Abstract
Background
Shift work is a necessary part of many industries; however, it can have detrimental effects on health over time.
Purpose
This study investigated the effect of a massage intervention on the cardiac autonomic activity and blood inflammatory markers of healthy medical residents working night shifts.
Setting
This trial was conducted at British Columbia Children’s and Women’s Hospital between February 2014 and June 2016.
Participants
Included participants were generally healthy medical residents and were working rotating night shifts on a regular basis.
Research Design
This was a randomized, controlled, crossover, open-label trial (NCT02247089).
Interventions
Participants received either a 30-min massage intervention or reading control after consecutive periods of night shift.
Main Outcome Measures
The primary outcome was high frequency, a proxy for the cardiac parasympathetic activity, measured via heart rate variability. Secondary outcomes included other heart rate variability measures, blood markers of inflammation, and blood pressure.
Results
Twelve participants were recruited (nine female) with median age of 28 years. There was no significant difference between the massage intervention and the reading control for the primary outcome, (median relative change between pre- and postmassage [interquartile range]: 62% [−1 to 150], pre- and postreading: 14% [−10 to 51], p = .16). Similarly, there was no difference with respect to blood inflammatory markers and blood pressure. Median high frequency significantly increased between pre- and postmassage (185 vs. 358 ms2, p = .04).
Conclusion
This pilot study found no statistically significant difference between the massage intervention and the reading control; however, we did observe a significant increase in median high frequency from before massage to after massage, indicative of increased parasympathetic activity. This study may help inform planning of larger trials evaluating massage interventions on the activity of the autonomic nervous system and managing shift work stress.
Keywords: autonomic nervous system, inflammation, massage, randomized controlled trial, shift work
INTRODUCTION
Shift work is common in the United States and Canada, with 29% and 28% of employees working alternating shifts (e.g., evening, night, or rotating shift), respectively.(1,2) Working outside normal daytime hours disrupts normal circadian rhythm, and has been associated with cardiovascular diseases(3–5) and increased risk of malignancy.(6–8) This has grave implications for sectors that provide around-the-clock services, such as health care.
Imbalances in the autonomic nervous system (ANS) have also been noted in night shift workers.(9–11) The ANS regulates unconscious bodily functions and keeps the body in homeostasis through the interplay between the sympathetic nervous system (SNS), responsible for “fight or flight”, and the parasympathetic nervous system (PNS), responsible for “rest and digest.” Both significant(9,10) and trend-wise(11) increases in SNS activity and decreases in PNS activity have been reported during night shift or when anticipating night shift.(12) Notably, significant dominance of the SNS and increased heart rate were observed in surgeons working 17-hour night shifts.(10) As well, shift work has been shown to elicit systemic inflammatory changes observed in studies on factory and male manual workers.(13,14)
As a complementary therapy regaining popularity,(15–17) massage therapy has been found to generally shift the ANS towards increased PNS activity and decreased SNS activity.(15,18–21) Additionally, single massage therapy sessions have been shown to reduce anxiety, blood pressure, and heart rate across a variety of populations.(15,20,22,23) Here, our goal was to determine whether a massage intervention could improve cardiovascular stress markers and blood inflammatory markers in medical residents after night shift work.
METHODS
Study Design
This randomized, controlled, crossover, open-label trial (NCT02247089) was conducted at British Columbia Children’s and Women’s Hospital between February 2014 and June 2016. Institutional research ethics board approval was given by the University of British Columbia (H13-01584). Informed consent was obtained from all participants at their initial visit prior to any data collection. We anticipated minimal risk for patients included in this study due to the nature of the intervention.
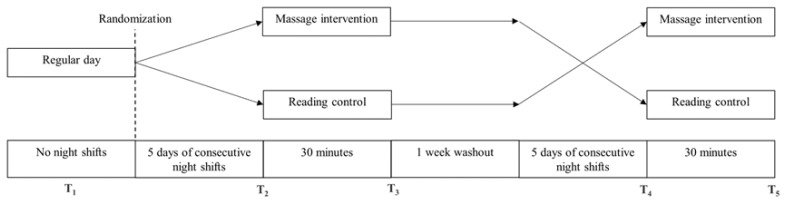
Participants were assessed for cardiovascular and inflammatory stress markers at five time points (Figure 1): at baseline, on a regular working day with no prior shifts for at least a week (T1); after five consecutive nights of shift work, immediately prior to (T2) and immediately after (T3) the massage intervention or the reading control; after a washout period of one week, and after another five consecutive nights of shift work, immediately prior to (T4); and immediately after (T5) the crossover intervention (i.e., from massage intervention to reading control, or vice versa). Prior to assessments, participants were asked not to perform heavy exercise within 24 hours, avoid drinking caffeinated beverages within 3 hours, and refrain from eating a heavy meal within 1.5 hours. Shift work began at 20:00 and ended at 08:00 on the following day. All five assessments were conducted at approximately the same time around 08:00 for consistency.
Figure 1.
Study design.
Population
Recruitment posters were displayed on hospital boards around the neonatal intensive care unit (NICU) and pediatric intensive care unit (PICU) at the hospital. Convenience sampling was done to recruit 12 participants. Participants were included in the study if they were generally healthy medical residents and were working rotating night shifts on a regular basis. Use of anti-depressants or other pharmacological agents that affect the cardiovascular system or the ANS, smoking more than 10 cigarettes per day, and inability to communicate or provide consent were criteria for exclusion.
Intervention
Participants were randomized between a 30-min massage intervention vs. a reading control. Randomization was concealed, and the sequence was created using an online computer software program (https://www.random.org). Administration of intervention and control could not be blinded due to the inherent nature of the procedures. The massage intervention included the back, shoulders, arms, and neck while the participant was seated comfortably in a standard massage chair. Massage was administered by a registered massage therapist (RMT) according to a predefined protocol (Appendix A). The RMTs had been in practice between one to three years at the time of the study. All had graduated from the Vancouver College of Massage Therapy (VCMT) and were taught by the same group of teachers throughout their training. The control consisted of journal reading in the same chair and environment as the intervention. The environment consisted of an empty room (doors kept closed), with stable room temperature, and typical office noise and light levels.
Outcome Measures
The primary outcome was high frequency (HF) as measured by heart rate variability (HRV). HF is widely accepted as a reliable marker of pure cardiac PNS modulation.(24,25) Secondary outcomes included other HRV and impedance cardiography (ICG) parameters: total power (TP), estimating the ability of the ANS to cope with stress; pre-ejection period, inversely correlated with SNS influence on the heart; and heart rate, influenced by both the SNS and PNS. Blood markers of inflammation including interleukin (IL)-1beta, IL-6, IL-8, tumor necrosis factor alpha (TNF-alpha), and blood pressure were other secondary outcomes. The markers of inflammation have been shown to play a significant role in chronic inflammatory conditions and oncological conditions.(26–28) Number of hours slept on the last night shift (i.e., at T2 and T4) was also captured.
Cardiovascular Stress Markers
Cardiovascular measures consisted of blood pressure obtained in a seated position, followed by 5 min of continued comfortable sitting, and then HRV and ICG recording for 5 min using six electrodes placed on the skin of the chest, neck, and lower back. At the end of HRV and ICG recording, a 5-ml sample of blood was collected intravenously for the assessments of blood inflammatory markers.
HRV and ICG recordings were collected by a BIOPAC MP150 data acquisition system (BIOPAC Systems Inc., Goleta, CA). Further details on HRV and ICG are provided in Appendix B. Systolic and diastolic blood pressures were obtained using a digital blood pressure monitor (Model UA-787, A&D Company, Tokyo, Japan), and the average of three measures is reported.
Inflammatory Blood Markers
Blood was collected in sodium heparin microcontainers. Plasma IL-1beta, IL-6, IL-8, and TNF-alpha were assessed using commercially available ELISA kits (V-PLEX Human Proinflammatory Panel II [4-Plex]) and a SECTOR Imager 2400A Model 1250. Samples were run in duplicate to ensure reproducibility. Analyses were done using DISCOVERY WORKBENCH 4.0 (Meso Scale Diagnostics (MSD), Rockville, MD).
Statistical Analysis
Wilcoxon signed-rank test was used for all within-group and between-group analyses. Results are reported as median (interquartile range). We present the change in outcomes from before to after intervention or control as relative percentage changes. This was done to adjust for baseline differences among participants. Relative percentage changes were calculated as follows: (i) relative change for each participant from pre- to postmassage intervention or reading control was obtained using the formula (postvalue - prevalue) / prevalue × 100%, and (ii) the median of the set of values from step (i) was obtained and presented in this paper.
Effect size (Cohen’s d) was calculated for HF using the methods described by Fritz et al.(29) for nonparametric data. For consistency, we also calculated the effect size by applying methods developed by Morris and DeShon for repeated measures design,(30) using the log-transformed HF data to increase normality.
To explore the effect of consecutive days of night shift, the regular day assessment (T1) and the average of the two night shift assessments (i.e., the average of T2 and T4) were compared. Spearman’s rank correlation was conducted using data collected at T1 to explore the association between the inflammatory markers and HRV and ICG parameters. Analyses were done on the per-protocol population, and a p value < .05 was considered statistically significant (SPSS® v.20.0, IBM, Armonk, NY).
RESULTS
Patient Characteristics and Recruitment
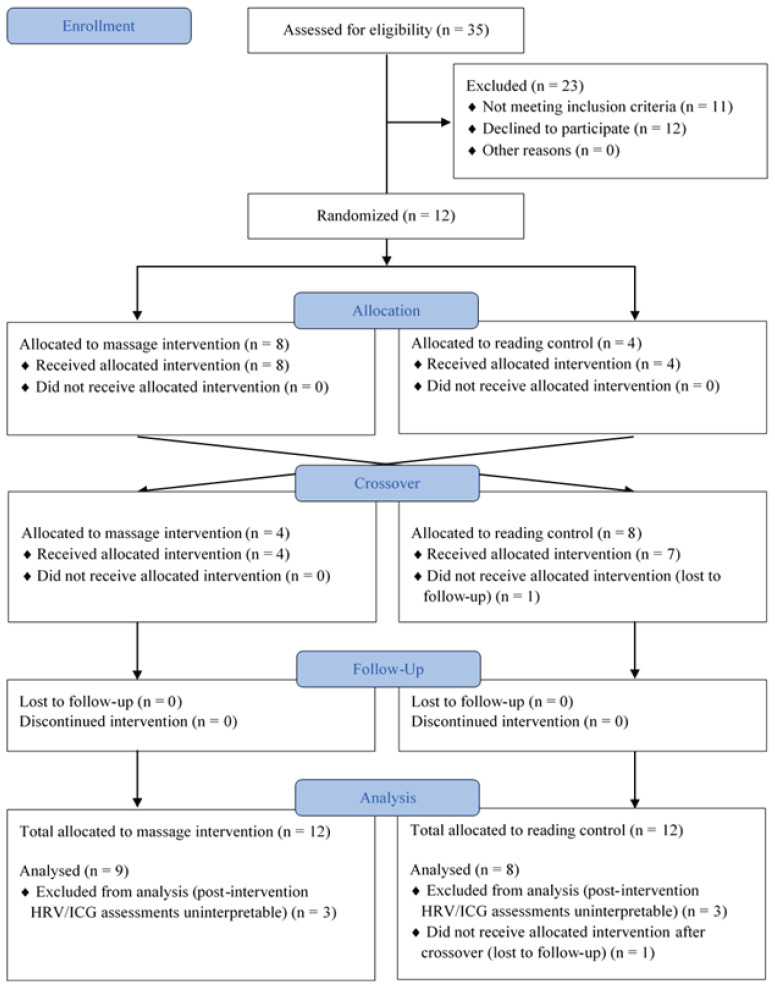
Twelve healthy adult participants were recruited (9 females; median age 28 years [26 to 30]). All were residents of the Pediatric Department at the British Columbia Children’s and Women’s Hospital. One participant was lost to follow-up after crossover, and data for this participant are available only for the massage intervention (Figure 2). Postintervention HRV and ICG assessments were uninterpretable for three participants due to the significant number of artifacts in the recorded data and were excluded from analyses. Consequently, nine participants in the massage intervention group and eight participants in the reading control group were included for the HRV and ICG analysis. No adverse events were reported.
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Effects of Night Shift on ANS Profile & Blood Markers of Inflammation
The baseline physiological and biochemical characteristics between the regular day assessment (T1) and the pooled night shift assessment (the average of T2 and T4) were not significantly different from one another, except for heart rate and IL-6 (Table 1). Median heart rate was lower for the pooled night shift assessment compared to regular day (70 vs. 77 beats/min, p = .05), while median IL-6 was greater (0.5 vs. 0.3 pg/ml, p = .01).
Table 1.
Demographic and Clinical Characteristics of Participantsa
| Female, n (%) | 9 (75) | ||
| Age (range in years) | 28 (26–30) | ||
|
| |||
| HRV/ICG Parameters (n = 11) | Regular Day (T 1 ) | Night Shift (average of T 2 and T 4 ) | P value b |
|
| |||
| HF (ms2) | 127 (80 to 277) | 265 (157 to 453) | .11 |
| TP (ms2) | 675 (287 to 818) | 1029 (319 to 1415) | .13 |
| Pre-ejection period (ms) | 120 (116 to 125) | 122 (116 to 125) | .86 |
| Heart rate (beats/min) | 77 (61 to 81) | 70 (58 to 82) | .05 |
|
| |||
| Blood Inflammatory Markers (n = 11) | Regular Day (T 1 ) | Night Shift (average of T 2 and T 4 ) | P value |
|
| |||
| IL-6 (pg/ml) | 0.3 (0.3 to 0.5) | 0.5 (0.3 to 0.8) | .01 |
| IL-8 (pg/ml) | 3.7 (3.4 to 4.4) | 4.2 (3.3 to 5.5) | .37 |
| TNF-alpha (pg/ml) | 1.6 (1.4 to 1.9) | 1.8 (1.5 to 2.1) | .29 |
|
| |||
| Blood Pressure (n = 11) | Regular Day (T 1 ) | Night Shift (average of T 2 and T 4 ) | P value |
|
| |||
| Systolic (mmHg) | 114 (102 to 118) | 114 (105 to 125) | .33 |
| Diastolic (mmHg) | 78 (74 to 82) | 78 (70 to 91) | .26 |
Values are median (interquartile range)
P values are reported using Wilcoxon signed-rank test
HRV = heart rate variability; HF = high frequency; ICG = impedance cardiography; IL = interleukin; TNF-alpha = tumor necrosis factor alpha; TP = total power.
Effect of Massage Intervention and Reading Control
No significant differences between the massage intervention and the reading control were seen for any of the HRV/ICG parameters, blood inflammatory markers, and blood pressure (Table 2). Some within-group differences were observed (Table 3). Median HF significantly increased from before massage to after massage intervention (185 vs. 358 ms2, p = .04), and an increase in TP was observed from before reading to after reading (617 vs. 807, p = .04) (Table 3). The effect size for HF from before to after massage intervention was 1.12. IL-1beta was assessed; however, most of its data were below the lower limit of detection and are therefore not presented.
Table 2.
Comparison Between Massage Intervention and Reading Controla
| HRV/ICG Parameters | Before Massage(n = 9) | Relative Change From Before Massage to After Massage | Before Reading (n = 8) | Relative Change From Before Reading to After Reading | P value b |
|---|---|---|---|---|---|
| HF (ms2) | 185 (112 to 315) | 62% (−1 to 150) | 200 (88 to 370) | 14% (−10 to 51) | .16 |
| TP (ms2) | 675 (310 to 1910) | 44% (12 to 138) | 617 (217 to 1346) | 25% (15 to 85) | .40 |
| Pre-ejection period (ms) | 121 (116 to 126) | 1% (0 to 2) | 119 (115 to 128) | 0% (−1 to 1) | .24 |
| Heart rate (beats/min) | 78 (65 to 85) | −4% (−8 to 1) | 72 (67 to 91) | −3% (−5 to 0) | .89 |
|
| |||||
| Blood Inflammatory Markers | Before Massage (n = 12) | Relative Change From Before Massage to After Massage | Before Reading (n = 11) | Relative Change From Before Reading to After Reading | P value b |
|
| |||||
| IL-6 (pg/ml) | 0.5 (0.4 to 1.1) | −9.0% (−28.4 to 6.8) | 0.4 (0.3 to 0.6) | −4.2% (−12.9 to 5.7) | .37 |
| IL-8 (pg/ml) | 4.0 (3.2 to 6.7) | −5.4% (−26.3 to 8.0) | 3.4 (3.3 to 3.8) | −2.6% (−6.7 to 2.7) | .37 |
| TNF-alpha (pg/ml) | 1.8 (1.5 to 2.4) | −2.5% (−6.7 to 1.7) | 1.8 (1.5 to 2.0) | −1.5% (−6.6 to 3.6) | .18 |
|
| |||||
| Blood Pressure | Before Massage (n = 12) | Relative Change From Before Massage to After Massage | Before Reading (n = 11) | Relative Change From Before Reading to After Reading | P value b |
|
| |||||
| Systolic (mmHg) | 113 (105 to 119) | −1% (−3 to 3) | 109 (104 to 127) | −3% (−6 to −2) | .13 |
| Diastolic (mmHg) | 77 (73 to 89) | 1% (−2 to 4) | 78 (69 to 90) | −3% (−4 to 9) | .86 |
Values are median (interquartile range)
P value is for the comparison between massage and reading relative changes; values are reported using Wilcoxon signed-rank test
HRV = heart rate variability; HF = high frequency; ICG = impedance cardiography; IL = interleukin; TNF-alpha = tumor necrosis factor alpha; TP = total power.
Table 3.
Effect of Massage Intervention and Reading Control on Outcomesa
| HRV/ICG Parameters | Before Massage (n = 9) | After Massage (n = 9) | P value | Before Reading (n = 8) | After Reading (n = 8) | P value b |
|---|---|---|---|---|---|---|
| HF (ms2) | 185 (112 to 315) | 358 (157 to 476) | .04 | 200 (88 to 370) | 207 (95 to 531) | .78 |
| TP (ms2) | 675 (310 to 1910) | 868 (662 to 2560) | .07 | 617 (217 to 1346) | 807 (294 to 1740) | .04 |
| Pre-ejection period (ms) | 121 (116 to 126) | 123 (116 to 126) | .16 | 119 (115 to 128) | 121 (116 to 129) | .58 |
| Heart rate (beats/min) | 78 (65 to 85) | 73 (62 to 85) | .07 | 72 (67 to 91) | 73 (64 to 89) | .05 |
|
| ||||||
| Blood Inflammatory Markers | Before Massage (n = 12) | After Massage (n = 12) | P value | Before Reading (n = 11) | After Reading (n = 11) | P value |
|
| ||||||
| IL-6 (pg/ml) | 0.5 (0.4 to 1.1) | 0.6 (0.2 to 0.9) | .70 | 0.4 (0.3 to 0.6) | 0.4 (0.3 to 0.8) | .59 |
| IL-8 (pg/ml) | 4.0 (3.2 to 6.7) | 3.4 (2.8 to 4.5) | .27 | 3.4 (3.3 to 3.8) | 3.3 (3.0 to 3.8) | .42 |
| TNF-alpha (pg/ml) | 1.8 (1.5 to 2.4) | 1.8 (1.4 to 2.2) | .12 | 1.8 (1.5 to 2.0) | 1.7 (1.4 to 2.0) | .59 |
|
| ||||||
| Blood Pressure | Before Massage (n = 12) | After Massage (n = 12) | P value | Before Reading (n = 11) | After Reading (n = 11) | P value |
|
| ||||||
| Systolic (mmHg) | 113 (105 to 119) | 114 (102 to 118) | .67 | 109 (104 to 127) | 106 (100 to 120) | .05 |
| Diastolic (mmHg) | 77 (73 to 89) | 79 (74 to 87) | .62 | 78 (69 to 90) | 77 (73 to 89) | .79 |
Values are median (interquartile range)
P values are reported using Wilcoxon signed-rank test
HRV = heart rate variability; HF = high frequency; ICG = impedance cardiography; IL = interleukin; TNF-alpha = tumor necrosis factor alpha; TP = total power.
The mean amount of sleep reported by participants receiving massage intervention on their last night shift was 1.4 hours (95% CI: 0.7 to 2.2). Participants tended to report slightly less sleep on their last night shift before receiving the reading control (mean: 1.1 hours, 95% CI: 0.4 to 1.8). Individual participant data are available in Table 4.
Table 4.
Hours of Sleep Self-Reported by Individual Participants on Night Shift
| Participant | Before Massage (T 2 or T 4 ) | Before Reading (T 2 or T 4 ) |
|---|---|---|
| 1 | 0.7 | 2.5 |
| 2 | 0.0 | 0.0 |
| 3 | 3.0 | 0.8 |
| 4 | 1.5 | 0.0 |
| 5 | 0.0 | 0.0 |
| 6 | 1.5 | 0.0 |
| 7 | 0.0 | 0.5 |
| 8 | 4.0 | Not available (lost to follow-up) |
| 9 | 3.0 | 1.0 |
| 10 | 0.0 | 1.5 |
| 11 | 2.0 | 3.5 |
| 12 | 1.5 | 2.5 |
The Relationship Between ANS Parameters & Blood Markers of Inflammation
There was a negative correlation between TP and IL-6 (rs = −0.77, p = .005), and a positive correlation between TP and IL-8 (rs = 0.87, p < .01). HF and IL-8 were positively correlated (rs = 0.82, p = .002). No statistically significant correlation was found between TNF-alpha and any of the HRV and ICG parameters.
DISCUSSION
Although shift work is common and necessary in many professions including health workers, the adverse effects caused by the resulting stress effects are substantial and must be addressed to prevent serious long-term complications. To the best of our knowledge, this is the first randomized crossover trial objectively evaluating the effect of massage on the ANS, measured via HRV and ICG, and on blood inflammatory markers in night shift workers.
Effects of Night Shift on ANS Profile & Blood Markers of Inflammation
Comparing the regular day assessment and the pooled night shift assessment, we observed median heart rate to be significantly lower for the pooled night shift assessment. Previously, Adams et al.(9) measured HRV via a Holter monitor in emergency physicians pre-shift, during shift, and post-shift. Similar to our findings, they observed a lower heart rate post-shift compared to pre-shift work (71.5 vs. 81.3 beats/min, p < .001). Interestingly, the authors also reported greater HF post-shift work compared to pre-shift work (473 vs. 302 ms2, p = .04), which indicates a greater activity of the PNS. In our study, HF for the pooled night shift assessment was also greater compared to regular day; however, this did not reach statistical significance.
The drive towards greater PNS activity post-night shift, indicated by lowered heart rate and increased HF, may be explained with Hans Selye’s General Adaptation Syndrome.(31) There are three stages in this model of adaptation to stress: (1) Alarm, where an organism uses resources to resist a stressor; (2) Resistance, where the response to stress is intensified to return the organism to homeostasis; and (3) Recovery/Exhaustion, where either the stressor is overcome or the organism’s resources are depleted leading to loss of bodily function or illness. We hypothesize that the stress from consecutive nights of shift work stemming from sleep disturbances and staying alert led to an initial Alarm stage. Subsequently, to return to homeostasis, a parasympathetic response was initiated, leading to the Resistance stage. We likely assessed participants while they were in this Resistance stage; hence the decrease in heart rate (and trend-wise increase in HF) that were observed, which are indicative of greater parasympathetic activity.
We found IL-6 was significantly greater for the pooled night shift compared to the regular day. Previously, a small trial reported a significant increase in IL-6 following night shift work compared to day work.(32) It has been shown that IL-6 is elevated during acute sleep deprivation,(33) which is common in night shift workers and may also be the source of the IL-6 elevation we observed in our study. However, studies assessing changes to inflammatory markers in shift work are few and results can be inconsistent. A cross-sectional study of IL-6 levels in shift workers and daytime workers observed no difference between the two worker groups.(34) Similarly, another study found no difference in IL-6 between shift-working and daytime nurses at baseline or at 12 months of follow-up, although other markers (IL-1beta and TNF-alpha) were greater for night shift workers at baseline.(35) Further evidence is needed to determine the relationship between shift work and inflammatory markers.
Effects of Massage Intervention and Reading Control
When comparing HF between the massage intervention and the control we did not observe any statistically significant difference. This statistically non-significant finding may be due to a limitation that arises from the pilot nature of this study and lack of power to establish a difference between intervention and control. Two other factors may have contributed to observing a smaller difference between the two interventions: (1) at the end of night shift, the participants had already increased TP and HF as a stress compensatory mechanism (Table 1), therefore leaving a smaller postmassage marginal gain in these two parameters; and (2) the reading control was also a relaxing intervention that led to the same trends in increasing TP and HF, therefore reducing the difference with massage intervention.
We did observe greater relative change in HF for the massage intervention (62% vs. 14%), which may suggest a greater PNS response for massage as compared to the control. From before massage to after massage we observed a significant increase in median HF, indicative of increased PNS activity. Previous studies have observed an increase in PNS activity after massage. Diego and Field evaluated the effects of moderate pressure versus light pressure massage in healthy adults and reported that, during the initial part of a 15-minute massage, a parasympathetic response, indicated by an increase in HF, was observed.(18) As well, massage of other local body areas has been shown to elicit an ANS response; head massage in healthy adults significantly increased the overall ANS activity,(36) while hand and feet massage in critically ill children significantly increased parasympathetic activity.(37) The parasympathetic response observed may be attributed to the potential stimulating effects on vagal afferent fibers, that could activate the efferent fibers, and hence create the overall PNS response observed.(18)
However, further randomized clinical studies with larger sample sizes are warranted to establish any causal links between massage and changes in HF. Our findings provide a large effect size of 1.12 for HF from before to after massage. This estimate is also robust in that calculating it using formulas for repeated measures design yields only a slightly more conservative effect size of 1.096.
We observed a reduction of almost twofold in blood markers of inflammation postmassage intervention compared to the reading control; however, these differences did not reach statistical significance. Our pilot study was not powered to test these hypotheses, and we did not find similar studies assessing blood markers of inflammation after a massage intervention to be able to compare our findings. However, a study on the effect of massage on leg muscles with exercise-induced damage did find production of TNF-alpha and IL-6 was lower for the leg that received massage compared to the control leg.(38) Our findings are similar with regards to the direction of change postmassage.
The Relationship Between ANS Parameters & Blood Markers of Inflammation
Our exploratory analyses showed that TP has a strong negative correlation with IL-6, and both TP and HF displayed strong positive correlations with IL-8. Similar correlations for TP and IL-6 have been reported in two studies investigating patients with decompensated congestive heart failure, and in depressed patients with coronary heart disease.(39,40) It is interesting to note that our results in otherwise healthy participants who work night shifts indicate a similar relationship between TP and IL-6. The inverse relationship observed in our study between overall ANS activity characterized by TP and IL-6, a pro-inflammatory cytokine, may be explained in consideration of the cholinergic anti-inflammatory pathway, where increased cytokine production in response to stress stimulates afferent vagus nerves. This, in turn, leads to the inhibition of cytokine release, as well as increased HRV via efferent vagus nerve activity.(41)
Our study showed a strong positive correlation between HF and IL-8, and between TP and IL-8. However, two other studies reported no association between IL-8 and HRV parameters in people living with inflammatory conditions such as rheumatoid arthritis, fibromyalgia, and lupus.(42,43) Although these populations are not comparable to healthy adults, to the best of our knowledge these are the only studies evaluating associations between IL-8 and HRV parameters. Further work is needed to determine the cause of the positive correlation we observe between IL-8, a pro-inflammatory mediator, and the proxies for parasympathetic activity and overall ANS activity.
Strengths and Limitations
The strength of this study is its randomized and controlled design, with crossover allowing for comparison of participants with themselves to control for confounding covariates. Additionally, five different RMTs administered the intervention following one predefined protocol. Although this may have created some variation, we argue that our procedure provides a more pragmatic application of the intervention. A limitation of this pilot study is its small sample size, which decreases power. Also, HRV and ICG were measured after, rather than during, massage. The latter procedure provides a more accurate measurement of the effect of massage; however, the proximity of the RMT’s hand to the electrodes created artifacts in the recording and, hence, this procedure was not feasible.
CONCLUSION
Although shift work is common and necessary in many professions including health workers, the adverse effects caused by the resulting stress effects are substantial and must be addressed to improve health and prevent serious long-term medical complications. In this pilot study, we did not observe a statistically significant difference when comparing the massage intervention to the reading control. However, we did observe a significant increase in median HF from before massage to after massage, indicative of increased parasympathetic activity. These results provide potential effect sizes to inform larger trials evaluating the role of massage interventions in reducing the stress caused by repeated night shift work.
ACKNOWLEDGMENTS
We thank the participants of this study and the registered massage therapists for their time and cooperation. We thank Margaret Lin for her editorial support in preparation of this manuscript.
APPENDICES
Appendix A. Massage Protocol
Position subject on massage chair. Shoes remain on. No oil used during treatment. Treatment:
1 min of adaptation
2 min: Placement of therapist’s hands begins on upper trapezius/upper back area with slow, firm compressions, progress with compressions down the length of the spine (superior to inferior), first along spinalis, longissimus and iliocostalis muscles.
5 min: upper trapezius picking up, deep kneading and picking up around scapula muscle attachments (rhomboids, trapezius, teres major/minor, lat. dorsi), mobilization of the scapula in protraction, retraction, elevation and depression
4 min: progress from shoulder work on one side to same size mid to lower back, progressing to opposite size mid to lower back, deep kneading along erector spinae with pressure focus away from spinous processes. Apply firm pressure to rib cage at the area of the costovertebral joints, alternating right and left to encourage movement, working your way superiorly and into opposite shoulder. Repeat above protocol for shoulder/upper back area on opposite side.
5 min: other shoulder area
5 min (each arm: total 10 min): Deep kneading and picking up to deltoid, biceps brachii, triceps brachii, brachioradialis, extensor carpi radials, extensor digitorim, forearm flexors, intrinsic muscles of the hand. Passive range of motion humeroulnar/ humeroradial joint, mobilizations (grade 1/2) of radiocarpal joint, intercarpals, metacarpalphalangeal and proximal and distal interphalangeal joints.
5 min: Deep kneading through levator scapula/base of neck area, working into posterior neck with deep kneading and picking up into posterior neck muscles, suboccipitals, progress to head massage (focus on occipital-frontalis, auricular muscles and temporalis muscles)
2 min: Deep compressions from head down spine to sacrum, sacral decompression 30 sec to integrate and complete session
1 min of post-massage rest
Appendix B. Heart Rate Variability (HRV) and Impedance Cardiography (ICG)
For spectral analysis of HRV and ICG, data was collected by a Biopac MP150 data acquisition system (Biopac Systems Inc., Goleta, CA). HRV is a measure of the variability in the beat-to-beat heart rhythm (R-wave to R-wave in the QRST complex) and acts as a proxy for the status of the autonomic nervous system.(1) The interactions between the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS) can be measured through this precise and non-invasive procedure.(1) Data were recorded in 5-minute segments with signals sampled at 500 Hertz, and were reviewed manually by our HRV expert (MSF) for ectopic beats and analyzed using AcqKnowledge software version 4.2 manufactured by BIOPAC Systems Inc. Fast Fourier Transformation technique was used to perform frequency domain HRV analysis. The following frequency bands are reported: high frequency (0.15 to 0.4 Hertz), which is a marker of pure cardiac PNS modulation; and total power ( ≤0.4 Hertz), which represents an estimate of the overall autonomic nervous system regulatory ability in coping with physiological or non-physiological stress.(1) From ICG analysis we obtained pre-ejection period, which measures the time interval from the onset of the electromechanical systole to the onset of the opening of the aortic valve and is inversely correlated with the SNS influence upon the heart.(2,3) Heart rate, under the influence of both SNS and PNS, was also obtained from ICG analysis.
- 1.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 2.Newlin DB, Levenson RW. Pre-ejection period: measuring beta-adrenergic influences upon the heart. Psychophysiology. 1979;16(6):546–552. doi: 10.1111/j.1469-8986.1979.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 3.Sheps DS, Petrovick ML, Kizakevich PN, Wolfe C, Craige E. Continuous noninvasive monitoring of left ventricular function during exercise by thoracic impedance cardiography-automated derivation of systolic time intervals. Am Heart J. 1982;103(4):519–524. doi: 10.1016/0002-8703(82)90339-8. [DOI] [PubMed] [Google Scholar]
Footnotes
CONFLICT OF INTEREST NOTIFICATION
This study was financially supported by the Registered Massage Therapists’ Association of British Columbia. PML was supported by a career investigator award from the Michael Smith Foundation for Health Research and the British Columbia Children’s Hospital Research Institute. No other conflicts of interest exist.
REFERENCES
- 1.Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW, Calvert GM. Prevalence rates of work organization characteristics among workers in the US: data from the 2010 National Health Interview Survey. Am J Industrial Med. 2013;56(6):647–659. doi: 10.1002/ajim.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams C. Statistics Canada Catalogue No. 75-001-X. Ottawa, ON: Stats Canada; 2008. Work-Life Balance Of Shift Workers. Available from: https://www150.statcan.gc.ca/n1/en/pub/75-001-x/2008108/pdf/10677-eng.pdf?st=1qmpZU5C. [Google Scholar]
- 3.Boggild H, Knutsson A. Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health. 1999;25(2):85–99. doi: 10.5271/sjweh.410. [DOI] [PubMed] [Google Scholar]
- 4.Ellingsen T, Bener A, Gehani AA. Study of shift work and risk of coronary events. J Royal Soc Promot Health. 2007;127(6):265–267. doi: 10.1177/1466424007083702. [DOI] [PubMed] [Google Scholar]
- 5.Knutsson A, Jonsson BG, Akerstedt T, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;2(8498):89–92. doi: 10.1016/S0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 6.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164(6):549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 7.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41(13):2023–2032. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95(11):825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 9.Adams SL, Roxe DM, Weiss J, Zhang F, Rosenthal JE. Ambulatory blood pressure and Holter monitoring of emergency physicians before, during, and after a night shift. Acad Emerg Med. 1998;5(9):871–877. doi: 10.1111/j.1553-2712.1998.tb02816.x. [DOI] [PubMed] [Google Scholar]
- 10.Amirian I, Andersen LT, Rosenberg J, Gogenur I. Decreased heart rate variability in surgeons during night shifts. Can J Surg. 2014;57(5):300–304. doi: 10.1503/cjs.028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito H, Nozaki M, Maruyama T, Kaji Y, Tsuda Y. Shift work modifies the circadian patterns of heart rate variability in nurses. Int J Cardiol. 2001;79(2–3):231–236. doi: 10.1016/S0167-5273(01)00439-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang ML, Lin PL, Huang CH, Huang HH. Decreased parasympathetic activity of heart rate variability during anticipation of night duty in anesthesiology residents. Anesthesia & Analgesia. 2018;126(3):1013–1018. doi: 10.1213/ANE.0000000000002439. [DOI] [PubMed] [Google Scholar]
- 13.Sookoian S, Gemma C, Fernandez Gianotti T, Burgueno A, Alvarez A, Gonzalez CD, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261(3):285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim S-W, Jang E-C, Kwon S-C, Han W, Kang MS, Ham YH, et al. Night shift work and inflammatory markers in male workers aged 20–39 in a display manufacturing company. Ann Occup Environ Med. 2016;28(1):48. doi: 10.1186/s40557-016-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130(1):3–18. doi: 10.1037/0033-2909.130.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Centers for Disease Control & Prevention, CDC Stacks. Natl Health Stat Report No. 12. 2008;(12):1–23. [PubMed] [Google Scholar]
- 17.Esmail N. Complementary and alternative medicine in Canada: trends in use and public attitudes 1997–2006. Public Policy Sources. 2007;87:1–53. [Google Scholar]
- 18.Diego MA, Field T. Moderate pressure massage elicits a parasympathetic nervous system response. Int J Neurosci. 2009;119(5):630–638. doi: 10.1080/00207450802329605. [DOI] [PubMed] [Google Scholar]
- 19.Moraska A, Pollini RA, Boulanger K, Brooks MZ, Teitlebaum L. Physiological adjustments to stress measures following massage therapy: a review of the literature. Evid-based Complem Altern Med. 2010;7(4):409–418. doi: 10.1093/ecam/nen029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hand ME, Margolis J, Staffileno BA. Massage chair sessions: favorable effects on ambulatory cancer center nurses’ perceived level of stress, blood pressure, and heart rate. Clin J Oncol Nurs. 2019;23(4):375–381. doi: 10.1188/19.CJON.375-381. [DOI] [PubMed] [Google Scholar]
- 21.Seifert G, Kanitz J-L, Rihs C, Krause I, Witt K, Voss A. Rhythmical massage improves autonomic nervous system function: a single-blind randomised controlled trial. J Integrat Med. 2018;16(3):172–177. doi: 10.1016/j.joim.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Palma S, Keilani M, Hasenoehrl T, Crevenna R. Impact of supportive therapy modalities on heart rate variability in cancer patients—a systematic review. Disabil Rehabil. 2020;42(1):36–43. doi: 10.1080/09638288.2018.1514664. [DOI] [PubMed] [Google Scholar]
- 23.Givi M, Sadeghi M, Garakyaraghi M, Eshghinezhad A, Moeini M, Ghasempour Z. Long-term effect of massage therapy on blood pressure in prehypertensive women. J Edu Health Promot. 2018;7:54–54. doi: 10.4103/jehp.jehp_88_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 25.Thomas BL, Claassen N, Becker P, Viljoen M. Validity of commonly used heart rate variability markers of autonomic nervous system function. Neuropsychobiology. 2019;78(1):14–26. doi: 10.1159/000495519. [DOI] [PubMed] [Google Scholar]
- 26.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48(4):751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Shahzad A, Knapp M, Lang I, Köhler G. Interleukin 8 (IL-8)—a universal biomarker? Int Arch Med. 2010;3(1):11–11. doi: 10.1186/1755-7682-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 30.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7(1):105–125. doi: 10.1037/1082-989X.7.1.105. [DOI] [PubMed] [Google Scholar]
- 31.Selye H. Stress and the general adaptation syndrome. Br Med J. 1950;1(4667):1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khosro S, Alireza S, Omid A, Forough S. Night work and inflammatory markers. Ind J Occu Environ Med. 2011;15(1):38–41. doi: 10.4103/0019-5278.82996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metabol. 2010;24(5):775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Mark A, Weiler SW, Schröder M, Otto A, Jauch-Chara K, Groneberg DA, et al. The impact of shift work induced chronic circadian disruption on IL-6 and TNF-α immune responses. J Occu Med Toxicol. 2010;5(1):18. doi: 10.1186/1745-6673-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Copertaro A, Bracci M, Gesuita R, Carle F, Amati M, Baldassari M, et al. Influence of shift-work on selected immune variables in nurses. Ind Health. 2011;49(5):597–604. doi: 10.2486/indhealth.MS1210. [DOI] [PubMed] [Google Scholar]
- 36.Fazeli MS, Pourrahmat MM, Liu M, Guan L, Collet JP. The effect of head massage on the regulation of the cardiac autonomic nervous system: a pilot randomized crossover trial. J Altern Complement Med. 2016;22(1):75–80. doi: 10.1089/acm.2015.0141. [DOI] [PubMed] [Google Scholar]
- 37.Guan L, Collet J-P, Yuskiv N, Skippen P, Brant R, Kissoon N. The effect of massage therapy on autonomic activity in critically ill children. Evid-Based Complement Altern Med. 2014 doi: 10.1155/2014/656750. article ID 656750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crane JD, Ogborn DI, Cupido C, Melov S, Hubbard A, Bourgeois JM, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Translational Med. 2012;4(119):119ra113. doi: 10.1126/scitranslmed.3002882. [DOI] [PubMed] [Google Scholar]
- 39.Aronson D, Mittleman MA, Burger AJ. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J Cardiovasc Electrophysiol. 2001;12(3):294–300. doi: 10.1046/j.1540-8167.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 40.Carney RM, Freedland KE, Stein PK, Miller GE, Steinmeyer B, Rich MW, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosom Res. 2007;62(4):463–467. doi: 10.1016/j.jpsychores.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thanou A, Stavrakis S, Dyer JW, Munroe ME, James JA, Merrill JT. Impact of heart rate variability, a marker for cardiac health, on lupus disease activity. Arthritis Res Ther. 2016;18(1):197. doi: 10.1186/s13075-016-1087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosek E, Altawil R, Kadetoff D, Finn A, Westman M, Le Maitre E, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—Interleukin-8 in fibromyalgia and interleukin-1 beta in rheumatoid arthritis. J Neuroimmunol. 2015;280:49–55. doi: 10.1016/j.jneuroim.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]