Abstract
Purpose
The optimum systemic therapies for advanced/metastatic renal cell carcinoma (RCC) of favourable, intermediate and poor risk have not been established. We aimed to compare and rank the effects associated with systemic therapies in the first-line setting.
Methods
We searched PubMed, Cochrane databases, Web of Science and ClinicalTrials.gov for randomised controlled trials (RCT) published up to February 2020 of all available treatments for advanced/metastatic RCC. Analysis was done on a Bayesian framework.
Results
15 unique RCTs including 8995 patients were identified. For advanced/metastatic RCC of favourable risk, avelumab plus axitinib was associated with a significantly higher improvement in progression-free survival (PFS) than sunitinib (HR 0.57, 95% CI 0.34 to 0.96). For intermediate-risk patients, cabozantinib, nivolumab plus ipilimumab, pembrolizumab plus axitinib and avelumab plus axitinib were associated with significantly higher improvement in PFS than sunitinib (HR 0.63, 95% CI 0.44 to 0.97; HR 0.66, 95% CI 0.53 to 0.81; HR 0.58, 95% CI 0.44 to 0.80; HR 0.62, 95% CI 0.47 to 0.83, respectively); pembrolizumab plus axitinib and nivolumab plus ipilimumab were associated with significantly higher improvement in overall survival (OS) than sunitinib (HR 0.53, 95% CI 0.34 to 0.81; HR 0.66, 95% CI 0.50 to 0.87, respectively). For poor-risk patients, nivolumab plus ipilimumab and pembrolizumab plus axitinib were associated with significantly higher improvement in PFS than sunitinib (HR 0.57, 95% CI 0.43 to 0.76; HR 0.48, 95% CI 0.30 to 0.82, respectively); nivolumab plus ipilimumab and pembrolizumab plus axitinib were significantly more efficacious for OS than sunitinib (HR 0.57, 95% CI 0.39 to 0.883; HR 0.43, 95% CI 0.23 to 0.80, respectively). For OS, there were 81% and 78% probabilities that pembrolizumab plus axitinib was the best option for intermediate-risk and poor-risk patients, respectively.
Conclusion
Avelumab plus axitinib might be the optimum treatment for advanced/metastatic RCC of favourable risk. Pembrolizumab plus axitinib might be the optimum treatment for intermediate-risk and poor-risk patients.
Keywords: urological tumours, kidney tumours, immunology
Strengths and limitations of this study.
This is the first network analysis to compare systemic treatments for advanced/metastatic renal cell carcinoma separately by risk groups.
Various statistical models were applied to synthesise data. The reliability and accuracy of results were corroborated by the low statistical heterogeneity and excellent model fit.
Assessment of both efficacy and adverse events provides new insights into the benefit-harm balance of different systemic treatments.
Main limitation lies in the reporting quality of trials included.
Introduction
Renal cell carcinoma (RCC) comprises approximately 90% of renal cancer, and represents approximately 2%–3% of all new cancers worldwide.1 It was estimated that there would be 62 700 new cases of renal cancer and 14 240 renal cancer-related deaths in the USA in 2016.2 In the European Union, new renal cancer cases and deaths in 2012 were approximately 84 400 and 34 700, respectively.3 Up to 30% of patients were presented with advanced/metastatic RCC at the time of initial diagnosis.4 5 Advanced/metastatic RCC is not a single condition, but is actually a heterogeneous group of conditions with different prognosis. The most widely accepted prognostic model is from the Memorial Sloan Kettering Cancer Center (MSKCC) and stratifies patients into favourable, intermediate and poor-risk groups depending on the existence of well-characterised laboratory and clinical risk factors. The 2-year survival rates were 45%, 17% and 3% for favourable, intermediate and poor-risk groups, respectively.6 In this systematic review, we focus on favourable, intermediate and poor-risk patients with advanced/metastatic RCC.
In recent years, systemic treatment for advanced/metastatic RCC has changed from cytokines to drugs targeting angiogenesis. In 2007, results from two randomised controlled trials (RCT) have been published reporting progression-free survival (PFS) improvement of two newer targeted agents (sunitinib and sorafenib).7 8 To date, eight targeted drugs have been approved for treating advanced/metastatic RCC both in the USA and Europe: five tyrosine kinase inhibitors (TKI): sunitinib, sorafenib, pazopanib, cabozantinib and axitinib; two mammalian target of rapamycin (mTOR) complex 1 kinase inhibitors: temsirolimus and everolimus; and the recombinant humanised anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab combined with interferon-α (IFN-α). All eight targeted drugs showed significant survival benefit in randomised trials and established a prominent role in treating advanced/metastatic RCC.7 9–15 More recently, immune checkpoint antibodies have introduced a new treatment option. CheckMate 214 reported that nivolumab plus ipilimumab was associated with a significantly higher overall survival (OS) than sunitinib in the first-line setting.16 To further improve their efficacy, the combination of different classes of agents is currently evaluated in clinical trials.17–20 However, there are insufficient head-to-head RCTs to directly investigate the comparative effectiveness of all available therapies. Given the variety of treatment options for patients with advanced/metastatic RCC and the limited evidence regarding the optimum treatment strategy, it is a challenge for clinicians to make the best decision.
In the present study, we performed a Bayesian network meta-analysis to compare first-line systemic treatments for advanced/metastatic RCC of favourable, intermediate and poor risk, respectively. Network meta-analysis enables indirect comparisons based on a common comparator treatment when a head-to-head trial is unavailable and integrates direct and indirect comparisons to compare several treatment strategies while fully respecting randomisation.21 22 We aimed to summarise and compare the efficacy and safety associated with currently available systemic therapies for treating advanced/metastatic RCC of different risk categories using network meta-analysis.
Methods
Literature search strategy
A comprehensive literature search was performed in PubMed, Web of Science, ClinicalTrials.gov and Cochrane databases for RCTs of systemic therapies of advanced/metastatic RCC (see online supplementary appendix for all search terms). All the reference lists of identified trials and related reviews were examined to find potential trials. The search was conducted in February 2020. There were no publication date or language restrictions.
bmjopen-2019-034626supp001.pdf (1.4MB, pdf)
Inclusion and exclusion criteria
All studies were selected according to the search strategy based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria.23 Studies were included if they satisfied three criteria: (1) the study enrolled patients who had histologically or cytologically confirmed advanced/metastatic RCC of favourable, intermediate or poor risk; (2) patients were randomly assigned to receive systemic therapies alone or in combination. Relevant interventions included, but were not restricted to: sorafenib, sunitinib, pazopanib, cabozantinib, nivolumab, ipilimumab, axitinib, tivozanib, everolimus, temsirolimus, or bevacizumab plus IFN-α. Previous systemic therapy for advanced/metastatic RCC was not allowed; (3) one or more of the outcomes of interest mentioned below were reported. Non-original articles, duplicate reports and non-RCTs were excluded.
Data extraction and quality assessment
Two researchers (GC and XiW) examined the manuscripts of included trials independently, and extracted data into a structured form, including patient characteristics, treatment strategies and interest outcomes (PFS, OS, high-grade (grade ≥3) and overall drug-related adverse events (AE)). The patient characteristics, treatment strategies, PFS and OS were extracted at the study level for meta-analyses even if the patient level were available. For drug-related AEs, the patient-level data were extracted for meta-analyses. We gave priority to extracting data from intention-to-treat analyses. The methodological quality of included RCTs was assessed using the Cochrane risk of bias assessment tool.24 Disagreement between investigators was resolved by consensus.
Data synthesis and analysis
First, we performed traditional meta-analyses to compare the treatments using Stata V.12 (StataCorp, College Station, TX, USA). We applied the χ2 test and the I² statistic to investigate the possibility of heterogeneity among studies. A p value <0.10 or an I2 >50% suggested the presence of substantial heterogeneity.
Second, we did Bayesian network meta-analyses. For meta-analysis of PFS and OS, the reported adjusted HRs with 95% CIs were applied as the outcome measure. For studies not reporting HRs, we calculated them from Kaplan-Meier curve and information on follow-up with the pragmatic approach reported by Tierney et al.25 For drug-related AEs, we calculated ORs using the available patient-level data abstracted from the trials. Both random effects and fixed effects models were performed for all Bayesian network meta-analyses.26 Goodness of model fit was assessed using the deviance information criterion and between-study SD.26 27 Convergence was determined graphically according to the method described by Gelman and Rubin.28
It is believed that certain systemic treatments are effective in certain risk groups than others, for example, sunitinib is more effective in favourable-risk patients and nivolumab plus ipilimumab is more effective in intermediate and poor-risk patients,29 suggesting that there is a treatment-by-risk group (favourable, intermediate and poor-risk groups) interaction. Taking no account of this possible interaction in the analysis, transitivity assumption across all included trials would be violated. Therefore, we performed all network analyses separately by risk groups (favourable, intermediate and poor-risk groups) according to the MSKCC or International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk model to assure transitivity assumption.
One key assumption for network analysis is that direct and indirect comparisons do not disagree beyond chance.26 30 To explore for evidence of inconsistency in the network, investigators compared the estimated treatment effects from the entire network with traditional pairwise estimates.30 Sensitivity analyses were performed restricted to trials that assessed approved systemic therapies (sunitinib, sorafenib, pazopanib, cabozantinib, axitinib, everolimus, temsirolimus, bevacizumab plus IFN-α and nivolumab plus ipilimumab). Publication bias and small-study effects were assessed using funnel plots.31
We performed the Bayesian network analysis using OpenBUGS V.3.2.2 for PFS, and Gemtc V.0.14.3 (van Valkenhoef et al,32) for AEs. We performed fewer iterations for PFS to reduce computational burden without loss of convergence and model fit. For PFS, we applied 15 000 iterations obtained after a training phase of 10 000 iterations. In order to minimise autocorrelation, we applied a thinning interval of 50 for each chain. For AEs, we applied the 60 000 iterations after a training phase of 40 000 iterations. The treatments were ranked in terms of PFS, OS and high-grade AEs, respectively, using the surface under the cumulative ranking curve (SUCRA) and the distribution of the ranking probabilities.33
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Search results and study characteristics
The literature search yielded 2017 potentially eligible studies, of which 1873 were excluded based on screening titles and abstracts (figure 1). The full texts of 144 remaining studies were analysed, and finally 21 publications reporting 15 unique RCTs were included (table 1), involving 8995 participants randomly assigned to one of the 13 treatment strategies: sorafenib, sunitinib, pazopanib, cabozantinib, nivolumab plus ipilimumab, axitinib, tivozanib, everolimus, IFN-α, bevacizumab plus IFN-α, temsirolimus plus bevacizumab, avelumab plus axitinib, and pembrolizumab and axitinib. According to the MSKCC or IMDC criteria, there were 2783, 5474 and 721 participants who had favourable, intermediate and poor-risk disease, respectively.
Figure 1.
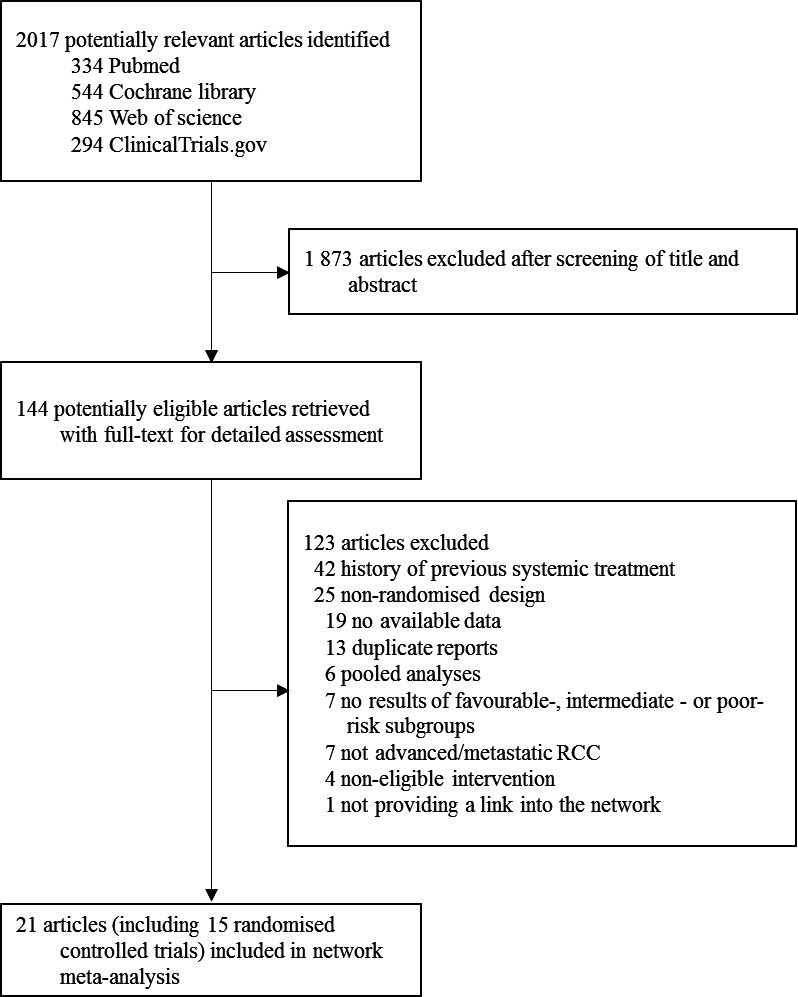
Literature search and selection. RCC, renal cell carcinoma.
Table 1.
Studies included in the multiple-treatment meta-analysis
| Study | Patients (n) | Age (years) median (range) | Sex (% male) | Favourable | MSKCC (%) Intermediate | Poor | Median PFS in months (95% CI) | HR (95% CI) |
| Motzer et al39 (RECORD-3) | ||||||||
| Everolimus | 238 | 62 (20–89) | 69.7 | 29 | 56 | 15 | 7.9 | 1.4 (1.2 to 1.8) |
| Sunitinib | 238 | 62 (29–84) | 73.9 | 30 | 56 | 14 | 10.7 | 1 (Ref) |
| Motzer et al43 (COMPARZ) | ||||||||
| Pazopanib | 557 | 61 (18–88) | 71 | 27 | 58 | 12 | 8.4 (8.3 to 10.9) | 1.05 (0.90 to 1.22) |
| Sunitinib | 553 | 62 (23–86) | 75 | 27 | 59 | 9 | 9.5 (8.3 to 11.1) | 1 (Ref) |
| Rini et al38 (INTORACT) | ||||||||
| Temsirolimus plus bevacizumab | 400 | 59 (22–87) | 72 | 28 | 65 | 8 | 9.1 (8.1 to 10.2) | 1.1 (0.9 to 1.3) |
| Bevacizumab plus IFN-α | 391 | 58 (23–81) | 69 | 27 | 65 | 8 | 9.3 (9.0 to 11.2) | 1 (Ref) |
| Procopio et al42 (ROSORC) | ||||||||
| Sorafenib plus interleukin-2 | 66 | 64 (57–69)* | 79 | 55 | 41 | 5 | NA | NA |
| Sorafenib | 62 | 62 (52–69)* | 69 | 55 | 39 | 6 | NA | NA |
| Hutson et al12 | ||||||||
| Axitinib | 192 | 58 (23–83) | 70 | 49 | 44 | 4 | 10.1 (7.2 to 12.1) | 0.77 (0.56 to 1.05) |
| Sorafenib | 96 | 58 (20–77) | 77 | 55 | 42 | 2 | 6.5 (4.7 to 8.3) | 1 (Ref) |
| Motzer et al13 | ||||||||
| Tivozanib | 260; treatment naive 181 | 59 (23–83) | 71 | 27 | 67 | 7 | 12.7 (9.1 to 15.0) | 0.756 (0.580 to 0.985) |
| Sorafenib | 257; treatment naive 181 | 59 (23–85) | 74 | 34 | 62 | 4 | 9.1 (7.3 to 10.8) | 1 (Ref) |
| Sternberg et al11 (VEG105192) | ||||||||
| Pazopanib | 290; treatment naive 155 | 59 (28–82) | 68 | 36 | 56 | 4 | 11.1 | 0.40 (0.27 to 0.60) |
| Placebo | n=145; treatment naive 78 | 62 (25–81) | 74 | 40 | 51 | 6 | 2.8 | 1 (Ref) |
| Motzer et al36 | ||||||||
| Sunitinib | 375 | 62 (27–87) | 71 | 38 | 56 | 6 | 11 (11 to 13) | 0.539 (0.451 to 0.643) |
| IFN-α | 375 | 59 (34–85) | 72 | 34 | 59 | 7 | 5 (4 to 6) | 1 (Ref) |
| Negrier et al10 (TARGET) | ||||||||
| Sorafenib | 451; treatment naive 77 | 60 | 63.6 | 53.2 | 46.8 | 0 | 5.8 | 0.48 (0.32 to 0.73) |
| Placebo | 452; treatment naive 84 | 60.5 | 69 | 45.2 | 54.8 | 0 | 2.8 | 1 (Ref) |
| Rini et al35 (CALGB 90206) | ||||||||
| Bevacizumab plus IFN-α | 369 | 61 (56–70) | 73 | 26 | 64 | 10 | 8.5 (7.5 to 9.7) | 0.71 (0.61 to 0.83) |
| IFN-α | 363 | 62 (55–70) | 66 | 26 | 64 | 10 | 5.2 (3.1 to 5.6) | 1 (Ref) |
| Escudier et al34 (AVOREN) | ||||||||
| Bevacizumab plus IFN-α | 327 | 61 (30–82) | 68 | 27 | 56 | 9 | 10.2 | 0.61 (0.51 to 0.73) |
| IFN-α | 322 | 60 (18–81) | 73 | 29 | 56 | 8 | 5.4 | 1 (Ref) |
| Motzer et al16 (CheckMate 214) | ||||||||
| Nivolumab plus ipilimumab | 550 | 62 (26–85) | 75 | 23 | 61 | 17 | 11.6 (8.7 to 15.5) | 0.82 (0.64 to 1.05)† |
| Sunitinib | 546 | 62 (21–85) | 72 | 23 | 61 | 16 | 8.4 (7.0 to 10.8) | 1 (Ref) |
| Choueiri et al15 | ||||||||
| Cabozantinib | 79 | 63 (40–82) | 83.5 | 0 | 81.0‡ | 19.0‡ | 8.2 (6.2 to 8.8) | 0.66 (0.46 to 0.95) |
| Sunitinib | 78 | 64 (31–87) | 73.1 | 0 | 80.8‡ | 19.2‡ | 5.6 (3.4 to 8.1) | 1 (Ref) |
| Motzer et al41 (JAVELIN Renal 101) | ||||||||
| Avelumab plus axitinib | 442 | 62 (29–83) | 71.5 | 21.7 | 64 | 11.5 | 13.8 (11.1-NE) | 0.69 (0.56 to 0.84) |
| Sunitinib | 444 | 61 (27–88) | 77.5 | 22.5 | 66 | 10.1 | 8.4 (6.9 to 11.1) | 1 (Ref) |
| Rini et al40 (KEYNOTE-426) | ||||||||
| Pembrolizumab and axitinib | 432 | 62 (30–89) | 71.3 | 31.9‡ | 55.1‡ | 13‡ | 15.1 (12.6 to 17.7) | 0.69 (0.57 to 0.84) |
| Sunitinib | 429 | 61 (26–90) | 74.6 | 30.5‡ | 57.3‡ | 12.1‡ | 11.1 (8.7 to 12.5) | 1 (Ref) |
RECORD-3, COMPARZ, INTORACT, ROSORC, TARGET, CALGB, AVOREN, JAVELIN, and KEYNOTE are names of the trials.
NE denotes could not be estimated.
*IQR.
†99.1% CI.
‡International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group.
CALGB, Cancer and Leukemia Group B; IFN-α, interferon-α; INTORACT, Investigation of Torisel and Avastin Combination Therapy trial; MSKCC, Memorial Sloan Kettering Cancer Center; NA, not available; PFS, progression-free survival; RECORD, Renal Cell Cancer Treatment With Oral RAD001 Given Daily; Ref, reference group (hence HR set to 1); TARGET, Treatment Approaches in Renal Cancer Global Evaluation Trial.
The main characteristics of included RCTs are summarised in table 1. The demographic characteristics of patients were well balanced across trials. Enrolled patients across trials were similar in terms of age, gender and risk classification. Across trials, the median age of patients ranged from 58 to 64 years. The participants were predominantly male (71.7%, 6451 of 8995). The included trials were designed similarly. Median follow-up ranged from 10.7 to 58 months. The mean sample sizes were 100, 192 and 32 patients per group for favourable, intermediate and poor-risk subtypes, respectively. Thirteen trials selected for clear-cell carcinoma subtypes,10–12 15 16 34–41 and two trials also included small subsets of non-clear-cell histotypes, each comprising 11% and 14% of the study population, respectively.42 43 All studies were two-arm trials. The dosages used in most of the trials were within the recommended dose ranges.
In this network meta-analysis, results are reported based on fixed effects models because they demonstrated better goodness of fit compared with random effects models. The results of random effects models are available in online supplementary appendix tables 1–5.
Progression-free survival
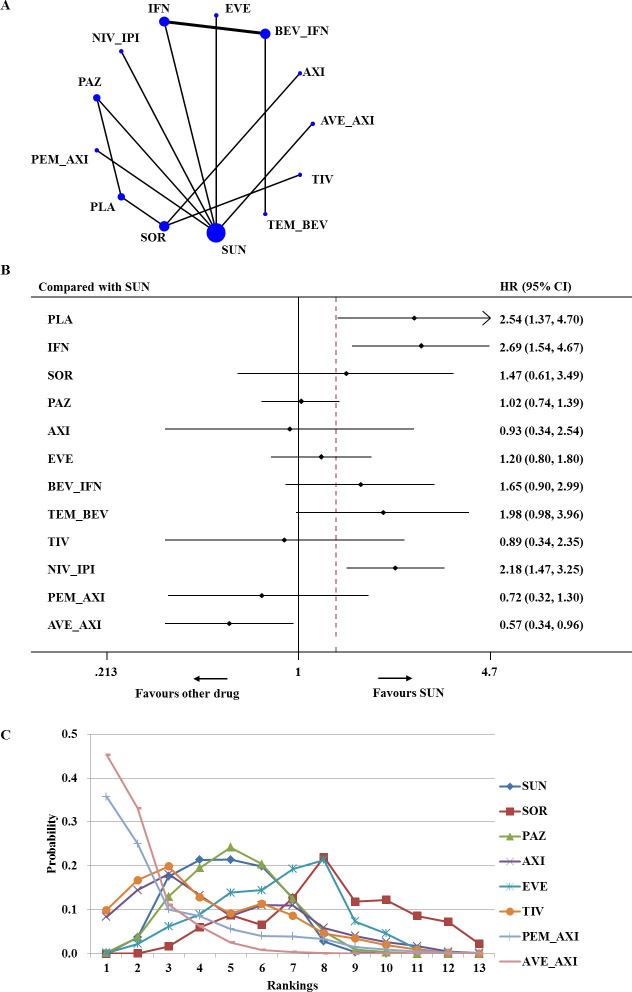
For advanced/metastatic RCC of favourable risk, 13 trials enrolling 2514 total patients reported adequate information on PFS and contributed to network meta-analysis (figure 2A).10–13 15 16 35–38 40–43 Figure 2B summarises the results of the network meta-analysis for PFS. Compared with sunitinib, IFN-α and nivolumab plus ipilimumab were associated with significantly worse PFS (HR 2.69, 95% CI 1.54 to 4.67 and HR 2.18, 95% CI 1.47 to 3.25, respectively). Network meta-analysis showed that only avelumab plus axitinib was associated with a significantly higher improvement in PFS than sunitinib (HR 0.57, 95% CI 0.34 to 0.96). Based on the results of ranking, there was a 45% chance that avelumab plus axitinib provided the greatest PFS benefit for patients with favourable-risk disease (SUCRA=92.3%) (figure 2C).
Figure 2.
Analysis of progression-free survival for patients with favourable-risk disease. (A) Network diagram: the size of every treatment node corresponds to the number of randomly assigned patients. The width of the lines is proportional to the number of trials. (B) Forest plot, with sunitinib as the comparator. (C) Ranking of treatments. Rankograms were drawn according to distribution of the ranking probabilities. Ranking indicates the probability to be the best treatment, the second best, the third best, and so on, in terms of progression-free survival (PFS), among the 13 treatments. Numbers in parentheses indicate 95% credible intervals. AVE_AXI, avelumab plus axitinib; AXI, axitinib; BEV_IFN, bevacizumab plus interferon; EVE, everolimus; IFN, interferon; NIV_IPI, nivolumab plus ipilimumab; PAZ, pazopanib; PEM_AXI, pembrolizumab plus axitinib; PLA, placebo; SOR, sorafenib; SUN, sunitinib; TEM_BEV, temsirolimus plus bevacizumab; TIV, tivozanib.
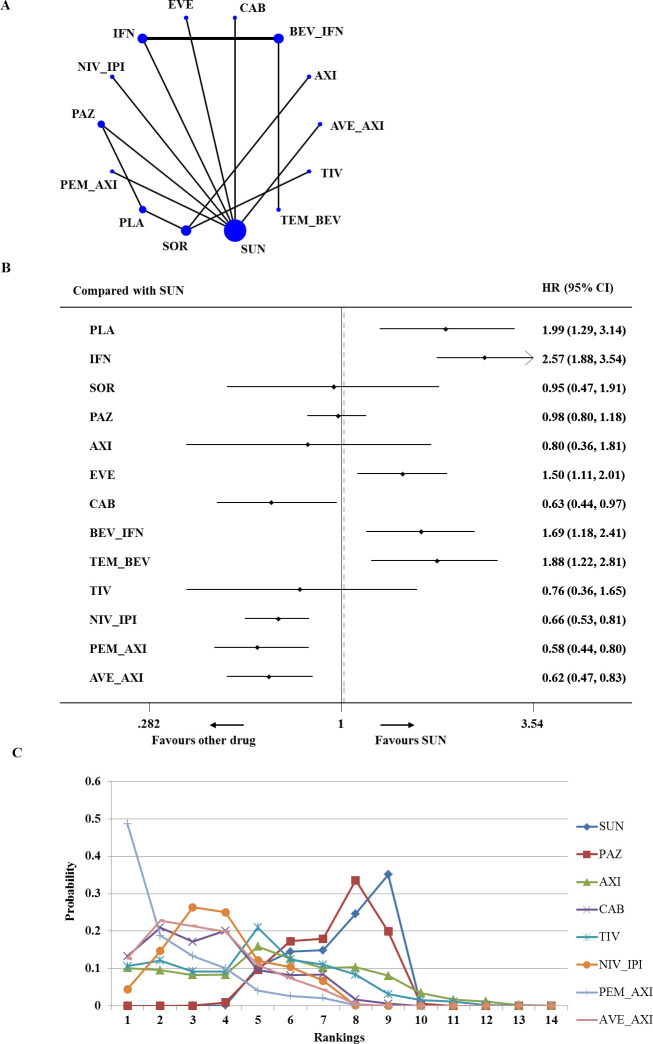
For advanced/metastatic RCC of intermediate risk, 14 trials enrolling 5473 total patients contributed to the analysis of PFS (figure 3A).10–13 15 16 34–38 40 41 43 Network meta-analysis demonstrated that cabozantinib, nivolumab plus ipilimumab, pembrolizumab plus axitinib and avelumab plus axitinib were associated with significantly higher improvement in PFS than sunitinib (HR 0.63, 95% CI 0.44 to 0.97; HR 0.66, 95% CI 0.53 to 0.81; HR 0.58, 95% CI 0.44 to 0.80; HR 0.62, 95% CI 0.47 to 0.83, respectively). Everolimus, bevacizumab plus IFN-α and temsirolimus plus bevacizumab were significantly less efficacious for PFS than sunitinib (HR 1.50, 95% CI 1.11 to 2.01; HR 1.69, 95% CI 1.18 to 2.41; HR 1.88, 95% CI 1.22 to 2.81, respectively) (figure 3B). Based on the analysis of ranking, pembrolizumab plus axitinib had the highest probability (49%) to be the best treatment for intermediate-risk patients (SUCRA=90.7%). Avelumab plus axitinib and cabozantinib had a similar likelihood of being the second-best option for patients with intermediate-risk disease (figure 3C).
Figure 3.
Analysis of progression-free survival for patients with intermediate-risk disease. (A) Network diagram. (B) Forest plot, with sunitinib as the comparator. (C) Ranking of treatments. Numbers in parentheses indicate 95% credible intervals. AVE_AXI, avelumab plus axitinib; AXI, axitinib; BEV_IFN, bevacizumab plus interferon; CAB, cabozantinib; EVE, everolimus; IFN, interferon; NIV_IPI, nivolumab plus ipilimumab; PAZ, pazopanib; PEM_AXI, pembrolizumab plus axitinib; PLA, placebo; SOR, sorafenib; SUN, sunitinib; TEM_BEV, temsirolimus plus bevacizumab; TIV, tivozanib.
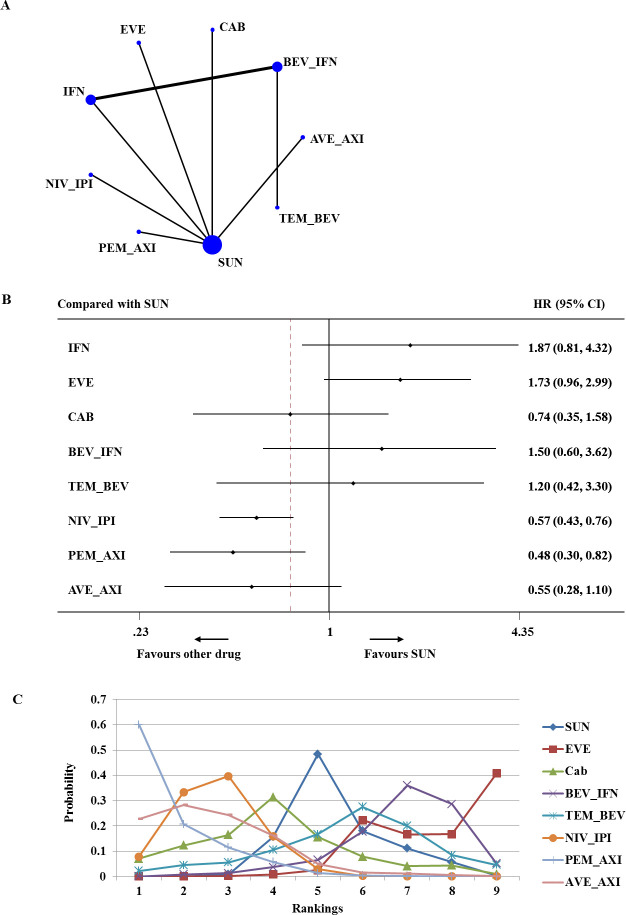
Based on data that were available for advanced/metastatic RCC of poor risk, the network involved seven trials comparing nine different treatments (721 total patients; figure 4A).15 16 34–36 38 40 41 43 Network meta-analysis demonstrated that nivolumab plus ipilimumab and pembrolizumab plus axitinib were associated with significantly higher improvement in PFS than sunitinib (HR 0.57, 95% CI 0.43 to 0.76; HR 0.48, 95% CI 0.30 to 0.82, respectively) (figure 4B). On the base of ranking analysis, there was a 60% probability that pembrolizumab plus axitinib had the greatest PFS for poor-risk patients (SUCRA=91.3%) (figure 4C).
Figure 4.
Analysis of progression-free survival for patients with poor-risk disease. (A) Network diagram. (B) Forest plot, with sunitinib as the comparator. (C) Ranking of treatments. Numbers in parentheses indicate 95% credible intervals. AVE_AXI, avelumab plus axitinib; BEV_IFN, bevacizumab plus interferon; CAB, cabozantinib; EVE, everolimus; IFN, interferon; NIV_IPI, nivolumab plus ipilimumab; PEM_AXI, pembrolizumab plus axitinib; SUN, sunitinib; TEM_BEV, temsirolimus plus bevacizumab.
Overall survival
Five RCTs reported OS according to risk subgroups, and data from three of them contributed to the network meta-analysis (572, 1801 and 407 patients for favourable, intermediate and poor risk, respectively).16 39 40 For advanced/metastatic RCC of favourable risk, there is no significant OS benefit between sunitinib and pazopanib (HR 0.88, 95% CI 0.64 to 1.21) or pembrolizumab plus axitinib (HR 0.64, 95% CI 0.24 to 1.70) (figure 5A). For intermediate-risk patients, pembrolizumab plus axitinib and nivolumab plus ipilimumab were associated with significantly higher improvement in OS than sunitinib (HR 0.53, 95% CI 0.34 to 0.81; HR 0.66, 95% CI 0.50 to 0.87, respectively) (figure 5B). For advanced/metastatic RCC of poor risk, pembrolizumab plus axitinib and nivolumab plus ipilimumab were significantly more efficacious for OS than sunitinib (HR 0.43, 95% CI 0.23 to 0.80; HR 0.57, 95% CI 0.39 to 0.83, respectively) (figure 5C). Based on the results of ranking, there were 81% and 78% probabilities for pembrolizumab plus axitinib to be the best choice for intermediate and poor-risk patients, respectively (SUCRA=93.1% and SUCRA=91.4%, respectively) (online supplementary appendix figures 1–3).
Figure 5.
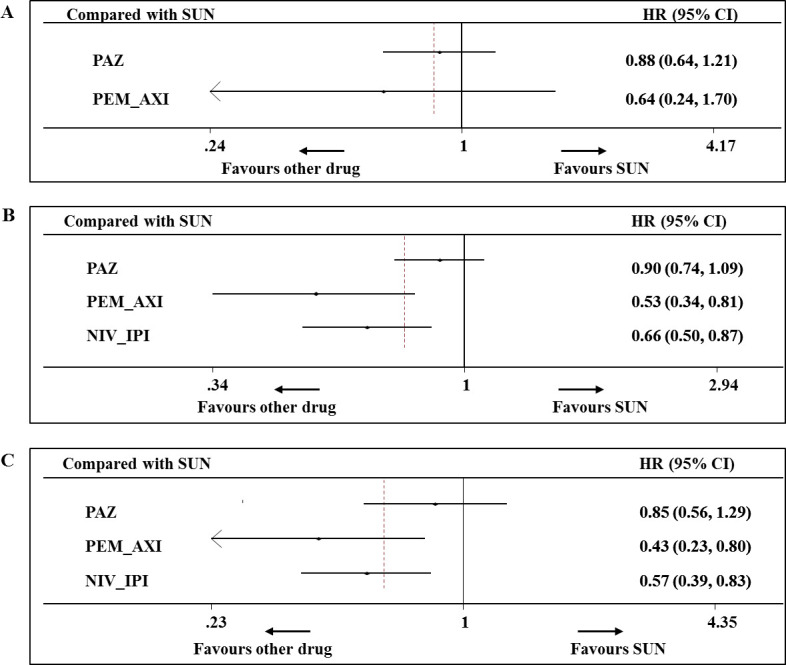
Analysis of overall survival for patients with favourable risk (A), intermediate risk (B) and poor risk (C). Numbers in parentheses indicate 95% credible intervals. NIV_IPI, nivolumab plus ipilimumab; PAZ, pazopanib; PEM_AXI, pembrolizumab plus axitinib; SUN, sunitinib.
Adverse events
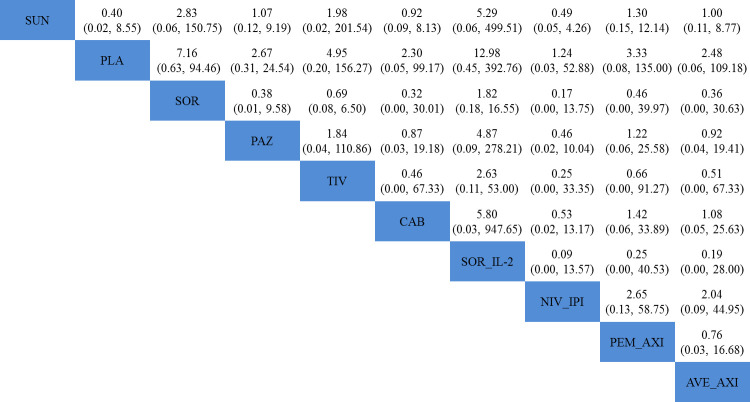
Nine trials contributed to our analysis of overall and high-grade drug-related AEs.10 11 13 15 16 37 40–42 All the nine trials did not provide AE data for different risk groups, so we extracted a summary of AE data. Results of comparisons of AEs of nine systemic treatments are presented in figure 6 and online supplementary appendix figure 4. Stepwise comparison of all the seven therapies did not find significant differences in rates of high-grade or overall AEs. The most common AEs included diarrhoea, hypertension, fatigue and decreased appetite.
Figure 6.
Pooled ORs for high-grade adverse events. The column treatment is compared with the row treatment. ORs lower than 1 favour the column-defining treatment. Numbers in parentheses indicate 95% credible intervals. Stepwise comparison of treatments did not find significant differences in rates of high-grade adverse events. AVE_AXI, avelumab plus axitinib; CAB, cabozantinib; NIV_IPI, nivolumab plus ipilimumab; PAZ, pazopanib; PEM_AXI, pembrolizumab plus axitinib; PLA, placebo; SOR, sorafenib; SOR_IL-2, sorafenib plus interleukin-2; SUN, sunitinib; TIV, tivozanib.
Network assumptions, sensitivity analysis, publication bias and risk of bias
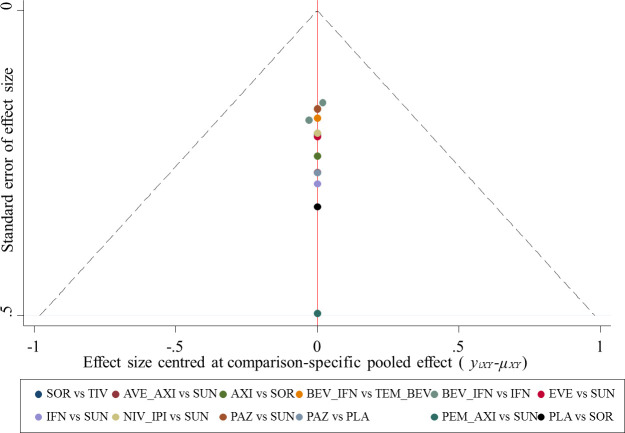
Consistencies between direct and indirect evidence were noted for any comparisons (online supplementary appendix figure 5 and online supplementary appendix tables 1–5). Results from the sensitivity analyses were in line with the primary analysis (online supplementary appendix table 6). The comparison-adjusted funnel plot (figure 7) for PFS was largely symmetric, indicating no obvious small-study effects and publication bias. The methodological quality was moderate in the included studies (online supplementary appendix figure 6). All trials were thought to have low risk of bias for random sequence generation, incomplete outcome data and selective reporting of outcomes. Ten trials had evidence of high risk of bias for masking.12 13 15 36–38 40–43
Figure 7.
Funnel plot of randomised controlled trials included in the meta-analysis for HRs of progression-free survival. AVE_AXI, avelumab plus axitinib; AXI, axitinib; BEV_IFN, bevacizumab plus interferon; EVE, everolimus; IFN, interferon; NIV_IPI, nivolumab plus ipilimumab; PAZ, pazopanib; PEM_AXI, pembrolizumab plus axitinib; PLA, placebo; SOR, sorafenib; SUN, sunitinib; TEM_BEV, temsirolimus plus bevacizumab; TIV, tivozanib.
Discussion
Our network meta-analysis of 15 RCTs including 8995 individuals assessed the efficacy and safety of all major systemic therapies for the treatment of advanced/metastatic RCC in the first-line setting. Findings of this meta-analysis might help choose among systemic agents for the management of patients with previously untreated advanced/metastatic RCC. In terms of PFS, avelumab plus axitinib was most likely to be the best treatment regimen for advanced/metastatic RCC of favourable risk, and pembrolizumab plus axitinib seemed to be the most efficacious treatment strategy for patients with intermediate and poor risk. In terms of OS, there were no significant differences among systemic therapies for advanced/metastatic RCC of favourable risk, and pembrolizumab plus axitinib was probably to be the best option for patients with intermediate and poor risk. In terms of drug-related AEs, there were no significant differences among systemic therapies.
In RCC with clear-cell subtype, hypoxia-inducible factor (HIF) accumulation due to loss of von Hippel-Lindau leads to overexpression of VEGF and platelet-derived growth factor (PDGF), which promotes tumour angiogenesis.44 45 This process substantially makes a contribution to the development and progression of clear-cell RCC. Inhibiting the VEGF signalling has been supposed as the key mechanism for antitumour effects in clear-cell RCC. To date, eight targeted drugs have been approved for treating advanced RCC: sunitinib, sorafenib, pazopanib, cabozantinib, axitinib, everolimus, temsirolimus and bevacizumab (in combination with IFN-α).
As shown in this analysis, for patients with intermediate risk, sunitinib resulted in a significant PFS benefit compared with everolimus. The varied clinical benefit could be associated with mechanisms of action of TKI and mTOR inhibitor. Sunitinib inhibits VEGF receptors 1, 2 and 3, which may be the most clearly relevant targets in RCC so far, and exhibits potent activity against PDGF receptor.11 46 It has been reported that PDGF plays a critical role in the recruitment of pericytes to sprouting tumour vessels, and pericyte-covered vessels are more likely resistant to antivascular therapy than those pericyte-negative vessels.47 48 The mTOR complex is the upstream of an intracellular signalling network regulating cell growth and angiogenesis, and it plays a key role in the pathogenesis of advanced/metastatic RCC.49 It has been demonstrated that rapamycin analogues, including everolimus and temsirolimus, inhibit only one of two signalling complexes of mTOR.50 The mTORC1 signalling is potently inhibited by everolimus and temsirolimus, while the mTORC2 signalling is not.51 Consequently, one downstream signalling of mTOR activation is unopposed. The relatively unsatisfactory efficacy should disable the mTOR inhibitors as more suitable therapies for the treatment of advanced/metastatic RCC than TKIs. Regarding TKIs, our results suggest that sunitinib was most likely to be the best treatment regimen for patients with favourable-risk disease.
A potentially additive benefit from combinations of targeted drugs has been suggested on the basis that they inhibit separate cellular pathways. However, our results show that temsirolimus plus bevacizumab and bevacizumab plus IFN-α provide little survival benefit compared with sunitinib, further confirming absence of evidence that combination treatment simultaneously inhibiting both VEGF and mTOR signalling results in therapeutic synergy.18 20 38
Immune checkpoint antibodies block the inhibitory T-cell receptor programmed death-1 (PD-1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4) signalling to augment tumour-specific immune response.52 Nivolumab (an anti-PD-1 antibody) is approved for the treatment of advanced RCC in the second-line setting. Ipilimumab (an anti-CTLA-4 antibody) is approved for the treatment of advanced melanoma. Nivolumab plus ipilimumab has been reported to have significant efficacy in multiple tumour types.53 54 In this analysis, pembrolizumab plus axitinib appeared to be the optimum treatment for intermediate and poor-risk patients. Single-agent antitumour activity of pembrolizumab and axitinib for mRCC has been reported in previous studies.12 55 Accordingly, axitinib in combination with pembrolizumab was assessed and contributed to objective response rate in 73% of patients in a phase 1b trial.56 Our result was in consistent with results of KEYNOTE-426 trial, demonstrating that pembrolizumab plus axitinib resulted in significant OS and PFS benefit compared with sunitinib.40 In addition, the survival benefit of pembrolizumab plus axitinib was observed independent of PD-L1 status.40
Pembrolizumab plus axitinib is a combination of anti-PD-1 monoclonal antibody and VEGF receptor TKI. Immune checkpoint inhibitors (ICI) block the inhibitory T-cell receptor PD-1 or CTLA-4-signalling to augment tumour-specific immune response.52 Besides antiangiogenic effects, VEGF inhibition could enhance the recruitment and infiltration of immune cells into the tumours.57 58 It was reported that simultaneous blockade of PD-1 and VEGF receptor-2 induced decreased tumour neovascularisation and tumour inhibition in a murine model.59 These studies suggested that the combination of ICI and VEGF receptor inhibitors could provide enhanced benefit for mRCC. Recently, in addition to pembrolizumab plus axitinib, avelumab (anti-PD-L1 antibody) plus axitinib and atezolizumab (anti-PD-L1 antibody) plus bevacizumab were respectively assessed in two phase 3 RCTs (IMmotion 151 and JAVELIN Renal 101), and both of them showed significant survival benefit for mRCC compared with sunitinib.40 60 However, there is no head-to-head trial comparing combinations of ICI and VEGF receptor inhibitors (pembrolizumab plus axitinib, avelumab plus axitinib and atezolizumab plus bevacizumab) directly. Consistent with our previous study, the present analysis revealed that pembrolizumab plus axitinib presented the highest OS benefit for intermediate and poor-risk patients. For advanced/metastatic RCC of favourable risk, only avelumab plus axitinib was associated with a significantly higher improvement in PFS than sunitinib, suggesting avelumab plus axitinib might be the optimum treatment for favourable-risk patients. Considering patients continued to be followed for OS in the JAVELIN Renal 101 trial,41 the real OS benefit for avelumab plus axitinib over sunitinib requires additional follow-up.
Recently, several network meta-analyses were attempted to investigate the comparative effects of different systemic agents for treatment of advanced/metastatic RCC.61–64 However, trials included in the meta-analyses enrolled patients with different risk groups. The analysis used aggregate data and did not perform subgroup analysis based on risk strata. In the present study, we performed a network meta-analysis to compare first-line systemic treatments for advanced/metastatic RCC of favourable, intermediate and poor risk, respectively, thus providing physicians with the optimal treatment for different risk groups.
The strengths of our study are as follows. To the best of our knowledge, this study is the first network analysis to compare systemic treatments for advanced/metastatic RCC separately by risk groups. We applied multiple rigorous search strategies to retrieve all potentially eligible RCTs. In the present study, we comprehensively compared and ranked all available first-line systemic therapies for advanced/metastatic RCC of favourable, intermediate and poor risk, respectively, thus providing physicians with an overall appraisal of systemic therapies for different risk groups. In addition, we used Bayesian network meta-analysis to synthesise data. This approach provides indirect effect estimates in the absence of head-to-head trial and incorporates all available information from RCTs while fully maintaining randomisation.21 22 We applied various statistical models to increase reliability of the results. Results were consistent across all analysed outcomes. Moreover, the reliability and accuracy of results were corroborated by the low statistical heterogeneity and excellent model fit. Finally, assessment of both efficacy and AEs provides new insights into the benefit-harm balance of different systemic treatments.
However, the limitations of our study must be taken into account. The major limitation of this network meta-analysis lies in the reporting quality of trials reviewed. Ten included trials were not masked, which might affect the validity of our findings. In addition, three included trials (CABOSUN, ROSORC and RECORD-3) are phase 2 RCTs with smaller sample size, and they may be less authoritative compared with phase 3 RCTs. Moreover, most of the trials did not perform the analysis of OS in risk subgroups, which made it impossible to assess the OS benefits of all the existing treatments for different risk patients. In addition, this meta-analysis was conducted based on summary statistics rather than individual patient-level data. There might be some confounding factors (eg, ethnic origin, prior nephrectomy, and so on) at the individual patient level that might influence the benefit of systemic treatments, but were not available; therefore analyses adjusted for these factors were impossible in our network meta-analysis. Access to patient-level longitudinal data would allow us to establish more robust and accurate conclusions in specific subgroups of patients. Moreover, the length of follow-up varied across studies, resulting in potential variations in survival benefits and AEs. Due to only eight trials reporting median follow-up, sensitivity analyses adjusted for this factor were impossible. Moreover, individual dosage varied across studies and data were too sparse to investigate the effects of different schedules, which might somewhat affect the generalisability of our findings. Since the analysis was based on highly selected RCTs and the results were based on fixed effects models, findings in this analysis may not be entirely generalised to real-world practice. Finally, findings in this meta-analysis were mainly based on patients with clear-cell advanced RCC, thus no robust recommendations can be provided for non-clear-cell subtypes. Two trials included small subsets of non-clear-cell histotypes (11% and 14% of the study population), which might somewhat damage the results of our analysis.
Conclusions
Our network meta-analysis suggested that: avelumab plus axitinib might be the optimum treatment for advanced/metastatic RCC of favourable risk; pembrolizumab plus axitinib was most likely to be the best option for intermediate and poor-risk patients. Further well-designed, large-scale RCTs are required to confirm and update the findings of this analysis.
Supplementary Material
Footnotes
Contributors: GC and XiW conceived and designed the meta-analysis. ZW and XT identified and acquired the reports of trials, and extracted the data. XuW and HZ analysed and interpreted the data. CZ and GJ contacted the authors of the trials for additional information. GC and XiW drafted the manuscript. TY critically reviewed the manuscript. All authors approved the final submitted version of the report.
Funding: This research was supported by the Henan Provincial Medical Scientific and Technological Research Project (Grant No 201702191).
Disclaimer: The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913–24. 10.1016/j.eururo.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 4.Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843–52. 10.1016/S0094-0143(03)00056-9 [DOI] [PubMed] [Google Scholar]
- 5.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005;353:2477–90. 10.1056/NEJMra043172 [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–96. 10.1200/JCO.2002.20.1.289 [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34. 10.1056/NEJMoa060655 [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008;26:5422–8. 10.1200/JCO.2008.16.9847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negrier S, Jäger E, Porta C, et al. Efficacy and safety of sorafenib in patients with advanced renal cell carcinoma with and without prior cytokine therapy, a subanalysis of target. Med Oncol 2010;27:899–906. 10.1007/s12032-009-9303-z [DOI] [PubMed] [Google Scholar]
- 11.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8. 10.1200/JCO.2009.23.9764 [DOI] [PubMed] [Google Scholar]
- 12.Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287–94. 10.1016/S1470-2045(13)70465-0 [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31. 10.1056/NEJMoa1303989 [DOI] [PubMed] [Google Scholar]
- 14.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280–9. 10.1200/JCO.2008.19.3342 [DOI] [PubMed] [Google Scholar]
- 15.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol 2017;35:591–7. 10.1200/JCO.2016.70.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonasch E, Corn P, Pagliaro LC, et al. Upfront, randomized, phase 2 trial of sorafenib versus sorafenib and low-dose interferon alfa in patients with advanced renal cell carcinoma: clinical and biomarker analysis. Cancer 2010;116:57–65. 10.1002/cncr.24685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Négrier S, Gravis G, Pérol D, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol 2011;12:673–80. 10.1016/S1470-2045(11)70124-3 [DOI] [PubMed] [Google Scholar]
- 19.Procopio G, Verzoni E, Bracarda S, et al. Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. Br J Cancer 2011;104:1256–61. 10.1038/bjc.2011.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravaud A, Barrios CH, Alekseev B, et al. RECORD-2: phase II randomized study of everolimus and bevacizumab versus interferon α-2a and bevacizumab as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol 2015;26:1378–84. 10.1093/annonc/mdv170 [DOI] [PubMed] [Google Scholar]
- 21.Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897–900. 10.1136/bmj.331.7521.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24. 10.1002/sim.1875 [DOI] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dias S, Welton NJ, Sutton AJ, et al. A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. Med Decis Making 2014. [PubMed] [Google Scholar]
- 27.Spiegelhalter DJ, Best NG, Carlin BP, et al. Bayesian measures of model complexity and fit. J Royal Statistical Soc B 2002;64:583–639. 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- 28.Gelman A, Rubin DB. Markov chain Monte Carlo methods in biostatistics. Stat Methods Med Res 1996;5:339–55. 10.1177/096228029600500402 [DOI] [PubMed] [Google Scholar]
- 29.Powles T, Albiges L, Staehler M, et al. Updated European association of urology guidelines: recommendations for the treatment of first-line metastatic clear cell renal cancer. Eur Urol 2018;73:311–5. 10.1016/j.eururo.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 30.Dias S, Welton NJ, Sutton AJ, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641–56. 10.1177/0272989X12455847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaimani A, Higgins JPT, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Valkenhoef G, Lu G, de Brock B, et al. Automating network meta-analysis. Res Synth Methods 2012;3:285–99. 10.1002/jrsm.1054 [DOI] [PubMed] [Google Scholar]
- 33.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 34.Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144–50. 10.1200/JCO.2009.26.7849 [DOI] [PubMed] [Google Scholar]
- 35.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 2010;28:2137–43. 10.1200/JCO.2009.26.5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90. 10.1200/JCO.2008.20.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791–9. 10.1200/JCO.2012.47.4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rini BI, Bellmunt J, Clancy J, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol 2014;32:752–9. 10.1200/JCO.2013.50.5305 [DOI] [PubMed] [Google Scholar]
- 39.Motzer RJ, Hutson TE, McCann L, et al. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med 2014;370:1769–70. 10.1056/NEJMc1400731 [DOI] [PubMed] [Google Scholar]
- 40.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27. 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 41.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–15. 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Procopio G, Verzoni E, Bracarda S, et al. Overall survival for sorafenib plus interleukin-2 compared with sorafenib alone in metastatic renal cell carcinoma (mRCC): final results of the ROSORC trial. Ann Oncol 2013;24:2967–71. 10.1093/annonc/mdt375 [DOI] [PubMed] [Google Scholar]
- 43.Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:2765–72. 10.1200/JCO.2013.54.6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patard J-J, Rioux-Leclercq N, Fergelot P. Understanding the importance of smart drugs in renal cell carcinoma. Eur Urol 2006;49:633–43. 10.1016/j.eururo.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 45.Costa LJ, Drabkin HA. Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist 2007;12:1404–15. 10.1634/theoncologist.12-12-1404 [DOI] [PubMed] [Google Scholar]
- 46.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327–37. [PubMed] [Google Scholar]
- 47.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998;125:1591–8. [DOI] [PubMed] [Google Scholar]
- 48.Gee MS, Procopio WN, Makonnen S, et al. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol 2003;162:183–93. 10.1016/S0002-9440(10)63809-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Creighton CJ, Morgan M, Gunaratne PH, et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–9. 10.1038/nature12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loewith R, Jacinto E, Wullschleger S, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 2002;10:457–68. 10.1016/S1097-2765(02)00636-6 [DOI] [PubMed] [Google Scholar]
- 51.Inoki K, Guan K-L. Complexity of the TOR signaling network. Trends Cell Biol 2006;16:206–12. 10.1016/j.tcb.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 52.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med 2012;366:2517–9. 10.1056/NEJMe1205943 [DOI] [PubMed] [Google Scholar]
- 53.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol 2017;35:3851–8. 10.1200/JCO.2016.72.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 55.McDermott DF, Lee J-L, Szczylik C, et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): results from cohort a of KEYNOTE-427. JCO 2018;36:4500 10.1200/JCO.2018.36.15_suppl.4500 [DOI] [Google Scholar]
- 56.Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1B trial. Lancet Oncol 2018;19:405–15. 10.1016/S1470-2045(18)30081-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shrimali RK, Yu Z, Theoret MR, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010;70:6171–80. 10.1158/0008-5472.CAN-10-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 2012;109:17561–6. 10.1073/pnas.1215397109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 2013;172:500–6. 10.1111/cei.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019;393:2404–15. 10.1016/S0140-6736(19)30723-8 [DOI] [PubMed] [Google Scholar]
- 61.Wallis CJD, Klaassen Z, Bhindi B, et al. First-Line systemic therapy for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol 2018;74:309–21. 10.1016/j.eururo.2018.03.036 [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Li X, Wu X, et al. Role of immune checkpoint inhibitor-based therapies for metastatic renal cell carcinoma in the first-line setting: a Bayesian network analysis. EBioMedicine 2019;47:78–88. 10.1016/j.ebiom.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hahn AW, Klaassen Z, Agarwal N, et al. First-Line treatment of metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol 2019;2:708–15. 10.1016/j.euo.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 64.Karner C, Kew K, Wakefield V, et al. Targeted therapies for previously treated advanced or metastatic renal cell carcinoma: systematic review and network meta-analysis. BMJ Open 2019;9:e024691. 10.1136/bmjopen-2018-024691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-034626supp001.pdf (1.4MB, pdf)