Abstract
Objectives
To evaluate the analytical and clinical performance of the automated Elecsys anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody (Elecsys Ab) assay on the Roche cobas e602 analyzer. With the ongoing global coronavirus disease 2019 (COVID-19) pandemic, widespread and routine serologic testing of SARS-CoV-2 remains a pressing need. To better understand its epidemiologic spread and to support policies aimed at curtailing further infections, reliable serologic testing is crucial for providing insight into the dynamics of the spread of COVID-19 on a population level.
Methods
The presence of anti–SARS-CoV-2 antibodies in polymerase chain reaction–positive, confirmed COVID-19 patient samples was determined using the Elecsys Ab assay on the Roche cobas e602 analyzer. The precision and cross-reactivity of the Elecsys Ab assay were characterized and its performance was compared against the EuroImmun IgA/IgG antibody (EuroImmun Ab) assay. Calculated sensitivity, specificity, and positive and negative predictive values were assessed.
Results
The Elecsys Ab assay demonstrated good precision, had no cross-reactivity with other viral samples, and showed 100% concordance with the EuroImmun Ab assay. Excellent clinical performance with respect to sensitivity, specificity, and positive and negative predictive values was observed.
Conclusions
The Elecsys Ab assay is a precise and highly reliable automated platform for clinical detection of seropositivity in SARS-CoV-2 infection.
Keywords: SAR-CoV-2 antibodies, COVID-19, Coronavirus, Immunoassay, Serology, IgM, IgG, IgA, Diagnostics, Surveillance
Key Points.
• The Elecsys Ab assay is a highly precise and reliable immunoassay platform.
• The clinical performance of the Elecsys Ab assay compares well against that of the EuroImmun IgA/IgG Ab assay.
• Ninety-five percent sensitivity, 100% specificity, and greater than 99% positive and negative predictive values were achieved in our study population and samples.
We are in the midst of a global coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus that emerged from Wuhan, China, in late 2019.1-4 The virus has spread rapidly around the globe, but its pattern of spread within and between nations has been divergent, relating partly to varied public health policies and societal responses.5-8 Testing capabilities and capacities have also been seriously challenged during this pandemic, resulting in inadequacies in many parts of the world and ultimately in the collective effort to accurately characterize the epidemiologic spread of SARS-CoV-2 both at local levels and on a global scale.9,10
Given that the virus consists of an RNA genome, initial testing for SARS-CoV-2 infection is based on reverse transcription quantitative polymerase chain reaction (RT-qPCR) for direct detection.11 RT-qPCR remains the gold standard diagnostic test, although questions remain concerning the persistence of viral genetic material postinfection or in asymptomatic carriers.12,13 However, due to consumable and reagent supply shortages that are required to perform SARS-CoV-2 direct detection methods, there have been considerable limitations to conducting widespread testing. As such, there have been significant recent efforts to develop serologic assays for clinical detection of SARS-CoV-2 using patient serum samples.14-16 In addition, serologic testing provides imperative epidemiologic data for surveilling populational seroprevalence and a means to assess antiviral activity and clinical efficacy of neutralization by convalescent plasma.
Compared with direct detection methods, serologic assays provide a means and an added advantage to assess the exposure history of an individual, as well as entire populations, to help guide public health policies.17 Seroconversion for immunoglobulin M (IgM), immunoglobulin G (IgG), and total antibodies against SARS-CoV-2 has been observed to occur several weeks after infection and peaking after 2 to 3 weeks, 3 to 6 weeks, and 2 to 3 weeks, respectively.18-21 However, given that there is significant observed variability in both IgM and IgG levels, most serologic assays are designed to detect both IgM and IgG simultaneously.22 The formation of neutralizing antibodies remains an active area of research.23-26
In this study, the qualitative Elecsys Ab assay (Roche Diagnostics) was evaluated analytically and clinically as a practical modality for widespread and routine serologic testing. The immunoassay is an automated assay based on recombinant nucleocapsid protein technology for the detection of SARS-CoV-2 seropositivity. Here we report that the Elecsys Ab assay performs reliably and consistently with both RT-qPCR and the EuroImmun immunoglobulin A (IgA)/IgG antibody (EuroImmun Ab) assay (PerkinElmer) with high precision. Moreover, 95% sensitivity, 100% specificity, and greater than 99% positive and negative predictive values were achieved in our study population and samples.
Materials and Methods
Patient Samples
Leftover heparinized plasma and serum samples from admitted PCR-positive, confirmed COVID-19 patients who had routine metabolic profiles and serologies ordered for clinical care were retrieved from the Clinical Chemistry and Immunology Laboratories for this evaluation. For longer-term storage, samples were frozen at –25°C until ready for use. Samples were collected under a quality assurance protocol that qualifies for institutional review board waiver, as no patient identifiers were used.
Analyzer Systems
The Elecsys Ab is an automated sandwich, double-antigen electrochemiluminescent immunoassay that employs recombinant protein representing the nucleocapsid antigen of the virus. This Ab assay has recently received emergency use authorization from the US Food and Drug Administration (FDA EUA) for the detection of total antibodies (IgG, IgM, and IgA) in COVID-19–infected patients.27 At our institution, this immunoassay is installed on the Roche cobas e602 module component of the cobas 8000 total automation system. This assay employs a cutoff index (COI) that is mathematically derived from the calibration using two standards—a COI of 1.0 or more is considered reactive (REACT)/positive, while a COI less than 1.0 is reported as nonreactive (NONREACT)/negative. The EuroImmun Ab assay is an enzyme-linked immunosorbent assay (ELISA) that is also authorized by the FDA (via EUA) for the detection of IgA and IgG Ab developed against the SARS-CoV-2 virus and has been previously described.28
Precision Study With Pooled Plasma
A positive antibody pool, approximately 20 mL, was prepared by combining leftover antibody-positive plasma samples with a COI value of approximately 2.0 to 3.0. Similarly, a negative antibody pool, approximately 20 mL, was prepared by diluting a positive antibody pool with Elecsys universal diluent to yield a COI of approximately 0.4 to 0.5. Aliquots of 0.25 mL were prepared from each pool and frozen at –25°C for longer-term storage. A within-run precision study was performed by assaying each pool 20 times. Between-day precision study was performed by thawing out each respective aliquot to room temperature and running over 38 days. The mean and SD were calculated for each pool, and coefficient of variation (CV) was determined as CV (%) = (SD × 100)/mean.
Method Comparison Study
A total of 66 RT-qPCR–confirmed, archived serum samples from the validation studies for the EuroImmun Ab assay28 were used for the method comparison study with the Elecsys Ab assay. Analysis of concordance was performed using the Microsoft Excel software.
Cross-Reactivity Studies
In total, 25 samples from patients with PCR-positive non–SARS-CoV-2 respiratory infections (via the BioFire FilmArray Respiratory Panel 2), 20 human immunodeficiency virus (HIV)–positive samples, 15 hepatitis B surface antigen (HepBS) Ab-positive samples, and 17 hepatitis C virus (HCV) Ab-positive samples were analyzed on the Elecsys Ab assay to assess the extent of cross-reactivity.
Clinical Performance Study
A total of 78 PCR-confirmed (Roche SARS-CoV-2 qualitative PCR assay) positive samples from COVID-19 patients that were collected either 0 to 13 days (40 samples) or 14 or more days (38 samples) after initial PCR positivity and 53 prepandemic samples (41 from a reference range study prior to 2018 and 12 from a banked respiratory viral panel from early 2019) were retrieved for assessment of clinical performance studies. Calculations of sensitivity, specificity, and positive and negative predictive values were performed using the SciStat software (https://www.scistat.com/statisticaltests/diagnostic_test.php), assuming a 5% prevalence.29
Results
Precision Studies
Readouts of the automated Elecsys Ab assay performed on the Roche cobas e602 analyzer are resulted as either positive or negative based on a COI of 1.0 or more or less than 1.0, respectively, thereby rendering the assay a binary, qualitative analysis. To evaluate the within-run precision of the Elecsys Ab assay, both a positive and a negative control sample were run consecutively (n = 20 replicates), yielding mean COI values of 2.47 (CV of 0.8%) and 0.49 (CV of 1.5%), respectively. The between-day precision analysis was repeated over 38 subsequent days (n = 60 replicates), showing a mean COI of 3.97 (CV of 2.9%) for the positive pool and a mean COI of 0.36 (CV of 2.4%) for the negative pool, respectively Table 1.
Table 1.
Precision Study of the Elecsys Anti–SARS-CoV-2 Antibody Assaya
| SARS-CoV-2 Controls | Within-Run | Between-Run | ||||
|---|---|---|---|---|---|---|
| Mean COI | CV, % | No. | Mean COI | CV, % | No. | |
| Negative pool | 0.49 | 1.3 | 20 | 0.36 | 2.4 | 60 |
| Positive pool | 2.47 | 0.8 | 20 | 3.97 | 2.9 | 60 |
COI, cutoff index; CV, coefficient of variation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aA positive antibody result is defined by a COI of 1.0 or more, whereas the CV (%) = (SD/mean) × 100. Two different sets of negative and positive pools were used for within-run and between-run studies respectively.
Comparison Between the Elecsys and Euroimmun Ab Assays
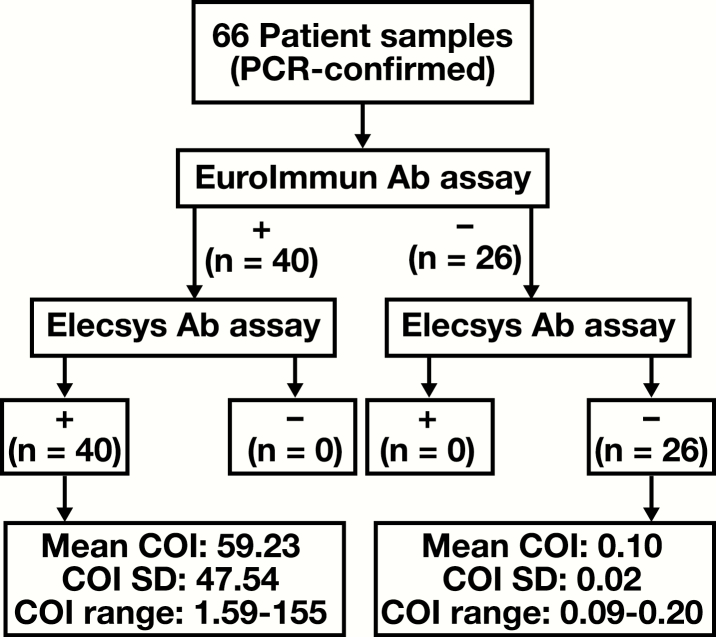
The performance of the Elecsys Ab assay was compared with that of the Euroimmun Ab assay, a well-established and broadly used test for direct detection of antibodies against SARS-CoV-2,28 for 66 patient samples Figure 1. All 40 samples that were reactive by the Euroimmun Ab (36 were positive for both IgA and IgG, whereas 4 were positive for only IgG) assay yielded COI values of 1.0 or more using the Elecsys Ab assay. Similarly, all 26 samples that were nonreactive by the Euroimmun Ab assay yielded COI values less than 1.0. Of note, there was significantly greater variation in the measured COI values (range, 1.59-155) for the positive Elecsys Ab assay samples than what was observed for the negative samples (COI range, 0.09-0.20). The overall concordance rate between the two assays was 100%.
Figure 1.
Method comparison of the EuroImmun vs Elecsys anti–severe acute respiratory syndrome coronavirus 2 antibody (Ab) assays. A total of 66 polymerase chain reaction (PCR)–confirmed patient samples were compared, resulting in zero discrepancies and an overall concordance rate of 100%. Reactive (+) and nonreactive (–) Elecsys Ab assay results are defined by a cutoff index (COI) of 1.0 or more or less than 1.0, respectively. Of the 40 (+) EuroImmun Ab assay samples, 36 were positive for both immunoglobulin A (IgA) and immunoglobulin G (IgG) and 4 were positive for only IgG. All 20 (–) EuroImmun Ab assay samples were negative for both IgA and IgG.
Specificity of the Elecsys Ab Assay
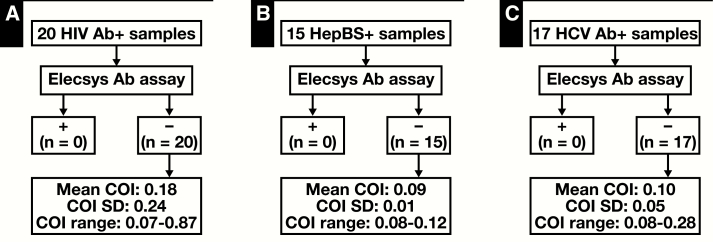
The specificity of the Elecsys Ab assay for SARS-CoV-2 was examined by testing for cross-reactivity against samples from patients who tested positive for non–SARS-CoV-2 respiratory and other viruses such as HIV, HBV, and HCV. A total of 25 non–SARS-CoV-2 respiratory viral samples, including several common strains of other coronaviruses (HKU1, OC43, NL63, and 229E), were tested using the Elecsys Ab assay, which consistently yielded a COI of less than 1.0 and thus zero cross-reactivity Table 2. Similarly, testing of 20 HIV serology-positive, 15 HepBS Ab-positive, and 17 HCV Ab-positive samples on the Elecsys Ab assay also demonstrated zero cross-reactivity, although several samples did yield relatively higher COI values while less than 1.0 Figure 2. Therefore, the Elecsys Ab assay demonstrates high specificity for qualitative detection of anti–SARS-CoV-2 antibodies.
Table 2.
Cross-Reactivity of Non–SARS-CoV-2 Respiratory Virusesa
| Sample | Description | Elecsys Ab | |
|---|---|---|---|
| COI | Result | ||
| 1 | HKU1 CV | 0.10 | NONREACT |
| 2 | HKU1 CV | 0.09 | NONREACT |
| 3 | HKU1 CV | 0.09 | NONREACT |
| 4 | HKU1 CV | 0.10 | NONREACT |
| 5 | HKU1 CV + RSV | 0.10 | NONREACT |
| 6 | NL63 CV | 0.09 | NONREACT |
| 7 | NL63 CV | 0.09 | NONREACT |
| 8 | NL63 CV | 0.09 | NONREACT |
| 9 | NL63 CV | 0.09 | NONREACT |
| 10 | NL63 CV | 0.10 | NONREACT |
| 11 | NL63 CV | 0.09 | NONREACT |
| 12 | NL63 CV | 0.09 | NONREACT |
| 13 | OC43 CV | 0.10 | NONREACT |
| 14 | OC43 CV | 0.09 | NONREACT |
| 15 | OC43 CV | 0.10 | NONREACT |
| 16 | OC43 CV | 0.10 | NONREACT |
| 17 | OC43 CV | 0.09 | NONREACT |
| 18 | OC43 CV | 0.10 | NONREACT |
| 19 | OC43 CV | 0.10 | NONREACT |
| 20 | OC43 CV | 0.09 | NONREACT |
| 21 | 229E CV | 0.09 | NONREACT |
| 22 | 229E CV | 0.10 | NONREACT |
| 23 | OC43 CV + 229E CV | 0.11 | NONREACT |
| 24 | Rhinovirus | 0.20 | NONREACT |
| 25 | Rhinovirus | 0.11 | NONREACT |
COI, cutoff index; CV, coronavirus; NONREACT, nonreactive; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aZero cross-reactivity was observed with 25 samples from patients positive for non–SARS-CoV-2 respiratory viruses. HKU1, OC43, NL63, and 229E are common strains of other coronaviruses.
Figure 2.
Cross-reactivity with other viral positive samples. A total of 20 human immunodeficiency virus (HIV) (A), 15 hepatitis B surface antigen (HepBS) (B), and 17 hepatitis C (HCV) (C) antibody (Ab)–positive samples were assayed. Reactive (+) and nonreactive (–) Elecsys Ab assay results are defined by a cutoff index (COI) of 1.0 or more or less than 1.0, respectively.
Clinical Performance of the Elecsys Ab Assay
As shown in Table 3 and Table 4, the overall clinical performance of the Elecsys Ab assay was excellent and is consistent with recently reported data30,31 and manufacturer specifications.27,29 Based on our study data, we determined that the Elecsys Ab assay showed a sensitivity of 77.50% (95% confidence interval [CI], 61.55%-89.16%) for samples collected 0 to 13 days after PCR positivity and a sensitivity of 94.74% (95% CI, 82.25%-99.36%) for samples collected 14 or more days after PCR positivity. The specificity of the assay was 100% (95% CI, 93.28%-100%), based on 53 prepandemic samples. Assuming a 5.0% disease prevalence (consistent with the prevalence the FDA assumes in its review of serology test performance for EUA), the calculated positive predictive value (PPV) is 100% and the negative predictive value (NPV) is 98.83% (95% CI, 97.94%-99.34%) with an overall accuracy of 98.88% (95% CI, 94.07%-99.97%) for samples collected 0 to 13 days and 99.72% (95% CI, 98.94%-99.93%) with an overall accuracy of 99.74% (95% CI, 95.51%-100%) for samples collected 14 or more days after PCR positivity, respectively (Tables 3 and 4). For comparison, Table 5 shows the calculated PPV, NPV, and accuracy at 3%, 5%, and 10% prevalence, respectively. Overall, the test showed high clinical performance at these various assumed prevalence percentages.
Table 3.
Clinical Performance of the Elecsys Anti-SARS-CoV-2 Antibody Assaya
| Test | Prepandemic | PCR Positive 0-13 Days | PCR Positive ≥14 Days |
|---|---|---|---|
| No Disease, No. | Disease, No. | Disease, No. | |
| Elecsys Ab positive | 0 | 31 | 36 |
| Elecsys Ab negative | 53 | 9 | 2 |
| Total | 53 | 40 | 38 |
PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aFifty-three prepandemic samples were collected from a normal range study on 41 volunteers from prior to 2019 and 12 respiratory viral samples from the early part of 2019.
Table 4.
Clinical Performance Test Characteristics of the Elecsys Anti-SARS-CoV-2 Antibody Assaya
| Performance Measure | Estimate of Performance (95% CI), % | ||
|---|---|---|---|
| PCR Positive 0-13 Days | PCR Positive ≥14 Days | ||
| Sensitivity | 77.50 (61.55-89.16) | 94.74 (82.25-99.36) | |
| Specificity | 100 (93.28-100) | 100 (93.28-100) | |
| Prevalence | 5 | 5 | |
| Positive predictive value | 100 | 100 | |
| Negative predictive value | 98.83 (97.94-99.34) | 99.72 (98.94-99.93) | |
| Accuracy | 98.88 (94.07-99.97) | 99.74 (95.51-100) |
CI, confidence interval; PCR, polymerase chain reaction.
aA prevalence of 5% was used for calculating positive and negative predictive values, consistent with the prevalence the US Food and Drug Administration assumes in its review of serology test performance for emergency use authorization.
Table 5.
Comparison of Clinical Performance of the Elecsys Anti–SARS-CoV-2 Antibody Assay Assuming 3%, 5%, or 10% Disease Prevalence
| PCR Positive | 3% | 5% | 10% | |||
|---|---|---|---|---|---|---|
| 0-13 Days, % | ≥14 Days, % | 0-13 Days, % | ≥14 Days, % | 0-13 Days, % | ≥14 Days, % | |
| Sensitivity | 77.50 | 94.74 | 77.50 | 94.74 | 77.50 | 94.74 |
| Specificity | 100 | 100 | 100 | 100 | 100 | 100 |
| Positive predictive value | 100 | 100 | 100 | 100 | 100 | 100 |
| Negative predictive value | 99.31 | 99.84 | 98.83 | 99.72 | 97.56 | 99.42 |
| Accuracy | 99.33 | 99.84 | 98.88 | 99.74 | 97.75 | 99.47 |
PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
Reliable and robust diagnostic platforms remain a pressing need in the ongoing COVID-19 pandemic caused by SARS-CoV-2.32 Although RT-qPCR remains the gold-standard diagnostic test for active infection, serology assays have become increasingly available, particularly through FDA EUA, for clinical use to help improve our understanding and tracking of both the natural immune response against SARS-CoV-2 and the epidemiologic spread of the virus on populational levels. As with all clinical assays, thorough validation of any novel diagnostic test by the clinical laboratory is critical prior to widespread or routine implementation of the test.
In late February 2020, after the onset of the ongoing COVID-19 pandemic, the FDA permitted immunoassay manufacturers to offer SARS-CoV-2 Ab kits prior to receiving EUA to mitigate the testing capability deficits in the United States at the time.33 However, this decision by the FDA ultimately led to the availability and utilization of several substandard Ab kits for COVID-19 surveillance, resulting in a higher incidence of false-positive Ab results and therefore a sense of unreliability. The FDA has since revised its policy34,35 to require manufacturers to seek EUA prior to marketing and distributing serologic tests and, as part of its efforts to tighten the quality of available Ab kits, stipulated that these serologic test characteristics ought to be sufficiently high and made publicly available. To date, 33 serologic tests (vs 19 in mid-June 2020), including the Elecsys Ab assay, have received EUA.29
Current RT-qPCR tests for active infection employ direct detection of viral genetic material, whereas serologic testing reveals a subject’s immune response toward the presence of an antigen. As such, serologic testing is not the preferred method for diagnosing COVID-19, and their routine use must also account for immunosuppressive states and antibody-level variations related to disease course and severity.36 On the other hand, serologic tests remain an invaluable diagnostic modality in that they provide still much-needed insight into the adaptive immune response against SARS-CoV-2, the exposure history of an individual, transmission patterns, and potential donors of convalescent plasma. Furthermore, as we combat the ongoing COVID-19 pandemic, the true prevalence of Ab positivity against SARS-CoV-2 remains unknown in the US population.
In this study, we analytically and clinically evaluated the qualitative, automated Elecsys Ab assay and report that it performs reliably, precisely, and consistently with both RT-qPCR and the EuroImmun Ab assay, with calculated 95% sensitivity, 100% specificity, and positive and negative predictive values approaching 100% for samples obtained 14 or more days after PCR positivity, consistent with manufacturer specifications.27,29 There were two PCR-positive, confirmed COVID-19 cases that showed negative Ab results due to the patients being in a clinically immunocompromised state. Thus, a negative Ab result in a COVID-19 patient could be due to blood drawn too early after infection or compromised immunity (eg, in patients on immunosuppressive regimen or newborns with an immature immune system).
Our studies demonstrate that the Elecsys Ab assay is a robust serologic diagnostic platform and is consistent with initial estimates of its performance measures.29 Importantly, our work provides independent external validation data in a major teaching hospital laboratory setting in the United States and therefore expands on recent studies addressing the clinical performance of the Elecsys Ab assay.30,31 Furthermore, whereas these other studies30,31 focused primarily on the relationship between the number of days after symptom onset vs the sensitivity of the Elecsys Ab assay, we provide additional data that specifically address the precision and cross-reactivity of the immunoassay when performed on the Roche cobas e602 analyzer.
Acknowledgments
We thank the Clinical Chemistry staff at the University of Chicago for providing excellent technical support for this project.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamel Boulos MN, Geraghty EM. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: how 21st century GIS technologies are supporting the global fight against outbreaks and epidemics. Int J Health Geogr. 2020;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5:e279-e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peak CM, Kahn R, Grad YH, et al. Individual quarantine versus active monitoring of contacts for the mitigation of COVID-19: a modelling study [published online May 20, 2020]. Lancet Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holshue ML, DeBolt C, Lindquist S, et al. ; Washington State 2019-nCoV Case Investigation Team First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822-3835. [DOI] [PubMed] [Google Scholar]
- 10. Esbin MN, Whitney ON, Chong S, et al. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26:771-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl). 2020;133:1039-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C, Gao G, Xu Y, et al. SARS-CoV-2-positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann Intern Med. 2020;172:832-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin Y, Wang M, Zuo Z, et al. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 2020;94:49-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with COVID-19 [published online April 19, 2020]. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffman T, Nissen K, Krambrich J, et al. Evaluation of a COVID-19 IgM and IgG rapid test; an efficient tool for assessment of past exposure to SARS-CoV-2. Infect Ecol Epidemiol. 2020;10:1754538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 28, 2020]. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845-848. [DOI] [PubMed] [Google Scholar]
- 20. Lou B, Li TD, Zheng SF, et al. Serology characteristics of SARS-CoV-2 infection since exposure and post symptom onset [published online March 27, 2020]. Eur Respir J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [DOI] [PubMed] [Google Scholar]
- 22. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao Y, Su B, Guo X, et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182:73-84.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11:2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490-9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corporation RD. Elecsys® Anti-SARS-CoV-2 Method Sheet. Indianapolis, IN: Roche Diagnostics; 2020. [Google Scholar]
- 28. Beavis KG, Matushek SM, Abeleda APF, et al. Evaluation of the EUROIMMUN anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J Clin Virol. 2020;129:104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. EUA authorized serology test performance 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance. Accessed August 9, 2020.
- 30. Favresse J, Eucher C, Elsen M, et al. Clinical performance of the Elecsys electrochemiluminescent immunoassay for the detection of SARS-CoV-2 total antibodies. Clin Chem. 2020;66:1104-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang MS, Hock KG, Logsdon NM, et al. Clinical performance of the Roche SARS-CoV-2 serologic assay. Clin Chem. 2020;66:1107-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann Intern Med. 2020;172:726-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA issues new policy to help expedite availability of diagnostics [press release]. https://www.fda.gov. Accessed June 16, 2020.
- 34.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides more regulatory relief during outbreak, continues to help expedite availability of diagnostics [press release] https://www.fda.gov. Accessed June 16, 2020.
- 35.US Food and Drug Administration. Policy for coronavirus disease-2019 tests during the public health emergency (revised) [press release] https://www.fda.gov. Accessed June 16, 2020.
- 36. Sun B, Feng Y, Mo X, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9:940-948. [DOI] [PMC free article] [PubMed] [Google Scholar]