Abstract
Objectives
Peripheral blood abnormalities in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have not been fully elucidated. We report qualitative and quantitative peripheral blood findings in coronavirus disease 2019 (COVID-19) patients and compare them with a control group.
Methods
We reviewed electronic medical records, complete blood counts, peripheral blood smears, and flow cytometry data in 12 patients with SARS-CoV-2. These were compared with 10 control patients with symptoms suspicious for SARS-CoV-2 but who tested negative.
Results
No significant differences were noted in blood counts, except that absolute lymphopenia was present frequently in the control group (P < .05). Acquired Pelger-Huët anomaly (APHA) was noted in all COVID-19 cases, in most cases affecting over 5% of granulocytes. This contrasted with APHA in only 50% of control cases, affecting fewer than 5% of granulocytes in all cases (P < .05). Monolobate neutrophils were exclusive to COVID-19 cases. COVID-19 patients had greater frequency of plasmacytoid lymphocytes (P < .05). Flow cytometry data revealed absolute CD3+ T-cell count reduction in 6 of 7 patients; all of them required mechanical ventilation.
Conclusions
Lymphopenia was infrequent in our COVID-19 cohort; however, flow cytometric analysis revealed absolute T-cell count reduction in most cases. COVID-19 cases had significant APHA with monolobate neutrophils and plasmacytoid lymphocytes as compared to controls.
Keywords: COVID-19, Peripheral smear, Hematologic, SARS-CoV-2, Peripheral blood, Coronavirus
Key Points.
• This is the first study where a detailed qualitative and quantitative examination of peripheral smear findings in COVID-19 patients was performed and compared against a control group.
• Absolute lymphopenia is not specific to COVID-19. Acquired Pelger-Huët anomaly with monolobate neutrophils is significantly more common in COVID-19 patients compared to the control group.
• A spectrum of variant lymphocytes is seen in COVID-19 cases, albeit constituting less than 10% of lymphocytes in most cases. Plasmacytoid lymphocytes are more common in COVID-19 cases.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the coronavirus disease 2019 (COVID-19) pandemic, is a highly contagious enveloped single-stranded RNA virus that belongs to the family of Betacoronaviruses.1 This virus shares genomic and clinical similarities with the other highly pathogenic coronaviruses, namely severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), which caused fatal epidemics in 2002 and 2012 respectively.2 SARS-CoV-2 infections can range from asymptomatic carriers to mild respiratory symptoms and fatal acute respiratory distress syndrome. The virus is thought to cause T-cell immune dysregulation, especially in immunocompromised patients, resulting in monocyte/macrophage activation, uncontrolled cytokine release, and fatal multiorgan dysfunction.3 Laboratory findings reported in association with COVID-19 include leukopenia, lymphopenia, monocytosis, neutrophilia, eosinopenia, and thrombocytopenia.4-6 To the best of our knowledge, peripheral blood smear morphologic findings are reported in detail in less than 5 publications to date in the literature.7-10 The reported abnormalities in peripheral blood smear include a range of reactive lymphocytes, abnormal platelet morphology, leukoerythroblastosis, and one publication on acquired Pelger-Huët anomaly (APHA). In this study, we report peripheral blood abnormalities, with emphasis on peripheral blood smear morphology, in 12 cases of SARS-CoV-2 infection and compare them with a control group.
Materials and Methods
The study was approved by the institutional review board. The list of all patients tested for SARS-CoV2 infection was retrieved. Clinical criteria recommended by the Centers for Disease Control and Prevention (CDC) were adopted by our institution for COVID-19 testing. Detection of SARS-CoV-2 was carried out using a modified CDC emergency use authorization–approved polymerase chain reaction (PCR) assay that had been successfully validated in our laboratory. This method involves nucleic acid extraction from nasopharyngeal swabs using Nuclisens easyMAG (bioMérieux) or Maxwell RSC 48 (Promega). Reverse transcription and then real-time PCR reactions were performed using 2 sets of CDC primers targeting the SARS-CoV-2 nucleocapsid genes N1 and N2 on the QuantStudio-3 Real-Time PCR system (ThermoFisher). Specimens were considered positive if either N1 or N2 was detected, and they were considered negative if neither was detected in the presence of appropriately detected controls.
As of April 22, 2020, a total of 1,584 cases were tested, among which SARS-CoV-2 virus was detected by PCR in 98 patients. Electronic medical records of the positive cases were reviewed for evidence of any hematologic tests performed. Twelve patients had peripheral blood smears available for review, and 6 of them had flow cytometric studies performed. Ten patients who met symptomatic criteria for COVID-19 testing, but who tested negative, had smears available for review. These 10 were reviewed as the control group. The peripheral blood smears were reviewed by 4 board-certified pathologists, which included 2 board-certified hematopathologists. The pathologists were blinded to the COVID-19 test result of patients during smear review. Statistical analysis was performed on the Microsoft Excel platform.
Complete blood cell counts (CBCs) were performed using Sysmex XN-9000. Leukocyte differential counts were performed using Sysmex DI-60 Cellavision software, and manual differential counts were performed when flagged for abnormalities. Peripheral blood smears were stained with Wright stain.
With regard to granulocytes, the presence of APHA, monolobate neutrophils, and toxic changes were recorded as a percentage of total granulocytes. These changes were reported as present if they were noted in more than 1% of granulocytes. Left shift of granulocytes is reported as present using the following cutoffs per total granulocyte count: bands, 10%; metamyelocytes and myelocytes, 5%; promyelocytes, 1%; and blasts, greater than 0%. Toxic changes in granulocytes were defined as presence of at least 2 of the following features: prominent cytoplasmic granulation, cytoplasmic vacuolization, and Döhle bodies, present in at least 5% of granulocytes.
The presence of atypical lymphocytes was noted, and atypical lymphocytes were further classified as Downey type I, Downey type II, Downey type III, plasmacytoid lymphocytes, large granular lymphocytes (LGL above normal ranges), and other atypical lymphocytes. The individual categories of atypical lymphocytes were reported as a percentage of total lymphocytes. Flow cytometric immunophenotyping for lymphocyte subsets was performed using BD FACSCanto II (Becton Dickinson). The antibodies analyzed included CD3, CD4, CD8, CD19, and CD16/56 with percentages and absolute counts reported.
Results
The 12 COVID-19 cases included 7 men and 5 women, with an age range of 25 to 100 years (mean, 55 years). The patients presented predominantly with respiratory symptoms (11/12) and fever (6/12) Table 1. Most patients had comorbidities at presentation, which included hypertension (3/12), asthma (3/12), HIV/AIDS (2/12), bacterial infections (2/12), malignancy (1/12), chronic kidney disease (1/12), and diabetes (1/12). Both patients with HIV/AIDS had less than 20 RNA copies/mL by PCR. Seven patients (58%) were admitted directly to the intensive care unit and required mechanical ventilation, of which 2 patients (16%) died. The demographics and symptoms in the control group were similar Table 2. The 10 patients in the control group included 8 men and 2 women, with an age range of 27 to 77 years (mean, 56 years). The comorbidities in this group of patients included bacterial infections (7/10), malignancy (3/10), hypertension (2/10), diabetes (2/10), HIV/AIDS (1/10), renal disease (1/10), congestive heart failure (1/10), and chronic obstructive pulmonary disease (1/10). Two patients (20%) required mechanical ventilation and died in this group.
Table 1.
Clinical and Laboratory Findings in Patients With COVID-19
| Patient | Clinical History | CBC Abnormalities on Admission | Flow Cytometry Abnormalities | Peripheral Smear Findings on Admission | Clinical Follow-up |
|---|---|---|---|---|---|
| 1 | 44 yo F with asthma presented with SOB and fever and found to have multifocal pneumonia. She was admitted to the ICU. Clinical condition deteriorated rapidly and she was pronounced dead shortly after. No OP medications were listed in the chart. | Absolute neutrophilia (12.13 × 103/μL) | CD3 abs: 4,824 H (723-2,737/μL) CD4 abs: 3,326 H (404-1,612/μL) CD8 abs: 1,631 H (220-1,129/μL) | APHA >10%, monolobate, PMNL, toxic changes, leukoerythroblastosis, left shift to blast, Pl-ly <5%, T2-ly <5%, Other-ly <5% | Progressed to respiratory failure, MV, deceased |
| 2 | 25 yo M presented with SOB. He was admitted to the ICU and went into respiratory failure requiring MV. No OP medications were listed in the chart. | Thrombocytosis (519 × 103/μL) | CD3 abs: 517 L CD4 abs: 239 L | APHA >10%, monolobate, PMNL, left shift to myelocytes, Pl-ly 5%-10%, T2-ly 5%-10%, LGL 5%-10% | Progressed to respiratory failure, MV, recovered and discharged |
| 3 | 52 yo M with hypertension, presented with SOB. He was admitted to the ICU and found to have hypoxic respiratory failure due to pneumonia. OP medications included oseltamivir, cetirizine, and benzonatate. | Leukocytosis (18.75 × 103/μL) Absolute neutrophilia (14.49 × 103/μL) | CD3 abs: 594 L CD8 abs: 113 L | APHA >10%, left shift to metamyelocyte, Pl-ly <5%, T2-ly 5%-10%, T3-ly 5%-10%, LGL <5% | Progressed to respiratory failure, MV, recovered and discharged |
| 4 | 50 yo M presented with SOB and fever. No OP medications were listed in the chart. | Absolute monocytopenia (0.16 × 103/μL) | — | APHA <5%, left shift to myelocytes, Pl-ly <5%, T2-ly 5%-10%, LGL >10%, Other-ly <5% | Stable clinical course, recovered and discharged |
| 5 | 32 yo F presented with cough, headache, and SOB. OP medications included acetaminophen and clindamycin. | Anemia (Hb: 10.9 g/dL) Thrombocytopenia (112 × 103/μL) | — | APHA 5%-10%, monolobate, PMNL, left shift to myelocytes, Pl-ly <5%, T1-ly <5%, T2-ly 5%-10%, T3-ly <5%, LGL <5%, Other-ly <5% | Stable clinical course, recovered and discharged |
| 6 | 67 yo M with HIV/AIDS and COPD was admitted to the ICU with hypoxemic respiratory failure. HIV RNA PCR <20 copies/mL. OP medications included Isentress, Descovy, metoprolol, clonazepam, and amlodipine. | Anemia (Hb: 11.9 g/dL) Leukocytosis (10.53 × 103/μL) Absolute neutrophilia (9.23 × 103/μL) | CD3 abs: 252 L CD4 abs: 108 L CD8 abs: 172 L | APHA >10%, monolobate, PMNL, left shift to myelocyte, PL-ly 5%-10%, T2-ly 5%-10%, T3-ly <5%, LGL <5%, Other-ly <5% | Progressed to respiratory and multiorgan failure, MV, deceased |
| 7 | 100 yo F with hypertension, thyroid and ovarian cancer, and chronic kidney disease presented with fever and cough. OP medications included metoprolol, sertraline, and cetirizine. | None | — | APHA >10%, left shift to bands, Pl-ly 5%-10%, T2-ly 5%-10%, T3-ly <5%, LGL 5%-10%, Other-ly <5% | Stable clinical course, recovered and discharged |
| 8 | 72 yo M presented with fever, SOB, and diarrhea. He was admitted to the ICU. No OP medications were listed in the chart. | Leukocytosis (35.6 × 103/μL) Absolute neutrophilia (32.68 × 103/μL) | CD3 abs: 244 L CD4 abs: 197 L CD8 abs: 62 L CD19 abs: 21 L (80-616/μL) | APHA <5%, monolobate, PMNL, toxic changes, left shift to bands, T2-ly <5%, T3-ly <5%, LGL <5%, Other-ly <5% | Progressed to respiratory failure, MV, recovered and discharged |
| 9 | 49 yo M with schizophrenia and history of polytrauma and Pseudomonas infection was admitted to the ICU for hypoxemic respiratory failure. OP medications included benztropine, escitalopram, and acetaminophen. | Anemia (Hb: 11.1 g/dL) Absolute lymphopenia (0.44 × 103/μL) | CD3 abs: 592 L CD4 abs: 353 L CD16/56 abs: 71 L (84-724/μL) | APHA 5%-10%, monolobate, PMNL, toxic changes,T2-ly 5%-10%, LGL <5% | Progressed to respiratory failure, MV, recovered and discharged |
| 10 | 61 yo M with HIV/AIDS, hypertension, COPD, and asthma presented with fever, cough, and diarrhea and was admitted to the ICU. HIV RNA PCR <20 copies/mL. OP medications included Triumeq, aspirin, amlodipine, Nexium, and Flonase. | Anemia (Hb: 11.6 g/dL) Leukocytosis (11.02 × 103/μL) Absolute lymphopenia (0.85 × 103/μL) Absolute neutrophilia (8.92 × 103/μL) | CD3 abs: 507 L CD4 abs: 145 L | APHA >10%, Pl-ly <5%, T2-ly <5%, LGL 5%-10% | Progressed to respiratory failure, MV, recovered and discharged |
| 11 | 77 yo F with type 1 diabetes, asthma, and CAD presented with SOB and was found to have viral and bacterial pneumonia. OP medications included Advair, aspirin, enalapril, amitriptyline, albuterol, Lipitor, and Macrobid. | Anemia (Hb: 10 g/dL) Leukocytosis (12.41 × 103/μL) Absolute neutrophilia (10.11 × 103/μL) | — | APHA <5%, T1-ly <5%, T2-ly <5%, LGL <5%, Other-ly <5% | Stable clinical course, recovered and discharged |
| 12 | 33 yo F presented with cough, SOB, and fever. No OP medications were listed in the chart. | None | — | APHA 5%-10%, toxic changes, Pl-ly <5%, T2-ly <5%, LGL <5% | Stable clinical course, recovered and discharged |
abs, absolute; APHA, acquired Pelger-Huët anomaly; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; F, female; H, high; Hb, hemoglobin; ICU, intensive care unit; L, low; LGL, large granular lymphocyte; M, male; MV, mechanical ventilation; OP, outpatient; Other-ly, other atypical lymphocytes; PCR, polymerase chain reaction; Pl-ly, plasmacytoid lymphocytes; PMNL, polymorphonuclear leukocytes; SOB, shortness of breath; T1-ly, Downey type I lymphocytes; T2-ly, Downey type II lymphocytes; T3-ly, Downey type III lymphocytes; yo, year old.
Table 2.
Clinical and Laboratory Findings in Control Group of Patients
| Patient | Clinical History | CBC Abnormalities on Admission | Flow Cytometry Abnormalities | Peripheral Smear Findings on Admission | Clinical Follow-up |
|---|---|---|---|---|---|
| 1 | 77 yo F with hypertension, type 2 diabetes, and breast cancer presented with SOB, fever, and hypotension. She was found to have metapneumovirus infection and Clostridium difficile colitis. OP medications included metformin, metoprolol, and levothyroxine. | Anemia (Hb: 10.2 g/dL) Absolute lymphopenia (0.67 × 103/μL)Absolute monocytopenia (0.19 × 103/μL) | — | APHA 5%-10%, toxic changes, left shift to myelocytes, T2-ly 5%-10%, LGL 5%-10% | Recovered and discharged |
| 2 | 39 yo M presented with SOB and new onset heart failure. He was found to have metapneumovirus and Rhinovirus by PCR. OP medications included acetaminophen, guaifenesin, albuterol, dicyclomine, and ondansetron. | None | — | Pl-ly <5%, T2-ly <5%, T3-ly <5%, Other-ly <5% | Recovered and discharged |
| 3 | 39 yo M presented with SOB and pneumonia. His tracheal swab was positive for Escherichia coli. OP medications included metoprolol, sertraline, buspirone, and trazodone. | Anemia (Hb: 7.3 g/dL)Leukocytosis (18.38 × 103/μL)Absolute lymphopenia (0.74 × 103/μL)Absolute neutrophilia (16.54 × 103/μL) | — | APHA <5%, toxic changes, left shift to bands, PL-ly <5%, T2-ly <5% | Progressed to respiratory and multiorgan failure, MV, deceased |
| 4 | 67 yo M with history of oral cancer presented with fever and cough. He was found to have Streptococcus agalactiae bacteremia. OP medications included prednisone, metoprolol, atorvastatin, levothyroxine, guaifenesin, and furosemide. | Anemia (Hb: 7.4 g/dL)Leukocytosis (12.04 × 103/μL)Absolute lymphopenia (0.24 × 103/μL)Absolute neutrophilia (8.67 × 103/μL) | CD3 abs: 118 L (723-2,737/μL)CD4 abs: <40 L (404-1,612/μL)CD8 abs: 87 L (220-1,129/μL) | APHA <5%, left shift to myelocytes, T2-ly <5% | Progressed to respiratory and multiorgan failure, MV, deceased |
| 5 | 63 yo M with hypertension, congestive heart failure, and COPD presented with fever and SOB. He was found to have metapneumovirus infection and secondary bacterial pneumonia. OP medications included metoprolol, lisinopril, aspirin, atorvastatin, cetirizine, and prednisone. | Anemia (Hb: 11.4 g/dL)Leukocytosis (23.97 × 103/μL)Absolute lymphopenia (0.46 × 103/μL)Absolute neutrophilia (21.89 × 103/μL) | — | Toxic changes, T2-ly <5%, LGL <5% | Recovered and discharged |
| 6 | 61 yo M with end-stage renal disease and type 2 diabetes presented with fever and SOB. He was found to have Staphylococcus aureus bacteremia. OP medications included amlodipine, atorvastatin, furosemide, and Keppra. | Anemia (Hb: 11.3 g/dL) | — | APHA <5%, toxic changes, left shift to metamyelocytes, Pl-ly <5% | Recovered and discharged |
| 7 | 27 yo M presented with fever and sore throat. Monospot test was positive. No OP medications were listed in the chart. | Leukocytosis (13.15 × 103/μL)Absolute lymphocytosis (5.65 × 103/μL)Absolute monocytosis (1.58 × 103/μL) | — | PL-ly <5%, T1-ly >10%, T2-ly >10%, T3-ly <5%, LGL >10% | Recovered and discharged |
| 8 | 62 yo M with chronic neutropenia and history of Kaposi sarcoma presented with fever and pneumonia. He was positive for rhinovirus/enterovirus on PCR. OP medications included metoprolol, amlodipine, and Zoloft. | Anemia (Hb: 9.9 g/dL)Leukopenia (1.85 × 103/μL)Absolute lymphopenia (0.39 × 103/μL)Absolute neutropenia (1.28 × 103/μL) | — | PL-ly 5%-10%, T1-ly <5%, T2-ly <5%, LGL <5% | Recovered and discharged |
| 9 | 59 yo M with HIV and autoimmune hemolytic anemia presented with fever. He was found to have rhinovirus/enterovirus on PCR and Pseudomonas in urine sample. HIV RNA PCR <20 copies/mL. OP medications included BIKTARVY. | Anemia (Hb: 11.6 g/dL)Absolute lymphopenia (0.24 × 103/μL)Thrombocytopenia (95 × 103/μL) | — | Toxic changes, left shift to bands, T1-ly 5%-10%, T2-ly <5%, LGL 5%-10% | Recovered and discharged |
| 10 | 64 yo F presented with cough and fever. She was found to have bacterial pneumonia. No OP medications were listed in the chart. | Leukocytosis (16.91 × 103/μL)Absolute neutrophilia (12.83 × 103/μL) | — | APHA <5%, toxic changes, left shift to metamyelocytes, PL-ly: <5%, T2-ly 5%-10%, T3-ly <5%, LGL <5% | Recovered and discharged |
abs, absolute; APHA, acquired Pelger-Huët anomaly; COPD, chronic obstructive pulmonary disease; F, female; L, low; LGL, large granular lymphocyte; M, male; MV, mechanical ventilation; OP, outpatient; Other-ly, other atypical lymphocytes; PCR, polymerase chain reaction; Pl-ly, plasmacytoid lymphocytes; SOB, shortness of breath; T1-ly, Downey type I lymphocytes; T2-ly, Downey type II lymphocytes; T3-ly, Downey type III lymphocytes; yo, year old.
Among the COVID-19 patients, CBCs revealed anemia in 5 cases (mean hemoglobin, 12.5 g/dL; range, 10-16.1 g/dL), leukocytosis in 4 cases (mean, 7.3 × 103/μL; range, 3.9-35.6 × 103/μL), thrombocytosis in 1 case, and thrombocytopenia in 1 case. Absolute lymphopenia was present in 2 cases; one of them had HIV/AIDS. Absolute neutrophilia was seen in 6 cases (mean, 9.24 × 103/μL; range, 2.3-32.6 × 103/μL). Absolute monocyte count was low in 1 patient and within reference ranges in others. Absolute eosinophil count was within reference ranges in all patients. The control group showed CBC findings similar to the COVID-19 group; however, absolute lymphopenia was present in 6 control group patients as compared to only 2 COVID-19 patients (P < .05).
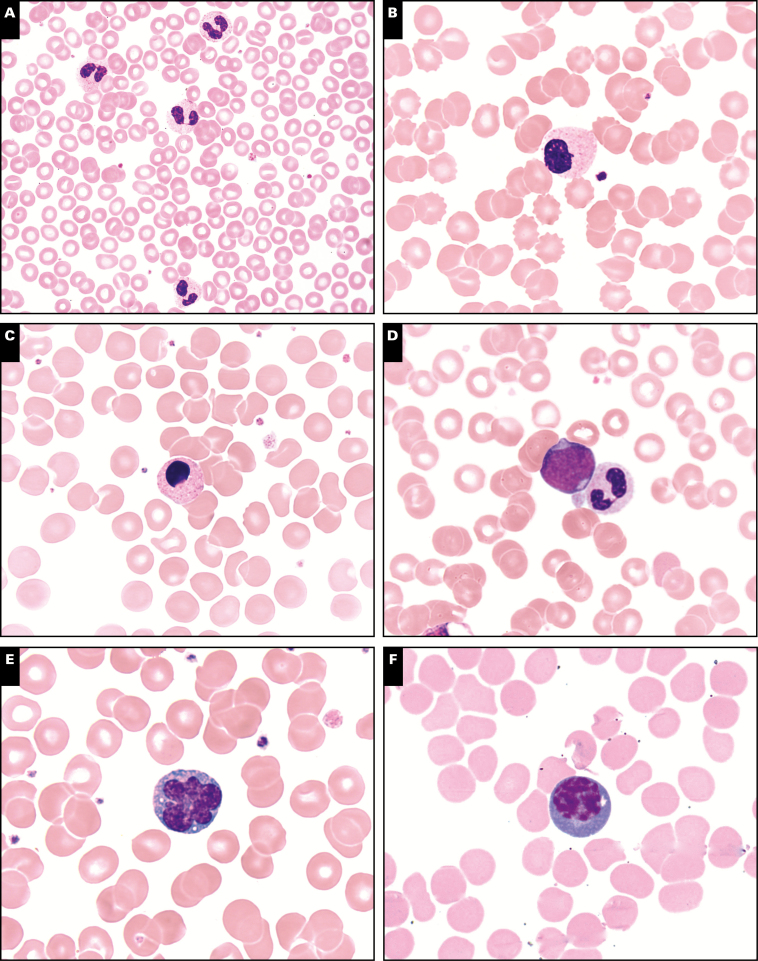
Peripheral blood smear review showed presence of APHA in all COVID-19 cases, with 50% of cases (6/12) having over 10% pelgeroid neutrophils, 25% cases (3/12) having 5% to 10%, and the remaining 25% cases (3/12) having less than 5%. Monolobate neutrophils were noted in 50% of COVID-19 cases (6/12). Rare neutrophils showed prominent apoptotic changes. In contrast, the control group showed APHA in 50% of cases (P < .05), and in 4 of 5 of these cases, pelgeroid cells comprised less than 5% of neutrophils. Notably, monolobate neutrophils were not present in any of the control cases. Morphologic findings in granulocytes are illustrated in Image 1A, Image 1B, Image 1C, and Image 1D. Two patients with HIV/AIDS had APHA greater than 10%; however, both patients had less than 20 HIV RNA copies/mL detected by PCR. None of these patients had a history of myeloid neoplasms. Review of outpatient medications revealed no definite evidence of medications reported to be associated with APHA. Inpatient medications were not pertinent, as the peripheral blood studied were drawn during hospital admission.
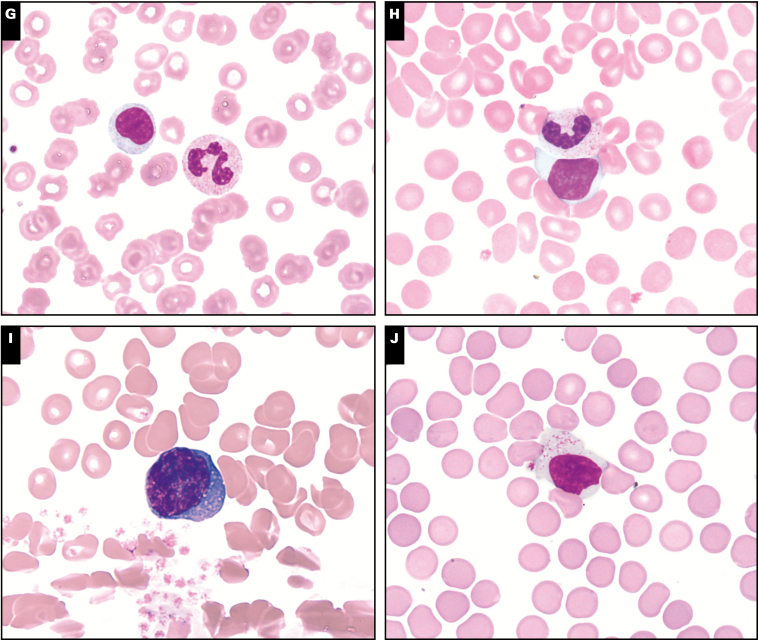
Image 1.
Morphologic findings in peripheral smears in coronavirus disease 2019 patients (Wright stain, ×100). A, Acquired Pelger-Huët anomaly. B, Monolobate neutrophil. C, Apoptotic neutrophil. D, Left shift to blast stage. E, Atypical lymphocyte with irregular nuclear contours. F, Plasmacytoid lymphocyte. G, Downey type I lymphocyte. H, Downey type II lymphocyte. I, Downey type III lymphocyte. J, Large granular lymphocyte.
In only a minority of COVID-19 cases did granulocytes show prominent toxic changes. In 1 severely ill patient with multifocal pneumonia and respiratory failure, the peripheral smear showed leukoerythroblastosis and toxic changes in greater than 10% neutrophils. The patient died soon after. In the remaining 11 cases, toxic changes were present in less than 10% of granulocytes. However, granulocytic left shift was a prominent feature in a majority of cases (8/12). Among the cases with left shift, 62% of cases (5/8) showed left shift to metamyelocyte/myelocyte stage, 25% of cases (2/8) showed presence of circulating bands, and the 1 case described above with leukoerythroblastosis showed rare circulating blasts (<1% blasts). In the control group, 6 of 10 cases showed extensive toxic changes and granulocytic left shift; however, most of those cases also had concurrent bacterial infections.
All COVID-19 cases showed varying types of atypical lymphocytes, albeit constituting less than 10% of lymphocytes in the majority of cases Image 1E, Image 1F, Image 1G, Image 1H, Image 1I, and Image 1J. The most common and least specific type of reactive lymphocyte, the Downey type II lymphocyte, was noted in all cases; however, it constituted less than 10% of lymphocytes in all cases. Large granular lymphocytes were noted in 11 of 12 cases, but only 1 case showed mildly increased numbers of LGLs comprising greater than 10% of lymphocytes. Plasmacytoid lymphocytes were noted in 9 of 12 cases, Downey type III lymphocytes in 5 of 12 cases, and Downey type I lymphocytes in 2 of 12 cases, with each individually comprising less than 10% of total lymphocytes. Rare atypical lymphocytes with markedly irregular nuclear contours and scant to moderate basophilic cytoplasm were noted in 7 of 12 cases but comprised less than 5% of total lymphocytes in each case. Plasmacytoid lymphocytes were seen in greater frequency in COVID-19 patients, compared to controls (P < .05), although they constituted less than 10% of lymphocytes in all cases. Otherwise, the control group had a comparable qualitative and quantitative spectrum of atypical lymphocytes, except 1 case of infectious mononucleosis.
Of the 12 cases, flow cytometric studies for lymphocyte subsets were performed in 7 cases. This included 2 patients with HIV/AIDS; however, both patients had less than 20 HIV RNA copies/mL detected by PCR. Decreases in absolute numbers of CD3+ T-cells, CD4+ T-cells, and CD8+ T-cells were identified in 6, 5, and 3 cases respectively. B cells and NK cells were enumerated only in 4 cases, of which the majority showed absolute counts in normal ranges. One case had a low B-cell count and another case had a low NK-cell count. Although the numbers are too few to draw meaningful comparisons, cytopenia of CD3+ T cells appears to be a common finding. All patients with decreased T cells required mechanical ventilation. The control group only had flow cytometry performed in 1 case.
Discussion
After its identification in December 2019 in Wuhan, China, COVID-19 has rapidly spread and evolved into a pandemic over a few months.11 The pathophysiology of this infection is not completely understood; however, the causative agent SARS-CoV-2 shares 79% genomic similarity with SARS-CoV.2 SARS-CoV infects host cells expressing the angiotensin-converting enzyme 2 (ACE-2) receptor, including airway epithelium, alveolar lining, endothelial cells, and alveolar macrophages. SARS-CoV-2 is postulated to infect cells via the ACE-2 and transmembrane protease serine 2 (TMPRSS2) receptors, leading to host cell death while triggering monocyte/macrophage activation, T-cell activation, cytokine release, and B-cell mediated antibody production.3 Furthermore, studies have demonstrated that SARS-CoV is able to infect and replicate in peripheral blood mononuclear cells.12,13 Transcriptomic analysis of peripheral blood in COVID-19 patients, however, has not demonstrated presence of SARS-CoV-2 RNA in hematopoietic cells.14 Activation of genes in the proapoptotic and p53 signaling pathway was identified in the peripheral blood mononuclear cells of patients with SARS-CoV-2.14
Laboratory abnormalities reported in COVID-19 patients include lymphopenia (>40% patients), leukocytosis, leukopenia, neutrophilia, monocytosis, and eosinopenia.4-6 These laboratory abnormalities were also reported in SARS-CoV patients.15 Lymphopenia is the predominant hematologic finding associated with SARS-CoV-2 in the literature and is reported to predict disease severity.16 Several hypotheses exist regarding mechanism of lymphopenia in COVID-19 patients, including direct viral toxicity due to ACE-2 receptor expression on lymphocytes, cytokine-induced lymphocyte apoptosis, and metabolic products causing lymphocyte inhibition.17 In our cohort of cases, absolute lymphopenia by CBC was only seen in 2 cases, but flow cytometric analysis revealed absolute T-cell count reduction in 6 of 7 cases. All patients with decreased T cells were admitted to the intensive care unit and required mechanical ventilation. Lymphocyte subset alterations are reported to be associated with disease activity in COVID-19 patients,18 and flow cytometry is a sensitive modality to detect that. No specific CBC abnormalities were identified in COVID-19 cases compared to the control group in this study.
There is limited literature on peripheral smear findings in COVID-19 patients. Zini et al8 reported presence of APHA, prominent abnormal neutrophilic granulation, monolobate neutrophils, granulocytic left shift, large abnormal platelet morphology, apoptotic cells, and few reactive lymphocytes. These abnormalities were not quantified in their study. Interestingly, the neutrophilic morphologic abnormalities almost entirely disappeared after 1 week of antiviral/anti-inflammatory treatment in a subset of cases. Our study is in concordance with that of Zini et al in that there is conspicuous presence of APHA including monolobate neutrophils in the peripheral blood of COVID-19 cases compared to control cases. Pelger-Huët anomaly is a benign hereditary condition where mutations in the lamin B receptor (LBR) result in hyposegmented neutrophils with dense chromatin.19 LBR plays an important role in maintaining the structure of nuclear membranes.20 Acquired causes of this anomaly include myelodysplastic syndrome, infections like tuberculosis, HIV/AIDS, influenza A, mononucleosis, parvovirus, and drugs such as immunosuppressive agents and some antibiotics.19 There was no evidence of medications associated with APHA identified in our patient cohort. In the 2 cases with HIV/AIDS, the viral loads were undetectable. The mechanism behind APHA is unclear. Some hypotheses include acquired mutations in LBR gene and accelerated apoptosis.21 In light of evidence regarding enriched apoptotic activity by SARS-CoV-2,14 that hypothesis is a possibility. APHA is not reported in association with SARS-CoV.
Barring myeloid neoplasms, left shift of granulocytes is conventionally interpreted as a sign of bacterial infection.22 Neutrophil kinetics in bacterial infections is thought to trigger mobilization of marrow reserves, resulting in left shift of granulocytes.22 Of the 8 patients with left shift in our study, only 2 patients had concurrent bacterial infections. SARS-CoV-2 induced cytokine release causing neutrophil migration akin to bacterial infections is a possibility. Direct myelotoxicity caused by the virus or marrow overproduction in a background of increased peripheral cell turnover are other hypotheses. Leukoerythroblastosis, defined as circulating immature granulocytes and nucleated RBCs with or without anemia, is typically associated with marrow infiltrative processes but may also represent marrow response to stressors like hypoxia, peripheral destruction/sequestration, or sepsis.23 Peripheral blood leukoerythroblastosis in a COVID-19 patient was reported by Mitra et al9 This was noted in 1 case in our cohort as well. Given the lack of any other evidence suggestive of an underlying myeloid neoplasm or malignancy causing a myelophthisic process, this may represent marrow stress and response to the viral infection.
Atypical/ reactive lymphocytes in the peripheral smear is the hallmark of some infections, such as infectious mononucleosis, Bordetella pertussis, and hantavirus.24-26 Infectious mononucleosis shows a spectrum of reactive/pleomorphic lymphocytes, while hantavirus shows predominantly Downey type III cells or immunoblasts. These are not specific findings and may be seen in varying numbers in other infections, autoimmune diseases, and malignancies. Peripheral smear review of 32 COVID-19 cases from Singapore reported presence of circulating reactive lymphocytes with predominantly lymphoplasmacytoid morphology in 72% of cases.7 Our study also shows a spectrum of atypical lymphocytes in almost all COVID-19 cases, with plasmacytoid lymphocytes present in greater frequency in COVID-19 cases compared to the control group. However, variant lymphocytes comprise a minority of lymphocytes (<10%) in most patients. This finding may be similar to SARS-CoV, where reactive lymphocytes were not identified in significant numbers by Chng et al27 and seen in only about 15% cases by Lee et al.28 Quantitation of reactive lymphocytes was not performed in other SARS-CoV or COVID-19 studies.
In summary, we report CBC and peripheral smear findings on admission in 12 symptomatic patients who tested positive for COVID-19 and compared them to a control group. To the best of our knowledge, this is the first study to quantify individual morphological findings on peripheral smear in COVID-19 cases. We acknowledge the limitations of our study, especially the number of peripheral smears available for review. Larger series of cases with peripheral smear review during and after treatment will be of interest, to study their potentially transient nature and correlation with disease activity.
Conclusion
Peripheral blood abnormalities are common in viral infections and can occasionally provide insight into underlying pathophysiologic processes. An increase in variant lymphocytes was observed in most COVID-19 patients, with plasmacytoid lymphocytes seen more often in COVID-19 patients than in controls. Morphologic abnormalities in the granulocytic series, namely APHA and left shift, were significantly more common in COVID-19 cases. The implications of these findings with respect to our understanding of SARS-CoV-2 infection are yet to be uncovered.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;1-12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30]. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chong VCL, Lim KGE, Fan BE, et al. Reactive lymphocytes in patients with Covid-19. Br J Haematol. 2020. doi: 10.1111/bjh.16690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zini G, Bellesi S, Ramundo F, et al. Morphological anomalies of circulating blood cells in COVID-19. Am J Hematol. 2020. doi: 10.1002/ajh.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitra A, Dwyre DM, Schivo M, et al. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J Hematol. 2020. doi: 10.1002/ajh.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 11. WHO. Coronavirus disease (COVID-2019) situation reports 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 12. Li L, Wo J, Shao J, et al. SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J Clin Virol. 2003;28:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiong Y, Liu Y, Cao L, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meo SA, Alhowikan AM, Al-Khlaiwi T, et al. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012-2019. [DOI] [PubMed] [Google Scholar]
- 16. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dusse LM, Moreira AM, Vieira LM, et al. Acquired Pelger-Huët: what does it really mean? Clin Chim Acta. 2010;411:1587-1590. [DOI] [PubMed] [Google Scholar]
- 20. Best S, Salvati F, Kallo J, et al. Lamin B-receptor mutations in Pelger-Huët anomaly. Br J Haematol. 2003;123:542-544. [DOI] [PubMed] [Google Scholar]
- 21. Cunningham JM, Patnaik MM, Hammerschmidt DE, et al. Historical perspective and clinical implications of the Pelger-Huët cell. Am J Hematol. 2009;84:116-119. [DOI] [PubMed] [Google Scholar]
- 22. Honda T, Uehara T, Matsumoto G, et al. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin Chim Acta. 2016;457:46-53. [DOI] [PubMed] [Google Scholar]
- 23. Burkett LL, Cox ML, Fields ML. Leukoerythroblastosis in the adult. Am J Clin Pathol. 1965;44:494-498. [DOI] [PubMed] [Google Scholar]
- 24. George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program. 2012;2012:475-484. [DOI] [PubMed] [Google Scholar]
- 25. Klein E, Kis LL, Klein G. Epstein-Barr virus infection in humans: from harmless to life endangering virus-lymphocyte interactions. Oncogene. 2007;26(9):1297-1305. [DOI] [PubMed] [Google Scholar]
- 26. Koster F, Foucar K, Hjelle B, et al. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am J Clin Pathol. 2001;116:665-672. [DOI] [PubMed] [Google Scholar]
- 27. Chng WJ, Lai HC, Earnest A, et al. Haematological parameters in severe acute respiratory syndrome. Clin Lab Haematol. 2005;27:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986-1994. [DOI] [PubMed] [Google Scholar]