Abstract
Introduction:
Acute respiratory distress syndrome (ARDS) is a severe form of acute lung injury common in critically ill patients and characterized by significant morbidity and mortality. It frequently manifests long-lasting effects beyond hospitalization, from cognitive impairment to physical weakness.
Areas covered:
Several complications of ARDS have been identified in patients after hospital discharge. The authors conducted literature searches to identify observational studies, randomized clinical trials, systematic reviews, and guidelines. A summary of is presented here to outline the sequelae of ARDS and their risk factors with a focus on the limited but growing research into possible therapies. Long term sequelae of ARDS commonly identified in the literature include long-term cognitive impairment, psychological morbidities, neuromuscular weakness, pulmonary dysfunction, and ongoing healthcare utilization with reduced quality of life.
Expert opinion:
Given the public health significance of long-term complications following ARDS, the development of new therapies for prevention and treatment is of vital importance. Furthering knowledge of the pathophysiology of these impairments will provide a framework to develop new therapeutic targets to fuel future clinical trials in this area of critical care medicine.
Keywords: acute respiratory distress syndrome, long-term outcomes
1. Introduction
Originally described in 1967 by Ashbaugh and colleagues, the acute respiratory distress syndrome, or ARDS, is a significant cause of mortality and morbidity in critically ill patients worldwide [1]. ARDS is defined as acute respiratory failure with bilateral opacities on imaging of the chest not due to cardiogenic sources, atelectasis, or volume overload, with significant hypoxemia, measured as a partial pressure of oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (PaO2/FiO2) ratio of ≤ 300 mmHg with PEEP or CPAP ≥ 5 mmHg while receiving invasive or noninvasive mechanical ventilation [2]. ARDS has a multitude of potential etiologies, including sepsis, pneumonia, massive transfusion, surgical insult, and trauma [2]. The incidence of the syndrome varies between studies depending on the care setting and the methods of measurement used. In a United States based cohort, the age-adjusted incidence was 86.2 cases per 100,000 person years, while in [3] the multinational Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG-SAFE), 10.4% of ICU admissions were due to ARDS, with the prevalence increasing to 23.4% of admissions for patients requiring mechanical ventilation [4]. The incidence of ARDS may be decreasing overall [5]. This fall in incidence of ARDS has been attributed to multiple improvements in critical care practice, including low tidal volume ventilation [6–8], restrictive blood product transfusion practices [9,10], and early resuscitation with appropriate antimicrobial administration in patients with sepsis [11].
In addition to decreased incidence of ARDS with clinical advances, mortality related to ARDS is also declining due to improvements in clinical care [12]. Despite these advances in the care of critically ill patients, survivors of ARDS experience significant long-term impairments (those continuing after hospital discharge) and high mortality in the first year beyond the initial critical illness. Common morbidities seen in survivors of ARDS include cognitive and psychological impairment, physical disability with reduced exercise capacity and muscle wasting, pulmonary function impairments, as well as poor quality of life and ongoing healthcare utilization (Figure 1). Most commonly, these issues arise from the downstream effects of treatment, such as mechanical ventilation, sedation, and immobility, or potentially as secondary effects from aspects of the disease process such as refractory hypoxemia. These impairments can persist for years following ARDS, with increased healthcare costs even in previously healthy persons [13,14]. To characterize these morbidities, a comprehensive PubMed search was performed using the following keywords or medical subject headings (MESH) in multiple iterations: ‘acute lung injury (ALI)’, ‘acute respiratory distress syndrome (ARDS)’, ‘acute hypoxemic respiratory failure’, ‘critical illness’, ‘outcomes’, ‘long-term mortality’, ‘cognitive impairment’, ‘neurocognitive’, ‘psychology’, “psychiatric, ‘depression’, ‘post-traumatic stress disorder’, ‘ICU-acquired weakness’, ‘critical illness myopathy’, ‘critical illness polyneuropathy’, ‘pulmonary function’, ‘fibrosis’, ‘exercise capacity’, ‘healthcare utilization’, ‘health-related quality of life’, and ‘employment’. This narrative review details the clinical evidence of the long-lasting effects of ARDS, describes the various patient domains that are impaired in survivors and the risk factors for these impairments (Table 1), discusses possible future treatment strategies, and outlines expectations for potential advances in clinical care and research in the ensuring years.
Figure 1.
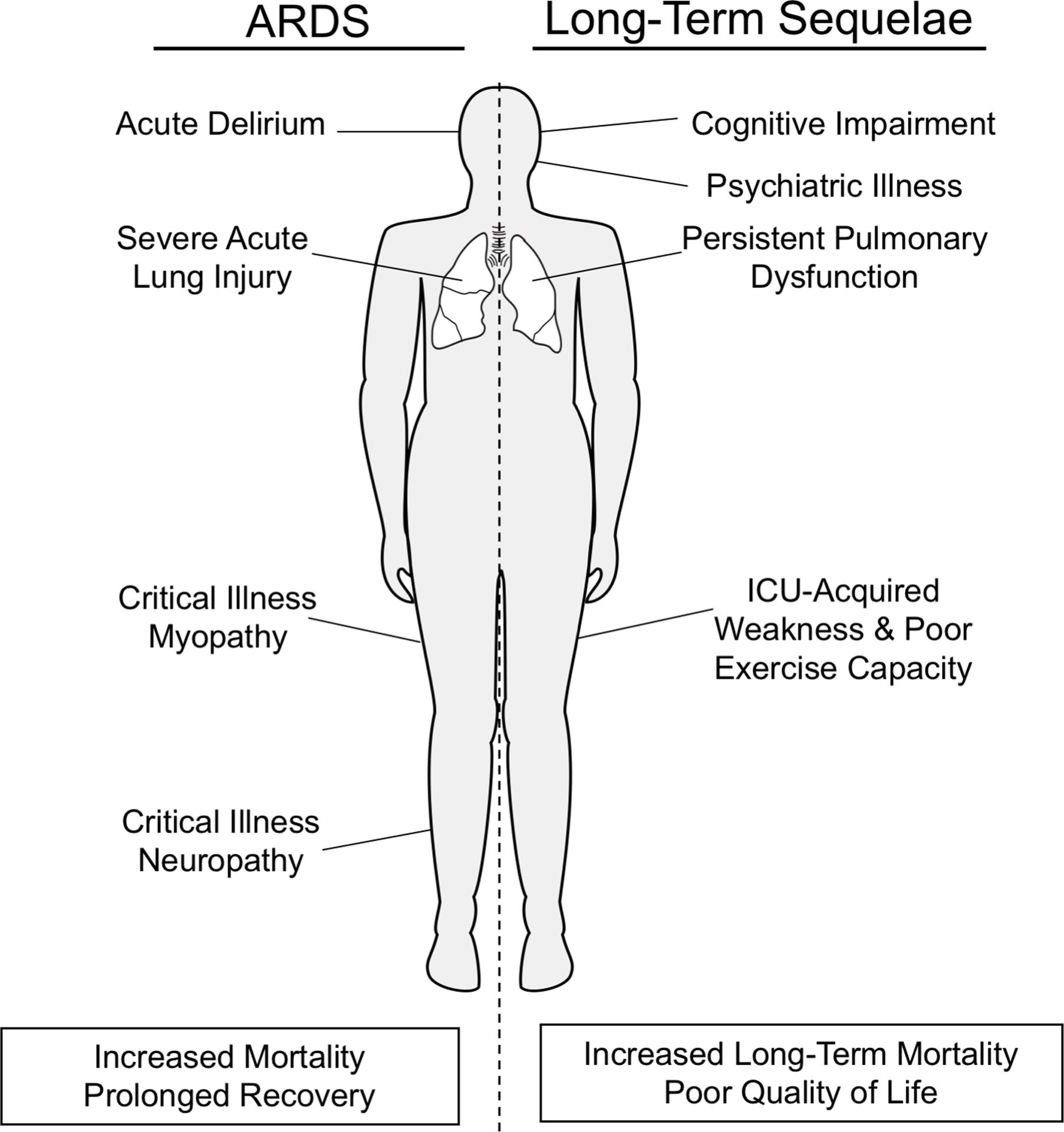
Relationship between acute manifestations of ARDS and its long-term sequelae.
Table 1.
Risk factors for long-term outcomes and complications from ARDS.
| Long-Term Outcome | Known or Suspected Risk Factors |
|---|---|
| Long-term mortality | Hospital-acquired ARDS, age, pre-morbid comorbidities, institutionalization prior to hospital admission |
| Cognitive impairment | Delirium (onset and duration), sedation with benzodiazepines sepsis, hypoxemia, pre-morbid cognitive impairment, veno-arterial ECMO rescue therapy |
| Psychiatric illness | Pre-morbid psychiatric disease, younger age, female, alcohol abuse, unemployment, inpatient opiate exposure |
| ICU-acquired weakness | Severity of illness, degree of organ failure, prolonged immobility, hyperglycemia, possible: paralytic use for refractory hypoxemia and corticosteroid use |
| Pulmonary dysfunction & radiographic abnormalities | Pulmonary causes of ARDS, duration of mechanical ventilation |
| Subsequent healthcare utilization | Inpatient length of stay, comorbid cardiovascular disease |
| Health-related quality of life | Persistent pulmonary dysfunction, ICU-acquired weakness, cognitive impairment, psychiatric illness |
2. Late mortality
In-hospital mortality rates for ARDS have been improving over the last several decades, from approximately 40% in early studies of the disease to now approximately 25% in more recent studies [12]. Despite declining in-hospital mortality in critically ill patients, ICU survivors continue to have an increased risk of death in the months to years following their illnesses as compared to their non-critically ill and healthy counterparts [15–17]. These trends are also demonstrated in ARDS patients. Herridge et al and Khandelwal et al demonstrated mortality rates of 11% at one-year and 15% at three-years post-ARDS, respectively [18,19]. The development of ARDS is clearly a risk factor for death after ICU discharge. For example, Biehl and colleagues evaluated the long-term survival of hospital-acquired ARDS patients as compared to matched controls without ARDS and found that survival was worse in those with hospital-acquired ARDS at both 90-days and 6-months (adjusted hazard ratio [HR] of 1.76; 95% CI: 1.2–2.5; P = 0.002) [20]. Similarly, Wang et al found that in patients hospitalized with ARDS, one-year mortality was substantially greater than in-hospital mortality (41% vs 24%, P < 0.01) [21]. Notably in this study, severity of illness measures, such as the Acute Physiology, Age, Chronic Health Evaluation II (APACHE II) score did not predict one-year mortality in multivariate models. However, age, chronic co-morbidities, such as renal disease and malignancy, and institutionalization prior to admission were all important predictors of late mortality, suggesting that mortality after discharge may be linked to processes beyond the immediate pathophysiology of ARDS.
The downstream complications of ARDS are predictive of long-term mortality as well. In a prospective evaluation of the 5-year outcomes of ARDS survivors, skeletal muscle weakness at discharge was independently associated with worse 5-year survival [22]. This finding was consistent whether muscle weakness persisted or resolved in the post-ICU period. Hermans and colleagues also demonstrated higher one-year mortality for critically ill patients with ICU-acquired muscular weakness (ICU-AW), a common complication of ARDS and critical illness [23].
The role of muscle weakness, aging, comorbidity, and functional dependency as biological precipitants of late mortality in ARDS is not well understood. Muscular weakness is associated with pharyngeal dysfunction leading to symptomatic aspiration and poor nutrition, which can contribute to significant morbidity and mortality [24]. More globally, it is likely that these sequelae are reflective of impaired home-ostasis and the complex interplay of individual patient vulnerability, acute insults, critical illness, and iatrogenesis. These pathways intersect in the phenomenon of frailty, a syndrome of reduced physiological reserve and increased susceptibility to acute stressors [25], which is linked to long-term mortality in survivors of critical illness [26]. This hypothesis is reinforced in a recent study by Hope and colleagues from the Lung Injury Prevention Study with Aspirin cohort [27]. This study demonstrated that pre-hospital vulnerability as assessed by the Vulnerable Elders Survey (VES) [28], which measures limitations in physical function, self-reported health, and disability, was a strong and significant predictor of one-year mortality in patients with ARDS. By contrast, there was no association between pre-hospital vulnerability and development of ARDS or with 28-day mortality. Preexisting vulnerabilities and limitations in physical function and activities of daily living, compounded by the sequalae of critical illness, seems to lead to an accumulation of worsening health and frailty in the months to years following ARDS. The development of these interrelated impairments may suggest a potential final common pathway to the late mortality seen in ARDS survivors.
3. Long-term cognitive impairment
Long-term cognitive impairment is a particularly debilitating morbidity in survivors of ARDS [29]. Estimates of the prevalence of cognitive impairment following ARDS vary. Wilcox and colleagues reported that over three-quarters of ARDS survivors had evidence of cognitive impairment at discharge, with over half continuing to have evidence of cognitive impairment at one-year follow-up and one in five having persistent deficits at five years following ARDS [29]. Similarly, Hopkins et al showed that at two years following ARDS, half of patients have significant deficits in memory, executive function, and learning, with half the cohort performing below the 6th percentile in testing [30]. These impairments impact patients similarly regardless of severity of illness, age, and education level [31].
The risk factors for the development of cognitive impairment following ARDS are multifactorial and inter-related (Table 1). These include pre-morbid clinical status such as baseline neurocognitive function, interventions provided in the intensive care unit such as deep sedation with benzodiazepines, and the underlying pathophysiology of critical illness and ARDS. Preexisting dementia or other neurocognitive pathology is a strong risk factor for post-ARDS cognitive impairment [32,33], though the actual prevalence of preexisting cognitive dysfunction is challenging to measure in critically ill patients and probably underestimated [34]. Similarly, hospitalization with acute illness is associated with a worse trajectory of cognitive function and dementia in subsequent follow-up as compared to patients who are not hospitalized or undergo non-urgent elective admissions [35,36]. The cognitive impairment that develops after ARDS impacts multiple cognitive domains, from memory to attention to visuo-spatial ability [30,33,37]. ARDS survivors also report significant deficits in memory, even up to 5 years following their illness [38,39]. Needham and colleagues found that a third of ARDS survivors had evidence of impaired memory along with deficits in executive function and attention at 6 months after their illness with a quarter of survivors having similar deficits at one year follow-up [40].
In addition to preexisting neurocognitive disease, the development of delirium, an acute, fluctuating disturbance in consciousness characterized by inattention and abnormal cognition and perception, during acute illness is strongly and independently associated with long-term cognitive impairment in both general critical illness and ARDS [41]. In critically ill patients, longer duration of delirium is associated with greater cognitive impairment at 3 and 12 month follow-up [33]. Preexisting neurological disease and the development of delirium are closely linked but the pathophysiology is not well understood; however, it has been demonstrated that patients with preexisting Alzheimer’s disease who suffered from delirium while hospitalized experienced an accelerated cognitive decline as compared to their non-delirious counterparts [42].
Girard and colleagues described several different clinical phenotypes of delirium in critical illness based on clinical insult, ranging from sedative-associated, to septic, to hypoxic delirium, with variability in the severity of cognitive impairment following each type [43]. Delirium associated with sedation, particularly when of longer duration and due to benzodiazepines, was strongly associated with long-term cognitive impairment, whereas delirium due to metabolic causes was not. Similarly, hypoxemia is a risk factor for the development of delirium, and the hypoxemic phenotype is related to worse long-term cognitive function [41]. It is likely that different risk factors for delirium are causally linked to the pathophysiology of long-term cognitive impairment following critical illness. For example, prolonged hypoxemia or sepsis may induce neuronal injury that is more severe than metabolic disturbances, pre-disposing patients with ARDS and delirium to long term cognitive sequelae. Lastly, the use of rescue therapies, such as extracorporeal membrane oxygenation (ECMO), may impact the risk of cognitive impairment. In a cohort of patients receiving ECMO therapy for severe respiratory failure, significant reductions in cognitive function were seen in patients who had evidence of cerebrovascular lesions at long term follow-up [44]. Notably, the patients with cerebrovascular lesions were more likely to have received veno-arterial ECMO. Cognitive dysfunction was not consistently noted in patients receiving veno-venous ECMO, and this is consistent with other studies of cognitive impairment in patients receiving veno-venous ECMO therapy for ARDS rescue therapy [45,46]. The complex interface between delirium, ARDS and its treatment, and long-term cognitive impairment likely represents a multifactorial pathophysiology that remains an urgently needed and important area of future research.
4. Psychiatric illness
In addition to cognitive impairment, psychiatric impairments are increasingly recognized as devastating complications of ARDS. Depression, anxiety, and post-traumatic stress disorder (PTSD) have been identified in survivors of ARDS [30,47]. Symptoms of psychiatric illness may include guilt, restlessness or psychomotor disturbances, overt sadness, nightmares or pervasive thoughts, emotional instability, and others. Mikkelsen and colleagues found that 36% of patients were clinically depressed, 62% had anxiety, and 39% had symptoms of PTSD at one-year after surviving ARDS [41]. Amongst over 100 ARDS survivors in the Toronto cohort, the prevalence of depressive symptoms at 2 and 5 year follow-up was 40% and 20%, respectively [13,48]. In the ARDSNet Long Term Outcomes Study (ALTOS), two-thirds of patients had significant psychiatric symptoms of depression (36%), anxiety (42%), or PTSD (24%) [49]. Often, patients in the ALTOS trial demonstrated overlap in these symptoms, with many have co-occurring symptoms. Similarly, Davydow and colleagues, in a systematic review of psychiatric morbidities in ARDS survivors, noted that the prevalence of depression, anxiety, or PTSD ranged from 17% to 48% [50]. Notably, these symptoms did not disappear after a year. In the same systematic review, the estimated prevalence of PTSD at 8-year follow-up was 24%. These results have been consistent across different studies with similar patient populations [51], implicating ARDS as a significant contributor to psychiatric symptoms in survivors of critical illness.
The pathophysiology of psychiatric disease following ARDS is poorly understood but is likely related to processes similar to those that give rise long-term cognitive impairment. Notably, severity of illness during ARDS does not appear to be related to psychological outcomes. For example, survivors of influenza-related ARDS that was severe enough to require extra-corporeal membrane oxygenation (ECMO) did not have a greater rate of anxiety, depression, or PTSD at one year compared to those who did not require ECMO [52].
Despite the lack of clear understanding of pathological mechanism, there have been some risk factors identified for the development of psychiatric symptoms following ARDS. in addition to pre-illness psychiatric disease, female sex, younger age, alcohol abuse, and unemployment were all notable social or demographic risk factors for the occurrence of post-ARDS psychiatric symptoms [53]. Additional in-hospital risk factors include a lower partial pressure of oxygen as well as greater opioid exposure while in the ICU [41,49]. As patients with ARDS often have prolonged hospitalizations with a high degree of severity illness and also endure long recoveries from both physical and cognitive impairments following their disease, it is not unexpected that psychological sequelae of the disease co-occur with other types of impairment following ARDS [54].
5. ICU acquired weakness
In addition to the cognitive outcomes experienced by survivors of ARDS, many are left with severe physical impairments, including persistent muscle wasting and weakness and reduced exercise capacity. These deficits may last for several years following the initial illness and can have a substantial impact on patients’ functional status, including disability in activities of daily living [13,18,48].
Intensive-care acquired weakness, or ICU-AW, is a broad term used to describe the syndrome of muscle weakness and wasting that often develops during critical illness and persists after discharge. The term is used broadly to describe multifactorial pathophysiological processes impacting both the intrinsic function and structure of skeletal muscle as well as the peripheral neural networks that drive muscular contraction, encompassing both critical illness myopathy and critical illness polyneuropathy. Herridge and colleagues originally described ICU-AW in ARDS in a cohort of survivors of severe ARDS, noting that impacted patients had a significant reduction in the expected distance walked during a six-minute walk test (6MWT) in the years following the initial illness [18]. Several other large studies of ARDS patients demonstrated the same phenomena as well [49,55]. The overall incidence of ICU-AW varies depending on the study population, and impacts critically ill patients both with and without ARDS. In ARDS patients, it is estimated that approximately one-third will have objective evidence of ICU-AW prior to hospital discharge, with at least 50% of patients having persistent or slowly resolving weakness over the subsequent years after hospitalization [22,56]. Risk factors for ICU-AW vary and remain an area of active investigation (Table 1). It is strongly associated with overall inpatient severity of illness and degree of organ failure across multiple studies [57,58]. Other risk factors commonly cited, such as the use of corticosteroids and neuromuscular blockade, have been inconsistently associated with ICU-AW, with some studies showing an association and others showing no association [57,59,60]. Intensive glycemic control has been demonstrated in one study to blunt abnormal EMG activity, suggesting that insulin therapy might limit ICU-AW, but additional studies are needed [61]. Lastly, prolonged immobility may also be associated with muscle wasting and potentially ICU-AW [62]. Critically ill patients already experience significant acute muscle wasting [63], so when coupled with prolonged immobility, high severity of illness, and possible iatrogenic precipitants, the risk of developing ICU-AW is high. Interventions such as early mobilization in the intensive care unit are being actively studied to reduce the development of ICU-AW.
The diagnosis of ICU-AW remains difficult, given both the multifactorial etiologies of the syndrome and diagnostic modalities used to assess weakness. Electromyographic and nerve conduction studies have been used to assess for evidence of muscular weakness or peripheral neuropathy, but the need for patient cooperation, need for specialized interpretation, and clinical factors such as tissue edema or coagulopathy limit the utility of these studies [64]. Instead, the diagnosis remains clinical in patients who have evidence of objective weakness without other obvious primary etiology, such as primary neuromuscular disease [65]. Most commonly, the diagnosis is confirmed in patients who are awake and participatory using the Medical Research Council scale to assess strength in various muscle groups in the upper and lower extremities, with scores less than 48 indicating ICU-AW [66]. Further, the diagnosis of ICU-AW can be subclassified into critical illness myopathy and critical illness polyneuropathy, though patients may exhibit diagnostic, electrophysiologic, and histologic characteristics of both myopathic and neuropathic processes.
Critical illness myopathy (CIM) is a common manifestation of ICU-AW, that presents as proximal limb and respiratory muscle weakness, classically with retention of sensory neural function. It is typically a non-necrotizing process that is diffuse in nature with concomitant fibrosis and fatty breakdown of muscle fibers with a preferential loss of thick myosin filaments [67,68]. Angel and colleagues demonstrated that the histological changes of CIM could be seen for up to two years in a case series of ARDS survivors [69]. Critical illness polyneuropathy (CIP) is a related pathology under the umbrella of ICU-AW that can present with identical symptoms as CIM. Peripheral neuronal pathology in the critically ill was originally described by Bolton and colleagues in patients with prolonged mechanical ventilation who were difficult to liberate from mechanical ventilation [70]. CIP is classically demonstrated to be a symmetric distal, mixed sensory-motor axonal polyneuropathy. It commonly affects limb and respiratory muscles, similar to CIM, but also can impact autonomic and sensory innervation [71]. CIP is characterized by abnormal nerve conduction testing and electromyography with reduced sensory action potential amplitude and compound motor action potential amplitude, whereas CIM demonstrates normal sensory action potential amplitude [68,71,72].
Ultimately, the presence and overlap of CIM and CIP in any given critically ill patient is common and each process shares common precipitating risk factors. The given degree of axonal degeneration and myopathy will vary between patients and their preexisting diseases as well as their individual critical illness phenotypes. Improved diagnostic testing would allow for more granular characterization of ICU-AW as well as potentially set the stage for the study of more targeted therapies for individual patients depending on their degree of neuronal and muscular pathology. Related complications due to immobility and positioning, such as peroneal nerve entrapment and subsequent foot drop, are not infrequent in ARDS, and they are preventable with the use of attention to positioning, braces and early mobilization [18].
6. Pulmonary dysfunction, radiological abnormalities, and exercise limitations
The extensive inflammatory insult to the lung inherent to the pathogenesis of ARDS often raises concern regarding the residual pulmonary function of ARDS survivors. Most often, ongoing pulmonary dysfunction is assessed using pulmonary function testing (PFTs), computed tomography (CT) imaging of the chest, and exercise capacity assessments such as the 6MWT (Table 2). Studies assessing PFTs following ARDS have shown heterogenous results, but most commonly show a mild to moderate reduction in diffusion capacity of the lungs for carbon monoxide (DLCO) with variable obstruction or restriction [73–75]. Herridge and colleagues noted that at 6-month follow-up, their cohort of ARDS survivors had normal spirometry and no evidence of restriction on PFTs. They did find mild to moderate reductions in DLCO following ARDS, with these impairments continuing through 4 years after the incident hospitalization with no further decrement in lung function after the initial few months following ARDS [13,18].
Table 2.
Clinical manifestations of persistent pulmonary abnormalities following ARDS.
| Persistent Pulmonary Abnormalities | Clinical Manifestation |
|---|---|
| Abnormal Pulmonary Function Testing |
|
| Radiographic Abnormalities |
|
| Reduced Exercise Capacity |
|
In the acute phase of ARDS, bilateral alveolar infiltrates on chest imaging are pathognomonic. Follow-up imaging in survivors varies. Infiltrates on chest x-ray may completely resolve but reticular infiltrates may persist. CT imaging of the chest provides the most detailed assessment of the pulmonary parenchyma following ARDS. Notably, three-quarters or more of survivors will have imaging evidence of fibrotic disease, manifesting as reticular fibrotic change, ground-glass opacification, traction bronchiectasis, etc (Figure 2) [74,76]. In these studies, the involved regions of the lung were pre-dominantly the non-dependent regions, and the overall extent of parenchymal injury and fibrosis was mild. Early research in ARDS survivors suggested that the degree of fibrosis was positively correlated with duration of mechanical ventilation, though this cohort of patients was recruited prior to the use of lung protective low-tidal volume ventilation [77]. Additionally, patients who presented with primary pulmonary etiologies of ARDS, such as pneumonia, versus non-pulmonary causes, such as pancreatitis, were more likely to have significant fibrosis [78].
Figure 2.
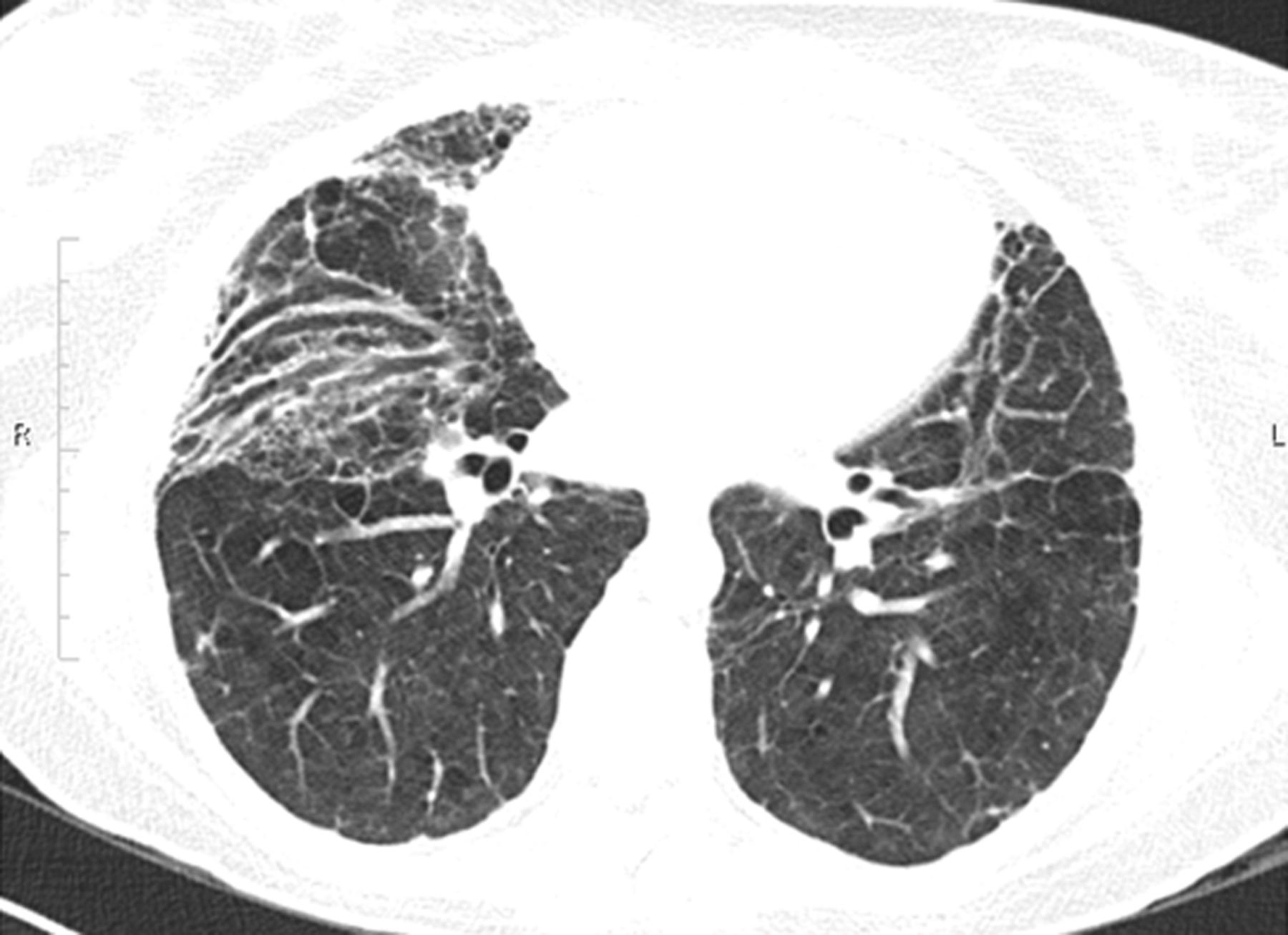
Computed tomography (CT) image of the chest of an ARDS survivor with fibrotic changes in the lungs at 18 months after ARDS.(Reproduced with permission of the © ERS 2020: European Respiratory Journal 43(1) 276,285; DOI: 10.1183/09031936.00196412 Published 31 December 2013).
In addition to persistent abnormalities in PFTs and chest imaging, many ARDS survivors have a significant exercise limitation. This has commonly been assessed through use of the 6MWT, with total distance as the main outcome. ARDS survivors have been shown to have significantly reduced 6MWT distances compared to age-adjusted means, ranging from approximately 50% of predicted at 3 months after hospitalization to 75% of predicted 5 years out from their illness [13]. Notably, while exercise limitation may be related to other etiologies, such as muscular weakness or cardiac disease, at one year follow-up, 6% of patients in the Toronto cohort had evidence of exercise-induced oxygen desaturation below 88%, suggesting ongoing pulmonary dysfunction. This is consistent with findings from Neff and colleagues, who found that half of ARDS survivors undergoing cardiopulmonary exercise testing (CPET), a more sensitive measure of pulmonary gas exchange, had evidence of reduced oxygen transfer [79]. The combination of abnormal diffusion capacity, persistent imaging findings, and reduced exercise capacity following ARDS are significant contributors, in addition to muscular weakness, mental health, and cognitive impairment, to functional limitations and reduced quality of life following ARDS.
7. Healthcare utilization, health-related quality of life, and return to work
The various physical and cognitive burdens that ARDS survivors experience following their illness can have substantial impact on their daily lives after hospitalization. Many patients experience significant ongoing healthcare needs and utilization as well as a reduced health related quality of life (HRQL). Increasingly, these outcomes are being researched in hopes of improving outcomes not just in the intensive care unit, but also for patients after they have left the hospital.
In the months to years following ARDS, survivors often have significant ongoing contact with the healthcare system. In a longitudinal follow-up study of ARDS patients from four different National Heart, Lung, and Blood Institute (NHLBI) ARDS Network clinical trials, 40% had a hospitalization in the 12 months following their initial hospitalization with ARDS [80]. In this cohort, comorbid cardiovascular disease and length of stay during the ARDS hospitalization predicted subsequent hospitalization in the next 12 months with a median cost of over 18,000 USD United States dollars for subsequent hospitalizations. Similarly, in a study of Medicare and commercial claims data evaluating patients hospitalized for ARDS, approximately 53% of survivors were re-hospitalized in the following year [81]. In the Toronto cohort of 109 ARDS survivors, 39% of patients were readmitted to the hospital within 2 years of discharge with 20% being admitted two or more times related to their hospitalization for ARDS and subsequent sequelae [48]. Repeat hospitalizations and subsequent rehabilitation were the predominant forms of healthcare use post-discharge with other costs including pharmaceutical use, physician outpatient visits, and home care. Despite surviving their severe illness, patients who experience ARDS continue to have significant healthcare needs in the months to years following.
Cognitive and physical impairments and frequent need for ongoing medical care can be burdensome and isolating for survivors of ARDS, leading to a reduced quality of life. Several studies in survivors of ARDS have analyzed health-related quality of life (HRQL), a multifactorial paradigm that evaluates physical and mental health dimensions in relation to a disease or treatment. Not unexpectedly, Herridge and colleagues found that ARDS survivors report worse physical and mental health and overall quality of life in the year following their acute illness as compared to the age and sex-matched general population [18]. In the same study, patient’s self-reported quality of life did improve over several assessments during a 12-month period, but remained below average. Hopkins and colleagues found that in the two years following ARDS, physical domains of HRQL improved up until one year despite remaining below expected for age and sex-matched controls, and then remained stable without further improvement at two years following hospitalization. There was no improvement in mental quality of life or overall general health during the two-years following ARDS [30]. Other studies have demonstrated similar reductions across multiple domains in the initial years following ARDS [48,52]. Despite evidence of initial improvement in the months following discharge, survivors of ARDS report persistent impaired HRQL beyond the initial 12–24 months after hospitalization [47]. These findings were confirmed in the Toronto cohort, where even at 5 years after hospitalization, patients reported reduced HRQL as compared to control population [13]. Notably, younger patients and those with fewer pre-ARDS comorbidities still reported significantly reduced HRQL, suggesting that ARDS contributes to poor functional status and quality of life beyond the impact of any preexisting limitations. In addition to self-reported quality of life, returning to work or employment is a vital surrogate of quality of life, linked to functional independence and a sense of well-being. Myhren et al found that in a general intensive care unit population, 55% of survivors had not returned to work at one-year [82]. In this same cohort, higher HRQL was associated with return to work by one year. In a five-year longitudinal cohort study of ARDS survivors, Kamdar and colleagues demonstrated that, of those previously employed, 31% never returned to work leading to a substantial loss of income [83]. The lack of the capacity to return to work is another substantial barrier to return to normalcy for ARDS survivors, and it is indicative of the broader growing concern regarding the outcomes of our most critically ill patients.
8. Conclusions and future directions
In the past few decades, our understanding and clinical management of ARDS has improved substantially, yet it remains a syndrome of substantial mortality and morbidity. Increasingly, the long-term impacts of ARDS are being recognized and studied. While some ARDS survivors will not experience limitations following their hospitalization, a significant number of them will be left with persistent impairments in a variety of organ systems, from cognitive impairment and psychiatric illness to physical weakness and neuromuscular pathology. Evidence of the long-lasting impact of ARDS can be seen in the imaging of the pulmonary system, with persistent pulmonary fibrosis and interstitial damage as well as ongoing limitations in exercise capacity. Lastly, patients who survive ARDS, especially those with significant comorbidities or a substantial burden of impairments, often suffer from recurrent hospitalizations and frequent healthcare usage. This constellation of long-term sequelae leads ARDS patients to suffer with reduced quality of life and limits their opportunities to return to employment.
Greater scientific understanding into both the pathophysiology and the epidemiology of these long-lasting effects of ARDS will be needed in order to develop novel prevention and treatment approaches to the long-term sequelae of ARDS. In addition to the focus on finding direct therapies to treat and prevent ARDS, critical care practice is evolving to focus on reducing risk factors for poor long-term outcomes for ARDS. For example, avoidance of benzodiazepines and utilization of light sedation, interventions that reduce delirium, are being implemented to help not only prevent delirium but also the downstream pathology of long-term cognitive impairment. Early mobility and physical rehabilitation have received substantial research attention as methods to reduce ICU-AW and physical impairment. Rehabilitation strategies after hospital discharge continue to be a source of significant research in hopes of improving both the physical and cognitive outcomes of ARDS. Treatment and management of post-ICU complications is also receiving greater focus, with growing interest in multi-disciplinary post-ICU clinics as a possible strategy to help patients receive the care they need to manage any aftereffects of ARDS. Post-ICU clinics provide coordinated, evidence-based care for ICU survivors, which can include physician and advanced practice provider visits, neuropsychological assessment, pharmacy services, and case management [84]. Provision of guideline recommended care for sepsis survivors improved morbidity and mortality after hospitalization [85]. Similar coordinated post-discharge care may also improve outcomes for ARDS survivors. Additionally, improved understanding of the pathophysiology of both the acute phase of ARDS and its long-term downstream effects, has great potential to reduce the consequences of ARDS by leading to new therapeutic strategies and by shifting the focus of clinical care to include not just the acute management of ARDS but also the management of the long-lasting effects of ARDS.
9. Expert opinion
The development of long-term sequelae from ARDS is a significant outcome for patients, one that has implications for many years following hospitalization. ARDS is a major risk factor for several outcomes, including long-term cognitive impairment, ICU-AW, persistent pulmonary dysfunction, and reduced quality of life. These outcomes are increasingly understood as significant, patient-centered outcomes, with increasing scientific effort devoted to combatting their development. Because of the major repercussions of the long-term outcomes from ARDS, further understanding of the pathophysiology of these outcomes and treatment strategies is paramount. The individual pathophysiology of the numerous long-term impairments seen after ARDS is complex and multifactorial. The field will continue to benefit from in-depth and focused basic science research into the mechanisms of cognitive impairment and mental illness after ARDS as well as research further elucidating the underlying mechanisms of ICU-AW. Further advances in this research will allow for the development of novel treatment approaches, which will be applicable both to patients with ARDS and other critically ill patients. Clinical trials of therapies such as early mobility and cognitive rehabilitation, have thus far demonstrated mixed results with unclear benefit. Focused identification of high-risk patients coupled with robust clinical trials of targeted interventions are needed to advance the field forward and reduce the physical and cognitive morbidities. As the research in these areas improves our understanding, increasing focus at the bedside should be to implement the current best-practice guidelines for ARDS with a goal of minimizing iatrogenesis that may contribute to these downstream sequelae. Given the significant burden of ARDS, both acutely and following hospital discharge, there is great opportunity to improve the long-term outcomes of our most critically ill patients.
In the next several years, we anticipate increasing research into the fundamental mechanisms of the complications of ARDS and critical illness more broadly. Currently, while we have started to understand the risk factors for long-term cognitive impairment and ICU-acquired weakness, further research, particularly at the basic level, will further elucidate these complex pathophysiologies. Additionally, In the last several years, ARDS has been increasingly recognized as a heterogenous syndrome with varying pathological and clinical characteristics. Calfee and colleagues have identified subphenotypes of ARDS based on inflammatory states, demonstrating that there are hyperinflammatory and hypoinflammatory subphenotypes with different clinical characteristics and outcomes [86]. These subphenotypes have been confirmed in other ARDS cohorts, as well, with similar outcomes [87–89]. Further study and characterization of these subphenotypes and their underlying mechanisms will set the stage for the development of biomarker-guided targeted therapy with the goal of personalizing ARDS treatment. Precision treatment of ARDS based on subphenotypes could potentially reduce the long-term complications that stem from the disease.
In addition to basic research at the bench, the development of biorepositories from human specimens will also help advance the field. Biobanks of brain, lung, and muscle tissue from patients with ARDS, including various subtypes, will advance translational research in the field, allowing observations made at the bench to be evaluated in human tissue and samples. In addition to biobanks, we also anticipate increasing interest in the genetic risk factors for complications from ARDS as well as continued focus on the identification of biomarkers and subphenotypes. Identifying high-risk patients and then studying novel treatments, coupled with the use of biomarkers to both help predict and follow disease-related outcomes, will help create more robust and informative clinical data. These data will allow for the development and implementation of clinical trials for therapies focused specifically on preventing the deleterious downstream effects of ARDS. Coupled with greater mechanistic understanding of individual disease processes, we anticipate the study of new therapies that will target specific etiologies of the morbidities of ARDS.
In summary, we anticipate that the future will bring greater understanding of the long-lasting effects of ARDS and the most effective management. Based on the current breadth of research, we anticipate more trials focused on therapeutic tools to prevent and limit or treat processes such as long-term cognitive impairment and ICU-AW. Ultimately, we anticipate that the critical care physician, astute in the care of the ARDS patient acutely, will merge this knowledge with the increasing understanding of the long-term effects of ARDS to improve the long-term outcomes of our sickest patients.
Article Highlights.
ARDS is a severe form of acute lung injury and critical illness, and its impact reaches beyond the immediate illness to impact survivors for years following hospitalization.
ARDS is associated with ongoing mortality risk following discharge from the hospital that may be related to other complications of critical illness, such as muscular weakness.
Patients who suffer from ARDS often develop a persistent form of cognitive dysfunction that can be severe and long-lasting. The strongest risk factor for this cognitive impairment is the development and duration of delirium during critical illness.
In addition to cognitive impairment, ARDS patients can suffer from significant psychological morbidities, including anxiety, depression, and PTSD.
One of the more debilitating impairments seen after ARDS is intensive care acquired weakness (ICU-AW), a broad term encompassing critical illness myopathy and polyneuropathy. These processes lead to muscle wasting and weakness that persists and is associated with greater long-term mortality following ARDS.
ARDS survivors also experience greater use of healthcare resources and re-hospitalizations following their illness, which is coupled with a significant reduction in quality of life following their disease that may not improve to pre-illness levels.
Funding
The authors are supported by funding from the NIH, including 5K24 HL103836 received by LB Ware and research training support received by MF Mart (NIH 5T32 HL087738). Additional research funding support through the Vanderbilt University Medical Center Arthur and Lisa Wheeler Critical Care Research Fund is received by MF Mart.
Declaration of interest
LB Ware has received advisory board fees from Bayer, Quark, Merck and CSL Behring and research support from CSL Behring and Genentech. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. [DOI] [PubMed] [Google Scholar]
- 2.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. Jama. 2012;307 (23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, et al. , Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. [DOI] [PubMed] [Google Scholar]; • Study outlining the important epidemiology and trends for ARDS internationally.
- 5.Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med. 2011;183(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ARDSNet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342(18):1301–1308. [DOI] [PubMed] [Google Scholar]; •• Seminal study showing benefit of low-tidal volume ventilation in ARDS.
- 7.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14(1):R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. Jama. 2012;308(16):1651–1659. [DOI] [PubMed] [Google Scholar]
- 9.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. transfusion requirements in critical care investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340 (6):409–417. [DOI] [PubMed] [Google Scholar]
- 10.Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176(9):886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–1522. [DOI] [PubMed] [Google Scholar]
- 12.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181(10):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herridge MS, Tansey CM, Matte A, et al. , Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. [DOI] [PubMed] [Google Scholar]; •• Large cohort demonstrating the long-term disability and morbidities of survivors of ARDS.
- 14.Chiumello D, Coppola S, Froio S, et al. What’s next after ARDS: long-term outcomes. Respir Care. 2016;61(5):689–699. [DOI] [PubMed] [Google Scholar]
- 15.Brinkman S, de Jonge E, Abu-Hanna A, et al. Mortality after hospital discharge in ICU patients. Crit Care Med. 2013;41(5):1229–1236. [DOI] [PubMed] [Google Scholar]
- 16.Wunsch H, Guerra C, Barnato AE, et al. Three-year outcomes for medicare beneficiaries who survive intensive care. Jama. 2010;303 (9):849–856. [DOI] [PubMed] [Google Scholar]
- 17.Lone NI, Gillies MA, Haddow C, et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med. 2016;194(2):198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herridge MS, Cheung AM, Tansey CM, et al. , One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. [DOI] [PubMed] [Google Scholar]; • One of the major studies that first described long-term outcomes of ARDS.
- 19.Khandelwal N, Hough CL, Bansal A, et al. Long-term survival in patients with severe acute respiratory distress syndrome and rescue therapies for refractory hypoxemia*. Crit Care Med. 2014;42(7):1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biehl M, Ahmed A, Kashyap R, et al. The incremental burden of acute respiratory distress syndrome: long-term follow-up of a population-based nested case-control study. Mayo Clin Proc. 2018;93(4):445–452. [DOI] [PubMed] [Google Scholar]
- 21.Wang CY, Calfee CS, Paul DW, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome [journal article]. Intensive Care Med. 2014;40 (3):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinglas VD, Aronson Friedman L, Colantuoni E, et al. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit Care Med. 2017;45(3):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermans G, Van Mechelen H, Clerckx B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. [DOI] [PubMed] [Google Scholar]
- 24.Mirzakhani H, Williams J-N, Mello J, et al. Muscle weakness predicts pharyngeal dysfunction and symptomatic aspiration in long-term ventilated patients. Anesthesiol J Am Soc Anesthesiologists. 2013;119(2):389–397. [DOI] [PubMed] [Google Scholar]
- 25.McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care. 2011;15(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186 (2):E95–E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hope AA, Chen JT, Kaufman DA, et al. The association between prehospital vulnerability, ARDS development, and mortality among At-risk adults. Results from the LIPS-A clinical trial. Ann Am Thorac Soc. 2019;16(11):1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saliba D, Elliott M, Rubenstein LZ, et al. The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691–1699. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox ME, Brummel NE, Archer K, et al. Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Crit Care Med. 2013;41(9 Suppl 1):S81–98. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–347. [DOI] [PubMed] [Google Scholar]; •• One of the early studies to identify cognitive dysfunction following ARDS.
- 31.Jackson JC, Hopkins RO, Miller RR, et al. Acute respiratory distress syndrome, sepsis, and cognitive decline: a review and case study. South Med J. 2009;102(11):1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross AL, Jones RN, Habtemariam DA, et al. , Delirium and long-term cognitive trajectory among persons with dementia. Arch Internal Med. 2012;172(17):1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Large observational cohort identifying long-term cognitive impairment after critical illness and its relationship to delirium.
- 33.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369 (14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pisani MA, Redlich C, McNicoll L, et al. Underrecognition of preexisting cognitive impairment by physicians in older ICU patients. Chest. 2003;124(6):2267–2274. [DOI] [PubMed] [Google Scholar]
- 35.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James BD, Wilson RS, Capuano AW, et al. Cognitive decline after elective and nonelective hospitalizations in older adults. Neurology. 2019;92(7):e690–e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adhikari NK, McAndrews MP, Tansey CM, et al. Self-reported symptoms of depression and memory dysfunction in survivors of ARDS. Chest. 2009;135(3):678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adhikari NK, Tansey CM, McAndrews MP, et al. Self-reported depressive symptoms and memory complaints in survivors five years after ARDS. Chest. 2011;140(6):1484–1493. [DOI] [PubMed] [Google Scholar]
- 40.Needham DM, Dinglas VD, Bienvenu OJ, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346(mar19 3):f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Bahr V, Kalzen H, Hultman J, et al. Long-term cognitive outcome and brain imaging in adults after extracorporeal membrane oxygenation. Crit Care Med. 2018;46(5):e351–e358. [DOI] [PubMed] [Google Scholar]
- 45.Sylvestre A, Adda M, Maltese F, et al. Long-term neurocognitive outcome is not worsened by of the use of venovenous ECMO in severe ARDS patients. Ann Intensive Care. 2019;9(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanfilippo F, Ippolito M, Santonocito C, et al. Long-term functional and psychological recovery in a population of acute respiratory distress syndrome patients treated with VV-ECMO and in their caregivers. Minerva Anestesiol. 2019;85(9):971–980. [DOI] [PubMed] [Google Scholar]
- 47.Schelling G, Stoll C, Haller M, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998;26(4):651–659. [DOI] [PubMed] [Google Scholar]
- 48.Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174(5):538–544. [DOI] [PubMed] [Google Scholar]
- 49.Needham DM, Wozniak AW, Hough CL, et al. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med. 2014;189(10):1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davydow DS, Desai SV, Needham DM, et al. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapfhammer HP, Rothenhausler HB, Krauseneck T, et al. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004;161(1):45–52. [DOI] [PubMed] [Google Scholar]
- 52.Luyt CE, Combes A, Becquemin MH, et al. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest. 2012;142(3):583–592. [DOI] [PubMed] [Google Scholar]
- 53.Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: a 1-year national multicenter study. Crit Care Med. 2016;44(5):954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown SM, Wilson EL, Presson AP, et al. Understanding patient outcomes after acute respiratory distress syndrome: identifying subtypes of physical, cognitive and mental health outcomes. Thorax. 2017;72(12):1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42(4):849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hough CL, Steinberg KP, Taylor Thompson B, et al. Intensive care unit-acquired neuromyopathy and corticosteroids in survivors of persistent ARDS. Intensive Care Med. 2009;35(1):63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. [DOI] [PubMed] [Google Scholar]
- 58.de Letter MA, Schmitz PI, Visser LH, et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med. 2001;29(12):2281–2286. [DOI] [PubMed] [Google Scholar]
- 59.Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27(8):1288–1296. [DOI] [PubMed] [Google Scholar]
- 60.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363 (12):1107–1116. [DOI] [PubMed] [Google Scholar]
- 61.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. [DOI] [PubMed] [Google Scholar]
- 62.Kortebein P, Ferrando A, Lombeida J, et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297 (16):1772–1774. [DOI] [PubMed] [Google Scholar]
- 63.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. [DOI] [PubMed] [Google Scholar]
- 64.Jolley SE, Bunnell AE, Hough CL. ICU-acquired weakness. Chest. 2016;150(5):1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 Suppl):S299–308. [DOI] [PubMed] [Google Scholar]
- 66.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. Jama. 2002;288(22):2859–2867. [DOI] [PubMed] [Google Scholar]
- 67.Latronico N, Fenzi F, Recupero D, et al. Critical illness myopathy and neuropathy. Lancet. 1996;347(9015):1579–1582. [DOI] [PubMed] [Google Scholar]
- 68.Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32(2):140–163. [DOI] [PubMed] [Google Scholar]
- 69.Angel MJ, Bril V, Shannon P, et al. Neuromuscular function in survivors of the acute respiratory distress syndrome. Can J Neurol Sci. 2007;34(4):427–432. [DOI] [PubMed] [Google Scholar]
- 70.Bolton CF, Gilbert JJ, Hahn AF, et al. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47(11):1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Batt J, Dos Santos CC, Cameron JI, et al. Intensive care unit-acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med. 2013;187(3):238–246. [DOI] [PubMed] [Google Scholar]
- 72.Witt NJ, Zochodne DW, Bolton CF, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99(1):176–184. [DOI] [PubMed] [Google Scholar]
- 73.Elliott CG, Rasmusson BY, Crapo RO, et al. Prediction of pulmonary function abnormalities after adult respiratory distress syndrome (ARDS). Am Rev Respir Dis. 1987;135(3):634–638. [DOI] [PubMed] [Google Scholar]
- 74.Masclans JR, Roca O, Munoz X, et al. Quality of life, pulmonary function, and tomographic scan abnormalities after ARDS. Chest. 2011;139(6):1340–1346. [DOI] [PubMed] [Google Scholar]
- 75.Linden VB, Lidegran MK, Frisen G, et al. ECMO in ARDS: a long-term follow-up study regarding pulmonary morphology and function and health-related quality of life. Acta Anaesthesiol Scand. 2009;53 (4):489–495. [DOI] [PubMed] [Google Scholar]
- 76.Wilcox ME, Patsios D, Murphy G, et al. Radiologic outcomes at 5 years after severe ARDS. Chest. 2013;143(4):920–926. [DOI] [PubMed] [Google Scholar]
- 77.Desai SR, Wells AU, Rubens MB, et al. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210(1):29–35. [DOI] [PubMed] [Google Scholar]
- 78.Kim SJ, Oh BJ, Lee JS, et al. Recovery from lung injury in survivors of acute respiratory distress syndrome: difference between pulmonary and extrapulmonary subtypes. Intensive Care Med. 2004;30 (10):1960–1963. [DOI] [PubMed] [Google Scholar]
- 79.Neff TA, Stocker R, Frey HR, et al. Long-term assessment of lung function in survivors of severe ARDS. Chest. 2003;123(3):845–853. [DOI] [PubMed] [Google Scholar]
- 80.Ruhl AP, Huang M, Colantuoni E, et al. Healthcare utilization and costs in ARDS survivors: a 1-year longitudinal national US multicenter study. Intensive Care Med. 2017;43(7):980–991. [DOI] [PubMed] [Google Scholar]
- 81.Wu N, Hanrahan J, Bornstein J, et al. Healthcare costs utilization and costs of patients hospitalized with acute respiratory distress syndrome (ARDS) in US commercially-insured individuals and medicare beneficiaries. Eur Respir J. 2015;46(suppl 59): PA2139. [Google Scholar]
- 82.Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med. 2010;38 (7):1554–1561. [DOI] [PubMed] [Google Scholar]
- 83.Kamdar BB, Huang M, Dinglas VD, et al. Joblessness and lost earnings after acute respiratory distress syndrome in a 1-year national multicenter study. Am J Respir Crit Care Med. 2017;196 (8):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bloom SL, Stollings JL, Kirkpatrick O, et al. Randomized clinical trial of an ICU recovery pilot program for survivors of critical illness. Crit Care Med. 2019;47(10):1337–1345. [DOI] [PubMed] [Google Scholar]
- 85.Taylor SP, Chou SH, Sierra MF, et al. Association between adherence to recommended care and outcomes for adult survivors of sepsis. Ann Am Thorac Soc. 2020;17(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2 (8):611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sinha P, Delucchi KL, Thompson BT, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44(11):1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Famous KR, Delucchi K, Ware LB, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195 (3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]


