To the Editor—A recent report by Rosenberg and colleagues highlights the importance of multiple sources of information for monitoring trends in the coronavirus disease 2019 (COVID-19) pandemic [1]. The authors used influenza-like illness (ILI) surveillance data and laboratory-confirmed influenza and COVID-19 cases to estimate population-based rates of illness during the beginning of the pandemic in New York state. Because severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was rapidly increasing during this period in many areas of the United States, numbers of COVID-19 cases were likely underestimated. This raises the question of whether COVID-19 contributed to ILI trends prior to widespread testing.
We recently examined the timing and extent of COVID-19 among patients with acute respiratory illness (ARI) enrolled in 2 US influenza vaccine effectiveness networks [2, 3]. We retrospectively tested specimens collected between late January 2020 and mid-March 2020, a time period during which genomic analyses of SARS-CoV-2 isolates suggested silent community spread in several US locations [4–6]. In the influenza networks, outpatients aged ≥6 months and inpatients aged ≥18 years with ARI (defined as cough or respiratory symptoms with onset ≤10 days earlier) were enrolled during the influenza season at healthcare facilities associated with study sites in 6 states (Michigan, Pennsylvania, Tennessee, Texas, Washington, and Wisconsin) [7]. During the influenza season, respiratory specimens, including nasal, throat, or nasopharyngeal swabs, were prospectively tested for influenza using reverse-transcription polymerase chain reaction (RT-PCR). We retrospectively tested a subset of stored specimens or extracted RNA at study sites for SARS-CoV-2 using RT-PCR designed to detect the SARS-CoV-2 nucleocapsid gene.
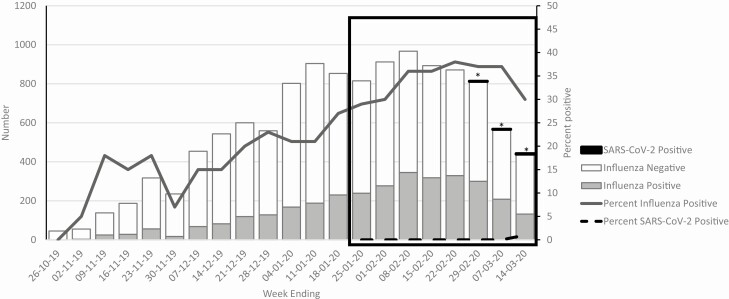
Although the number of confirmed influenza cases decreased after mid-February, influenza positivity among participants remained above 30% through early March when enrollment was interrupted due to the COVID-19 pandemic (Figure 1). Of 4961 specimens tested retrospectively, 5 (0.1%) specimens from patients at 3 study sites tested positive for SARS-CoV-2, all from patients enrolled within 1 week of the first COVID-19 cases reported in surveillance counties (Table 1). None of the patients had been previously identified as having COVID-19. Although few SARS-CoV-2–positive patients were identified before facility-based enrollment was halted, the timing of initial reports of COVID-19 cases in these surveillance areas coincided with detection of SARS-CoV-2–positive cases among outpatients and inpatients with respiratory symptoms.
Figure 1.
Number and percent of specimens from ambulatory patients and hospitalized adults with acute respiratory illness who tested positive for influenza or SARS-CoV-2, October 2019–March 2020. Box indicates weeks included in retrospective testing for SARS-CoV-2. *Week of first reported coronavirus disease 2019 case associated with community transmission in surveillance counties. Abbreviation: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Number of Enrolled Patients With Acute Respiratory Illness Tested, Positive Tests and Enrollment Dates for Severe Acute Respiratory Syndrome Coronavirus 2–Positive Patients, and Date of First Reported Nontravel-related Coronavirus Disease 2019 by Study Population, January 2020–March 2020
| Severe Acute Respiratory Syndrome Coronavirus 2 | |||||||
|---|---|---|---|---|---|---|---|
| Study Site | Surveillance Counties | Time Period | No. Enrolleda | No. Tested | No. Positive (%) | Date of Enrollment | Date of First Reported Coronavirus Disease 2019 Caseb |
| Ann Arbor and Detroit, Michigan | 10 counties in southeast Michigan | 23 January–13 March | 1026 | 848 | 2 (0.2) | 9 March, 11 March | 10 March |
| Pittsburgh, Pennsylvania | Allegheny | 27 January–22 March | 1294 | 1260 | 2 (0.2) | 3 March, 11 March | 7 March |
| Nashville, Tennessee | 7 counties in central Tennessee | 1 February–21 March | 226 | 226 | 0 (0) | NA | 5 March |
| Temple, Texas | 8 counties in central Texas | 25 January–5 March | 875 | 584 | 0 (0) | NA | 13 March |
| Seattle, Washington | King, Pierce, and Snohomish | 31 January–29 February | 1619 | 1214 | 1 (0.1) | 25 February | 21 February |
| Marshfield, Wisconsin | Clark, Marathon, and Wood | 5 February–13 March | 1367 | 660 | 0 (0) | NA | 16 March |
| All sites | 23 January–22 March | 6407 | 4792 | 5 (0.1) | |||
Abbreviation: NA, not applicable.
aIncludes ambulatory patients aged ≥6 months and hospitalized adults aged ≥18 years who presented to healthcare facilities with acute respiratory illness.
bDate of first reported COVID-19 case associated with community transmission in surveillance county.
Rapid increases in COVID-19 cases may have contributed to peaks in ILI activity observed in many states near the end of the influenza season, as suggested by computer modeling [8], but changes in ambulatory care utilization may have also contributed. Given overlap between ARI/ILI and symptoms of mild/moderate COVID-19 [9], systematic testing for SARS-CoV-2 and influenza will be needed during the upcoming influenza season to interpret trends in ILI surveillance and determine contributions of each viral illness to the burden of respiratory disease. Testing for both pathogens (and other respiratory viruses) may become routine for inpatients with respiratory illness but will depend on availability and access to testing among patients with mild illness. Facility-based surveillance for ILI and research studies will have to adapt. We agree with Rosenberg et al that alternatives to facility-based specimen collection, including home-based nasal swabs or saliva collection, will be needed.
Notes
US Influenza Vaccine Effectiveness Network and Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN) investigators: Edward A. Belongia, MD, Huong Q. McLean, PhD, Marshfield Clinic Research Institute; Richard K. Zimmerman, MD, MPH, M. Patricia Nowalk, PhD, Monika Johnson, MS, University of Pittsburgh; Arnold S. Monto, MD, Lois E. Lamerato, PhD, Anurag N. Malani, MD, University of Michigan; C. Hallie Phillips, Med, Erika Kiniry, MPH, Stacie Wellwood, LPN, Kaiser Permanente Washington Health Research Institute; Marcus Volz, Kimberly Walker, Arundhati Rao, MD, Baylor Scott & White Health; Dayna Wyatt, Christopher Trabue, MD, Yuwei Zhu, MD, MS, Vanderbilt University; Jessie R. Chung, MPH, Sara S. Kim, MPH, Jill M. Ferdinands, PhD, Manish M. Patel, MD. Centers for Disease Control and Prevention (CDC).
Acknowledgments. The authors thank contributors from the US Flu Vaccine Effectiveness (for complete list, please see Dawood et al [7]) and HAIVEN networks and members of the Centers for Disease Control and Prevention (CDC) COVID-19 Response. None of these persons received any compensation for their contributions.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by the CDC through cooperative agreements (IP15–002, IP16–002) and by the National Institutes of Health (UL1TR001857) to the University of Pittsburgh.
Potential conflicts of interest. J. V. W. serves on the Quidel Scientific Advisory Board and on the Independent Data Monitoring Committee for GlaxoSmithKline. E. T. M. has received research funds from Merck and Roche Pharmaceuticals. M. L. J. has received grants from Sanofi Pasteur. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
US Influenza Vaccine Effectiveness Network and the Hospitalized Adult Influenza Vaccine Effectiveness Network:
Edward A Belongia, Huong Q McLean, Richard K Zimmerman, M Patricia Nowalk, Monika Johnson, Arnold S Monto, Lois E Lamerato, Anurag N Malani, C Hallie Phillips, Erika Kiniry, Stacie Wellwood, Marcus Volz, Kimberly Walker, Arundhati Rao, Dayna Wyatt, Christopher Trabue, Yuwei Zhu, Jessie R Chung, Sara S Kim, Jill M Ferdinands, and Manish M Patel
References
- 1. Rosenberg ES, Hall EW, Rosenthal EM, et al. Monitoring COVID-19 through trends in influenza-like illness and laboratory-confirmed influenza and COVID-19—New York State, excluding New York City, January 1–April 12, 2020. Clin Infect Dis 2021; 72:144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jorden MA, Rudman SL, Villarino E, et al. ; CDC COVID-19 Response Team . Evidence for limited early spread of COVID-19 within the United States, January-February 2020. MMWR Morb Mortal Wkly Rep 2020; 69:680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rha B, Lively JY, Englund JA, et al. SARS-CoV-2 infections in children—multi-center surveillance, United States, January-March 2020. J Pediatric Infect Dis Soc 2020:piaa075. doi: 10.1093/jpids/piaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu HY, Englund JA, Starita LM, et al. ; Seattle Flu Study Investigators . Early detection of Covid-19 through a citywide pandemic surveillance platform. N Engl J Med 2020; 383:185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalez-Reiche AS, Hernandez MM, Sullivan MJ, et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science 2020; 369:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng X, Gu W, Federman S, et al. A genomic survey of SARS-CoV-2 reveals multiple introductions into northern California without a predominant lineage. medRxiv. 2020. doi: 10.1101/2020.03.27.20044925. [DOI] [Google Scholar]
- 7. Dawood FS, Chung JR, Kim SS, et al. Interim estimates of 2019-20 seasonal influenza vaccine effectiveness—United States, February 2020. MMWR Morb Mortal Wkly Rep 2020; 69:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silverman JD, Hupert N, Washburne AD. Using influenza surveillance networks to estimate state-specific case detection rates and forecast SARS-CoV-2 spread in the United States. medRxiv. doi: 10.1101/2020.04.01.20050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kong W, Li Y, Peng M, et al. SARS-CoV-2 detection in patients with influenza-like illness. Nat Microbiol 2020. doi: 10.1038/s41564-020-0713-1. [DOI] [PubMed] [Google Scholar]