Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has caused substantial morbidity and mortality worldwide. Few reports exist in Latin America, a current epicenter of transmission. Here, we aim to describe the epidemiology and outcomes associated with coronavirus disease 2019 (COVID-19) in Honduras.
Methods
Baseline clinical and epidemiological information of SARS-CoV-2 reverse transcriptase polymerase chain reaction–confirmed cases detected between 17 March–4 May in the San Pedro Sula Metropolitan area was collected; for hospitalized cases, clinical data were abstracted. Logistic regression models were fit to determine the factors associated with hospitalization.
Results
We identified 877 COVID-19 cases, of which 25% (n = 220) were hospitalized. The 19–44-year age group (57.8%) and males (61.3%) were predominant in overall COVID-19 cases. Of the cases, 34% (n = 299) had at least 1 preexisting medical condition. Individuals aged 45–69 years (adjusted odds ratio [aOR] = 4.05; 95% confidence interval [CI], 2.85–5.76) or ≥70 years (aOR = 9.12; 95% CI, 5.24–15.86), of male sex (aOR = 1.72; 95% CI, 1.21–2.44), and those with a preexisting condition (aOR = 2.12; 95% CI, 1.43–3.14) had higher odds of hospitalization. Of inpatients, 50% were hospitalized more than 7 days. The median length of hospitalization was 13 days (interquartile range [IQR], 8–29) among individuals aged 19–44 years, and 17 days (IQR, 11–24.6) among those aged 45–69. Of the fatal cases, 42% occurred among adults under 60 years old.
Conclusions
Our findings show that a high proportion of COVID-19 cases in Honduras occurred among younger adults, who also constituted a significant proportion of severe and fatal cases. Preexisting conditions were associated with severe outcomes independently from age and were highly prevalent in Honduran COVID-19 cases.
Keywords: Latin America, COVID-19, epidemiology, SARS-CoV-2, associated factors
Our study of clinical and epidemiologic features of coronavirus disease 2019 (COVID-19) in San Pedro Sula, Honduras, found frequent cases among younger adults, with severe outcomes, including death. Comorbidities and age were independently associated with disease severity and hospitalization.
On March 2020, the World Health Organization declared coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a pandemic [1]. Although we are still in the early months of the pandemic, it has already caused a substantial number of severe cases and deaths worldwide [2]. Globally, the United States, Brazil, and India have reported the largest number of cases to date, with the United States accounting for the largest number of deaths. However, Latin America and the Caribbean (LAC) is quickly becoming the new epicenter of the pandemic, with severe outbreaks occurring throughout the region [3]. The clinical course of the disease and its epidemiology have been described previously outside LAC [4–6].
In LAC, 1 of the first cases of COVID-19 was reported in Brazil on 21 February [7]. Furthermore, in Bolivia, 12 imported cases and their clinical characteristics were described in March [8]. Honduras, in Central America, reported 1 of its first COVID-19 cases on 9 March [9]. As of June 2020, the Honduran Ministry of Health reported more than 9000 reverse transcriptase polymerase chain reaction (RT-PCR)–confirmed cumulative cases, with the San Pedro Sula Metropolitan Area accounting for approximately 50% of the cases nationwide [10].
Although previous research has described the epidemiology of COVID-19 and the clinical features of disease, limited reports exist on the epidemiology of COVID-19 in LAC countries. This study aims to describe the epidemiology of COVID-19 in the Metropolitan Area of San Pedro Sula, Honduras.
METHODS
Population
Recent estimates show that the Honduras population is composed of almost 9 000 000 inhabitants [11]. The city of San Pedro Sula is located in the department of Cortés in the Northwestern region of the country. As of the 2018 census, the Metropolitan Area of San Pedro Sula’s population was estimated to be 777 877 inhabitants. This census estimated that 39.7% of the population were 0–18 years old, 42.2% of the population were 19–44 years old, 11.2% were 45–69 years old, and 6.9% were aged 70 years or older [12]. Upon identification of the first COVID-19 case in the city of San Pedro Sula, Honduras, on 17 March, 2 public, tertiary-care hospitals and a social security hospital created COVID-19 units for cases that required hospitalization. Combined, these units had 170 beds, of which 31 were for severe cases that might require mechanical ventilation.
Data Collection/Data Sources
Testing for SARS-CoV-2 in the San Pedro Sula Metropolitan Area uses the Charité RT-PCR protocol (Berlin, Germany) [13] as recommended by the Pan American Health Organization [14], and began the week of 3 March 2020. During early March and late April 2020, samples were processed at Laboratorio Nacional de Virología in the city of Tegucigalpa, while the RT-PCR testing capacity was established in San Pedro Sula. RT-PCR testing in San Pedro Sula began in late April.
During the first weeks of the pandemic, criteria for testing was limited to individuals with COVID-19 symptoms and recent travel to a country with an ongoing COVID-19 outbreak. During the week of 30 March, with the number of COVID-19 cases increasing significantly, and taking into account testing capacity, individuals who had contact with a positive case and had symptoms were also tested, regardless of their travel history. Further, testing was extended to suspected cases of COVID-19, regardless of contact with a positive case or travel history. Beginning on 22 April, as RT-PCR was available at San Pedro Sula laboratories, asymptomatic individuals who had contact with a positive case were also tested at times when there was sufficient testing [15]. These testing criteria were applied at both the primary care and hospital levels. As of 4 May, the city of San Pedro Sula was testing at public hospitals, private hospitals, and 2 primary care clinics for individuals with mild symptoms.
Data for all COVID-19 cases occurring between 9 March and 4 May in the San Pedro Sula Metropolitan area were compiled. The complete data set included data from the Metropolitan Area Public Health Office and local hospital medical records.
For all COVID-19 suspected cases, baseline epidemiologic and clinical data were collected using a modified influenza-like illness surveillance form. This includes demographic data, including age, sex, preexisting medical conditions, and travel history, and a clinical history of the disease, including the date of onset of disease. The clinical section of the form collects baseline signs and symptoms that are associated with respiratory illness, number of days the case was symptomatic, and whether the case required hospitalization or ambulatory care.
Clinical and outcome data for hospitalized cases were collected by public health office physicians at each of the COVID-19 units using medical records. Data included: date of hospitalization, presence of pneumonia, whether mechanical ventilation (either invasive or noninvasive) was needed, and the date of outcome (discharge/death).
Ethical Statement
All data were collected as a part of the public health response to COVID-19 in Honduras, and thus the study was determined by the Honduran Ministry of Health and the University of Michigan Institutional Review Board not to be human subjects research. Since data collection occurred through essential disease surveillance activities, informed consent was waived. Data were anonymized prior to the analysis.
Statistical Analysis
We stratified COVID-19 cases by hospitalization status and performed descriptive statistics. Age groups were stratified according to the groups used in the latest census estimates at the San Pedro Sula Metropolitan Area [12]. The P values were calculated using a Fisher’s exact test, Wilcoxon rank-sum test, or chi-square test, as appropriate. We fitted a logistic regression model, adjusting for age and sex, to calculate odds ratios (ORs) and 95% Wald confidence intervals (95% CIs) for the assessment of factors associated with hospitalization. Logistic regression models to assess multimorbidity’s association with hospitalization were fitted using the Centers for Disease Control and Prevention’s list of risk factors with a high level of evidence that contribute to severe COVID-19, which include obesity, diabetes, chronic kidney disease, serious heart conditions, cancer, sickle cell anemia, and solid organ transplantation [16]. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc.). Figures were designed with the “ggplot2” package using R version 3.4.2.
RESULTS
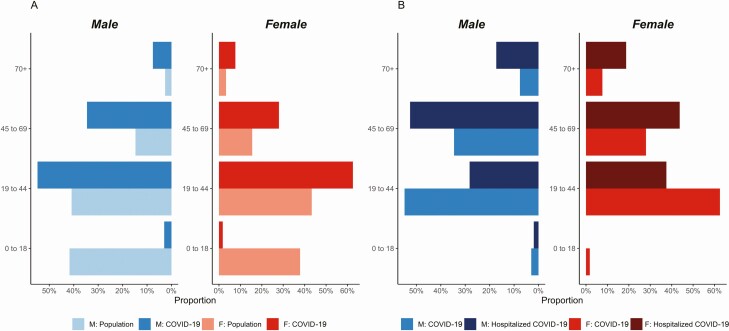
During 9 March to 4 May, 1934 individuals were tested for SARS-CoV-2 using RT-PCR in the Metropolitan Area of San Pedro Sula. Of these, 964 (49.8%) tested positive (Supplementry Figure S1). Out of the RT-PCR–confirmed COVID-19 cases, 877 (90.1%) had complete baseline information. Hospitalization was required for 25% (n = 220) of the cases. Male sex was predominant among cases (61.3%; n = 538; P < .01). Healthcare workers accounted for 10.7% (n = 96) of all COVID-19 cases (Table 1). Among both males and females, adults aged 19–44 years constituted the largest proportion of COVID-19 cases. The age distribution of COVID-19 cases roughly followed the population age distribution of the San Pedro Sula Metropolitan Area, except for in children aged 0–18 (Figure 1A).
Table 1.
Baseline Epidemiological and Clinical Characteristics
| Total, n = 877 | Nonhospitalized, n = 657 (%) | Hospitalized, n = 220 (%) | P c |
|---|---|---|---|
| Age groups, years | … | … | <.01 |
| 0–18 | 19 (2.9) | 3 (1.4) | |
| 19–44 | 439 (66.8) | 68 (30.9) | |
| 45–69 | 171 (26.0) | 110 (50.0) | |
| ≥70 | 28 (4.3) | 39 (17.7) | |
| Male sex | 382 (58.1) | 156 (70.9) | <.01 |
| Symptoms at baseline | |||
| Fever | 455 (69.3) | 186 (84.6) | <.01 |
| Cough | 454 (69.1) | 186 (84.6) | <.01 |
| Headache | 339 (51.6) | 109 (49.6) | .64 |
| Myalgia | 308 (46.9) | 100 (45.5) | .76 |
| Rhinorrhea | 244 (37.1) | 73 (33.2) | .33 |
| Dysphagia | 232 (35.1) | 66 (30.0) | .16 |
| Malaise | 229 (34.9) | 119 (54.1) | <.01 |
| Dyspnea | 128 (19.5) | 153 (69.6) | <.01 |
| Nausea | 52 (7.9) | 24 (10.9) | .16 |
| Loss of smell | 46 (9.6) | 2 (1.2) | .02 |
| Diarrhea | 27 (5.6) | 10 (5.7) | .86 |
| Loss of taste | 24 (4.9) | 1 (.6) | .01 |
| Adenopathy | 11 (1.7) | 4 (1.8) | .88 |
| Intercostal retraction | 10 (1.5) | 50 (23.6) | <.01 |
| Cyanosis | 4 (.6) | 20 (9.1) | <.01 |
| Symptoms duration at baseline, days [IQR] | 5 [3-8] | 5.5 [3-8] | .41 |
| Case type | … | … | .45 |
| Local | 648 (98.6) | 216 (98.2) | |
| Imported | 9 (1.4) | 4 (1.8) | |
| Comorbiditiesa | |||
| Hypertension | 67 (10.2) | 59 (26.8) | <.01 |
| Diabetes mellitus | 59 (8.9) | 67 (30.5) | <.01 |
| Obesity | 28 (4.3) | 28 (12.7) | <.01 |
| Coronary heath disease | 11 (1.7) | 23 (10.5) | <.01 |
| Asthma | 20 (3.0) | 11 (5.0) | .29 |
| Chronic obstructive pulmonary disease | 4 (.6) | 13 (5.9) | <.01 |
| Cancer | 3 (.5) | 3 (1.4) | .17 |
| Pregnancyb | 9 (4.4) | 3 (11.1) | .10 |
| Healthcare workers | 78 (11.9) | 16 (7.3) | .06 |
Data are of RT-PCR–confirmed COVID-19 cases.
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; RT-PCR, reverse transcriptase polymerase chain reaction.
aComorbidities are ordered by prevalence.
bTotal number of women between 15–47 years old (n = 232).
cChi-square/Fisher’s exact/Wilcoxon rank-sum test, as needed.
Figure 1.
Distribution of COVID-19 cases and hospitalized cases. A, San Pedro Sula population distribution with distribution of COVID-19 cases. M: Population represents the proportion of males in the population bins (blue) and F: Population represents the proportion of females in the population (red). M: COVID-19 and F: COVID-19 represent the proportions of males and females with confirmed COVID-19 cases. B, Hospitalized COVID-19 proportion of cases compared to COVID-19 proportions of cases overall. Abbreviation: COVID-19, coronavirus disease 2019.
Clinical and Epidemiologic Features of COVID-19 Cases at Presentation
Fever (69.3%), cough (69.1%), and headache (51.6%) were the 3 most frequent clinical features at presentation among individuals who did not require hospitalization. Similarly, in individuals who required hospitalization, fever (84.6%), cough (84.6%), and shortness of breath (69.6%) were most frequent (Table 1). For both sexes, those aged 45–69 years accounted for the largest proportion of hospitalizations (females: 43.8%; males: 52.6%; Supplementary Table S1). Additionally, women patients aged 70 years and older had a similar proportion of cases requiring hospitalization when compared to men in the same age group (18.8% vs 17.3%, respectively; Figure 1B; Supplementary Table S2).
Out of the 877 cases, 10.9% (n = 96) reported no symptoms and are thus considered asymptomatic. For those cases who reported symptoms, the median time from symptom onset to testing was similar in ambulatory (5 days; interquartile range [IQR], 3–8) and hospitalized cases (5.5 days; IQR, 3–8).
The proportion of cases with at least 1 preexisting medical condition was 34.1% (n = 299). Diabetes and hypertension were the 2 most frequent preexisting conditions among both hospitalized and nonhospitalized cases (Table 1). Overall, the median age of cases with at least 1 preexisting medical condition was 52 years (IQR, 41–64 years). Conversely, for those cases with no history of a preexisting condition, the median age was 34 years (IQR, 27–45 years).
Factors Associated With Hospitalization
Hospitalization was required for 25% (n = 220 of 877) of the cases. Individuals aged 70 or older and individuals aged 45–69 years were more likely to be hospitalized than individuals aged 19–44, after adjusting for sex (OR, 9.12 [95% CI, 5.24–15.86] and OR, 4.05 [95% CI, 2.85–5.76], respectively). Moreover, male sex was associated with hospitalization when adjusting for age (OR, 1.72; 95% CI, 1.21–2.44). After adjusting for age and sex, having at least 1 preexisting medical condition was associated with hospitalization (OR, 2.12; 95% CI, 1.43–3.14), and having 2 or more comorbidities further increased the odds of hospitalization (OR, 6.23; 95% CI, 2.99–12.94). In models adjusted for age and sex, the preexisting medical conditions that were associated with hospitalization were diabetes mellitus (OR, 2.55; 95% CI, 1.66–3.92), obesity (OR, 4.51; 95% CI, 2.47–8.22), coronary heart disease (OR, 4.23; 95% CI, 1.91–9.03), chronic obstructive pulmonary disease (OR, 5.17; 95% CI, 1.54–17.27), asthma (OR, 2.78; 95% CI, 1.24–6.26), and hypertension (OR, 1.83; 95% CI, 1.18–2.83; Table 2).
Table 2.
Logistic Regression Analysis, Hospitalization
| Unadjusted OR, (95% CI) | Adjusted ORa (95% CI) | P Value b | |
|---|---|---|---|
| Age groups, years | |||
| 0–18 | 1.02 (.29–3.54) | .95 (.27–3.30) | .04 |
| 19–44 | Reference | Reference | |
| 45–69 | 4.15 (2.93–5.89) | 4.05 (2.85–5.76) | .01 |
| 70+ | 8.99 (5.19–15.56) | 9.12 (5.24–15.86) | <.01 |
| Male sex | 1.76 (1.26–2.44) | 1.72 (1.21–2.44) | .01 |
| Symptoms duration at baseline, per additional day | 1.01 (.98–1.05) | .99 (.96–1.04) | .93 |
| Comorbiditiesc | |||
| Hypertension | 3.23 (2.18–4.77) | 1.83 (1.18–2.83) | .01 |
| Diabetes mellitus | 4.44 (2.99–6.57) | 2.55 (1.66–3.92) | <.01 |
| Obesity | 3.28 (1.89–5.67) | 4.51 (2.47–8.22) | <.01 |
| Coronary heart disease | 6.85 (3.28–14.31) | 4.23 (1.91–9.34) | .01 |
| Asthma | 1.68 (.79–3.56) | 2.78 (1.24–6.26) | .01 |
| Chronic obstructive pulmonary disease | 10.25 (3.30–31.78) | 5.17 (1.54–17.27) | .01 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aModels adjusted for age and sex.
bWald chi-square test.
cDefined as currently having a diagnosis of a preexisting medical condition at baseline.
Hospitalized Cases Characteristics and Outcomes
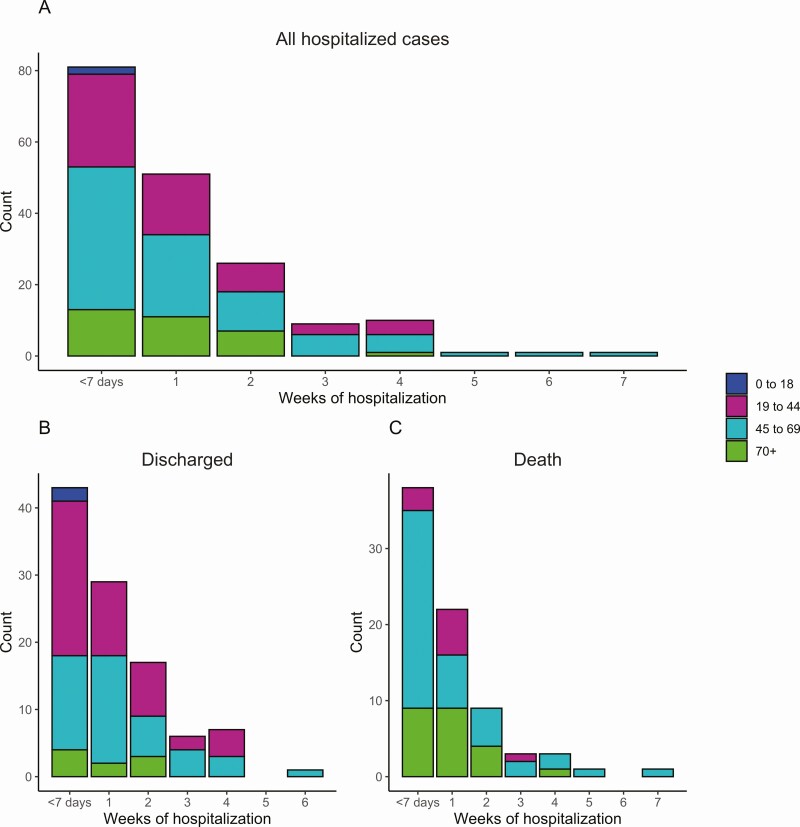
Out of the 220 hospitalized cases, medical records from 180 inpatients (81.8%) were available, and these were included in subsequent analyses. Male sex was predominant (71.1%; n = 128). The majority of inpatients received a diagnosis of pneumonia (83.7%; n = 144). Half (50.0%; n = 99 of 180) of the cases were hospitalized for more than 7 days (Figure 2A; Supplementary Table S3). In cases aged 19–44 years, the median length of hospitalization was 7.5 days (IQR, 3–14). For individuals aged 45–69 and 70 years or older, the median hospitalization lengths were 8 (IQR, 3–15.5) and 7 (IQR, 3–13) days, respectively. The median length of hospitalization among nonfatal cases was 8 days (IQR, 3–15). Furthermore, when assessing the length of hospitalization in nonfatal cases who spent more than 7 days at the hospital, those aged 19–44 years had a hospitalization median of 15 days (IQR, 9–19 days). Individuals aged 45–69 years spent a median of 11.5 days (IQR, 8–21 days) hospitalized, and those aged 70 years or older spent a median of 15 days (IQR, 11–15 days). Interestingly, the proportion of all cases that required extended hospitalizations of 4 weeks or longer was higher (12.5%) in adults aged 45–69 than in adults aged 19–44 years or 70+ years (8.6% and 3.1%, respectively; P = .20; Figure 2A; Supplementary Table S3). This trend was similar among individuals who were discharged (Figure 2B; Supplementary Table S4) and cases that died at the hospital (Figure 2C).
Figure 2.
Length of hospitalization by age and outcome. A, Length of stay in weeks for all cases by age group. Cases with less than 1 week of hospital stay were categorized as <7 days. B, Length of hospitalization among cases who were discharged by age group. C, Length of hospitalization among cases who died by age group.
Either invasive or noninvasive mechanical ventilation was required in 21% (n = 38) of individuals. The median age of those who did not receive mechanical ventilation was 53 years (IQR, 39–62), and the median was also 53 years (IQR, 41–65) for cases who were under any type of mechanical ventilation. Hospitalized cases who received mechanical ventilation had a longer median duration of hospitalization (14.5 days; IQR, 8–21) than those who did not require mechanical ventilation (6 days; IQR, 2–11; P < .01).
A total of 77 (42.8%) of the hospitalized cases died. Of the deaths, 72% were among men (n = 55 of 77; P = .93), and individuals aged 45–69 years old accounted for 57.1% of the deaths (n = 44 of 77; Supplementary Table S5). Interestingly, 42.8% (n = 33) of deaths among all hospitalized cases were among individuals aged less than 60 years old. Further, the median age of fatal COVID-19 cases was 62 years (IQR, 52–71). When compared to the median age of hospitalized cases overall (53 years; IQR, 40–65), age was significantly higher (P < .01) among fatal cases. Those cases not under mechanical ventilation accounted for 81.6% (n = 31 of 38) of the fatalities during the first week of hospitalization and 64.9% (n = 50 of 77) of the overall fatalities. Hospitalized cases who received any type of mechanical ventilation were more likely to have fatal outcomes (P < .01; Table 3).
Table 3.
Hospitalized Confirmed Coronavirus Disease 2019 Cases
| Total, n = 180 | No Mechanical Ventilation n = 142 | In Mechanical Ventilation n = 38 | P Valuea |
|---|---|---|---|
| Age in yrs, median [IQR] | 53 [39–65] | 53 [41–65] | .80 |
| Male sex | 96 (67.6) | 32 (84.2) | .04 |
| Symptoms at baseline | |||
| Cough | 123 (86.6) | 29 (76.3) | .13 |
| Fever | 119 (83.9) | 34 (89.5) | .45 |
| Shortness of breath | 100 (70.4) | 30 (78.9) | .41 |
| Malaise | 78 (54.9) | 20 (52.6) | .86 |
| Headache | 69 (48.6) | 21 (55.3) | .58 |
| Myalgia | 61 (42.9) | 20 (52.6) | .35 |
| Dysphagia | 50 (35.2) | 10 (26.3) | .33 |
| Rhinorrhea | 48 (33.8) | 12 (31.6) | .84 |
| Retraction | 28 (19.7) | 16 (42.1) | <.01 |
| Symptom duration at baseline, days [IQR] | 5.0 [3–7] | 5.0 [3–8] | .80 |
| Hospitalization duration, median days [IQR] | 6.0 [2–11] | 14.5 [8–21] | <.01 |
| Comorbidities | |||
| Diabetes | 49 (34.5) | 8 (21.1) | .12 |
| Hypertension | 36 (25.4) | 13 (34.2) | .31 |
| Obesity | 18 (12.7) | 6 (15.8) | .59 |
| Coronary heath disease | 16 (11.3) | 4 (10.5) | .89 |
| Chronic obstructive pulmonary disease | 9 (6.3) | 3 (7.9) | .71 |
| Asthma | 7 (4.9) | 3 (7.8) | .44 |
| Pneumonia | 106 (79.1) | 38 (100.0) | <.01 |
| Healthcare worker | 11 (7.8) | 2 (5.3) | .59 |
| Death | 50 (35.2) | 27 (71.1) | <.01 |
Values are shown as n (%) unless otherwise noted.
Abbreviations: IQR, interquartile range.
aChi-square/Fisher’s exact/Wilcoxon rank-sum test, as appropriate.
Fatal cases had a median hospitalization time of 7 days (IQR, 2–12), with 49% (n = 38) of the fatalities occurring in the first 7 days of hospitalization. Further, 42.1% (n = 16 of 38) of these cases died within 24 hours of hospital admission. For those fatal cases who spent more than 7 days at the hospital, the median lengths of hospitalization were 8 days (IQR, 8–13), 16 days (IQR, 9–21), and 11.5 days (IQR, 7–14) among age groups 19–44, 45–69, and 70 years or older, respectively.
DISCUSSION
Few data exist on the epidemiology of COVID-19 in LAC. Previous case series have addressed the course of this disease and its outcomes [4, 5, 17, 18] outside LAC. Our findings show that a high proportion of COVID-19 cases in Honduras are males and are aged 19–44 years old. Although the majority of cases were young, the prevalence of preexisting medical conditions affected outcomes, and severe cases of COVID-19 were frequent.
Our findings were similar to other reported case series in several areas. First, the Honduran cases reported in this study showed a high proportion of males infected with SARS-CoV-2, which was similar not only to a Bolivian case series [8], but to other series reported in the United States [5, 18], China [4, 19–22], and Italy [6]. Further, the clinical course of COVID-19 in Honduran cases was not different when compared to case series worldwide, in which fever, cough, and shortness of breath are prevalent [4, 5]. Cases that required mechanical ventilation had a significantly higher proportion of fatalities in our study, which is similar to previous described reports [6, 23].
After adjusting for age and sex, hospitalization was associated with diabetes, obesity, coronary heart disease, chronic obstructive pulmonary disease, asthma, and hypertension in Honduran COVID-19 cases. Obesity and diabetes particularly have been highly associated with developing severe disease in COVID-19 cases [23, 24], and thus our findings are consistent with what has been previously reported. Further, our findings show that multimorbidity was strongly associated with hospitalization and severe COVID-19 among the Honduran population.
In contrast to other settings, Honduran COVID-19 cases were more frequent among the age group between 19–44 years old; however, this distribution of cases roughly followed the population age distribution of the Metropolitan Area of San Pedro Sula [12], and also the country population’s age distribution [25]. When comparing the Honduras to LAC [26], the population age distributions are similar, suggesting that similar patterns may be seen in other LAC countries. This younger population distribution may explain why COVID-19 cases are more frequent in younger individuals in Honduras and why our findings differ from the previously described reports outside LAC [4–6, 18, 27], where older adults constitute a higher proportion of the population [11].
Unlike the vast majority of previous research, where severe cases and those admitted to intensive care units had preexisting conditions at older ages [5, 6, 23, 28], Honduran COVID-19 cases had a high prevalence of preexisting medical conditions at younger ages. This supports a public health concern raised by the Pan-American Health Organization, which recently stated that 3 out of every 10 people living in the region of the Americas had at least 1 preexisting medical condition, and the populations are therefore at a higher risk of severe COVID-19 [29].
While length of stay at the hospital has previously been reported to have a median of 7 days if intensive care unit treatment was not required [23], Honduran cases had longer hospitalization durations, even in younger adults. Indeed, while the median time of hospitalization for all adult age groups in our study was 7–8 days, adult nonfatal cases aged 19–44 who were hospitalized for over a week spent a median of 15 days hospitalized, creating a substantial burden on the healthcare system. The proportion of fatal cases associated with COVID-19 appears to be higher among individuals at older ages [6], potentially due to their higher risk of having a preexisting condition and older age [23]. Importantly, we found that in Honduras, nearly 50% of the fatal cases were among those aged less than 60 years old who also had a high prevalence of preexisting conditions.
A major strength of this study relies on the inclusion of cases that did not require hospitalization. Unlike most of the research undertaken to describe the epidemiology of COVID-19, this study assessed the severity and outcomes of COVID-19 in both hospitalized and nonhospitalized cases, therefore giving a broader perspective of the disease. Due to the population age distribution in this study, it was possible to examine the severe outcomes of COVID-19 in a younger population. This becomes crucial from a public health perspective when determining interventions in low- and middle-income countries, in which resources are limited and the extrapolation of data from other countries could bias their allocation.
Our research has certain limitations. First, this report was drafted during an ongoing outbreak; thus, we do not have outcome data on all hospitalized cases. Second, over the time period covered, the testing criteria changed and resources sometimes limited testing capacity. Third, our sample size is limited, and thus may not have enough power to measure the effect of all comorbidities in outcomes like hospitalization/death. Further, we have no information regarding the severity of preexisting conditions, which could potentially limit our assessment of the association between comorbidities and hospitalization. Lastly, we were not able to abstract data from inpatients that included the therapy received for the disease, which could have been used to assess the impacts of therapy on the course of disease, severity, and mortality.
In this study, we describe the epidemiology of COVID-19 from a large number of cases in a Latin American country. Our work shows that COVID-19 cases are frequent among a young population in Honduras, with fatalities also being frequent in younger individuals. Urgent public health interventions are needed to prevent hospitalizations, severe disease, and deaths not only for older individuals, but also for younger individuals in LAC countries with similar population distributions and comorbidity prevalences.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Drs Abril Dominguez, Cesia Rivera, Maria F. Diaz, Polette Rivera, Kevin Euceda, Andrea Caraccioli, Allan Zelaya, and Roberto Chacon for providing hospital outcome data; Dr Hannah Maier for assisting with figure creation; and Dr Lionel Gresh for his assistance with manuscript editing.
Financial support. This work was supported by the University of Michigan Office of Global Public Health, Gelman Global Scholars, and by the National Institute for Allergy and Infectious Disease (contract number HHSN272201400006C).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 21 July 2020.
- 2. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. 2020. Available at: https://covid19.who.int. Accessed 23 June 2020.
- 3. World Health Organization. Coronavirus disease (COVID-19) pandemic. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 21 July 2020.
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 10022:E1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez-Morales A, Gallego V, Escalera-Antezana J, et al. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis 2020; 35:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Escalera-Antezana JP, Lizon-ferrufino NF, Maldonado-Alanoca A, et al. Clinical features of the first cases and a cluster of coronavirus disease 2019 (COVID-19) in Bolivia imported from Italy and Spain. Travel Med Infect Dis 2020; 35:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zambrano L, Fuentes-Barahona I, Bejarano-Torres D, et al. A pregnant woman with COVID-19 in Central America. Travel Med Infect Dis 2020; 1–2. doi: 10.1016/j.tmaid.2020.1016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sistema Nacional de Gestion de Riesgos. Coronavirus COVID-19 en Honduras.2020. Available at: https://covid19honduras.org. Accessed 23 June 2020.
- 11. United Nations. World population prospects 2019. 2019. Available at: https://population.un.org/wpp/DataQuery/. Accessed 26 July 2020.
- 12. Instituto Nacional de Estadistica. San Pedro Sula, Cortes: informacion general /2018. Available at: https://www.ine.gob.hn/V3/imag-doc/2019/08/San-Pedro-Sula-Cortes.pdf. Accessed 23 June 2020.
- 13. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020; 25:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan-American Health Organization. Laboratory guidelines for the detection and diagnosis of COVID-19 virus infection sample collection and proper shipment laboratory testing. 2020. Available at: https://iris.paho.org/bitstream/handle/10665.2/52140/PAHOPHEIMSCOVID-19200003_eng.pdf?sequence=1&isAllowed=y. Accessed 22 June 2020.
- 15. Secretaria de Salud Honduras. Lineamientos para la vigilancia epidemiológica, manejo, control y prevención de COVID-19. Tegucigalpa, Honduras: Secretaria de Salud Honduras, 2020. [Google Scholar]
- 16. Centers for Disease Control and Prevention. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html. Accessed 31 July 2020. [PubMed]
- 17. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA 2020; 323:2195–8. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020; 55: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA 2020; 3:e205619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020; 71:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 2020; 369:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab 2020; 105:2752–61. [Preprint]. doi: 10.1210/clinem/dgaa346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan American Health Organization. Country report: Honduras. 2015. Available at: https://www.paho.org/salud-en-las-americas-2017/?page_id=133. Accessed 25 July 2020.
- 26. Pan American Health Organization. Population distribution by age and sex. 2015. Available at: https://www.paho.org/salud-en-las-americas-2017/?p=1864. Accessed 25 July 2020.
- 27. Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis 2020; 20:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lian J, Jin X, Hao S, et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis 2020; 71:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan American Health Organization. Three out of 10 people in the Americas are at increased risk of severe COVID-19 because of underlying conditions, PAHO Director says. 2020. Available at: https://www.paho.org/en/news/21-7-2020-three-out-10-people-americas-are-increased-risk-severe-covid-19-because-underlying. Accessed 24 July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.