Abstract
Objective
To illustrate the posterior longitudinal ligament is one of the tissue candidates who can contribute to low back pain (LBP).
Methods
This is a retrospective study. A series of 72 patients who underwent single‐level percutaneous endoscopic lumbar discectomy performed for lumbar disc herniation with LBP from June 2014 to June 2016 were examined. There are 42 males and 30 females. The ages of patients were 40 to 57 years, and the mean age was 49.8 years. The symptomatic disc level was at L4‐5 in 43 patients and L5S1 in 29 patients. Thirty‐two patients (19 patients in L4‐5 disc level, 13 patients in L5S1 disc level) had LBP (which was limited to the lower back and buttock area) before the operation. All of the operative approaches were performed under local anesthesia. A posterior body diagram (15 cm × 10 cm) was made for this study to record the pain distribution. The centered foci of low back pain were subjectively recorded before, during, and after the operation. The transforaminal endoscopic spine system technology was used in this study. Radiological examinations (X‐ray, computed tomography, and magnetic resonance imaging) were performed prior to and after surgery. The Visual Analogue Score (VAS) and Oswestry Disability Index (ODI) scores were taken before and after the surgery to observe the degree of pain. The VSA and ODI score before and after operation were expressed as mean ± SD, and compared by t‐test for statistical analysis.
Results
When inciting the posterior longitudinal ligament during the operation, all 72 patients had provoked low back pain. Forty‐three patients with symptomatic discs at L4‐5 had pain foci in the lower back and upper gluteal region under the L4 spinous process. Twenty‐nine patients with symptomatic discs at L5S1 had pain foci in the gluteal region under the S1 spinous process. The pain localizations of L4‐5 and L5S1 were different. After the surgery, the provoked low back pain disappeared, and had not returned in any of the patients at the 6‐month follow‐up. After the operation, one patient suffered from lower limb pain that he did not have before the operation, and the lower limb pain abated a few days later. Three patients had cerebrospinal fluid leakage and were treated with higher pressure applied on the incision and bed rest for 10 days. During the 6‐months follow‐up period, the mean VAS decreased from 5.97 ± 1.10 to 2.13 ± 0.78. The mean ODI score decreased from 23.14 ± 3.28 to 7.92 ± 1.85.
Conclusions
The intervertebral posterior longitudinal ligament may be one of the tissues from which low back pain originates.
Keywords: Low back pain, Lumbar spine, Percutaneous endoscopic lumbar discectomy, Posterior longitudinal ligament
Introduction
Low back pain (LBP) is the most common cause for chronic or permanent impairment in adults under the age of 65 years, and the most common cause of activity limitations in persons under the age of 45 years 1 . LBP is defined as pain on the posterior aspect of the body from the lower margin of the twelfth rib to the lower gluteal folds with or without pain referred into one or both lower limbs that lasts for at least 1 day 1 , 2 . LBP is extremely common, and is the leading cause of years lived with disability in both developed and developing countries, and is sixth in terms of overall disease burden (disability adjusted life‐years) 3 . Though estimates vary widely, studies in developed countries report point prevalence of 12% to 33%, one‐year prevalence of 22% to 65%, and lifetime prevalence of 11% to 84% 4 . LBP is a symptom‐related reason which is second to upper respiratory problems for patients to visit a physician.
LBP is recognized as a complex, challenging condition with widespread adverse consequences for patients including physical disability, disturbed sleep, psychosocial disruption and increased use of healthcare resources 5 , 6 . Some study indicated that LBP is more trouble as a symptom rather than a disease, even several lumbar or sacral disorders were confirmed contributing to LBP but it does not have a known pathoanatomical cause 1 . Many lumbar or sacral diseases are confirmed to give rise to LBP, such as lumbar disc herniation, lumbar instability or deformity, spondylolisthesis, malalignment, and spondyloarthropathy. Experimental studies suggested that LBP may originate from many spinal structures, including ligaments, facet joints, the vertebral periosteum, the paravertebral musculature and fascia, blood vessels, the anulus fibrosus, and spinal nerve roots 5 . Perhaps most common are musculoligamentous injuries and age‐related degenerative processes in the intervertebral discs and facet joints. Other common problems include spinal stenosis and disk herniation 5 . Some patients with LBP can have no pathological change. While LBP in a patient can be a symptom associated with several diseases mentioned above. Clinical practice guidelines state that the tissue source of LBP cannot be specified in the majority of patients. If the tissue‐source of LBP could be identified, this may lead to more logical, and effective, interventions. That will be of great significance for the pain management of the elderly, with the development of an aging society.
Our understanding of the causes of LBP has evolved over the past century 7 , 8 , 9 . Nevertheless, identifying a definitive source of LBP between the intervertebral discs, the zygapophysial (facet) joints, and sacroiliac joints is still a major challenge, and few study focus on the posterior longitudinal ligament (PLL) 8 , 10 , 11 , 12 , 13 , 14 . Discography, MRI, CT, and ultrasound had been used to identify the source of LBP 15 , 16 , 17 , 18 , but few studies have explored the origin of LBP through the operation 14 .
Common back surgeries include fusion for nonradicular low back pain with degenerative changes (most frequently degenerative disc disease with presumed discogenic back pain), discectomy for radiculopathy with herniated lumbar disc, and decompressive laminectomy (with or without fusion) for symptomatic spinal stenosis with or without degenerative spondylolisthesis. Other surgical techniques include artificial disc replacement as an alternative to fusion, and an interspinous spacer device as an alternative to decompressive laminectomy 19 . In common lumbar decompression surgery, the reason why the PLL associated pain had not been found might be that part of the facet joint needs to be removed and nerve roots might be stimulated when we resecting the PLL during surgery, and the pain caused by PLL resection was covered by facet joint pain or nerve roots stimulation. In this study, the percutaneous transforaminal endoscopic system can avoid nerve roots stimulation and can incite PLL without resecting facet joint. Advantages of endoscopic spine surgeries are less tissue dissection and muscle trauma, reduced blood loss, less damage to the epidural blood supply and consequent epidural fibrosis and scarring, reduced hospital stay, early functional recovery and improvement in the quality of life, and better cosmesis.
Therefore, this retrospective study explored the LBP origination. The aim of the present study was: (i) to recorded the LBP distribution of different disc levels before, during, and after operation; (ii) to compare the LBP distribution before, during, and after operation; (iii) to explore the original tissue of LBP and provide doctors with a target for LBP treatment.
In this study, we reviewed an entire 2‐year series of 72 patients who underwent single‐level PLL incision during a percutaneous endoscopic lumbar discectomy (PELD) under local anesthesia. We recorded the LBP distribution before the operation, the provoked pain distribution during PLL incision in a body diagram; observed patient recovery by recording whether the provoked pain remained or not after operation, and whether the LBP remained or not during the follow‐up period and radiological examinations; compared the pain distribution before the operation to the pain distribution after the operation; and illustrated that PLL is one of the candidates that can contribute to the LBP.
Materials and Methods
Patient Information
From June 2014 to June 2016, the inclusion for patients in this study were the following: (i) patients had neurogenic claudication with or without LBP; (ii) magnetic resonance imaging findings of lumbar disc herniation; (iii) conservative treatment failed to relieve neurogenic claudication; (iv) received PLL incision during PELD surgery under local anesthesia; and (v) pain distribution could be recorded before, during, and after the surgery. Exclusion criteria: (i) the patients had sciatica; (ii) the LBP pain was caused by a problem outside of the lumbar spine (e.g., leaking aortic aneurysm); (iii) a specific, known disorder affecting the lumbar spine (e.g., epidural abscess, compression fracture, spondyloarthropathy, malignancy, cauda equina syndrome); and (iv) radiculopathy.
Finally, 72 patients (42 males and 30 females; 40 to 60 years old, mean 49.8 years old) who underwent PELD under local infiltration anesthesia without intravenous sedatives were included in this study. The symptomatic disc level was at L4‐5 in 43 patients and L5S1 in 29 patients. Thirty‐two patients (19 in L4‐5, 13 in L5S1) had LBP (which was limited to the lower back and buttock area) before the operation. Magnetic resonance imaging (MRI), computed tomography (CT), and plain X‐rays were performed on all the patients before and after surgery. Authors had access to information that could identify individual participants during or after data collection.
Recording Procedure
A posterior body diagram (15 cm × 10 cm) was made for this study. The doctors interviewed each patient and recorded the location of their pain‐centered foci on the body diagram before the operation. The provocation of pain during the incision of PLL was described by patients. After the surgery, the pain center foci distribution during PLL incision was also recorded on a body diagram. Each patient could only indicate one spot (the most painful area, 5 mm in diameter on the body diagram) on the diagram to point out the focus of the provoked LBP. The localization of the pain focus from each disc level was collected. The patients were asked to make a mark on the body diagram again after surgery and at follow‐up to identify any remaining LBP. All of the pain foci diagrams were overlaid one by one after removing the background color with Photoshop CS6.
Surgical Technique
Step 1: The patients were placed in prone position under local infiltration anesthesia. The local anesthetic was 60 mL 0.5% lidocaine. The symptomatic disc was localized using C‐arm fluoroscopy. The location of the skin incision was marked.
Step 2: The percutaneous posterior‐lateral approach was conducted under orthogonal radiologic control in two planes under local infiltration anesthesia and the patients were awake for the procedure. The PLL were not anesthetized to avoid the lumbar cord block. The PLL was exposed through a posterolateral work approach via the intervertebral foramen with the transforaminal endoscopic spine system (TESSYS) technology.
Step 3: The decompression was then performed under visual control and gravity‐controlled liquid flow (Fig. 1A,B). The PLL was incited to expose the lumbar disc (Fig. 1C). Then the protruded nucleus pulposus in the spinal canal was removed with a nucleus pulposus clamp through the intervertebral foramen (Fig. 1D). The compressed epidural and nerve root adhesion were released, and the salient fiber ring was ablated with a radiofrequency electrode. During the whole visually controlled resection, there was no pain except during the PLL incision. The patients were allowed to get out of bed the next morning with protection of the waistline.
Fig. 1.
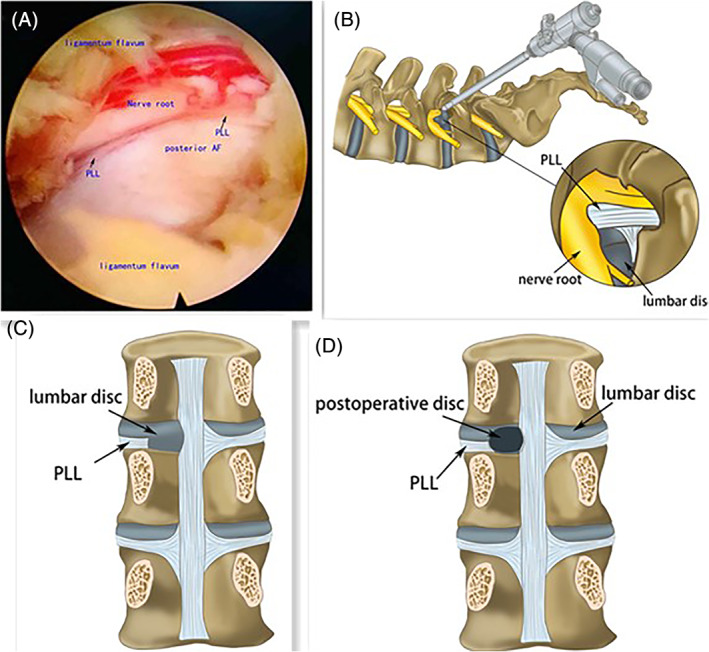
Decompression under visual control. (A) and (B) showed the image of PLL before the decompression. The decompression was performed under visual control and gravity‐controlled liquid flow. To expose the lumbar disc, the PLL need to be incited. (C) showed the PLL was incited and only during the PLL incision, the patients had pain provoked. (D) showed the operated disc and the protruded nucleus pulposus has been removed.
Postoperative Evaluation
Surgical outcomes were assessed by reviewing the clinical data and imaging examinations from before surgery, and 6 months postoperatively. The Visual Analogue Scale (VAS) and Oswestry Disability Index (ODI) were taken before and after the surgery to observe the degree of pain. A postoperative MRI within 2 days after surgery was taken to see whether or not the herniated disc disappeared outside of the disc boundary.
Visual Analogue Scale (VAS)
The VAS is a measure made that using a ruler, the score is determined by measuring the distance (cm) on the 10‐cm line between the “no pain” anchor and the patient's mark, providing a range of scores from 0–10. A higher score indicates greater pain intensity. Based on the distribution of VAS scores in post‐surgical patients who described their postoperative pain intensity as none, mild, moderate, or severe, the following cut points on the pain VAS have been recommended: no pain (0–2), mild pain (3–5), moderate pain (6–8), and severe pain (8–10). Normative values are not available. The scale has to be shown to the patient otherwise it is an auditory scale not a visual one.
Oswestry Disability Index (ODI)
ODI is a principal condition‐specific outcome measure used in the management of spinal disorders and to assess patient progress in routine clinical practice. The ODI score system includes 10 sections: pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and traveling. For each section of six statements the total score is 5. Intervening statements are scored according to rank. If more than one box is marked in each section, take the highest score. If all 10 sections are completed the score is calculated as follows: total scored out of total possible score × 100. If one section is missed (or not applicable) the score is calculated: (total score/(5 × number of questions answered)) × 100%. Scores of 0%–20% are considered mild dysfunction, 21%–40% is moderate dysfunction, 41%–60% is severe dysfunction, and 61%–80% is considered as disability. For cases with score of 81%–100%, either long‐term bedridden, or exaggerating the impact of pain on their life.
Statistical Analysis
Data were entered and analyzed using the IBM SPSS 20.0 (International Business Machines Corporation, Armonk, New York, USA). The VSA and ODI score before and after operation were expressed as mean ± SD, and compared by t‐test for statistical analysis. A P‐value less than 0.05 was considered as statistically significant (P < 0.05).
Results
Pain Recordation and Distribution
Seventy‐two patients who received a single‐level decompression had LBP responses only when inciting the PLL. Thirty‐two patients (19 in L4‐5, 13 in L5S1) had LBP before the operation. The distribution of LBP foci before the operation is shown in Fig. 2, and the distribution of LBP foci provoked during PLL resections is shown in Fig. 3. For purposes of illustration only, pain is depicted as unilateral to the left. Forty‐three patients with symptomatic discs at L4‐5 had pain foci in the lower back and upper gluteal region under the L4 spinous process. Twenty‐nine patients with symptomatic discs at L5S1 had pain foci in the gluteal region under the S1 spinous process. The incision taken during the operation was unilateral, and examination of the data showed that the locations of the pain foci during the operation were all unilateral. When the incision was over, the provoked LBP disappeared in all patients, and all stated that there was no remaining provoked pain after the operation and no remaining LBP during the follow‐up period.
Fig. 2.
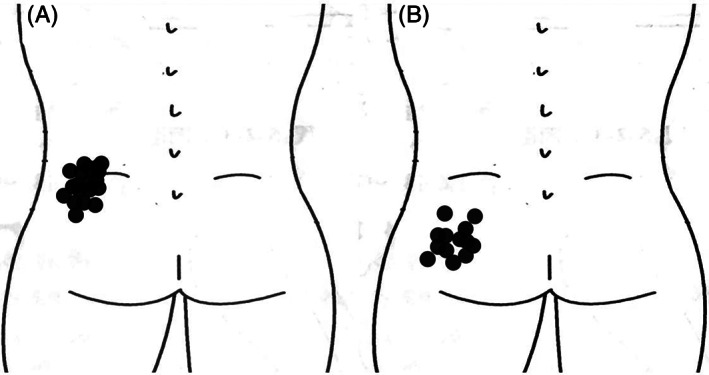
L4‐5 Foci of the pain recorded before the operation. (A) Foci of the pain from L4‐5. (B) Foci of the pain from L5S1. For purposes of illustration only, pain is depicted as unilateral to the left. The distribution of LBP foci from L4‐5 was in lower back and upper gluteal region under the L4 spinous process, while L5S1 had pain foci in the gluteal region under the S1 spinous process.
Fig. 3.
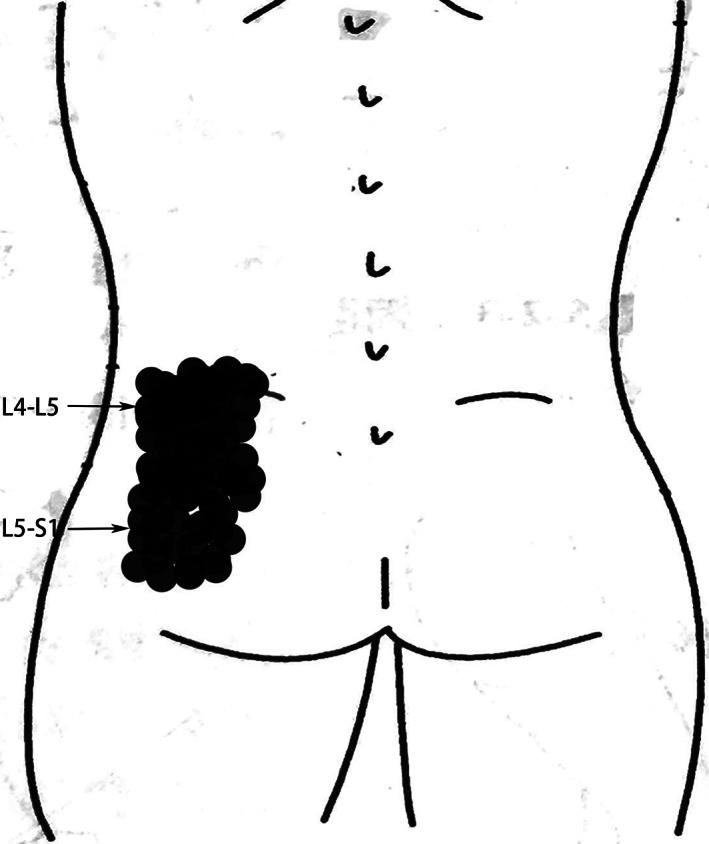
Foci of the pain provoked by PLL incision at each lumbar level. The distribution of LBP foci from L4‐5 was in lower back and upper gluteal region under the L4 spinous process, while L5S1 had pain foci in the gluteal region under the S1 spinous process. There is an overlapping region between the pain foci of L4‐5 and L5S1. For purposes of illustration only, pain is depicted as unilateral to the left.
Functional Evaluation
After 6‐months follow‐up, neurogenic claudication was improved in 72 patients (100%). The mean VAS scores of post‐operation patients were significantly better than that of pre‐operation patients (2.13 ± 0.78 vs 5.97 ± 1.10, P < 0.001). The mean ODI scores of post‐operation patients were better than that of pre‐operation patients (7.92 ± 1.85 vs 23.14 ± 3.28, P < 0.001). The percutaneous transforaminal endoscopic lumbar discectomies the patients received in this study were successful. The MRIs of three patients before and after the operation are shown in Figs 4, 5, 6.
Fig. 4.
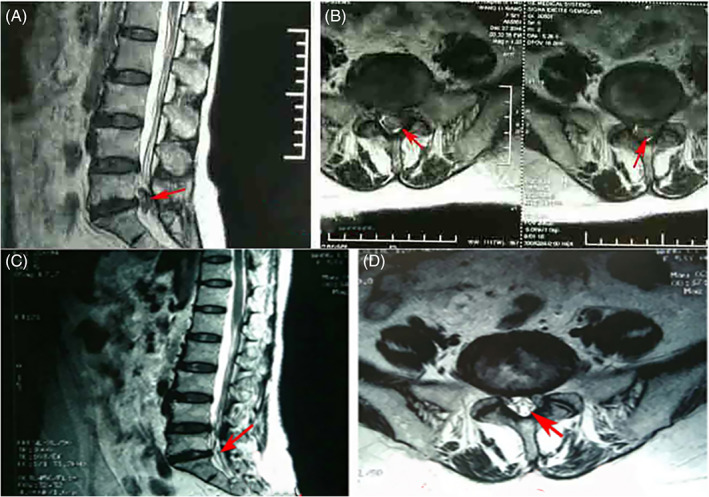
The patient (male, 46 years old) who received PELD (L5S1) and had LBP and neurogenic claudication before the operation. (A) and (B) Preoperative MRI of sagittal and axial of the lumbar spine showed the L5S1 disc herniation and spinal stenosis. The patient indicated the pain provocation during the PLL incision and the pain is similar to his LBP before the operation. (C) and (D) Postoperative MRI indicated that the disc herniation was cleared the decompression was successful.
Fig. 5.
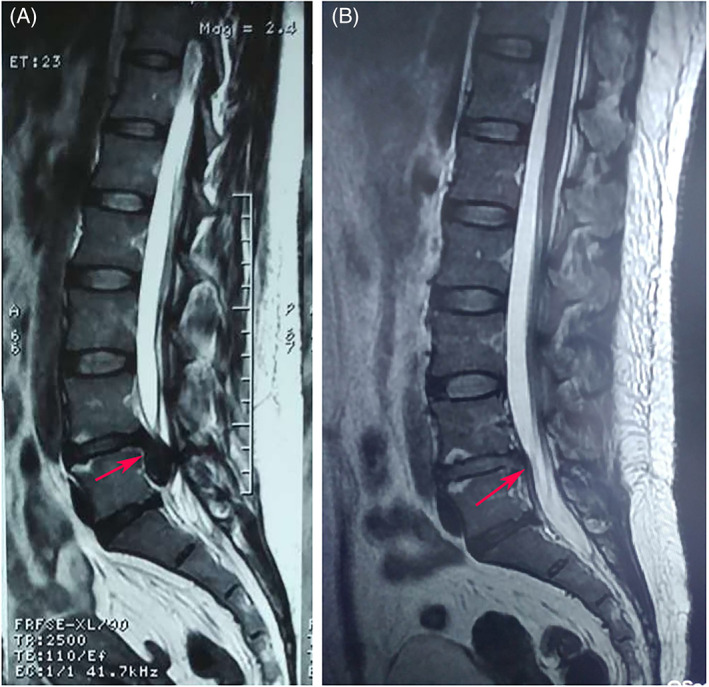
A 42 years old male patient who under PELD (L4‐5) and had neurogenic claudication without LBP before the operation. (A) Sagittal preoperative MRI of the lumbar spine showed the L4‐5 disc herniation and spinal stenosis. The patient also indicated the pain provocation during the PLL incision and the pain disappeared when the PLL incision is over. (B) Postoperative MRI indicated that the decompression was successful. On the first postoperative day, the patient was able to get out of bed with protection of the waistline.
Fig. 6.
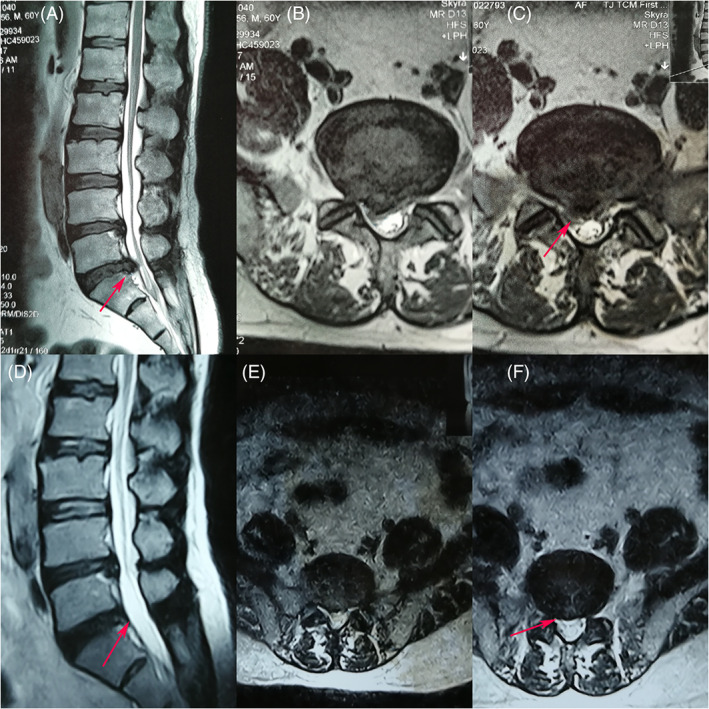
A 60 years old male patient who under PELD (L5S1) and had LBP and neurogenic claudication before the operation. (A), (B) and (C) Sagittal and axial preoperative MRI of the lumbar spine showed the L5S1 disc herniation and spinal stenosis. (D), (E) and (F) Postoperative MRI indicated that the spinal canal is clear and the herniated disc had been removed clearly. On the first postoperative day, the patient suffered from right lower limb pain that he did not have before the operation, and the lower limb pain abated in 1 week.
Complications
One patient suffered from lower limb pain that he did not have before the operation, and the lower limb pain abated a few days later. Three patients had cerebrospinal fluid leakage and were treated with higher pressure applied on the incision and bed rest for 10 days. No patients developed a surgical site infection, epidural hematoma, or other serious complications.
Discussion
Historical Background
The origination tissues of LBP have been explored in many studies in the past years 7 , 8 , 9 . Zygapophysial (facet) joints, disc and sacroiliac joints are still considered the major original tissues of LBP 8 , 10 , 11 , 12 , 13 , 14 . Discography, MRI, CT, and ultrasound have been used to identify the source of LBP 15 , 16 , 17 , 18 . Schwarzer et al. 11 found that a diagnosis of internal disc disruption can be made in a significant proportion of patients with chronic LBP, but no conventional clinical test can discriminate patients with internal disc disruption from patients with other conditions. Falco et al. 12 used facet joint nerve blocks to study the facet joint pain. In the previous studies, few studies focused on PLL.
Pain Distribution
The distribution of LBP foci from L4‐5 was in lower back and upper gluteal region under the L4 spinous process, while L5‐S1 had pain foci in the gluteal region under the S1 spinous process. The distributions were similar to the pain pattern which McCall et al. 20 made for facet joints in L1‐2 and L4‐5, while our patients did not complaint about the pain distribution in the anterior part of thigh.
Pain Disappearance
The immediate disappearance of provoked pain after operation and the absence of LBP during follow‐up indicated that the percutaneous transforaminal endoscopic lumbar discectomies were successful. However, further studies are needed to compare the surgical outcomes between the patients who underwent PLL resection and patients who kept the PLL in the lumbar decompression.
LBP Originates from PLL
Due to the provoked LBP that appeared only when inciting the PLL and disappeared post‐operation, and the pain provoked during the PLL incision was localized in a similar way to before the operation, this study of PLL incision in percutaneous endoscopic lumbar discectomy shows fair evidence that the PLL is one of the tissue candidates who can contribute to the LBP. Bogduk found that the sinuvertebral nerves supplied the PLL 21 . The sinuvertebral nerve divides into ascending, descending, and transverse branches. The transverse and descending branches supply the PLL at the level entry of the nerve while the ascending branch passes to the next higher level where it overlaps with the supra‐adjacent nerve. This may explain why the pain localizations from the adjacent disc level are partially overlapped and indicate that the LBP during PLL incision may be provoked by the incision of the sinuvertebral nerves. The sinuvertebral nerves also supplied the same level of the facet joints. In further studies, it might be feasible to study LBP by focusing on the sinuvertebral nerves.
Limitation of Study
The limitation of this study was that while it could identify that the PLL were sources of LBP, it could not identify other tissues from which LBP may have originated, such as the sacroiliac joints and facet joints. The relationship of all tissues associated with LBP need more studies to explore it fully. And it is expected that imaging can diagnose PLL associated LBP before the operation with the development of medical imageology. It was unclear whether the local anesthetic may have affected the judgment of patients or not. In the further research, a more objective measurement will be needed to identify the whole range of LBP distribution in patients.
Conclusions
LBP, which was similar to the pain patients had before the operation, was aggravated intraoperatively only during the PLL incision, and disappeared postoperatively, which implied that the intervertebral PLL may be a source of LBP.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethical Committee of Tianjin Medical University General Hospital, Ethical NO. IRB2014‐YX‐047) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Disclosure: National Natural Science Foundation of China (81871124) funds were received in support of this work.
References
- 1. Maher C, Underwood M, Buchbinder R. Non‐specific low back pain. Lancet, 2017, 389: 736–747. [DOI] [PubMed] [Google Scholar]
- 2. Hoy D, March L, Brooks P, et al The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis, 2014, 73: 968–974. [DOI] [PubMed] [Google Scholar]
- 3. Global Burden of Disease Study C . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet, 2015, 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golob AL, Wipf JE. Low back pain. Med Clin North Am, 2014, 98: 405–428. [DOI] [PubMed] [Google Scholar]
- 5. Jones LD, Pandit H, Lavy C. Back pain in the elderly: a review. Maturitas, 2014, 78: 258–262. [DOI] [PubMed] [Google Scholar]
- 6. Weiner DK, Sakamoto S, Perera S, Breuer P. Chronic low back pain in older adults: prevalence, reliability, and validity of physical examination findings. J Am Geriatr Soc, 2006, 54: 11–20. [DOI] [PubMed] [Google Scholar]
- 7. Freburger JK, Holmes GM, Agans RP, et al The rising prevalence of chronic low back pain. Arch Intern Med, 2009, 169: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manchikanti L, Boswell MV, Singh V, et al Comprehensive evidence‐based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician, 2009, 12: 699–802. [PubMed] [Google Scholar]
- 9. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine (Phila Pa 1976), 2009, 34: 49–59. [DOI] [PubMed] [Google Scholar]
- 10. Wolfer LR, Derby R, Lee JE, Lee SH. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta‐analysis of false‐positive rates. Pain Physician, 2008, 11: 513–538. [PubMed] [Google Scholar]
- 11. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976), 1995, 20: 1878–1883. [DOI] [PubMed] [Google Scholar]
- 12. Falco FJ, Manchikanti L, Datta S, et al An update of the systematic assessment of the diagnostic accuracy of lumbar facet joint nerve blocks. Pain Physician, 2012, 15: E869–E907. [PubMed] [Google Scholar]
- 13. Simopoulos TT, Manchikanti L, Singh V, et al A systematic evaluation of prevalence and diagnostic accuracy of sacroiliac joint interventions. Pain Physician, 2012, 15: E305–E344. [PubMed] [Google Scholar]
- 14. Hancock MJ, Maher CG, Latimer J, et al Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J, 2007, 16: 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yrjama M, Tervonen O, Kurunlahti M, Vanharanta H. Bony vibration stimulation test combined with magnetic resonance imaging. Can discography be replaced?. Spine (Phila Pa 1976), 1997, 22: 808–813. [DOI] [PubMed] [Google Scholar]
- 16. Young S, Aprill C, Laslett M. Correlation of clinical examination characteristics with three sources of chronic low back pain. Spine J, 2003, 3: 460–465. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida H, Fujiwara A, Tamai K, Kobayashi N, Saiki K, Saotome K. Diagnosis of symptomatic disc by magnetic resonance imaging: T2‐weighted and gadolinium‐DTPA‐enhanced T1‐weighted magnetic resonance imaging. J Spinal Disord Tech, 2002, 15: 193–198. [DOI] [PubMed] [Google Scholar]
- 18. Yrjama M, Tervonen O, Vanharanta H. Ultrasonic imaging of lumbar discs combined with vibration pain provocation compared with discography in the diagnosis of internal anular fissures of the lumbar spine. Spine (Phila Pa 1976), 1996, 21: 571–575. [DOI] [PubMed] [Google Scholar]
- 19. Chou R, Baisden J, Carragee EJ, Resnick DK, Shaffer WO, Loeser JD. Surgery for low back pain: a review of the evidence for an American pain society clinical practice guideline. Spine (Phila Pa 1976), 2009, 34: 1094–1109. [DOI] [PubMed] [Google Scholar]
- 20. McCall IW, Park WM, O'Brien JP. Induced pain referral from posterior lumbar elements in normal subjects. Spine (Phila Pa 1976), 1979, 4: 441–446. [DOI] [PubMed] [Google Scholar]
- 21. Bogduk N. The innervation of the lumbar spine. Spine (Phila Pa 1976), 1983, 8: 286–293. [DOI] [PubMed] [Google Scholar]


