Abstract
Pheochromocytomas, arising from chromaffin cells, produce catecholamines, epinephrine and norepinephrine. The tumor biochemical phenotype is defined by which of these exerts the greatest influence on the cardiovascular system when released into circulation in high amounts. Action on the heart and vasculature can cause potentially lethal arrhythmias, often in the setting of comorbid blood pressure derangements. In a review of electrocardiograms obtained on pheochromocytoma patients (n = 650) treated at our institution over the last decade, severe and refractory sinus tachycardia, atrial fibrillation, and ventricular tachycardia were found to be the most common or life-threatening catecholamine-induced tachyarrhythmias. These arrhythmias, arising from catecholamine excess rather than from a primary electrophysiologic substrate, require special considerations for treatment and complication avoidance. Understanding the synthesis and release of catecholamines, the adrenoceptors catecholamines bind to, and the cardiac and vascular response to epinephrine and norepinephrine underlies optimal management in catecholamine-induced tachyarrhythmias.
Keywords: blood pressure, catecholamines, pheochromocytoma, sinus tachycardia, tachyarrhythmias, ventricular tachycardia
Cardiac complications, including serious arrhythmias and blood pressure (BP) derangements, occur in patients with tumors arising from chromaffin cells of the adrenal medulla and extra-adrenal sympathetic paraganglia (1), collectively referred to as pheochromocytomas. These produce excess catecholamines, particularly epinephrine (EPI) and norepinephrine (NE) with continuous or episodic release (2). Here, we focus on arrhythmias due to catecholamine excess in pheochromocytomas.
Although there are a variety of clinical manifestations of pheochromocytoma, cardiovascular manifestations, arrhythmias, and BP derangements account for 71% of the mortality (3–6). Arrhythmias with pheochromocytoma, with sinus tachycardia (ST) being the most common (7,8). Excluding ST, 1 study found arrhythmias in 10% of patients with pheochromocytoma (6). Of these patients, atrial fibrillation was the most common (60%), followed by bradyarrhythmias (20%) and ventricular tachycardia (13%); the remaining patients had unspecified supraventricular tachycardias (6). In total, 90% of patients with pheochromocytoma have hypertension; 75% of these patients have paroxysms of hypertension at least once a week (7).
We retrospectively reviewed patients seen at our institution from 2004 to 2019 and found that 650 patients had electrocardiograms. In our patient set, 71 (10.9%) patients experienced tachyarrhythmia. Of these, 70 of 71 (98.6%) at one time experienced ST (heart rate [HR] greater the 100 beats/min in normal sinus rhythm), 8 of 71 (11.3%) atrial fibrillation, 4 of 71 (5.6%) atrial flutter; and 3 of 71 (4.2%) ventricular tachycardia (VT). Of the patients with VT, none had structural heart disease. Of the patients with atrial fibrillation/flutter, all but 1 patient had a prior history and all but 1 were anticoagulated. None of these patients had recurrence of their arrhythmia after appropriate surgical and/or pharmacological treatment. These tachyarrhythmias occurred despite treatment with standard ß-adrenoceptor blocking agents.
Management of catecholamine-induced tachyarrhythmias, which are common and sometimes refractory to conventional medical therapy, is complex; without intervention, these can deteriorate to cardiac arrest (9). Treatment of HR in the state of catecholamine excess alters BP significantly; its treatment failure can lead to serious complications including hypertensive crises, hypotensive shock, and death (10–14).
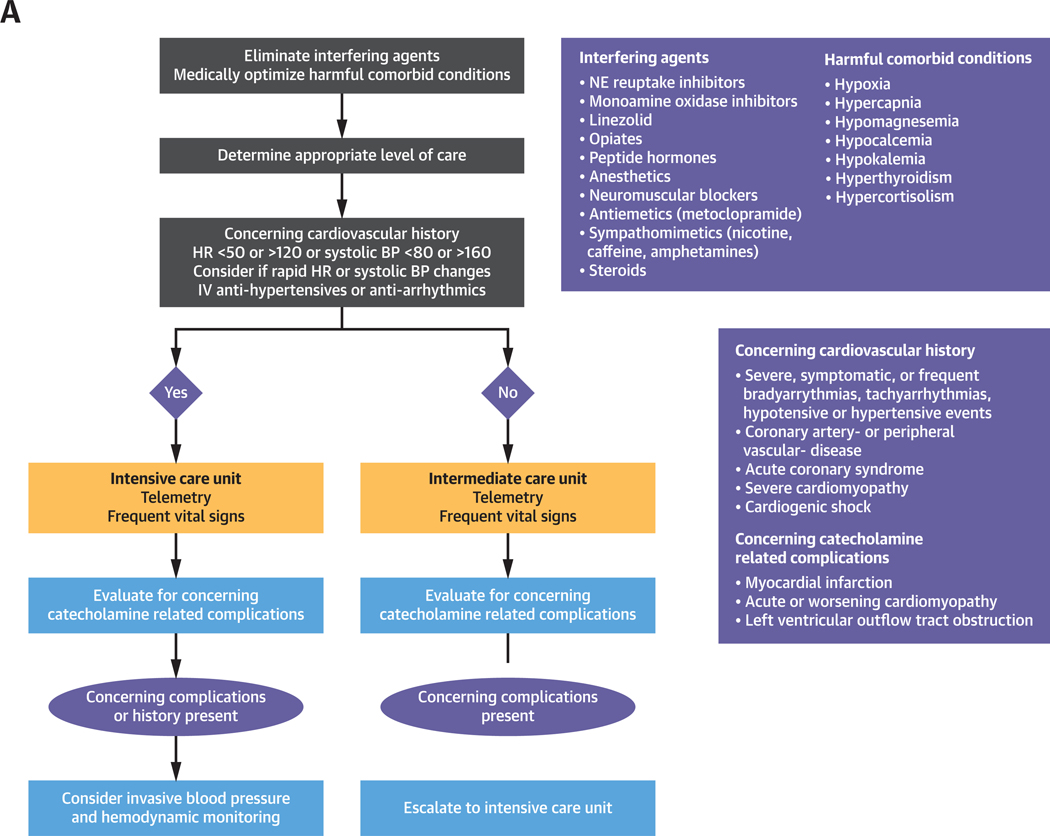
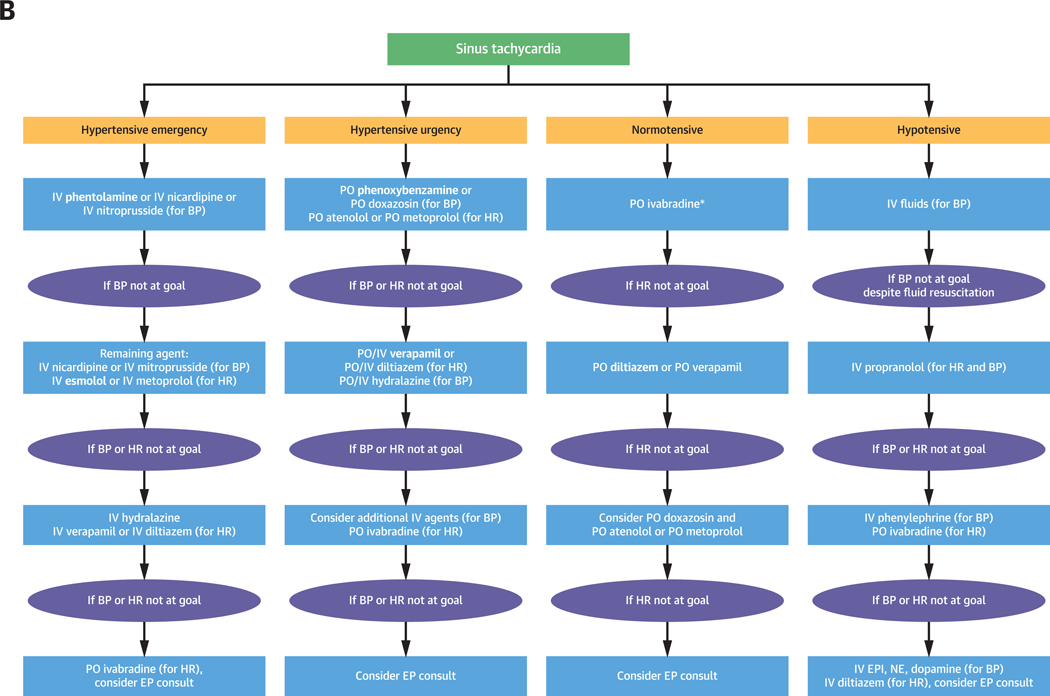
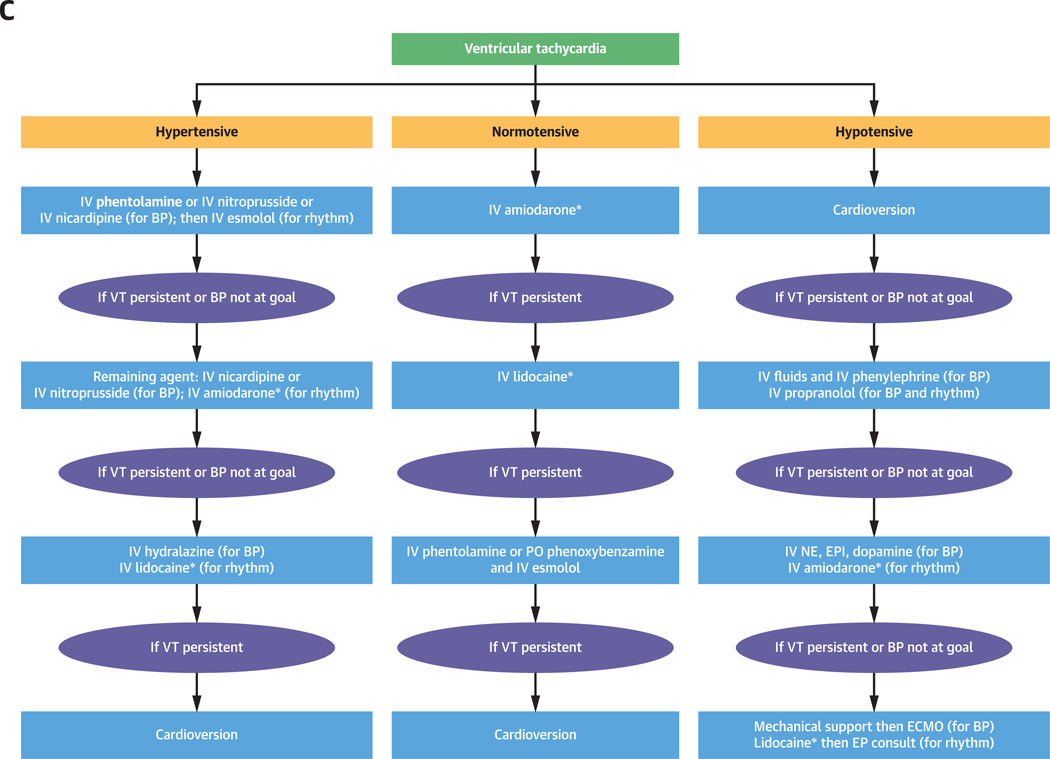
Here, we describe the management of catecholamine-induced tachyarrhythmias and comorbid BP derangements (Figures 1A to 1C). This review is timely given newly published European guidelines for the management of patients with supraventricular tachycardia (15), which call for changes in conventional management of tachyarrhythmias and emphasize the use of ß-adrenoceptor blocking agents and ivabradine in ST treatment (15). Given these cardiovascular complications and the complexity of their management, a cardiologist is essential in management, especially in light of new treatment guidelines for arrhythmia.
FIGURE 1. Management of Catecholamine-Induced Tachyarrhythmias.
General management of catecholamine-induced tachyarrhythmias (A), management of ST (B), and VT (C). Refer to text for elaboration. BP = blood pressure; EP = electrophysiologist; EPI = epinephrine; HR = heart rate; IV = intravenous; NE = norepinephrine; PO = oral; VT = ventricular tachycardia.
*If ivabradine is unavailable or too expensive, agents like diltiazem, verapamil, and metoprolol or atenolol after α-adrenoceptor blockade can be used but beware of hypotension
*Sotalol, propafenone and procainamide can also be used as add on agents in the management of ventricular tachycardia
CLINICAL SCENARIOS: THE PATIENT
Identifying the biochemical phenotype of a pheochromocytoma, defined by catecholamine production/release and resultant cardiovascular response, is an important first step in evaluating catecholamine-induced tachyarrhythmias. These phenotypes—noradrenergic, adrenergic, or mixed—determine the frequency of arrhythmias, BP derangements, and the risk to a given patient. We present 2 prototypical cases (Case 1 and 2) illustrating the different clinical presentations of 2 biochemical phenotypes. These cases illustrate pertinent aspects of catecholamine physiology relevant to management.
CASE 1 (NORADRENERGIC PHENOTYPE).
A middle-aged man with a history of metastatic extra-adrenal pheochromocytoma presents to the emergency department with frequent palpitations, persistent headache, nausea, and vomiting. BP and HR at presentation were 160/108 mm Hg and 124 beats/min (ST), respectively. Two weeks prior, his laboratory results showed elevated serum norepinephrine of 1,404 pg/ml (range 80 to 499 pg/ml), normetanephrine of 1,148 pg/ml (range 18 to 112 pg/ml), epinephrine of 14 pg/ml (range 0 to 57 pg/ml), and metanephrine of 56 pg/ml (range 12 to 61 pg/ml). He was given 2 mg of doxazosin once daily and atenolol 12.5 mg twice daily, admitted to the telemetry care unit, and given metoclopramide 10 mg orally (PO) for nausea and labetalol 200 mg PO for hypertension. BP and HR improved to 151/89 mm Hg and 110 beats/min, respectively. After several hours, BP remained high due to provision of the catecholamine-releasing agent metoclopramide (16,17), which was discontinued.
Labetalol was also discontinued, and intravenous (IV) diltiazem was titrated from 5 to 10 mg/h. BP and HR improved to 130/85 mm Hg and 88 beats/min, respectively.
CASE 2 (ADRENERGIC PHENOTYPE).
A middle-aged woman with a history of hyperthyroidism and a known 3.2-cm left adrenal pheochromocytoma presents to the emergency department with a 2-week history of worsening palpitations and -diaphoresis. She had consistently elevated serum catecholamines, most recently: epinephrine of 382 pg/ml (range 0 to 57 pg/ml), metanephrine of 801 pg/ml (range 12 to 61 pg/ml), norepinephrine of 441 pg/ml (range 80 to 499 pg/ml), and normetanephrine of 101 (range 18 to 112 pg/ml). She stopped taking her home nicardipine (60 mg/day). HR and BP at presentation were 118 beats/min (ST) and 143/66 mm Hg, respectively. Phenoxybenzamine 20 mg PO was given. Subsequently, BP decreased to 94/56 mm Hg and HR increased to 135 beats/min; she became obtunded and was transferred to the intensive care unit; phenoxybenzamine was discontinued. IV NE was started and titrated to 150 μg/min. BP increased to 112/64 mm Hg, but HR remained unchanged. Subsequently, she had premature ventricular contractions and paroxysms of nonsustained VT. A cardiologist was immediately consulted. Propranolol 1 mg IV was given twice. BP improved to 138/66 mm Hg, episodes of VT subsided, and HR returned to 82 beats/min.
CATECHOLAMINE SYNTHESIS, PHYSIOLOGY, ACTION, CARDIAC, AND VASCULAR RESPONSE
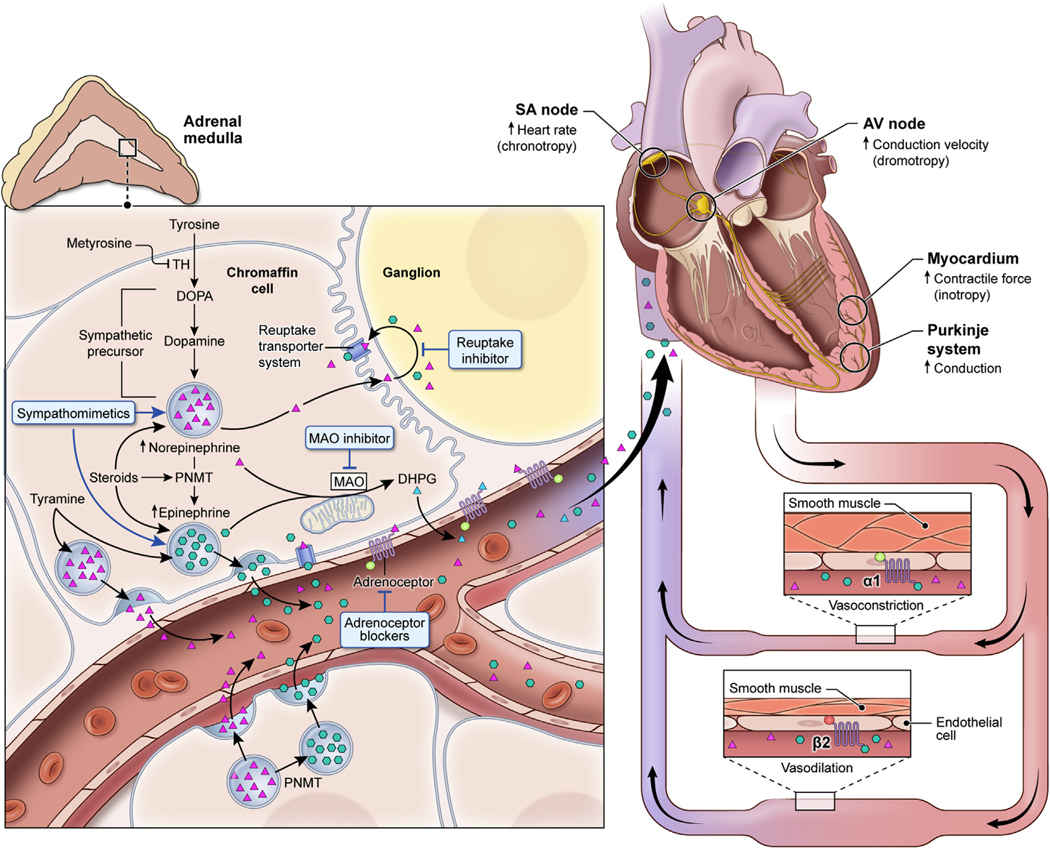
The mechanisms underlying NE and EPI synthesis, action, and cardiovascular response provide the framework for selecting treatments in the management of catecholamine-induced tachyarrhythmias (Figure 2) (2,18). The agent α-methyl-L-tyrosine, or metyrosine (Demser, Bausch Health, West Laval, Quebec, Canada), has proven very useful in our experience because it can dramatically reduce the production of NE and EPI by inhibiting tyrosine hydroxylase, the rate-limiting enzyme of catecholamine biosynthesis (18,19).
FIGURE 2. Catecholamine Synthesis and Interfering Agents.
Catecholamines (dopamine, NE, and EPI) are produced in chromaffin cells through a common synthetic pathway (left), beginning with tyrosine hydroxylase (TH) regulating the conversion of tyrosine to dihydroxyphenylalanine, the rate-limiting step. Dihydroxyphenylalanine is converted to dopamine, which is converted to NE; phenylethanolamine-N-methyltransferase (PNMT) converts NE to EPI. Common foods and medications interfere with 3 mechanisms in catecholamine synthesis and turnover that are relevant to triggering and worsening of tachyarrhythmias. First, synthesized NE and EPI sequestered into storage vesicles can be displaced by prescribed drugs (e.g., sympathomimetics) and tyramine found in fermented, aged, and smoked foods (cheese, wine, beer, soy sauce, avocado, banana, and so on). Second, reuptake inhibitors (serotonin and/or NE-related antidepressants) block catecholamine reuptake. Third, following reuptake, NE and EPI are deactivated by monoamine oxidase and are eventually metabolized to dihydroxyphenylglycol (DHPG). Monoamine oxidase is inhibited by monoamine oxidase inhibitors, including oxazolidinedione antibiotics (linezolid). Additional agents also contribute to catecholamine excess by alternative, multiple, or less-well-known mechanisms and are not shown. These agents include opiates, peptide hormones (vasopressin, glucagon, steroids), neuromuscular blockers, anesthetics, and antiemetics (notably metoclopramide). Ultimately, catecholamines enter the circulation and act upon the heart and vasculature via adrenoceptors (right). Glucocorticoids and thyroid hormones either increase the number of adrenoceptors or their affinity to catecholamines. AV = atrioventricular; SA = sinoatrial; other abbreviations as in Figure 1.
In contrast, sympathomimetics, catecholamine reuptake inhibitors, monoamine oxidase inhibitors, opiates, anesthetics, steroids, antiemetics (notably metoclopramide as in Case 2), linezolid, peptide hormones, and neuromuscular blockers interfere with catecholamine turnover leading to elevated catecholamine levels in systemic circulation (Figure 2) (2,10,11,18). These agents and their actions are outlined in Figures 1A and 2, respectively.
Following synthesis, catecholamines are stored and released from pheochromocytomas in paroxysmal, continuous, or mixed patterns (2,18,20). EPI-releasing pheochromocytomas store and episodically release life-threatening amounts of EPI causing paroxysms of short-lasting tachyarrhythmias (18,20,21). NE-releasing pheochromocytomas release stored catecholamines continuously, often causing persistent hypertension and tachyarrhythmias as opposed to sudden cardiovascular decompensation (18,20,21). Mixed phenotype pheochromocytomas, releasing both NE and EPI, cause both continuous and episodic tachyarrhythmias (20,21).
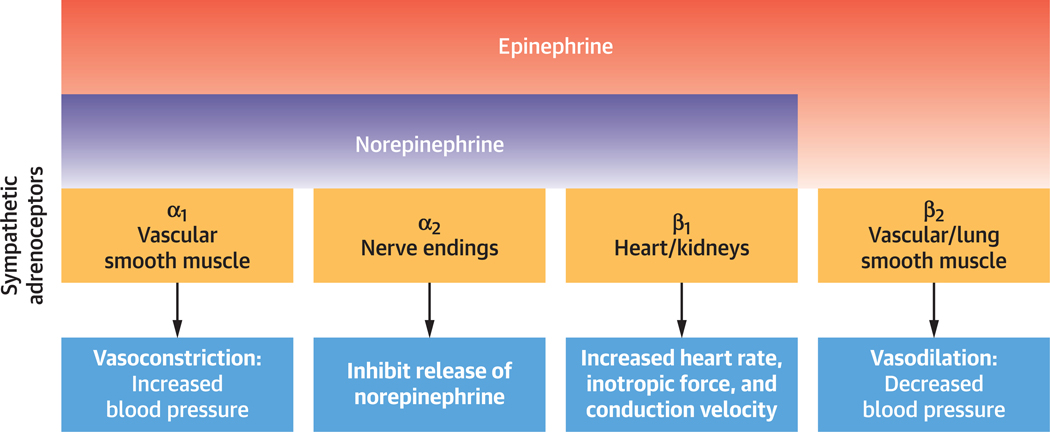
Catecholamines, released from pheochromocytomas bind to adrenoceptors distributed in multiple organs, especially the heart and vasculature with varying affinities (Figure 3) (18), causing tachyarrhythmias and BP changes (20). Although cardiac β1-adrenoceptor stimulation by both NE and EPI causes tachyarrhythmias, the effect on the vasculature is determined by the distribution and ranging affinities of adrenoceptors for NE and EPI (Figure 3) (20,22). EPI has a tendency to cause tachyarrhythmias and, less so, hypertension given its higher affinity for ß1-compared with α1-adrenoceptors; the opposite is true for NE, causing hypertension and less often tachyarrhythmias (Figures 2 and 3) (1,18,22–25). Furthermore, peripheral β2-adrenoceptor stimulation leads to vasodilation and in excess hypotension (Figure 2) (20,23,25). This is seen in EPI-secreting pheochromocytomas or during treatment of pheochromocytomas with sole α1-adrenoceptor blockade due to a relative excess of catecholamines available to β2-adrenoceptors (20,23,25).
FIGURE 3. Catecholamine Binding Affinities and Adrenoceptor Actions.
α(1,2), β(1,2) adrenoceptors present on end organs bind EPI and NE with differing affinities. α1-adrenoceptors are present on vascular smooth muscle and result in vasoconstriction and hypertension when activated by NE or EPI. α2-adrenoceptors are present on synaptic nerve terminals and inhibit the release of NE. β1-adrenoceptors are present on the heart and kidney and, when activated by EPI or NE, result in increased heart rate, inotropic force, and conduction velocity within the heart, and renin release from the kidney. β2-adrenoceptors, present on certain vessels and lung smooth muscle; when activated by EPI, result in vasodilation and in excess can lead to hypotension. Abbreviations as in Figure 1.
Stimulation of cardiac β1-adrenoceptors leads to a rise in cyclic adenosine monophosphate (cAMP), activating calcium and hyperpolarization-activated cyclic nucleotide–gated (HCN) channels as elaborated in the Central Illustration (18,22,26–28), producing tachyarrhythmias. Glucocorticoids, thyroid hormone, and additional abnormalities increase adrenoceptor expression and sensitivity worsening BP and HR derangements (Figure 1A) (29–31).
CENTRAL ILLUSTRATION. Tachyarrhythmias in Catecholamine Excess.
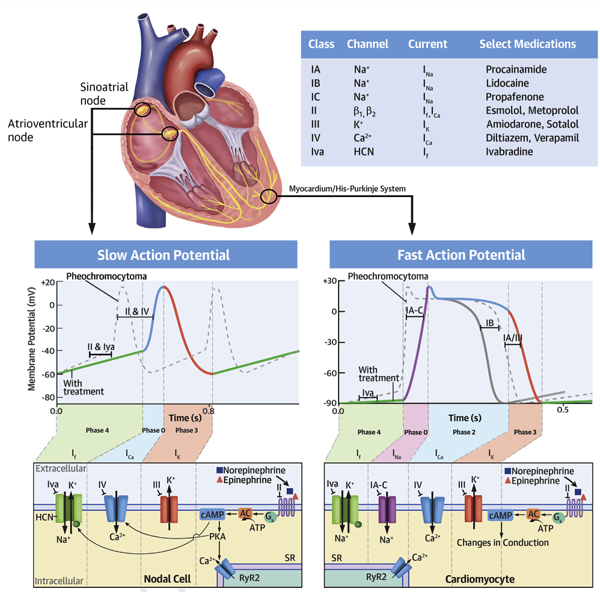
Pheochromocytomas produce excess catecholamines, which bind adrenoceptors and act on the sinoatrial node (SAN), atrioventricular node (AVN), His-Purkinje system, and myocardium (top). Cardiac β1-adrenoceptor stimulation leads to stimulatory guanine triphosphate protein-coupled receptor (Gs) activation, which activates adenylate cyclase (AC) to convert adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA). Nodal cells of the SAN and AVN have slow APs (bottom left). In the SAN, cAMP activates the HCN channel. This increases the slope of diastolic depolarization in phase 4 (green) and increases chronotropy (dashed line vs. solid line). Ivabradine opposes this. In the AVN, cAMP-activated PKA phosphorylates L-type calcium channels, which increases the slope of phase 0 (orange) and increases dromotropy (dashed line vs. solid line). Nondihydropyridine calcium channel and β1-adrenoceptor blocking agents oppose this. In fast-action potentials of the myocardium (bottom right), β1-adrenoceptor stimulation increases conduction velocity, enhances automaticity, and shortens refractoriness (phase 0 to the end of phase 3) by a variety of mechanisms mediated by an elevation in cAMP. β1-adrenoceptor blockade prolongs refractoriness and may decrease spontaneous depolarization (dashed line vs. solid line). Class IA-C antiarrhythmics acting on phase 0 (purple) of depolarization prolong refractoriness, while class III antiarrhythmics do so by acting on phase 3 (pink) of depolarization (dashed line vs. solid line). Ryanodine 2 receptors (RyR2) present on the sarcoplasmic reticulum (SR) of cardiac myocytes and cells of the His-Purkinje system are phosphorylated by PKA and cause calcium efflux into the cytosol; calcium binds the troponin-tropomyosin complex revealing myosin binding sites on actin, enhancing cross-bridge cycling, and increasing inotropy (not shown).
Given the integral role of adrenoceptor stimulation in causing tachyarrhythmias, adrenoceptor blockade is the initial approach in treatment of catecholamine-induced tachyarrhythmias; however, inappropriate adrenoceptor blockade can lead to cardiovascular decompensation and death (12,32). Appropriate biochemical phenotype determination therefore guides treatment approach, particularly in patients presenting with catecholamine-induced tachyarrhythmia and hypotension (18,20,25,33).
MANAGEMENT OF CATECHOLAMINE-INDUCED ARRHYTHMIAS
Bradyarrhythmias are also encountered in pheochromocytoma. They usually are due to either a baroreceptor-mediated reflex response to hypertension or due to a given treatment (e.g., β-adrenoceptor blockade) (34,35). These bradyarrhythmias improve with treatment of hypertension or removal of the offending agent and are not discussed in this text. Catecholamine-induced tachyarrhythmias, on the other hand, are often associated with abrupt BP changes that cannot be overlooked in the selection of appropriate treatments and are further discussed (Figure 1A to 1C).
As described in the previous text, catecholamines stimulate cardiac β1-adrenoceptors, consequently affecting the HCN and calcium channels (22,26,36,37), resulting in increased automaticity, conduction velocity, inotropy, and excitability (Central Illustration) (26,38,39). Thus, the initial treatment strategy in catecholamine-induced tachyarrhythmias relies heavily on β1-adrenoceptor blockade, followed by the use of membrane-active agents that reduce calcium overload and normalize excitability (Central Illustration, Figures 1B and 1C) (37).
TREATMENT OF CATECHOLAMINE-INDUCED ST.
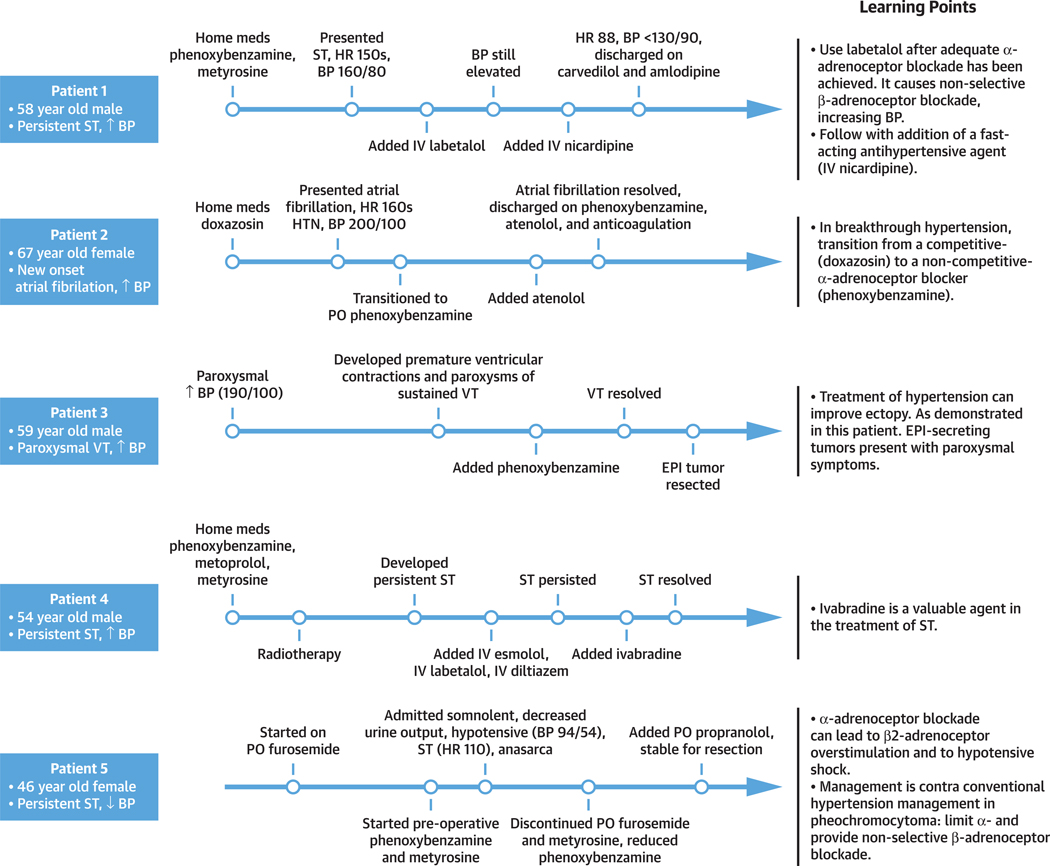
Once the patient is receiving the appropriate level of care, the ST can be treated. We recommend the intensive care unit for patients with prolonged HR <50 or >120 beats/min or systolic BP <80 or >160 mm Hg, a cardiovascular history of catecholamine-related complications, a need for IV antihypertensive or antiarrhythmic agents, or rapid and recurrent HR and BP changes; the remainder of patients can be treated in the intermediate care environment (Figure 1A). To supplement the discussion of ST management, we present patient cases from our institution with clinically relevant points highlighted (Figure 5, Patients 1 to 5).
FIGURE 5. Learning Points in our Clinical Experience.
Timelines detailing patient care and learning points for 5 patients cared for at our institution. Abbreviations as in Figures 1 and 4.
Excessive activation of adrenoceptors in the sinoatrial node leads to calcium and HCN channel activation, producing ST (Central Illustration) (26–28,39,40). Calcium influx via voltage-gated calcium channels due to adrenoceptor activation, increases conduction velocity in the atrioventricular node; β-adrenoceptor blocking and non-dihydropyridine calcium-channel blocking agents oppose this (e.g., diltiazem and verapamil). HCN-channel activation is also blocked by ivabradine (Central Illustration, Supplemental Table 1) (26,27,35).
In treating ST, the choice of whether to use ß-adrenoceptor blocking agents, nondihydropyridine calcium-channel blocking agents, or ivabradine depends on clinical context, the urgency with which the condition must be addressed, and medication-specific properties and adverse effects (27). Although ß-adrenoceptor blockade is a crucial initial step for treatment, nonselective ß-adrenoceptor blockade can precipitate hypertensive crises (Central Illustration, Figure 2) (12,32). This is caused by blocking ß2-adrenoceptor–mediated vasodilation which allows unopposed α-adrenoceptor mediated vasoconstriction (12,32). Initial α-then ß-adrenoceptor blockade is, therefore, strongly recommended except for normotensive/hypotensive patients as α-adrenoceptor blockade leads to hypotension (Figure 1B).
CHOICE OF ADRENOCEPTOR ANTAGONISTS.
We prefer cardio-selective adrenoceptor blocking agents for the treatment of catecholamine-induced tachyarrhythmias, as nonselective ß-adrenoceptor blockade can cause hypertension via ß2-adrenoceptor blockade (Central Illustration, Figure 2) (41). Esmolol is ideal in emergent circumstances as it has rapid onset and short-half life, allowing for titration in the acute setting with rapid fluctuations in hemodynamics (Supplemental Table 2, Figure 1B) (42). IV metoprolol is an alternative. Note, in high concentrations, ß1-adrenoceptor blocking agents may also block ß2-adrenoceptors; therefore, initial α-adrenoceptor blockade is important to prevent hypertension (13). We do not recommend the use of labetalol, which provides nonselective ß-adrenoceptor blockade with relatively weak α-blockade (ratio 1:7), as initial therapy; it may be used after adequate α-adrenoceptor blockade has been established (Figure 5, Patient 1) (2,12,20).
Oral (PO) ß1-adrenoceptor blocking agents can be used in less emergent settings. Controlled-release metoprolol (succinate), immediate-release metoprolol (tartrate), or, possibly, atenolol are preferred agents (Supplemental Table 2) (18). In patients with renal dysfunction, atenolol can accumulate leading to bradycardia; metoprolol tartrate/succinate is preferred, with metoprolol succinate favored for lessfrequent dosing (Supplemental Table 2) (35). Bisoprolol is more ß1-selective and can also be used (35). Propranolol and carvedilol are nonselective ß-adrenoceptor blocking agents (35), with carvedilol having a weak α1-adrenoceptor blocking effect (α:ß = 1:10) (43).
If α-followed by ß-adrenoceptor blockade is ineffective, or if it is contraindicated as in the hypotensive or normotensive patient, we recommend the use of verapamil or diltiazem (Supplemental Table 2, Figure 1B). In patients with hypertension and ST, verapamil is favored as a more potent antihypertensive (Supplemental Table 2, Figure 1B) (44). Given the negative inotropic effects of diltiazem and verapamil, these agents should be avoided in patients with heart failure due to LV systolic dysfunction (Supplemental Table 2) (35). Finally, ivabradine is an ideal agent in hypotensive and normotensive patients with ST or in patients refractory to or intolerant of ß-adrenoceptor and/or nondihydropyridine calcium-channel blocking agents (Figures 1 and 5, Patient 4) (27,45). We frequently recommend the use of ivabradine at outside institutions; within our cohort of 71 patients, 2 patients were treated with ivabradine in the acute setting. The high cost of ivabradine may, however, mitigate against its use as a first line agent.
BP CONSIDERATIONS IN ST.
In hypertensive patients with catecholamine-induced tachyarrhythmia, α-adrenoceptor blockade is the preferred initial treatment. Some of the following recommended medications may not be widely available. As such, we provide alternative management. IV administration is preferred in hypertensive emergency, whereas PO regimens are appropriate for hypertensive urgency or less emergent circumstances (Figure 1B). Oral selective α1-adrenoceptor–blocking agents like doxazosin/terazosin/prazosin can be used (Supplemental Table 2) (1,18,24). However, these competitive α1-inhibitors (Figure 5, Patient 2) are not always effective, as they can be displaced by surges of catecholamines, allowing for breakthrough hypertension (1,18). Use PO phenoxybenzamine, a noncompetitive α-adrenoceptor blocking agent, in this case because it cannot be displaced, allowing prevention of breakthrough hypertension (1,18). When rapid α-adrenoceptor blockade is warranted, consider escalating the level of care to the intensive care unit where IV phentolamine, a nonselective α-adrenoceptor blocking agent, can be given with appropriate invasive BP monitoring (Supplemental Table 2, Figures 1A, 1B, and 5, Patient 2). IV nitroprusside, nicardipine, verapamil, diltiazem, and lastly, hydralazine are suitable add-on agents (1,18,35).
In hypotensive patients with ST, rule out alternative forms of shock, provide appropriate fluid resuscitation, and use agents that control HR without a substantial effect on BP such as ivabradine, verapamil, and diltiazem (Supplemental Table 2, Figures 1A and 1B). If hypotension persists, consider potential β2-overstimulation and administer a non-selective β2-adrenoceptor blocking agent (e.g., propranolol) (Supplemental Table 2, Figures 1B and 5, Patient 5) (25). NE and EPI may be ineffective in providing adequate BP support (46). IV phenylephrine may be beneficial in supporting BP during the state of β2-adrenoceptor overstimulation with hypotension (Figure 1B). Ivabradine or esmolol, unless contraindicated, may be used if HR slowing is desired, but caution must be exercised in hypotensive patients dependent upon HR for sustaining BP. Corticosteroids and vasopressin can worsen underlying tachyarrhythmias by causing catecholamine release and should be avoided (except, e.g., in patients with adrenal insufficiency) (10,46). In hemodynamically unstable patients, follow advanced cardiac life support algorithms (47).
TREATMENT OF CATECHOLAMINE-INDUCED VT.
Catecholamine excess can result in monomorphic (48), bidirectional (49), or polymorphic VT (50,51). Although the precise mechanism is unclear, calcium overload due to cAMP-mediated effects on calcium influx, storage, and spontaneous release likely explains the majority of cases (52). Increased myocardial oxygen consumption due to tachycardia, augmented inotropy, and heightened wall stress from elevated BP contribute to pro-arrhythmic ischemia (31). Consider ischemia when ST-segment shifts precede the onset of VT, particularly polymorphic VT.
The therapeutic approach to catecholamine-induced VT focuses on reducing β1-adrenoceptor–driven cAMP accumulation effects, particularly calcium-overload mediated after-depolarizations, and avoiding inadvertent vasoconstriction that may incur ischemia in ventricular muscle (Central Illustration) (27,38,52). β1-adrenoceptor stimulation activates calcium and potassium channels within the His-Purkinje system and cardiac myocytes, increasing spontaneous depolarization and resulting in VT (52,53). The impact on action potential duration can be quite profound and unpredictable, resulting in prolongation of the QT interval in some and shortening in others (Central Illustration, Supplemental Table 1) (52). These changes contribute to ectopy and conditions that may give rise to re-entrant rhythms (Supplemental Table 1) (38,41). Catecholamine-induced VT is treated initially with β1-adrenoceptor blockade; amiodarone and lidocaine are second- and third-line therapies (Supplemental Table 1) (35,38,54).
We prefer the initial use of α-followed by ß-adrenoceptor blockade for VT in hypertensive patients (Figure 1C). We favor IV esmolol (or IV metoprolol if it is not available) as the ß-adrenoceptor blocking agent of choice. Refractory VT should be treated with amiodarone and lidocaine additionally, except for patients with ß2-adrenoceptor overstimulation leading to hypotension, in whom ß2-adrenoceptor blockade is necessary (e.g., propranolol) (Supplemental Table 2, Figure 1C). In patients who remain in VT despite interventions, cardioversion or venoarterial extracorporeal membrane oxygenation (ECMO) can be considered (47,55).
BP CONSIDERATIONS IN VT.
The same principles in BP management of ST apply to VT (Figure 1C). Further, the treatment of hypertension in patients with VT can reduce arrhythmia due to stretch-induced ectopy (Figure 5, Patient 3) (31). In hypertensive patients, start with α- then β-adrenoceptor blockade with IV esmolol or metoprolol (Supplemental Table 2, Figure 1C). IV propranolol has been used in the past successfully, but may incur unwanted hypertensive crises (Supplemental Table 2, Figure 1C, right) (56). Amiodarone is the preferred second-line agent, as it is indiscriminate in its effects, blocking the α- and β-adrenoceptor non-competitively, as well as membrane sodium, potassium, and calcium channels (Supplemental Table 2, Figure 1C) (35). Lidocaine causes greater hypotension compared with amiodarone (Supplemental Table 2, Figure 1C) (57).
In normotensive patients with VT, start with amiodarone, as it has the least likelihood of incurring hypotension. Then, if needed, consider lidocaine, α-adrenoceptor blockade, and propranolol sequentially (Supplemental Table 2, Figure 1C). In hemodynamically unstable patients with VT, follow advanced cardiac life support algorithms (47). After resuscitation, rule out alternative forms of shock and fluid resuscitate patients without cardiogenic shock (Figure 1A). Give IV propranolol without initial α-adrenoceptor blockade if β2-overstimulation is suspected followed by amiodarone, then lidocaine (Supplemental Table 2, Figure 5, Patient 5, Figure 1C) (23,25).
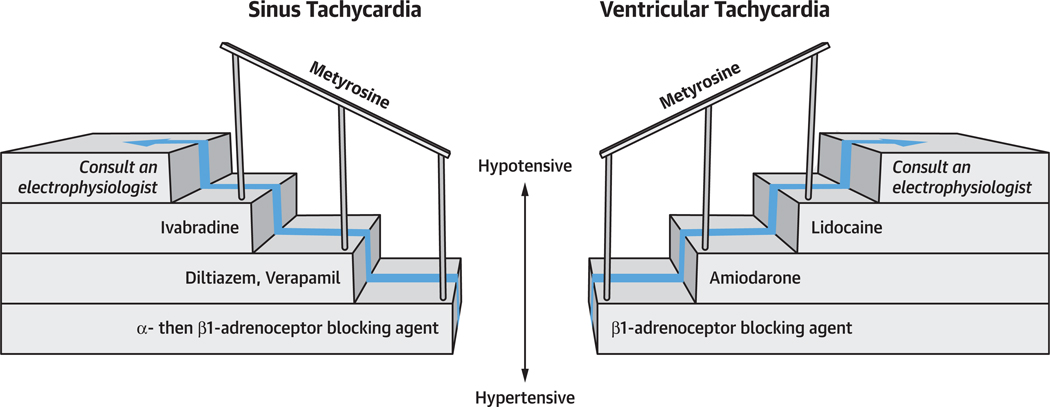
The approach to treatment of tachyarrhythmias thus relies principally on ß1-adrenoceptor blockade. Subsequent management of ST relies on the addition of ivabradine and diltiazem/verapamil, while subsequent management of VT relies on the addition of amiodarone and lidocaine (Figure 4). Sotalol, propafenone, and procainamide can also be used if not contraindicated.
FIGURE 4. Core Principles in Treatment of Catecholamine-Induced Tachyarrhythmias.
The principal treatment strategy for sinus tachycardia (ST) and ventricular tachycardia (VT) include ß1-adrenoceptor blockade especially in hypertensive patients (bottom). In ST, diltiazem, verapamil, and ivabradine are useful add-on agents (left). In VT, lidocaine and amiodarone are useful add-on agents (right). The order in which these agents are used should be considered in the context of comorbid BP derangements (center). Metyrosine (top) can also be considered as an adjunctive treatment in decreasing catecholamine synthesis. If ST and VT remain refractory after adding on these agents, consider consultation with a cardiologist or an electrophysiologist.
ATRIAL FIBRILLATION AND FLUTTER
In the hemodynamically unstable patient, follow advanced cardiac life support algorithms (47). In patients with underlying atrial fibrillation/flutter, ß-adrenoceptor/nondihydropyridine calcium-channel blocking agents provide atrioventricular node blockade to slow the ventricular response. Amiodarone, flecainide, propafenone, or sotalol may be used to try to convert the rhythm to sinus rhythm. Ivabradine is not recommended in the management of atrial fibrillation/flutter (35,45,58).
SPECIAL CONSIDERATIONS: CATECHOLAMINE-RELATED COMPLICATIONS
Patients with catecholamine-induced tachyarrhythmias may have concerning catecholamine-related complications, such as myocardial infarction or superimposed cardiomyopathies, which may be further complicated by left ventricular outflow tract obstruction (46,59). These conditions can lead to cardiogenic shock (59). Thus, they have a high index of suspicion, so obtain serial cardiac troponin measurements, electrocardiograms, and echocardiography to aid in identification and to optimize management, as these patients are at high risk of hemodynamic collapse (Figure 1A).
TACHYARRHYTHMIAS AND MANAGEMENT: BACK TO THE PATIENT
After stabilization, initial management of patients with catecholamine-induced tachyarrhythmias involves eliminating interfering agents, optimizing medical management of harmful comorbid conditions, identifying catecholamine-related complications (as detailed in the previous text), and directing the patient to the appropriate level of care with appropriate invasive BP and hemodynamic monitoring (elaborated in Figures 1A and 2). Once stabilized, the biochemical phenotype then guides and informs management, contingency plans for potential complications, and prognosis (Figure 1A).
We now return to our prototypical cases to evaluate interventions, examine what could be improved, and to highlight similar steps in management of patients taken care of at our institution (Figure 5).
CASE 1 (NORADRENERGIC PHENOTYPE).
This patient had ST and was admitted to the intensive care unit for stabilization. Dehydration from nausea and vomiting likely contributed to ST. Treatment with metoclopramide led to catecholamine release, worsening hypertension and ST (Figure 1A) (16,17). In addition, the nonselective β-adrenoceptor–blocking agent labetalol also worsened the underlying hypertension due to predominant β-adrenoceptor blockade (Figure 5, Patient 1) (18). Treatment with diltiazem corrected the hypertension and ST.
CASE 2 (ADRENERGIC PHENOTYPE).
This patient had hypertension and ST on presentation, which was complicated by hypotensive shock after treatment with phenoxybenzamine. The paroxysmal nature of this patient’s symptoms and the adrenal location of the tumor were consistent with an EPI-secreting pheochromocytoma (Figure 5, Patient 3). In the setting of α-adrenoceptor blockade (phenoxybenzamine), circulating EPI bound to the β2-adrenoceptor, leading to β2-adrenoceptor overstimulation, vasodilation, and hypotension (25). We have encountered this phenomenon of β2-adrenoceptor overstimulation in our patients (see Figure 5, Patient 5). These patients exemplify the need for a thorough evaluation to rule out alternative causes of shock. The addition of IV propranolol blocked β2-adrenoceptor overstimulation, correcting vasodilation and hypotensive shock while treating the underlying VT, a life-saving intervention (as seen in in our patient in Figure 5, Patient 5).
ADDITIONAL CONSIDERATIONS
In the management of patients with pheochromocytoma who have a partial response to treatment or who have persistent symptoms despite adrenoceptor blockade, metyrosine can be added to their medical management (1,18,19). Metyrosine decreases circulating catecholamines and mitigates tachyarrhythmias and BP derangements. It should be considered unless contraindicated (e.g., underlying depression or suicidal ideation) (18). Metyrosine, however, may not be readily available in many institutions given its prohibitive cost.
Acute management of catecholamine-induced tachyarrhythmias requires a coordinated approach among cardiologists, endocrinologists, oncologists, internists, pediatricians, intensivists, and others. Cardiologists play a pivotal role in the management of these tachyarrhythmias.
Many institutions do not have ready access to IV phentolamine or PO phenoxybenzamine. Additionally, ivabradine has a relatively high cost. In these circumstances, we suggest a repertoire of 5 more readily accessible agents: ST and VT can be treated with 1) IV esmolol, 2) IV nicardipine, or 3) IV nitroprusside added to control hypertension; in hypotensive patients, use fluid resuscitation and 4) IV phenylephrine; and in patients with refractory hypotension, assess for β2-adrenoceptor overstimulation and treat with 5) IV propranolol. Additional useful medications are identified in Figure 1 and Supplemental Table 2.
Supplementary Material
HIGHLIGHTS.
Pheochromocytomas cause life-threatening tachyarrhythmias; however, no up-to-date published data exists about their management.
Understanding catecholamine physiology ensures appropriate intervention while avoiding mismanagement and consequent poor cardiovascular outcomes.
Cardiologists are pivotal in implementing up-to-date care for pheochromocytoma-induced tachyarrhythmias.
Additional studies are needed to further optimize management strategies of pheochromocytoma-induced tachyarrhythmias.
Acknowledgments
This work was supported by the intramural research programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, and National Heart, Lung, and Blood Institute, National Institutes of Health.
ABBREVIATIONS AND ACRONYMS
- BP
blood pressure
- EPI
epinephrine
- HCN
hyperpolarization-activated cyclic nucleotide–gated
- HR
heart rate
- NE
norepinephrine
- ST
sinus tachycardia
- VT
ventricular tachycardia
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC author instructions page.
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet 2005;366:665–75. [DOI] [PubMed] [Google Scholar]
- 2.Berends AMA, Eisenhofer G, Fishbein L, et al. Intricacies of the molecular machinery of catecholamine biosynthesis and secretion by chromaffin cells of the normal adrenal medulla and in pheochromocytoma and paraganglioma. Cancers (Basel) 2019;11:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin Proc 1981;56:354–60. [PubMed] [Google Scholar]
- 4.Stolk R, Bakx C, Mulder J, Timmers H, Lenders J. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013;98:1100–6. [DOI] [PubMed] [Google Scholar]
- 5.Prejbisz A, Lenders JWM, Eisenhofer G, Januszewicz A. Mortality associated with phaeochromocytoma. Horm Metab Res 2013;45:154–8. [DOI] [PubMed] [Google Scholar]
- 6.Zelinka T, Petrak O, Turkova H, et al. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res 2012;44: 379–84. [DOI] [PubMed] [Google Scholar]
- 7.Prejbisz A, Lenders JWM, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens 2011;29: 2049–60. [DOI] [PubMed] [Google Scholar]
- 8.Schürmeyer TH, Engeroff B, Dralle H, von zur Mühlen A. Cardiological effects of catecholamine-secreting tumours. Eur J Clin Invest 1997;27: 189–95. [DOI] [PubMed] [Google Scholar]
- 9.Loscalzo J, Roy N, Shah RV, et al. Case 8–2018: a 55-year-old woman with shock and labile blood pressure. N Engl J Med 2018;378: 1043–53. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhofer G, Rivers G, Rosas AL, Quezado Z, Manger WM, Pacak K. Adverse drug reactions in patients with phaeochromocytoma. Drug Saf 2007;30:1031–62. [DOI] [PubMed] [Google Scholar]
- 11.Neary NM, King KS, Pacak K. Drugs and pheochromocytoma—don’t be fooled by every elevated metanephrine. N Engl J Med 2011;364:2268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf K, Santos J, Pacak K. Why take the risk? We only live once: the dangers associated with neglecting a pre-operative alpha adrenoceptor blockade in pheochromocytoma patients. Endocr Pract 2019;25:106–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenders JWM, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915–42. [DOI] [PubMed] [Google Scholar]
- 14.Young WF. Adrenal causes of hypertension: pheochromocytoma and primary aldosteronism. Rev Endocr Metab Disord 2007;8:309–20. [DOI] [PubMed] [Google Scholar]
- 15.Brugada J, Katritsis DG, Arbelo E, et al. , for the ESC Scientific Document Group. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. The Task Force for the Management of Patients With Supraventricular Tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2019. August 31 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Leonard JB, Munir KM, Kim HK. Metoclopramide induced pheochromocytoma crisis. Am J Emerg Med 2018;36 1124.e1–2. [DOI] [PubMed] [Google Scholar]
- 17.Guillemot J, Compagnon, Cartier D, et al. Metoclopramide stimulates catecholamine- and granin-dervided peptide section from pheochromocytoma cells through activation of serotonin type 4 (5-HT4) receptors. Endocr Relat Cancer 2009;16:281–90. [DOI] [PubMed] [Google Scholar]
- 18.Pacak K Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab 2007;92:4069–79. [DOI] [PubMed] [Google Scholar]
- 19.Sjoerdsma A, Engelman K, Spector S, Udenfriend S. Inhibition of catecholamine synthesis in man with α-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. Lancet 1965;2:1092–4. [DOI] [PubMed] [Google Scholar]
- 20.Gupta G, Pacak K, for the AACE Adrenal Scientific Committee. Precision medicine: an update on genotype/biochemical phenotype relationships in pheochromocytoma/paraganglioma patients. Endocr Pract 2017;23:690–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhofer G, Huynh T, Elkahloun A, et al. Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am J Physiol Endocrinol Metab 2008;295:E1223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacak K, Kaiser H, Eisenhofer G. Pheochromocytoma In: Jameson JL, De Groot LJ, editors. Endocrinology. 5th Edition Philadelphia: Elsevier Saunders, 2008:2501–34. [Google Scholar]
- 23.Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf) 1992;37: 304–6. [DOI] [PubMed] [Google Scholar]
- 24.Zuber S, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: characteristics and treatment. Endocrinol Metab Clin North Am 2011;40: 295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantorovich V, Pacak K. A New Concept of unopposed β-adrenergic overstimulation in a patient with pheochromocytoma. Ann Intern Med 2005;142:1026–8. [DOI] [PubMed] [Google Scholar]
- 26.Issa ZF, Miller JM, Zipes DP. Chapter 3: electrophysiological mechanisms of cardiac arrhythmias In: Issa ZF, Miller JM, Zipes DP, editors. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease. Second Edition New York: Elsevier, 2012:36–61. [Google Scholar]
- 27.Malaza G, Brofferio A, Lin F, Pacak K. Ivabradine use in refractory catecholamine-induced tachycardia in paraganglioma. N Engl J Med 2019;380:1284–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koruth JS, Lala A, Pinney S, Reddy VY, Dukkipati SR. The clinical use of ivabradine. J Am Coll Cardiol 2017;70:1777–84. [DOI] [PubMed] [Google Scholar]
- 29.Motulsky HJ, Insel PA. Adrenergic receptors in man: direct identification, physiologic regulation, and clinical alterations. N Engl J Med 1982;307: 18–29. [DOI] [PubMed] [Google Scholar]
- 30.Ciaraldi TP, Marinetti GV. Hormone action at the membrane level. VIII. Adrenergic receptors in rat heart and adipocytes and their modulation by thyroxine. Biochim Biophys Acta 1978;541: 334–46. [DOI] [PubMed] [Google Scholar]
- 31.Leon AS, Abrams WB. The role of catecholamines in producing arrhythmias. Am J Med Sci 1971;262:9–13. [DOI] [PubMed] [Google Scholar]
- 32.Sibal L, Jovanovic A, Agarwal SC, et al. Phaeochromocytomas presenting as acute crises after beta blockade therapy. Clinical Endocrinology 2006;65:186–90. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhofer G, Lenders JWM, Linehan M, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med 1999;340:1872–9. [DOI] [PubMed] [Google Scholar]
- 34.Forde TP, Yormak SS, Killip T 3rd. Reflex bradycardia and nodal escape rhythm in pheochromocytoma. Am Heart J 1968;76: 388–92. [DOI] [PubMed] [Google Scholar]
- 35.Eschenhagen T Chapter 28: Treatment of hypertension, chapter 29: therapy of heart failure In: Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e. New York: McGraw-Hill, 2018:507–46. [Google Scholar]
- 36.Moore EN, Morse HT, Price HL. Cardiac arrhythmias produced by catecholamines in anesthetized dogs. Circ Res 1964;15:77–82. [DOI] [PubMed] [Google Scholar]
- 37.Sharma PL. Effect of propranolol on catecholamine-induced arrhythmias during nitrous oxide-halothane anaesthesia in the dog. Br J Anaesth 1966;38:871–7. [DOI] [PubMed] [Google Scholar]
- 38.Broadley K Autonomic Pharmacology. First edition Bristol, PA: Taylor & Francis, Inc, 1996: 241. [Google Scholar]
- 39.Hoffman BF, Singer DH. Appraisal of the effects of catecholamines on cardiac electrical activity. Ann N Y Acad Sci 1967b;139:914–39. [DOI] [PubMed] [Google Scholar]
- 40.DiFrancesco D The role of the funny current in pacemaker activity. Circ Res 2010;106:434–46. [DOI] [PubMed] [Google Scholar]
- 41.Kirubakaran S, Jaswinder G. Exercise-induced arrhythmias Chapter 53. In: Saksena S, editor. Camm J. Electrophysiological Disorders of the Heart. Second edition New York: Elsevier, 2011: 783–94. [Google Scholar]
- 42.Gray RJ. Managing critically ill patients with esmolol. An ultra short-acting beta-adrenergic blocker. Chest 1988;93:398–403. [DOI] [PubMed] [Google Scholar]
- 43.Ruffolo RR Jr., Gellai M, Hieble JP, Willette RN, Nichols AJ The pharmacology of carvedilol. Eur J Clin Pharmacol 1990;38 Suppl 2: S82–8. [DOI] [PubMed] [Google Scholar]
- 44.Phillips BG, Gandhi AJ, Sanoski CA, Just VL, Bauman JL. Comparison of intravenous diltiazem and verapamil for the acute treatment of atrial fibrillation and atrial flutter. Pharmacotherapy 1997;17:1238–45. [PubMed] [Google Scholar]
- 45.Yancy CW, Januzzi JL Jr., Allen LA, et al. ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;71: 201–30. [DOI] [PubMed] [Google Scholar]
- 46.Santos JRU, Brofferio A, Viana B, Pacak K. Catecholamine-induced cardiomyopathy in pheochromocytoma: how to manage a rare complication in a rare disease? Horm Metab Res 2019;51: 458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122: S729–67. [DOI] [PubMed] [Google Scholar]
- 48.Michaels RD, Hays JH, O’Brian JT, et al. Pheochromocytoma associated ventricular tachycardia blocked with atenolol. J Endocrinol Invest 1990;13:943–7. [DOI] [PubMed] [Google Scholar]
- 49.Traykov VB, Kotirkov KI, Petrov IS. Pheochromocytoma presenting with bidirectional ventricular tachycardia. Heart 2013;99:509. [DOI] [PubMed] [Google Scholar]
- 50.Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circulation Circ Arrhythm Electrophysiol 2012;5:1044–52. [DOI] [PubMed] [Google Scholar]
- 51.Methe H, Hinterseer M, Wilbert-Lampen U, et al. Torsades de Pointes: a rare complication of an extra-adrenal pheochromocytoma. Hypertens Res 2007;30:1263–6. [DOI] [PubMed] [Google Scholar]
- 52.Cameron JS, Han J. Effects of epinephrine on automaticity and the incidence of arrhythmias in Purkinje fibers surviving myocardial infarction. J Pharmacol Exp Ther 1982;223:573–9. [PubMed] [Google Scholar]
- 53.Tsien RW. Effects of epinephrine on the pacemaker potassium current of cardiac Purkinje fibers. J Gen Physiol 1974;64:293–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018;138:e272–391. [DOI] [PubMed] [Google Scholar]
- 55.Guglin M, Zucker MJ, Bazan VM, et al. Venoarterial ECMO for adults. J Am Coll Cardiol 2019;73:698–716. [DOI] [PubMed] [Google Scholar]
- 56.Manger WM, Gifford RW. Clinical and Experimental Pheochromocytoma. Second edition. Cambridge, Massachusetts: Blackwell Science, 1996. [Google Scholar]
- 57.Somberg JC, Bailin SJ, Haffajee CI, et al. Intravenous lidocaine versus intravenous amiodarone (in a new aqueous formulation) for incessant ventricular tachycardia. Am J Cardiol 2002; 90:853–9. [DOI] [PubMed] [Google Scholar]
- 58.Fox K, Ford I, Steg PG, et al. Bradycardia and atrial fibrillation in patients with stable coronary artery disease treated with ivabradine: an analysis from the SIGNIFY study. Eur Heart J 2015;36: 3291–6. [DOI] [PubMed] [Google Scholar]
- 59.Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol 2015;173: 757–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.