ABSTRACT
The whole world is confronting the pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Unfortunately, there is no vaccine to prevent novel coronavirus infection. Besides several experimental drugs, the strong immune responses and convalescent sera are the current two potential options to tackle coronavirus disease 2019 (COVID-19) infection. Innate immune-mediated antiviral responses are initiated by the recognition of viral invasion through pathogen-associated molecular patterns (PAMPs). In coronavirus, the PAMPs are recognized by Toll-like receptors 3 and 7, endosomal ribonucleic acid receptors, RNA in cytosol, and by pattern recognition receptor (RIG-1) in the alveolar cells and site of invasion. Nuclear factor-κB and interferon regulatory transcription factor (IRF3) are activated in response to the above recognition episode and translocate to nucleus. These transcription factors in the nucleus initiate the expression of interferon type 1 and pro-inflammatory cytokine storm, which leads to first line of defense at the site of viral entrance. The effectiveness of innate immune system is greatly relies on type 1 interferons and its cascade, because of their role in the inhibition of viral replication and initiation of adaptive immune responses. The successful interferon type 1 response put down the viral replication and transmission at prompt point. Passive immunization is the administering of antibodies into infected patients, which is taken from recovered individuals. The convalescent sera of the recovered COVID-19 patients are containing antiviral neutralizing antibodies and are used therapeutically for infected individuals by SARS-CoV-2 and for the purpose of prophylaxis in exposed individuals. The convalescent sera is found effective when administered early at the onset of symptoms.
Keywords: innate immune responses, convalescent sera, passive immunization, antiviral responses, SARS-CoV-2, COVID-19
Statement of Significance
The current article emphasizes on the importance of innate immune-mediated antiviral responses to SARS-CoV-2. In the absence of vaccine and effective drugs to treat coronavirus disease 2019 (COVID-19), the options to compete with COVID-19 are: immune antiviral responses and use of convalescent sera. The innate immune-mediated antiviral responses greatly rely on the activation of interferon type 1 and its downstream cascade mechanism. The interferon and its downstream cascade mechanism stop the viral replication. The current article also signifies the procedure of taking, screening and use of convalescent sera to treat COVID-19 and associated risks and benefits with it. Convalescent sera have neutralizing antibodies and higher titer of neutralizing antibodies help in viral neutralization. In addition, the current article also addresses the immunopathology and immune evasion mechanism of COVID-19.
INTRODUCTION
As of December 2019, human population is facing pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for causing coronavirus infection, named as coronavirus disease 2019 (COVID-19). SARS-CoV-2 affected billions of people and still spreading rapidly throughout the world. Though the mortality of COVID-19 is far lower than SARS-CoV and MERS, the transmission rate is very high [1]. The deaths and confirmed cases are increasing day by day [2]. Currently, there is no specific vaccine, monoclonal antibody, and drugs for treatment, as this virus seems to be novel [3]. Vaccines for COVID-19 are in rapid developing stage and will be available soon [4]. The innate immune responses have a key role in slowing down the viral replication; the innate immune responses are stronger in young population than aged population. Therefore the mortality rate of SARS-CoV-2 is higher in old age population than adult. The current situation proclaims that passive immunization or passive antibody therapy is the best option for the treatment and prevention of SARS-CoV-2 disease. It would be possible only if large number of recovered individuals donate their immunoglobulin containing sera immediately [4, 5]. The current article focused on how innate immune responses and convalescent sera get to grip with SARS-CoV-2. The current article also addresses associated risks and benefits with the use of convalescent sera, immunopathology, and immune evasion mechanism of COVID-19.
Immunopathology of COVID-19
The COVID-19 initial site of infection and pathogenesis is still under investigation; however, in most cases, the lungs might be affected. Person-to-person transmission by droplets, cough, sneeze, talk, and close contact is prime mode of transmission. The incubation period is 2–14 days [6], and the rapid reproduction rate is 2.2–2.6 [7]. In total, 80% of the cases are asymptomatic or showing mild symptoms, whereas 20% cases are critical, which means that fatality and severity of SARS-CoV-2 are less than MERS and SARS, however the SARS-CoV-2 presents similar symptoms with SARS and MERS such as fever and respiratory symptoms. The percentage of gastrointestinal symptoms such as diarrhea in MERS and SARS is 20–25%, but in COVID-19 these symptoms are rare [8]. Mostly COVID-19 infected patients develop lymphopenia along with pneumonia [9]. IL-2, IL-7, IL-10, G-CSF, IP-10, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1A, and tumor necrosis factor alpha (TNFα) were also reported in severe infected patients of COVID-19 [9]. These pro-inflammatory cytokine storm may initiate viral sepsis and inflammatory-induced lung injury, which results in acute respiratory distress syndrome, pneumonitis, respiratory failure, shock, multiple organ failure, and eventually death. The mortality rate of SARS-CoV-2 is 2.4% [9]; this mortality rate is due to organ failure in aged peoples having past complications of hypertension, diabetes, and cardiopulmonary problems.
Potential immune evasion mechanisms of coronaviruses
The coronavirus have longer incubation period of 2–14 days as compared with influenza, which is 1–4 days [10]. It is due to the reason that coronaviruses dampen the immune responses and adapt to evade the immune barrier. Most evasion mechanisms involve the inhibition of interferon type 1 protiens. The M protein of coronavirus has a key role in immune modulation. The analysis of two MERS cases revealed that infected patients have low titer of type 1 interferons, whereas the recovered individuals have high titer of type 1 interferons [11]. When the dendritic cells and macrophages get infected by coronavirus, it leads to the down regulation of antigen presentation via MHC class I and II and stop or diminish the activation of T-cells; thus there will be no adaptive immunity to the virus [12].
INNATE IMMUNE-MEDIATED ANTIVIRAL RESPONSES TO SARS-COV-2 INFECTION
Innate immune responses of host are the by birth immunity, which present the first barrier to any kind of pathogen. Although there is limited information on the innate immune responses to COVID-19, but increase in neutrophils (38%), reduction in lymphocytes (35%), increased serum interleukin-6 (52%), and increased C-reactive protein (84%) were also investigated in a study in Wuhan on 99 patients [13]. In another study of 41 patients in Wuhan, increase in neutrophils and decrease in lymphocytes were reported significantly different in intensive care unit (ICU) and non-ICU patients. The increase and decrease in total neutrophils and lymphocytes are correlated with fatality of COVID-19 [14]. However, patients in ICU were found having high level of innate cytokines, CXCL-10 or IP-10, CCL2 or MCP-1, MIP-1A, and TNFα [15]. The above information indicates the involvement of pro-inflammatory cytokine storm in the severity and progression of COVID-19. The effectiveness of innate immune system greatly relies on type 1 interferons and its cascade, because of their role in inhibition of viral replication and adaptive immune responses. ACE-2 is the primary receptor site of SARS-CoV-2 and expressed on type 2 alveolar cells of lungs [9]. Innate immune-mediated antiviral responses is initiated by the recognition of viral invasion through PAMPs; in coronavirus the PAMPs are recognized by Toll-like receptors3 and 7, endosomal ribonucleic acid receptors, RNA in cytosol, and by pattern recognition receptor (RIG-1). Nuclear factor-κB, and interferon regulatory transcription factors (IRF3) are activated in response to the above recognition episode and translocate to the nucleus. These transcription factors in the nucleus initiate the expression of interferon-1 and pro-inflammatory cytokine storm, which leads to first line of defense responses at site of viral entrance [16]. The Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway is activated by interferon type 1 via interferon alpha/beta receptor, where phosphorylation of STAT1 and 2 occurs by JAK1, and tyrosine protein kinase 2. Interferon regulatory factor 9 and STAT 1 and 2 form a complex together, translocate to nucleus and start the transcription of interferon-stimulated genes under the regulation of interferon-stimulated response element [17]. The successful interferon type 1 response put down the viral replication and transmission at prompt point. SARS-CoV-2 shares 79% genomic similarity with SARS-CoV-1 and 50% with MERS. 10b, 13, and 14 are the additional gene regions reported in the genome of SARS-CoV-2. Although some amino acid sequences of SARS-CoV-2 shows 68% similarity with SARS-CoV [18]. Therefore, by the comparison of similar gene regions, it can be predicted that how the novel virus (SARS-CoV-2) messes with innates immune system of the host. However, it provides partial explanation to the speculation that SARS-CoV-2 uses same game plan to interfere with host immunity, may be it have some unrevealed mechanism. Innate immune responses play a major role in protective, destructive responses, and immune mediation. Interferon type 1, macrophages, and neutrophils are overproduced due to the active viral replication and thus results in cytokine storm. During COVID-19 the increase and decrease in total volume of neutrophils and lymphocytes, probably SARS-CoV-2 induces delayed interferon type 1 responses and thus loss of viral control occurs in prompt stage of infection. Elderly people are more susceptible to COVID-19, but some severe cases have also been reported in young population. Fig. 1.
Figure 1.

Innate immune responses to SARS-CoV-2.
PASSIVE IMMUNIZATION OR PASSIVE ANTIBODY THERAPY
Administration of antibodies to the susceptible individuals to a disease for the purpose of prevention and treatment is called passive antibody therapy, and the process is called passive immunization. In active immunization or vaccination, the triggering of immune responses to develop took much time and differs according to the recipient. Thus, the immediate way to prevent the susceptible population is passive antibody therapy. In 1890 foregoing to the happening of antimicrobial therapy in 1940, the passive immunization was considered the sole mean of handling defined infectious diseases [18, 19]. In case of SARS-CoV, it found that the convalescent sera contain nAbs to the concern virus [20]. The mechanism of action is same for SARS-CoV-2, the passive immunization initiate protection by viral neutralization. However, Ab-dependent cellular cytotoxicity and phagocytosis pathways are also to be considered. For SARS-CoV-2 the possible source of antibodies are human convalescent sera or to generate it in animal host, just like genetically engineered cow that produces human Abs [21]. The only immediate available antibody is human convalescent sera; however, many preparatory methods will be available soon (Fig. 2). The number of likely and potential donors is directly proportional to the number of recovered individuals. The effectiveness of passive antibodies is more for prophylaxis than for disease treatment. The antibody therapy is efficient when injected just after the appearance of signs and symptoms. The causes for temporary differences in effectiveness are not known properly but passive immunization works by viral neutralization [22], the antibody also works by modification of initial inflammatory responses [23]. The passive antibody therapy was found effective for pneumococcal pneumonia, when administered immediately just after the appearance of signs and symptoms, the antibody is found non-effective when administered after 3 days of onset of symptoms [24]. The effectiveness of antibody also depends on its amount to be administered. The administered antibody circulates in the blood of susceptible person and when reach tissues it initiates protection against infection. The duration of affectivity can last from weeks to months depending on the antibody composition and quantity.
Figure 2.
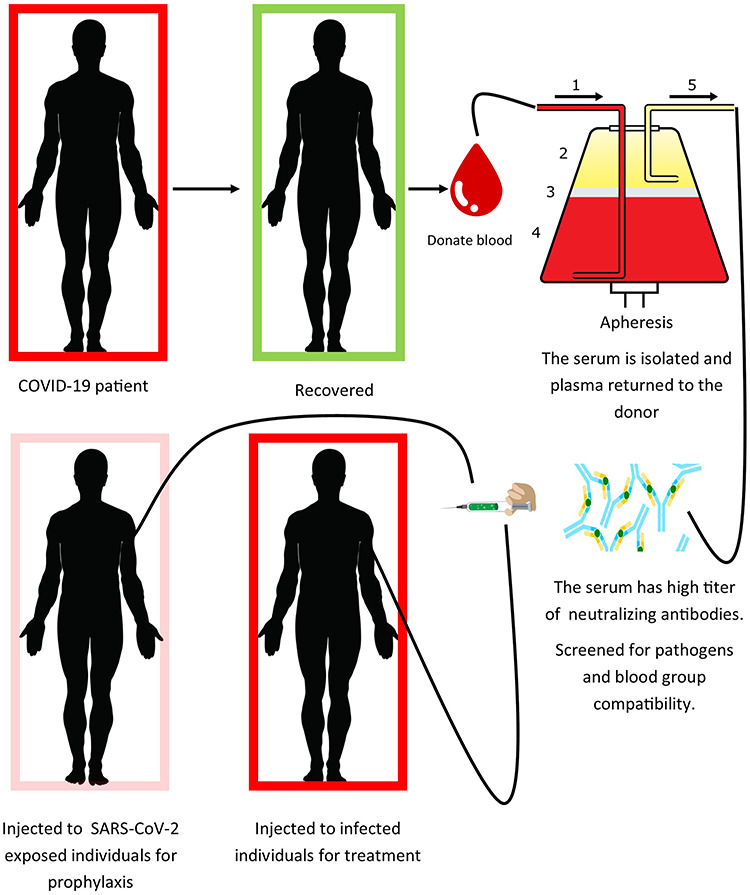
Mechanism of processing and injection of convalescent sera.
HISTORICAL EXAMPLES AND EVIDENCES WITH THE USE OF PASSIVE IMMUNIZATION
Outbreaks of viral diseases in onset of the 20th century, convalescent sera were used against viral infection of measles [25, 26], poliomyelitis [27], influenza [28], and mumps [29]. During 1918 H1N1 influenza pandemic, the convalescent sera were found effective in lowering the mortality rate [30]. In 2009–10 H1N1 influenza pandemic, convalescent sera were taken by apheresis and were utilize to cure individuals required intensive care, treated individuals were noted with reduced viral load, inflammatory responses, and mortality [31]. In 2013, West African Ebola epidemic, patients treated with convalescent sera showed longer survival [32], two patients in the USA survived when cured with a combinational therapy of convalescent sera and experimental drug [33]. Convalescent sera were found effective and saved life of many patients in the H5N1 [34, 35] and H7N9 [36] outbreaks. Although there are differences in every viral disease and epidemic, the important historical approaches give evidences and will be useful to protect population that is confronting COVID-19 pandemic.
CONVALESCENT SERA BASED PASSIVE IMMUNIZATION AGAINST CORONAVIRUSES: SOME FORMER EXPERIENCES
There have been two epidemics in the 21st century the SARS-CoV-1 and MERS. The SARS epidemic is continued but the MERS is endemic now. Due to the mortality and unavailability of vaccine, it leads to the use of convalescent sera in both outbreaks. In SARS-CoV-1, epidemic the largest study was held in Hong Kong in which 80 patients were treated with convalescent sera before day 14 and discharged from hospital before day 22 with improved prognosis; it revealed that the early administration of convalescent sera is more effective than later [37]. Three SARS-CoV-1 infected patients were treated with 500 mL of convalescent sera, the reduction in viral titer and mortality were recorded [38]. Three MERS infected patients were also treated with convalescent or passive antibody therapy, two of them produce nAbs and remaining one not [39], this study highlights the limitation in using of convalescent sera it means that the recovered individual may not have enough titer of nAbs [40]. In continuance with the above point, in a study neutralizing antibodies were found in 87 cases out of 99 with a geometric mean titer of 1:61 that were analyzed for the presence of neutralizing antibodies [20]. This study suggests that some patients produce high-titer responses or antibody decline with time. Possibility is there for the production of non-nAbs [41–43]. In china, convalescent sera were used for the treatment of COVID-19 [44]. In other countries, trials are started on convalescent sera. The available information on the use of convalescent sera or passive immunization for the treatment of SARS-CoV-2 suggests that early administration of convalescent serum reduces viral abundance and was found safe.
PASSIVE IMMUNIZATION AND CONVALESCENT SERA DEPLOYMENT AND ITS PROPOSED USE FOR COVID-19
Some conditions are required for the deployment of passive immunization which are the following: (i) recovered donor population, (ii) facilities for processing serum, (iii) facilities for serological and virological assays, (iv) a well-established molecular lab, (v) facilities for clinical trials, and (vi) regulatory resources. All the facilities and conditions should be available in the area of deployment. Takeda pharmaceutical company aims to isolate and prepare highly purified antibody from convalescent serum against COVID-19 [45]. Safer and high titer of nAbs in convalescent sera are preferable against COVID-19. COVID-19 recovered individuals should be screened for viral nucleic acids of SARS-CoV-2, if virus not detected, considered the individual for the donation of sera. The sera must be isolated by apheresis approach shown in Fig. 2 and scanning must be done for possible residing pathogens in the donor sera. It should be make sure that the sera have high titer of neutralizing antibodies. Similarly, we do not know that what doses would be therapeutically effective. A total of 10–40 cc doses were used against measles for the sake of prevention [26]. On the other hand, in 1918 influenza outbreak, 100 mm of convalescent serum was used to cure soldiers having severe infectious diseases [46]. In past studies, convalescent sera were found effective without knowing the antibody titer. This makes a sense that the prophylactic doses could be smaller than dose uses therapeutically. Convalescent sera could be utilized for prophylaxis and to cure disease. However, it could be administered at early onset of symptoms. Our health care providers are at high of exposure to SARS-CoV-2; if some get infection, he/she must be quarantined that will affect the health care system in turn. Convalescent serum can help to prevent SARS-CoV-2 infection if administered to health care providers and members of the family taking care of SARS-CoV-2 infected patients at homes, so this may allow them to continue their health care duties. It is clear that convalescent sera prevent infection and we are already confronting the COVID-19 pandemic. The federal and local government institutions should start the preparation for passive immunization immediately, as we have no vaccine till date. Some other proposed treatment options are shown in Tables 1 and 2.
Table 1.
Some Proposed treatments options and their Mechanism of Action for COVID-19 [48]
| Drug | Classification | Mechanism of action against SARS-CoV-2 |
|---|---|---|
| Lopinavir | HIV protease inhibitor | Suppress coronavirus replication by binding to enzyme Mpro |
| Chloroquine | Antimalarial | Not understood |
| Hydroxychloroquine | Antimalarial | Not understood |
| Azithromycin | Macrolide Antibacterial | Inhibition of mucus hypersecretion, decreased production of Reactive oxygen specie (ROS), accelerating neutrophil apoptosis, and blocking the activation of nuclear transcription factor (TF). |
| Remdesivir | Nucleoside Analogue | Inhibitor of RNA-dependent RNA polymerases |
| Tocilizumab | Interleukin-6 (IL-6) Receptor-Inhibiting Monoclonal Antibody | Inhibits IL-6-mediated signaling by competitively binding to both soluble and membrane-bound IL-6 receptors. |
HIV, human immunodeficiency virus.
Table 2.
Some under Processing Vaccines for COVID-19 [5]
| Vaccine | Phase | Clinical trial number |
|---|---|---|
| DNA | Phase II | (NCT03721718) |
| Viral vector | Phase I | (NCT03399578, NCT03615911) |
| Subunit | Preclinical stage | Not assigned |
| Inactivated | Preclinical stage | Not assigned |
| Live-attenuated virus | Preclinical stage | Not assigned |
| Virus-like particles | Preclinical stage | Not assigned |
RISKS AND BENEFITS ASSOCIATED WITH THE USE OF CONVALESCENT SERA AND PASSIVE IMMUNIZATION
The convalescent serum from COVID-19 recovered individuals can be either used for prophylaxis or the treatment of disease. Benefits in prophylaxis, the administration of convalescent sera can halt infection and prevent getting of disease in those individuals who are at higher risk for infection, such as patients with intensive care or serious health conditions, paramedical staff and health care providers because of their higher exposure to COVID-19. Passive antibody administration is already in use such as hepatitis B immune globulin (HBIG) for hepatitis B and human rabies immune globulin (HRIG) for rabies virus. Passive antibody is used as a treatment in respiratory syncytial virus (RSV) infected high-risk infants, polyclonal RSV-IG were prepared from donors samples having high serum titers of respiratory syncytial virus nAbs and given to infected individuals at higher risk to get disease. The respiratory syncytial immunoglobulin has now been replaced with palivizumab, a humanized murine monoclonal antibody. Convalescent sera therapy would be utilize in clinical infection to reduce the rate of mortality and to eliminate signs and symptoms. Controlled clinical trial would be perform to evaluate the efficacy of these approaches. Historical experiences suggest that antibody administration show more effectiveness in prevention from infections rather than treatment of diseases [28]. Risks of passive immunization are known and theoretical. Known risks are associated with blood transfusion, which includes pathogens in blood, non-compatibility of blood groups and immunological disturbance such as serum sickness. Modern techniques of blood screening reduce the chances of infection, non-compatibility of blood groups and serum sickness. The theoretical risk includes antibody-dependent enhancement phenomena. Antibody-dependent enhancement (ADE) involves the enhancement of disease due to the presence of certain antibodies it occurs in certain viral diseases. There is a theoretical approach for coronaviruses that Abs to one type could enhance infection to other strain of coronavirus. As proposed for MERS the risk of Ab-dependent enhancement of SARS-CoV-2 may be possible experimentally [45]. The proposed use of passive immunization for SARS-CoV-2 and minimizing of ADE would depend on its composition and preparations, having high quantity of nAbs against it. Evidences are there that the use of convalescent antibodies in infected individuals with SARS-CoV-1 and the Middle East respiratory syndrome [47], an anecdotal evidence is there from utilizing of convalescent serum in 245 patients infected with SARS-CoV-2 [44] suggests it is safe. Attenuation of immune responses is another theoretical risk, antibody administration to SARS-CoV-2 exposed individuals to prevent disease, and it leads such individuals susceptible to reinfection. Passive antibody administration before vaccination with RSV was reported to attenuate antibody mediated or humoral immunity rather than cellular or cell mediated immunity [47]. It could be investigated by the assessment of immune responses of SARS-CoV-2 exposed individuals and convalescent sera treated individuals in clinical trials. If the risk exists, the patients could be immunized against COVID-19 as the vaccine get at hand. Historical experiences and current available information suggest that passive immunization is safe for COVID-19 patients at higher risk and elderly people with chronic diseases. However, for all cases, convalescent serum therapy and its administration are considered a risk–benefit assessment.
CONCLUSION
The innate immune-mediated antiviral responses against coronavirus greatly rely on downstream cascade mechanisms of type 1 interferon, increase, and decrease in total blood neutrophils and lymphocytes.
Pro-inflammatory storm IL-2, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1A, and TNFα in severe infected patients of COVID-19 may initiate viral sepsis and inflammatory-induced lung injury, which results in acute respiratory distress syndrome, pneumonitis, respiratory failure, shock, multiple organ failure, and eventually death. The innate immune responses are stronger in young age individuals than old age; therefore, the severity and fatality is more in aged individuals > 57 years.
The fatality is also associated with chronic diseases. Historical precedents and former experiences with the use of convalescent sera proved its prophylactic and therapeutic efficacy. The convalescent sera are more effective when administered just after the onset of symptoms.
The neutralizing antibody titer must be higher to cope with the virus. The convalescent sera are the safe and proved way to tackle SARS-CoV-2. However, a large number of recovered patients and expertise are required.
Local and federal governments are advised to start immediate trials on this type of treatment because we have no vaccine yet.
ACKNOWLEDGMENTS
We are grateful to Department of Microbiology, Abdul Wali Khan University, Mardan, KPK, Pakistan for providing assistance and guideline.
Contributor Information
Abdullah, Department of Microbiology, Abdul Wali Khan University, Mardan, 23000, KPK, Pakistan; Department of Life Sciences, Abasyn University, Peshawar, 25000, KPK, Pakistan.
Shah Faisal, Department of Biotechnology, Bacha Khan University, Charsadda, 24420, KPK, Pakistan.
Komal Aman, Department of Life Sciences, Abasyn University, Peshawar, 25000, KPK, Pakistan.
Anees ur Rahman, Department of Life Sciences, Abasyn University, Peshawar, 25000, KPK, Pakistan.
CONFLICT OF INTEREST STATEMENT
None declared.
AUTHORS CONTRIBUTION
Abdullah contributed to the idea, conceptualization, writing, and creating figures and tables. F.S organized and formats the data. K.M and A.U.R helped in proofreading.
REFERENCES
- 1. Abdullah . A perspective study on oral-fecal transmission of COVID-19, its prevention and management. OSF Preprints, 1 May 2020. 10.31219/osf.io/r79v6. [DOI] [Google Scholar]
- 2. Worldometers . Coronavirus update (live). https://www.worldometers.info/coronavirus/ (27 May 2020, last accessed).
- 3. Ullah, M. (2020). The pandemic of novel coronavirus disease 2019 (COVID-19): need for an immediate action. 10.31219/osf.io/a83zh. [DOI]
- 4. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 2020;12(3):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020;38(1):1–9. [DOI] [PubMed] [Google Scholar]
- 6. Lu, R, Zhao, X, Li, J et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Center for Disease Control and Prevention . Symptoms of novel coronavirus (2019-nCoV) [about 1 screen]. Atlanta: CDC; c2020. https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html (10 February 2020, last accessed). [Google Scholar]
- 8. Chan, JF, Yuan, S, Kok, KH et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395: 514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang, C, Wang, Y, Li, X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lessler, J, Reich, NG, Brookmeyer, R et al. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis 2009; 9: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faure, E, Poissy, J, Goffard, A et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One 2014; 9: e88716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shokri, S, Mahmoudvand, S, Taherkhani, R et al. Modulation of the immune response by Middle East respiratory syndrome coronavirus. J Cell Physiol 2019; 234: 2143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest 2020;130(4):1545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou P, Yang XL, Wang XG et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature [Preprint]. 2020: 15. 10.1038/s41586-020-2012-7 (15 February 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu F, Zhao S, Yu B et al. A new coronavirus associated with human respiratory disease in China. Nature [Preprint]. 2020: 19. 10.1038/s41586-020-2008-3 (16 February 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu, N, Zhang, D, Wang, W et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Wit, E, van Doremalen, N, Falzarano, D et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016; 14: 523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis 1995;21(1):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol 2004;2(9):695–703. [DOI] [PubMed] [Google Scholar]
- 20. Zhang JS, Chen, JT, Liu, YX et al. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol 2005;77(2):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beigel JH, Voell, J, Kumar, P et al. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis 2018;18(4):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis 1995;171(6):1387–1398. [DOI] [PubMed] [Google Scholar]
- 23. Casadevall A, Pirofski LA. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol 2003;24(9):474–478. [DOI] [PubMed] [Google Scholar]
- 24. Casadevall A, Scharff MD. Serum therapy revisted: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother 1994; 38(8):1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park WH, Freeman RG. The prophylactic use of measles convalescent serum. JAMA 1926;87(8):556–558. [Google Scholar]
- 26. Gallagher JR. Use of convalescent measles serum to control measles in a preparatory school. Am J Public Health Nations Health 1935;25(5):595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park, WH. Therapeutic use of antipoliomyelitits serum in preparalytic cases of poliomyelitis. JAMA 1932; 99: 1050–3. [Google Scholar]
- 28. Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med 2010;38(4 suppl):e66–e73. [DOI] [PubMed] [Google Scholar]
- 29. Rambar, AC. Mumps; use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Child 1946; 71: 1–13. [PubMed] [Google Scholar]
- 30. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006;145(8):599–609. [DOI] [PubMed] [Google Scholar]
- 31. Hung IF, To, KK, Lee, CK et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza a (H1N1) 2009 virus infection. Clin Infect Dis 2011;52(4):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sahr F, Ansumana, R, Massaquoi, TA et al. Evaluation of convalescent whole blood for treating Ebola virus disease in Freetown, Sierra Leone. J Infect 2017;74(3):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kraft CS, Hewlett, AL, Koepsell, S et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis 2015;61(4):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kong LK, Zhou BP. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med J 2006;12(6):489. [PubMed] [Google Scholar]
- 35. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza a (H5N1) infection. N Engl J Med 2007;357(14):1450–1451. [DOI] [PubMed] [Google Scholar]
- 36. Wu, XX, Gao, HN, Wu, HB et al. Successful treatment of avian-origin influenza a (H7N9) infection using convalescent plasma. Int J Infect Dis 2015; 41: 3–5. [DOI] [PubMed] [Google Scholar]
- 37. Cheng Y, Wong, R, Soo, YOY et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005;24(1):44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yeh KM, Chiueh, TS, Siu, LK et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother 2005;56(5):919–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ko JH, Seok, H, Cho, SY et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single Centre experience. Antivir Ther (Lond). 2018;23(7):617–622. [DOI] [PubMed] [Google Scholar]
- 40. Arabi YM, Hajeer, AH, Luke, T et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerging Infect Dis 2016;22(9):1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Erp, EA, Luytjes, W, Ferwerda, G et al. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol 2019; 10: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jenks, JA, Goodwin, ML, Permar, SR. The roles of host and viral antibody fc receptors in herpes simplex virus (HSV) and human cytomegalovirus (HCMV) infections and immunity. Front Immunol 2019; 10: 2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gunn BM, Yu, WH, Karim, MM et al. A role for fc function in therapeutic monoclonal antibody-mediated protection against ebola virus. Cell Host Microbe 2018;24(2):221–233.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. China puts 245 COVID-19 patients on convalescent plasma therapy. News release. Xinhua, 28 February 2020. http://www.xinhuanet.com/english/202002/28/c_138828177.htm (10 March 2020).
- 45. Wan Y, Shang, J, Sun, S et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol 2020;94(5):e02015–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mair-Jenkins J, Saavedra-Campos, M, Baillie, JK et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015;211(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crowe JE, Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol 2001; 167(7):3910–3918. [DOI] [PubMed] [Google Scholar]
- 48. Jan H, Faisal S, Khan A, Khan S, Usman H, Liaqat R, Shah SA. COVID-19: review of epidemiology and potential treatments against 2019 novel coronavirus. Discoveries 2020, 8(2): e108. DOI: 10.15190/d.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


