ECG challenge
A 21-year-old man with a previous history of stage I sarcoidosis with mild lung impairment was admitted to the emergency department for 2-week exertional dyspnea and bilateral leg edema. He had no fever or cough and decided not to consult previously because of coronavirus disease 2019 (COVID-19) lockdown. At physical examination, there were bilateral rales. Nt-proBNP (N-terminal pro–B-type natriuretic peptide) was 7737 pg/mL and hs-T-Troponin (high-sensitivity troponin T) was 24 ng/L (normal, <14 ng/L), with the rest of his blood test parameters unremarkable. Initial ECG can be observed in Figure 1. A transthoracic echocardiography was performed, showing a severely dilated right ventricle (RV) with systolic dysfunction, systolic interventricular septum deviation, and an eccentricity index of 1.4; these findings are suggestive of pressure overload (Figure 2). The estimated systolic pressure of the pulmonary artery was 66 mm Hg. A computed tomography angiography was performed, revealing bilateral pulmonary infiltrates and ground-glass opacities, bilateral hilar lymphadenopathies that related to the previous diagnosis of sarcoidosis, and main pulmonary artery and RV enlargement in the context of the presumed pulmonary hypertension (PH; Figure 3). Pulmonary embolism (PE) was excluded. Considering the high prevalence of COVID-19 infection in the area of Madrid (Spain) at the time of the patient’s admission, a nasopharyngeal swab was obtained and a real-time polymerase chain reaction test for this virus was carried out, with negative results. The patient was hospitalized for further study.
Figure 1.
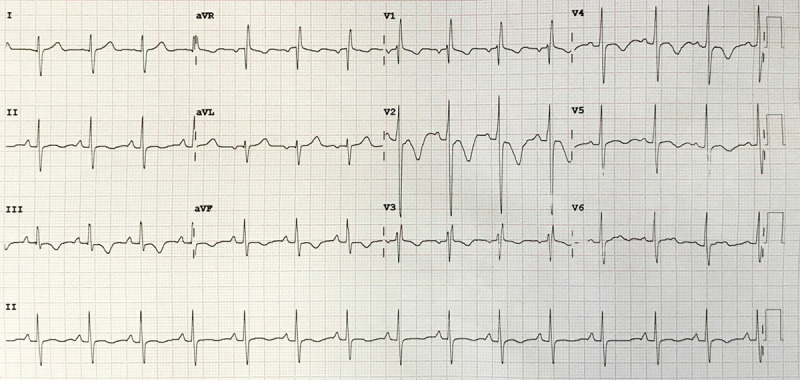
Baseline ECG in a man with dyspnea and signs of right heart failure. The baseline ECG demonstrates a normal sinus rhythm with heart rate of 90 beats per minute, right bundle-branch block, right axis deviation, and profound inversion of T waves among right precordial (V1–V4) and inferior frontal leads (III-aVF), with a corrected QT (cQT) interval of 489 ms by Bazzet formula.
Figure 2.
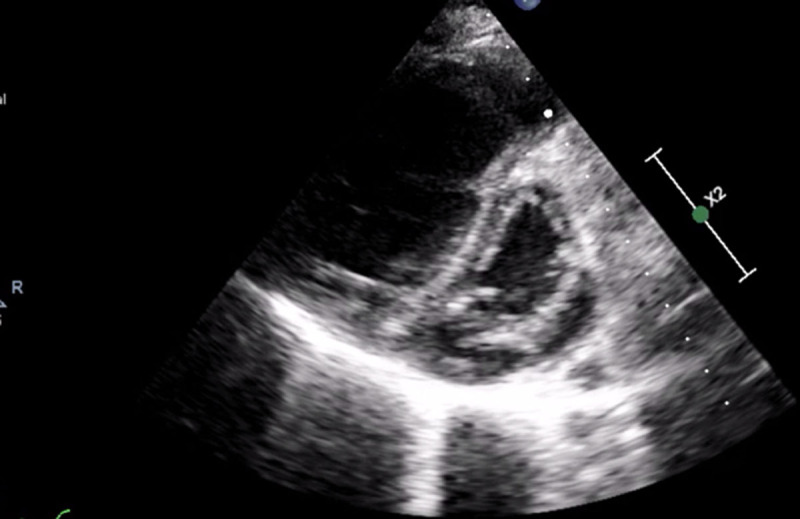
Baseline transthoracic echocardiography demonstrating severe dilation of the right ventricle and interventricular septum shift toward left ventricle in a short axis view.
Figure 3.
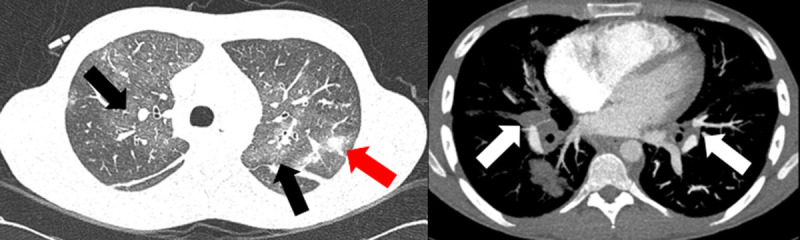
Computed tomography angiography. Pulmonary infiltrates (gray arrows), ground-glass opacities (black arrows), and bilateral hiliar lymph node enlargement (white arrows).
What is the most likely diagnosis? What does this ECG pattern show?
Please turn the page to read the diagnosis.
Response to ECG Challenge
The ECG in figure 1 shows right bundle-branch block, right axis deviation, and T wave inversion in right precordial and inferior leads with QT prolongation, findings related to RV strain pattern.
Although the diagnostic approach must first discard PE, other causes must be considered. Initially, computed tomography angiography ruled out PE, demonstrating bilateral infiltrates and signs of relevant PH. A second real-time polymerase chain reaction test sample for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was acquired, and was negative. Following medical advice, the patient had stopped taking corticosteroids 2 months previously. Consequently, a plausible explanation could be sarcoidosis reactivation after corticosteroids tapering. Additional work-up included right heart catheterization, cardiac magnetic resonance, pulmonary function tests, and a polysomnographic study. Right heart catheterization confirmed precapillary PH with left ventricular end-diastolic pressure of 6 mm Hg, mean pulmonary artery pressure of 35 mm Hg, and pulmonary vascular resistance of 5.5 Wood Units. In cardiac magnetic resonance, the RV was severely dilated, without edema or late gadolinium enhancement. On the other hand, pulmonary function tests were unremarkable, except for notable overnight desaturation. Absence of myocardial scar in cardiac magnetic resonance and arrhythmias on ECG monitoring, in addition to lung impairment along with precapillary PH as causes of heart failure symptoms, were enough to discard cardiac sarcoidosis. With the diagnosis of stage III pulmonary sarcoidosis and pressure overload–induced RV remodeling, high doses of Prednisone, intravenous loop diuretics, and oxygen supplementation were initiated. It was a surprise that the series of ECGs revealed progressive shortening of cQT interval, slight QRS axis deviation, and gradual correction of T wave inversion (Figures 4 and 5). Subsequent transthoracic echocardiography showed signs of significant hemodynamic improvement (eccentricity index of 1.3) and partial restoration of RV diameters. The patient was discharged 10 days later, having lowered Nt-proBNP levels to 567 pg/mL, and without requiring pulmonary vasodilators at that moment.
Figure 4.
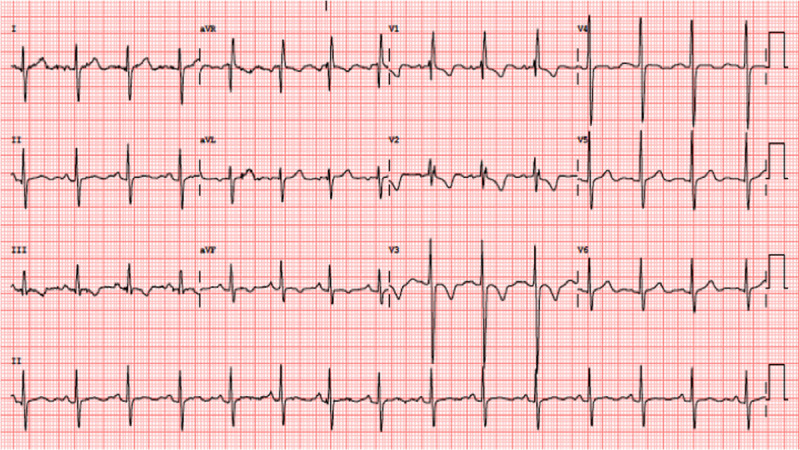
Subsequent ECG during hospitalization. A progressive amelioration of T wave inversion in precordial and inferior leads can be seen.
Figure 5.
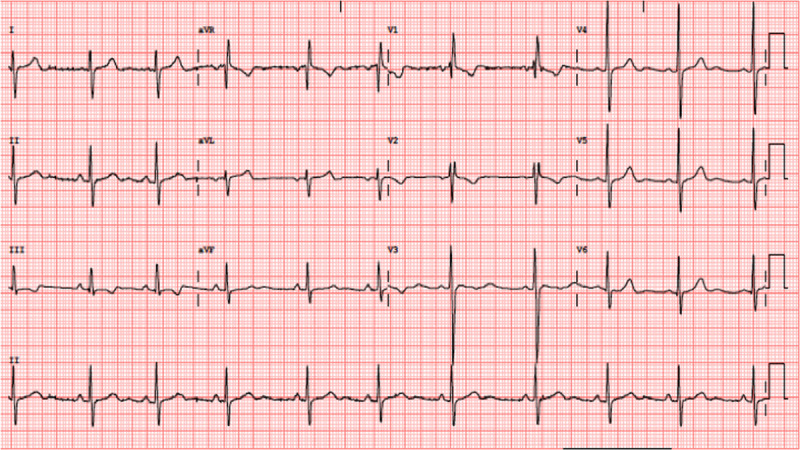
ECG performed before discharge. There is a normalization of the cQT interval and subtle changes of T wave in precordial and inferior leads.
Giant T wave inversion with QT prolongation has been described in a variety of clinical conditions: myocardial ischemia, PE, stress-induced (Takotsubo) cardiomyopathy, nonischemic pulmonary edema, or during acute stroke. COVID-19 has also been associated, mostly related with RV overload in PE or severe respiratory distress cases. Right bundle-branch block and right axis deviation oriented here to PH, however COVID-19 or PE were primarily ruled out.
Sarcoidosis-induced PH is a rare and severe entity with multiple causes; the progression of sarcoidosis lung involvement is the most frequent. Other causes have been described, such as granulomatous invasion of pulmonary vessels or extrinsic pulmonary vasculature compression by lymphatic nodes. Treatment thus varies according to the causative mechanism. Immunosuppressive treatment and pulmonary vasodilators are common options.1
It is well known that frontal plane QRS complex mean electric axis can be useful to follow RV widening.2 Also, as suggested in rodent models of PH with RV hypertrophy, cQT prolongation in surface ECG could be related with ionic remodeling, and restoration of the oxidative glucose metabolism can normalize cQT interval.3
Certainly, in this case, corticosteroids along with diuretics and oxygen therapy ameliorated RV hemodynamics and possibly RV-cell metabolism. Moreover, this ECG challenge enlightens how useful the assessment of ECG changes could be during PH treatment.
Acknowledgments
The authors acknowledge Ana Pérez and Ana Lareo for their help during patient hospitalization and for the manuscript editing.
Disclosures
None.
Footnotes
References
- 1.Boucly A, Cottin V, Nunes H, Jaïs X, Tazi A, Prévôt G, Reynaud-Gaubert M, Dromer C, Viacroze C, Horeau-Langlard D, et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J. 2017;50:1700465. doi: 10.1183/13993003.00465-2017. [DOI] [PubMed] [Google Scholar]
- 2.Bruner LH, Hilliker KS, Roth RA. Pulmonary hypertension and ECG changes from monocrotaline pyrrole in the rat. Am J Physiol 1983245H300–H306doi: 10.1152/ajpheart.1983.245.2.H300 [DOI] [PubMed] [Google Scholar]
- 3.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014115176–188doi: 10.1161/CIRCRESAHA.113.301129 [DOI] [PMC free article] [PubMed] [Google Scholar]


