoglobin levels on long-term kidney function in kidney transplant recipients: a randomized controlled trial. Nephrol Dial Transplant. 2018.** A open label multi-center randomized clinical trial that confirms the potential renoprotective of targeting hemoglobin by using erythropoietin-stimulating agent to lowering progression of renal allograft dysfunction by in kidney transplant recipients with chronic allograft dysfunction.
- 42.Jindal RM, Hariharan S. Chronic rejection in kidney transplants. An in-depth review. Nephron. 1999;83(1):13–24. [DOI] [PubMed] [Google Scholar]
- 43.Denton MD, Davis SF, Baum MA, et al. The role of the graft endothelium in transplant rejection: evidence that endothelial activation may serve as a clinical marker for the development of chronic rejection. Pediatr Transplant. 2000;4(4):252–60. [DOI] [PubMed] [Google Scholar]
- 44.Labarrere CA, Lee JB, Nelson DR, et al. C-reactive protein, arterial endothelial activation, and development of transplant coronary artery disease: a prospective study. Lancet. 2002;360(9344):1462–7. [DOI] [PubMed] [Google Scholar]
- 45.Grotz W, Siebig S, Olschewski M, et al. Low-dose aspirin therapy is associated with improved allograft function and prolonged allograft survival after kidney transplantation. Transplantation. 2004;77(12):1848–53. [DOI] [PubMed] [Google Scholar]
- 46.Cottone S, Palermo A, Vaccaro F, et al. Inflammation and endothelial activation are linked to renal function in long-term kidney transplantation. Transpl Int. 2007;20(1):82–7. [DOI] [PubMed] [Google Scholar]
- 47.Bakri RS, Afzali B, Covic A, et al. Cardiovascular disease in renal allograft recipients is associated with elevated sialic acid or markers of inflammation. Clin Transplant. 2004;18(2):201–4. [DOI] [PubMed] [Google Scholar]
- 48.Varagunam M, Finney H, Trevitt R, et al. Pretransplantation levels of C-reactive protein predict all-cause and cardiovascular mortality, but not graft outcome, in kidney transplant recipients. Am J Kidney Dis. 2004;43(3):502–7. [DOI] [PubMed] [Google Scholar]
- 49.Ducloux D, Kazory A, Chalopin JM. Predicting coronary heart disease in renal transplant recipients: a prospective study. Kidney Int. 2004;66(1):441–7. [DOI] [PubMed] [Google Scholar]
- 50.Winkelmayer WC, Lorenz M, Kramar R, et al. C-reactive protein and body mass index independently predict mortality in kidney transplant recipients. Am J Transplant. 2004;4(7):1148–54. [DOI] [PubMed] [Google Scholar]
- 51.Winkelmayer WC, Schaeffner ES, Chandraker A, et al. A J-shaped association between high-sensitivity C-reactive protein and mortality in kidney transplant recipients. Transpl Int. 2007;20(6):505–11. [DOI] [PubMed] [Google Scholar]
- 52.Abedini S, Holme I, Marz W, et al. Inflammation in renal transplantation. Clin J Am Soc Nephrol. 2009;4(7):1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez-Gomez JM, Perez-Flores I, Jofre R, et al. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol. 2004;15(9):2494–501. [DOI] [PubMed] [Google Scholar]
- 54.Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004;13(1):93–9. [DOI] [PubMed] [Google Scholar]
- 55.Campise M, Bamonti F, Novembrino C, et al. Oxidative stress in kidney transplant patients. Transplantation. 2003;76(10):1474–8. [DOI] [PubMed] [Google Scholar]
- 56.Raj DS, Lim G, Levi M, et al. Advanced glycation end products and oxidative stress are increased in chronic allograft nephropathy. Am J Kidney Dis. 2004;43(1):154–60. [DOI] [PubMed] [Google Scholar]
- 57.Parra Cid T, Conejo Garcia JR, Carballo Alvarez F, de Arriba G. Antioxidant nutrients protect against cyclosporine A nephrotoxicity. Toxicology. 2003;189(1–2):99–111. [DOI] [PubMed] [Google Scholar]
- 58.Tariq M, Morais C, Sobki S, et al. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant. 1999;14(4):923–9. [DOI] [PubMed] [Google Scholar]
- 59.Bostom AG, Lathrop L. Hyperhomocysteinemia in end-stage renal disease: prevalence, etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int. 1997;52(1):10–20. [DOI] [PubMed] [Google Scholar]
- 60.Friedman AN, Rosenberg IH, Selhub J, et al. Hyperhomocysteinemia in renal transplant recipients. Am J Transplant. 2002;2(4):308–13. [DOI] [PubMed] [Google Scholar]
- 61.Shemin D, Bostom AG, Selhub J. Treatment of hyperhomocysteinemia in end-stage renal disease. Am J Kidney Dis. 2001;38(4 Suppl 1):S91–4. [DOI] [PubMed] [Google Scholar]
- 62.Rangaswami J, Mathew RO, Parasuraman R, et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant. 2019;34(5):760–73.* This articles demonstrates all common cardiovascular diseases in kidney transplant recipients with current available evidence especially management for each cardiovascular diseases.
- 63.Mathew AT, Obi Y, Rhee CM, et al. Incremental dialysis for preserving residual kidney function-Does one size fit all when initiating dialysis? Semin Dial. 2018;31(4):343–52.* This article provides extensive review topics related to residual kidney function and incremental dialysis in non-kidney transplant patients.).
- 64.Marquez IO, Tambra S, Luo FY, et al. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol. 2011;6(2):290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dorairajan S, Chockalingam A, Misra M. Myocardial stunning in hemodialysis: what is the overall message? Hemodial Int. 2010;14(4):447–50. [DOI] [PubMed] [Google Scholar]
- 66.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4(12):1925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shafi T, Jaar BG, Plantinga LC, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suda T, Hiroshige K, Ohta T, et al. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant. 2000;15(3):396–401. [DOI] [PubMed] [Google Scholar]
- 70.Penne EL, van der Weerd NC, Grooteman MP, et al. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(2):281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Termorshuizen F, Korevaar JC, Dekker FW, et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis. 2003;41(6):1293–302. [DOI] [PubMed] [Google Scholar]
- 72.Jassal SV, Lok CE, Walele A, Bargman JM. Continued transplant immunosuppression may prolong survival after return to peritoneal dialysis: results of a decision analysis. Am J Kidney Dis. 2002;40(1):178–83. [DOI] [PubMed] [Google Scholar]
- 73.Andrews PA, Standards Committee of the British Transplantation S. Summary of the British Transplantation Society Guidelines for Management of the Failing Kidney Transplant. Transplantation. 2014;98(11):1130–3. [DOI] [PubMed] [Google Scholar]
- 74.Kalantar-Zadeh K, Kovesdy CP, Streja E, et al. Transition of care from pre-dialysis prelude to renal replacement therapy: the blueprints of emerging research in advanced chronic kidney disease. Nephrol Dial Transplant. 2017;32(suppl_2):ii91-ii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varas J, Perez-Saez MJ, Ramos R, et al. Returning to haemodialysis after kidney allograft failure: a survival study with propensity score matching. Nephrol Dial Transplant. 2019;34(4):667–72. [DOI] [PubMed] [Google Scholar]
- 76.Lin YF, Huang JW, Wu MS, et al. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrology (Carlton). 2009;14(1):59–64. [DOI] [PubMed] [Google Scholar]
- 77.Fernandez-Lucas M, Teruel-Briones JL, Gomis-Couto A, et al. Maintaining residual renal function in patients on haemodialysis: 5-year experience using a progressively increasing dialysis regimen. Nefrologia. 2012;32(6):767–76. [DOI] [PubMed] [Google Scholar]
- 78.Zhang M, Wang M, Li H, et al. Association of initial twice-weekly hemodialysis treatment with preservation of residual kidney function in ESRD patients. Am J Nephrol. 2014;40(2):140–50. [DOI] [PubMed] [Google Scholar]
- 79.Caria S, Cupisti A, Sau G, Bolasco P. The incremental treatment of ESRD: a low-protein diet combined with weekly hemodialysis may be beneficial for selected patients. BMC Nephrol. 2014;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldman L, Shani M, Efrati S, et al. N-acetylcysteine improves residual renal function in peritoneal dialysis patients: a pilot study. Perit Dial Int. 2011;31(5):545–50. [DOI] [PubMed] [Google Scholar]
- 81.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20(1):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perl J, Hasan O, Bargman JM, et al. Impact of dialysis modality on survival after kidney transplant failure. Clin J Am Soc Nephrol. 2011;6(3):582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molnar MZ, Ichii H, Lineen J, et al. Timing of return to dialysis in patients with failing kidney transplants. Semin Dial. 2013;26(6):667–74. [DOI] [PubMed] [Google Scholar]
- 84.Molnar MZ, Ojo AO, Bunnapradist S, et al. Timing of dialysis initiation in transplant-naive and failed transplant patients. Nat Rev Nephrol. 2012;8(5):284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Douzdjian V, Rice JC, Carson RW, et al. Renal retransplants: effect of primary allograft nephrectomy on early function, acute rejection and outcome. Clin Transplant. 1996;10(2):203–8. [PubMed] [Google Scholar]
- 86.Khakhar AK, Shahinian VB, House AA, et al. The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcome. Transplant Proc. 2003;35(2):862–3. [DOI] [PubMed] [Google Scholar]
- 87.Ahmad N, Ahmed K, Mamode N. Does nephrectomy of failed allograft influence graft survival after re-transplantation? Nephrol Dial Transplant. 2009;24(2):639–42. [DOI] [PubMed] [Google Scholar]
- 88.Kassakian CT, Ajmal S, Gohh RY, et al. Immunosuppression in the failing and failed transplant kidney: optimizing outcomes. Nephrol Dial Transplant. 2016;31(8):1261–9. [DOI] [PubMed] [Google Scholar]
- 89.Ajmal S, Bayliss GP, Machan JT, et al. Impact of transplant nephrectomy on HLA-sensitization and re-transplantation. J Am Soc Nephrol 2013; 24: 598A. [Google Scholar]
- 90.Sibbel SP, Koro CE, Brunelli SM, Cobitz AR. Characterization of chronic and acute ESA hyporesponse: a retrospective cohort study of hemodialysis patients. BMC Nephrol. 2015;16:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scornik JC, Meier-Kriesche HU. Blood transfusions in organ transplant patients: mechanisms of sensitization and implications for prevention. Am J Transplant. 2011;11(9):1785–91. [DOI] [PubMed] [Google Scholar]
- 92.Scornik JC, Schold JD, Bucci M, Meier-Kriesche HU. Effects of blood transfusions given after renal transplantation. Transplantation. 2009;87(9):1381–6. [DOI] [PubMed] [Google Scholar]
- 93.Cheigh JS, Suthanthiran M, Fotino M, et al. Minimal sensitization and excellent renal allograft outcome following donor-specific blood transfusion with a short course of cyclosporine. Transplantation. 1991;51(2):378–81. [DOI] [PubMed] [Google Scholar]
- 94.Flye MW, Burton K, Mohanakumar T, et al. Donor-specific transfusions have long-term beneficial effects for human renal allografts. Transplantation. 1995;60(12):1395–401. [DOI] [PubMed] [Google Scholar]
- 95.Sawinski D, Trofe-Clark J, Leas B, et al. Calcineurin Inhibitor Minimization, Conversion, Withdrawal, and Avoidance Strategies in Renal Transplantation: A Systematic Review and Meta-Analysis. Am J Transplant. 2016;16(7):2117–38. [DOI] [PubMed] [Google Scholar]
- 96.Kumar J, Reccia I, Kusano T, et al. Systemic meta-analysis assessing the short term applicability of early conversion to mammalian target of rapamycin inhibitors in kidney transplant. World J Transplant. 2017;7(2):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Traitanon O, Gallon L. Chimerism and tolerance induction in kidney transplantation. Nephron. 2015;129(1):34–8. [DOI] [PubMed] [Google Scholar]
- 98.Dafoe DC, Tantisattamo E, Reddy U. Precision Medicine and Personalized Approach to Renal Transplantation. Semin Nephrol. 2018;38(4):346–54.* This article reviews a relevant and practicle point for caring kidney transplant recipient by emphasize an individualized and presice practice.